Abstract
Tissue engineering provides a promising approach to treat degenerative disc disease, which usually requires a large quantity of seed cells. A simple and reliable in vitro culture system to expand seed cells in a timely fashion is necessary to implement the application clinically. Here, we sought to establish a cost-effective culture system for expanding human annulus fibrosus (AF) cells using extracellular matrix (ECM) proteins as culture substrates. Cells were cultured onto a plastic surface coated with various types of ECMs, including fibronectin, vitronectin, collagen type I, gelatin, and cell-free matrix deposited by human nucleus pulposus (NP) cells. AF cell morphology, growth, adhesion, and phenotype (anabolic and catabolic markers) were assessed by microscopy, real-time RT-PCR, western blotting, zymography, immunofluorescence staining and biochemical assays. Fibronectin, collagen and gelatin promoted cell proliferation and adhesion in a dose-dependent manner. Fibronectin elevated mRNA expression of proteoglycan and enhanced glycosaminoglycan (GAG) production. Both collagen and gelatin increased protein expression of type II collagen. Consistent with increased cell adhesion, collagen and fibronectin promoted formation of focal adhesion complexes in the cell-matrix junction, suggesting enhanced binding of actin network with both ECM substrates. On the other hand, fibronectin, collagen and gelatin decreased expression of matrix metalloproteinase-2, and matrix metalloproteinase-9 in media. Finally, a mixture of fibronectin (1.7 μg/mL) and collagen (1.3 μg/mL) was identified as the most promising in vitro culture substrate system in promoting proliferation and maintaining anabolic-catabolic balance. Our method provides a simple and cost-effective platform for tissue engineering applications in intervertebral disc research.
Keywords: intervertebral disc degeneration, extracellular matrix, cell proliferation, in vitro culture, tissue engineering
Introduction
Intervertebral disc (IVD) degeneration has a lifetime prevalence of 70% to 85% and contributes predominantly to low back pain, which constitutes a leading source of disability among those under 45 years old (Andersson 1999). By nature, the IVD is a connective tissue adjoining two vertebrae that provides cushion for various motions of the spine. The IVD is composed of a gelatinous nucleus pulposus (NP) in the center, a lamella fibrocartilage annulus fibrosus (AF) in the surroundings, and cartilage endplates connecting IVD to vertebral bodies. IVD degeneration is a chronic process of ECM degradation and destruction. Emerging evidence has suggested a strong connection between ECM and disc integrity. Patients with degenerative disc disease have been found with dysregulated elastic fiber system of the ECM, disoriented or ruptured disc structure, NP collapse, reduced disc height, and dramatically decreased ECM content (Loreto et al. 2011). Additionally, ECM breakdown fragments and microcrystals may trigger the inflammatory response associated with IVD degeneration and low back pain. As a result, incorporating appropriate ECM protein substrates into cell culture is a practical approach to preserve native IVD cell phenotype for downstream applications such as tissue engineering.
In the last decade, tissue engineering has proved to be a promising solution for replacing structure and restoring function of degenerated IVD (Yang et al. 2009; Park et al. 2012; Jin et al. 2013). However, such approaches usually require a large number of seed cells, and thus the slow proliferation rate and consequential phenotypic alternation are key limiting factors (Gruber et al. 1997). Even in the presence of high nutritional supplementation (20% FBS) human disc cells still grow relatively slowly, generally requiring about 1 month for P1 cultures to proliferate (Hanley and Gruber 2008). To counter this, researchers have developed various techniques that fall into either a two dimensional (2D) or three dimensional (3D) culture system. It is understood that 2D cultures generally yield greater numbers of cells, but phenotypic stability is compromised (Gruber et al. 2000). By contrast, 3D culture systems may favor disc matrix production and phenotype maintenance by incorporating various scaffolds such as collagen sponge, fibrin gel, agarose, and alginate (Yang et al. 2009; Park et al. 2012; Jin et al. 2014). However, 3D culture procedures may be time consuming, technically demanding, and costly. For instance, it may take up to six weeks to obtain sufficient porcine disc cells when cultured on a biphasic silk composite scaffold (Park et al. 2012). For this reason, a simple, reliable, and cost-effective method would be highly desirable for fast expansion of human IVD cells (Jin et al. 2013).
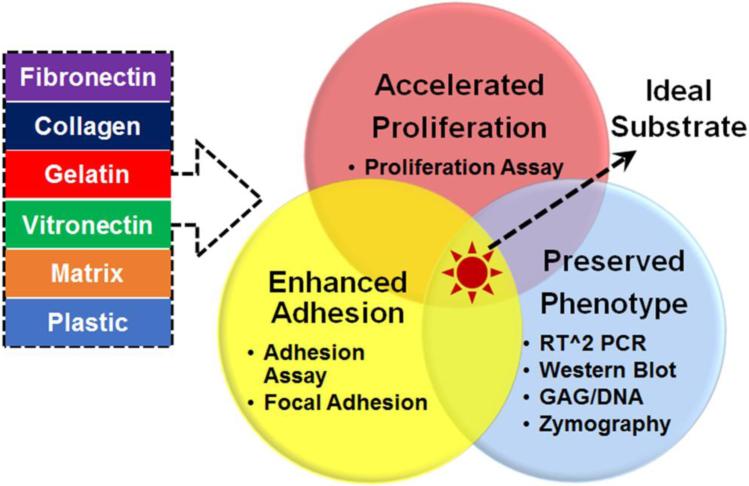
In the current study, we sought to screen selected ECM proteins as in vitro culture substrates on which human AF cells were cultured and expanded with preserved phenotype. As shown in Fig. 1, fibronectin, collagen type I, gelatin, vitronectin, and human NP cell deposited matrix (referred to as “matrix”) were implemented as cell culture substrates and their effects in regulating cell proliferation, adhesion, and phenotype (anabolic vs. catabolic activities) were sequentially evaluated.
Figure 1.
Experimental design for optimized human AF cell culture system. Five ECM substrates were screened including fibronectin, collagen, gelatin, vitronectin, human NP cell deposited matrix (untreated plastic surface as control). Time- and dose-dependent proliferation assays, adhesion assay, real-time RT-PCR, GAG and DNA assays, gelatinolytic zymography and western blot were performed to evaluate ECM effects on cell proliferation, adhesion, anabolic and catabolic activities. Expression and location of focal adhesion protein were assessed using immunofluorescence staining.
Materials and Methods
Chemicals and reagents
Triton X-100, L-ascorbic acid phosphate, papain, chondroitin sulfate-C, Hoechst dye, gelatin (type I), deoxyribonucleic acid (DNA) from calf thymus, bovine serum albumin (BSA), fibronectin, and vitronectin, ammonium hydroxide, anti-vinculin monoclonal antibody and monoclonal anti-β-actin antibody were purchased from Sigma-Aldrich (St. Louis, MO). PureCol Collagen (97% Type I, 3% Type III) was purchased from Advanced Biomatrix (Carlsbad, CA). Cell culture supplies, such as Dulbecco's Modified Eagle Medium Nutrient Mixture F-12 (DMEM/F12), Dulbecco's Modified Eagle Medium (high glucose 4.5 g/L), fetal bovine serum (FBS), Dulbecco's phosphate-buffered saline (DPBS) and penicillin-streptomycin were purchased from Gibco Invitrogen (Carlsbad, CA). Trizol Reagent, Alexa Fluo594 Goat-anti-mouse IgG (H+L) and Alexa Fluor 488 phalloidin were purchased from Life Technologies (Grand Island, NY). Biotinylated anti-collagen II antibody was purchased from Abcam (Cambridge, MA).
Human intervertebral disc cell isolation and culture
All procedures involving human materials were approved by the Institutional Review Board for Health Science Research (IRB-HSR) at the University of Virginia. Human intervertebral disc samples were obtained from three individuals who had undergone discectomy for surgical management of scoliosis at the University of Virginia Hospital, following guidelines of the U.S. National Institutes of Health Office of Human Subjects Research for use of surgical waste. The NP and AF were dissected and the cells were isolated by a collagenase/trypsin digestion procedure previously described by our group (Jin et al. 2014). Both human AF and NP cells were cultured in complete growth medium DMEM/F12 containing 10% FBS and 1% penicillin-streptomycin at 37°C in a humidified incubator with 5% CO2. This complete growth medium was used throughout following experiments unless specifically mentioned. Culture media were changed every other day. Cells at passages 2–6 were used in the current study.
Preparation of decellularized ECM deposited by human NP cells
Extracellular matrix proteins deposited by human NP cells were obtained following published procedures (Pei et al. 2011) with slight modification. In brief, human NP cells were cultured in complete growth medium until 80% confluence. L-ascorbic acid phosphate (50 μg/mL) was supplemented in media with additional culture for 9 days. Cell-free extracellular matrix was then obtained by incubating the cells with 0.5% Triton X-100 (3 mL) containing 20 mM ammonium hydroxide in DPBS for 30 min at 37°C. Supernatant was collected, centrifuged at 2000 rpm for 8 min, and stored at −70°C until use. Total protein concentration (0.31 mg/mL) of harvested NP deposited ECM mixture (referred to as “matrix” in the following text) was determined with Detergent-compatible (Dc) protein assay kit (Bio-Rad, Hercules, CA) according to the manufacturer's instruction.
Dose- and time-dependent proliferation assays
For dose-dependent proliferation assay, fibronectin, collagen, gelatin and vitronectin solutions were added at serial concentrations of 0.1, 1, 2, 5, and 10 μg/mL. ECM deposited by human NP cells was added at various dilutions of the original mixture (1:50, 1:20, 1:10, 1:5, 1:2 v/v diluted). Plastic wells were used as controls. Plates were coated with ECM protein solutions and stored at 4°C overnight, washed with ice-cold DPBS (×3) to remove the immobilized protein, and air-dried for 15 min on ice. Subsequently, human AF cells were seeded in 96-well plate at a density of 6,000 cells per well and cultured for 72 hrs. Cell growth was examined microscopically at 24 hrs, 48 hrs and 72 hrs post incubation. At the end of 3 days incubation, cell proliferation was determined using CellTiter Aqueous Non-Radioactive Cell Proliferation Assay Kit (Promega, Madison, WI) following manufacture's instruction. OD490 was measured using VERSA max microplate reader (Molecular Devices, Sunnyvale, CA) and plotted against the ECM concentration with Origin software. Optimal dose for each ECM was defined as the lowest effective concentration in promoting significant amount of cell growth within 72 hrs.
For time-dependent proliferation assay, 96-well plates were coated with ECM protein (150 μL/well) at their optimized doses. Human AF cells were seeded and cultured for various time (24 hrs, 48 hrs, 96 hrs, and 6 days). MTS assay was performed to evaluate cell growth at each time point. OD490 was measured, plotted against incubation time with Origin software to determine the optimal incubation time.
Adhesion assay
Adhesion assay was performed following previously published protocols (13). 96-well plates were coated with ECM protein (150 μL/well) at their optimized doses. Human AF cells were seeded in at a density of 5×104 per well in 100 μL serum-free DMEM. BSA-treated (0.5% in DPBS) and non-treated plastic wells served as controls. The plate was incubated at 37°C in CO2 incubator for 1 hr to allow cell adhesion. Medium was discarded by inverting and gently tapping the plate. Non-adhesive cells were washed off by warm DMEM for three times. Gross cell morphology was examined under microscope before and after medium washing. Cells were then fixed with 4% paraformaldehyde (PFA) for 15 min, stained with Genian Violet (1% Alcoholic, LabChem, Inc, Pittsburg, PA) for 10 min, washed with distilled water, and air-dried for 15 min. Stained cells were counted by Image J software, which was then plotted against ECM concentration to delineate their effects in promoting AF cell adhesion.
Immunofluorescence staining
For immunofluorescence staining, glass bottom culture dishes (MatTek Corporation, Ashland, MA) were coated with ECM solutions at optimized concentrations at 4°C overnight, washed with cold DPBS and air-dried for 15 min on ice. Human AF cells were seeded at a density of 5×104 cells, and incubated at 37°C for 4 days. Cells were fixed with 4% PFA for 30 min, permeablized with 0.3% Triton X-100 for 5 min, and blocked with 3% BSA for 1 hr at room temperature. Cells were incubated with anti-vinculin Monoclonal Antibody (1:1000 dilutions) at 4°C overnight, visualized with Alexa Fluo594 Goat-anti-mouse IgG (1:1000 dilutions). Actin was stained with Alexa Fluo 488-phalloidin (1:5000) for 30 min. Following incubation, the cover glasses were mounted onto slides using Aqua Poly/Mount Mounting Medium (Polyscience, Inc., Warrington, PA). IgG was used as a negative control. Fluorescence images were taken with Olympus FV300 confocal laser scanning microscope (Olympus Corporation, Center Valley, NJ).
Quantification of vinculin-positive focal adhesions
Focal adhesion structures can be classified into focal complexes (FC), focal adhesion (FA) and fibrillar adhesions (FBA) based on their size and subcellular location (Diener et al. 2005; Biggs et al. 2007). The small FC (<2 μm) are primary cell-ECM adhesion structures at the cell periphery, which either disassemble rapidly or mature into larger FAs (2-5 μm) at the cell peripheral or more centrally. And the centrally located FA then evolve into FBAs (> 5 μm). In our study, axial lengths of all visible adhesion structures were measured using ImageJ and categorized as followed: FC <2 μm at the cell periphery; FA 2-5 μm at both periphery and cytosolic compartments; FBA >5 μm in the cytosol. For each substrate condition, around 15-20 cells were assessed.
Real-time reverse transcription polymerase chain reaction
Human AF cells were seeded onto 6-well plates (~1×105 per well) coated with ECM substrates at their optimized concentrations and cultured in growth medium for 4 days. RNA was isolated with Trizol Reagent, and cDNA were synthesized with iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) following the manufacture's instruction. Real-time reverse transcription polymerase chain reaction (RT-PCR) was performed with RT^2 SYBR Green Fluo FAST Mastermix (Qiagen Sciences, Germantown, MA) using an iQ5 multicolor real-time PCR Detection System (Bio-Rad Laboratories, Hercules, California). The mRNA expression of target genes (aggrecan, type I and type II collagens) were analyzed and normalized to 18S. The same primer sequences were used as our prior studies (Yang et al. 2011).
Glycosaminoglycan (GAG) and DNA assays
Human AF cells were cultured at the same density as RT-PCR experiments for 4 days, and digested with papain extraction buffer (125 μg/mL in 1×PBE buffer, pH 6.5) at 60 °C overnight. Amino sugars, as a measure of glycosaminoglycan (GAG), were determined using a dimethylmethylene blue (DMMB) (Crescent Chemicals Corp., Islandia, NY) colorimetric assay with Chondroitin Sulfate-C as standard. DNA content was analyzed using the Hoechst dye with a calf thymus DNA standard (Jin et al. 2013).
Western blotting
Human AF cells were cultured at the same density as RT-PCR experiments for 4 days, and lysed with RIPA buffer. Equal amounts of protein were subjected to SDS-PAGE and transferred to a nitrocellulose membrane (Yeh et al. 2016). After incubation with Odyssey Blocking Buffer (LI-COR, Lincoln, NE) for 1 hr, the membranes were incubated with biotinylated anti-collagen II (1:1000) or anti-β-actin (1:2000) antibodies at 4 °C overnight, followed by incubation with a 1:2000 dilution of streptavidin-conjugated Alexa Fluor 680 (ThermoFisher Scientific) and goat-anti-mouse Alexa Fluor 680 for 1 hr, respectively. Membranes were scanned and analyzed with Odyssey Infrared Imaging System (LI-COR Biosciences).
Gelatinolytic zymography
Total protein concentration of culture media was determined by electrophoresis with Coomassie Blue staining. Gelatinolytic zymography was performed on 10% polyacrylamide resolving gels containing 1 mg/mL gelatin to evaluate enzymatic activities of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9). Gels were renatured, developed, stained with Coomassie Blue solution and de-stained following conventional protocols (Hu et al. 2010). Gels were scanned with a Chemidoc Imaging system (Bio-Rad) and analyzed by ImageJ.
Statistics
Human AF cells obtained from three individuals were used in the current study.Experimental data were presented as mean ± standard error of the mean (SEM). A two-tailed Student's t-test was performed between any given ECM substrate and plastic control with Origin Software. A p value less than 0.05 was considered statistically significant.
Results
Multiple ECM substrates promoted human AF cell proliferation in dose- and time-dependent manners
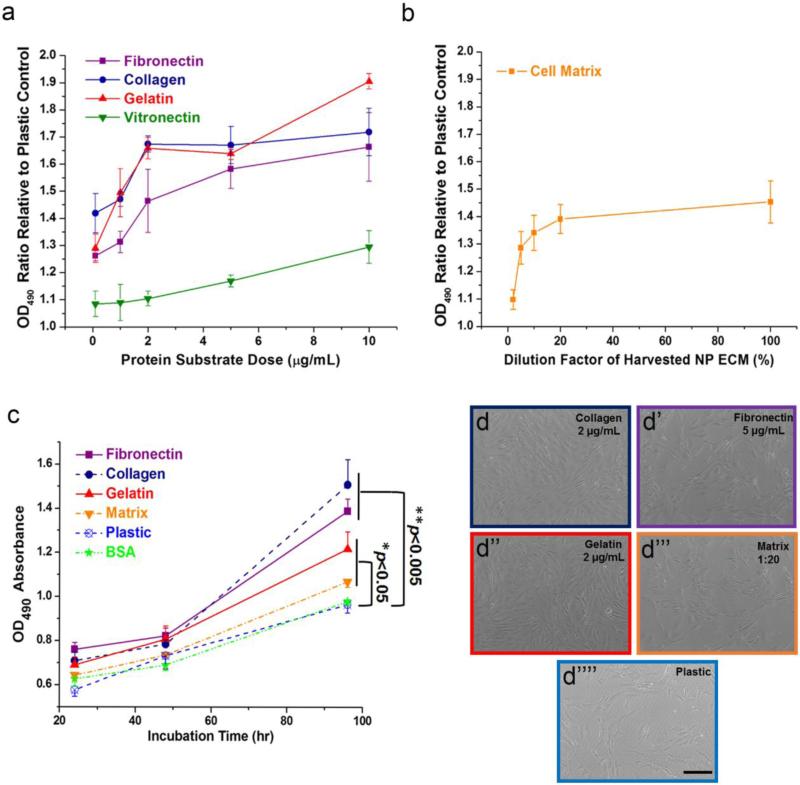
As shown in Fig. 2a, cells cultured on collagen and gelatin coated surface achieved a peak growth (~1.7 fold vs plastic control) at a low dose of 2 μg/mL and remained as a plateau thereafter. At a dose of 5 μg/mL, fibronectin effectively increased cell proliferation by 50%. The human NP deposited matrix slightly promoted AF cell proliferation by around 30% at a dilution factor 1:20 of the original solution (0.31 mg/mL as original concentration) (Fig. 2b). The dilution factor in Fig. 2b described the percentage concentration of the originally harvested NP ECM (larger value on x-axis corresponded to more concentrated solution). Vitronectin exhibited only a moderate proliferative effect which was not as promising as other substrates and thus was excluded from later experiments. As shown in Fig. 2c and Fig. 2d-d’’’, all ECM significantly promoted human AF cell growth (**p<0.005 for fibronectin and collagen, *p<0.05 for gelatin and matrix, compared to plastic and BSA groups) after culture for 4 days at their optimized doses. Data in Fig. 2c only showed measurements through 4 days as cells were already confluent by day 6.
Figure 2.
Dose- and time-dependent proliferation assay of human AF cells cultured on ECM substrates. (a) Fibronectin, collagen, gelatin and vitronectin promoted cell proliferation in a dose-dependent manner, however vitronectin has less proliferative effect compared to other ECM. (b) NP deposited extracellular matrix slightly enhanced cell proliferation in a dose-dependent manner. The dilution factor described the percentage concentration of the originally harvested NP ECM. (c) Various ECMs significantly improved cell growth rate in a time-dependent manner up to 4 days of culture with the most dramatic effect shown in fibronectin and collagen. (d-d’’’’) Microscopic images of cell population and morphology at the end of 4 days of culture on various ECM substrates. Note: *p<0.05 vs Plastic or BSA group; **p<0.005 vs. Plastic or BSA group. For (a) and (b), OD490 values of various ECM groups were normalized to those of plastic control. Scale bar represented 100 μm.
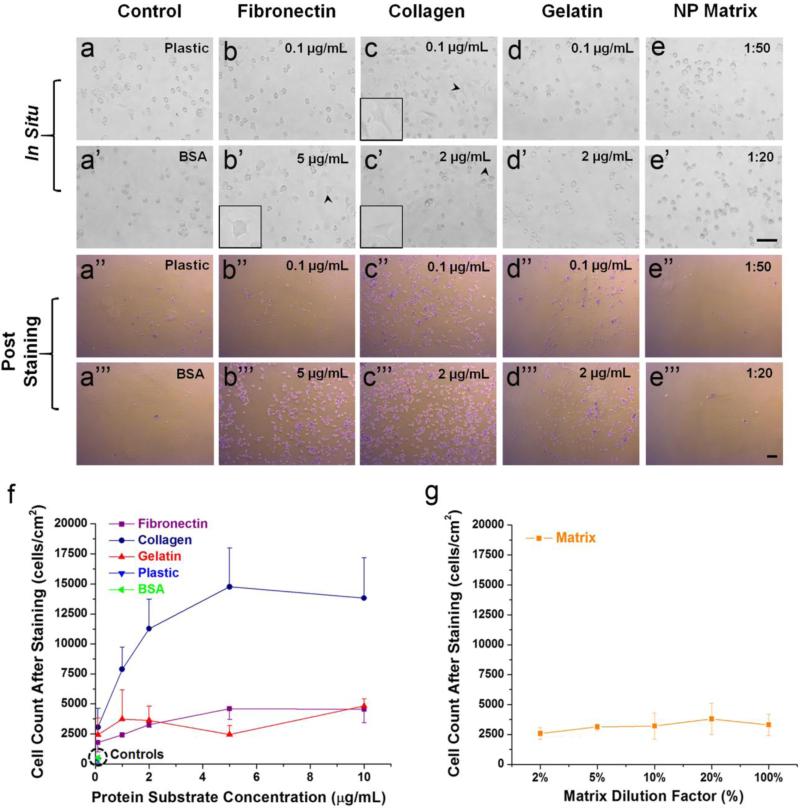
Fibronectin and collagen promoted human AF cell adhesion in a dose-dependent manner
As shown in Fig. 3, human AF cells started to adhere on collagen and fibronectin coated surfaces at doses of 0.1 μg/mL and 5 μg/mL as early as 30 min after incubation respectively, while gelatin only slightly increased cell adhesion and NP deposited matrix did not show any effect. The initial spreading of AF cells upon seeding onto fibronectin and collagen were demonstrated in the insets of Fig. 3b’, 3c, and 3c’.
Figure 3.
Dose-dependent adhesion assay of human AF cells cultured on ECM substrates. (a-e, a’-e’) In situ microscopic images of cells either undergoing adhesion (arrow head in fibronectin and collagen groups) or showing no adhesive effect (Plastic, BSA, Gelatin and Matrix groups) to corresponding ECM at the end of 30 min incubation. The initial spreading of AF cells upon seeding onto fibronectin and collagen were demonstrated in the insets images indicated by black arrow heads. The same scale bar (100 μm) was applied for a-e and a’-e’ images. (a’’-e’’, a’’’-e’’’) Microscopic images of stained adhering human AF cells after removing non-adhered cells. The same scale bar (100 μm) was applied for a’’-e’’ and a’’’-e’’’ images. (f) Collagen significantly improved cell-ECM adhesion in a dose-dependent manner while fibronectin and gelatin showed slight enhancement compared to Plastic and BSA controls. (g) Little concentration-dependent adhesion effect was observed in the Matrix coated group.
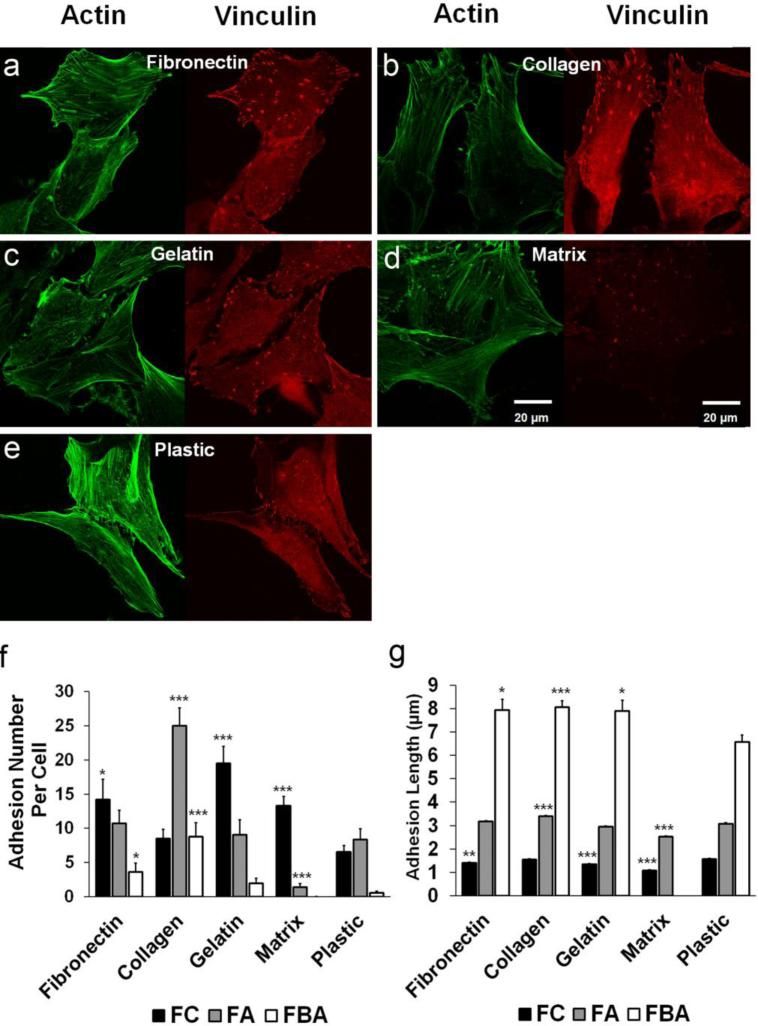
Fibronectin, collagen and gelatin promoted formation of focal adhesion structures in human AF cells
Focal adhesion plays a critical role in many cellular biological functions, specifically in adhesion, migration and proliferation. Vinculin is a well-known component of focal adhesion linking actin cytoskeleton to integrin adhesion molecules. As seen in Fig. 4a-e, fluorescence intensity of vinculin-positive focal adhesions (red) was relatively weaker in cells on the NP matrix compared to others. The overall adhesion structures appeared to be more abundant in fibronectin, collagen and gelatin groups compared to plastic control. Based on the size and sub-cellular location of adhesions (criteria described in the Materials and Methods section), numbers and lengths of FC, FA and FBA were quantified respectively for each substrate. As demonstrated in Fig. 4f, fibronectin (14.2±2.9 per cell, *p<0.05), gelatin (19.5±2.5***p<0.001) and matrix (13.3±1.4, ***p<0.001) presented more FC than plastic control (6.5±0.96). While collagen significantly increased the FA number per cell (25.0±2.6, ***p<0.001), matrix presented much less FA per cell (1.4±0.5, ***p<0.001) compared to plastic (8.3±1.6). Both fibronectin (3.6±1.3, *p<0.05) and collagen (8.8±2.1, ***p<0.001) significantly elevated FBA number per cell compared to plastic (0.5±0.2), however, matrix presented almost no detectable FBA. As shown in Fig. 4g, mean axial length of FC were relatively smaller in fibronectin (1.41±0.03 μm, **p<0.01), gelatin (1.34±0.03 μm, ***p<0.001) and smallest in matrix (1.07±0.04 μm, ***p<0.001) compared to plastic (1.56±0.04 μm). Mean axial length of FA was significantly longer in collagen group (3.39±0.04 μm, ***p<0.001), but was much shorter in the matrix group (2.52±0.05 μm, ***p<0.001) compared to plastic (3.08±0.05 μm). Moreover, FBA appeared much larger in fibronectin (7.94±0.03 μm, *p<0.05), collagen (8.06±0.26 μm, ***p<0.001) and gelatin (7.89±0.47 μm, *p<0.05) groups compared to plastic control (6.56±0.30 μm).
Figure 4.
Expression profile of vinculin-positive focal adhesions in human AF cells cultured on ECM substrates. (a-e) Immunofluorescence staining of vinculin (red fluorescence) illustrated varied expression profiles (sub-cellular location, size and number) of focal adhesion structures in human AF cells on different ECM substrates. Actin stress fiber was counterstained as green fluorescence. Scale bar represented 20 μm. (f) Mean adhesion number per cell was quantified in terms of focal complex (FC, with axial length no more than 2 μm and located at cell periphery), focal adhesion (FA, with axial length in the range of 2-5 μm and located at either periphery or more centrally) and fibrillar adhesion (FBA, longer than 5 μm presented inside cell cytosol) for each substrate. (g) Mean adhesion length was plotted for FC, FA and FBA respectively for each substrate. Note: *p<0.05, **p<0.005, ***p<0.001 compared to the corresponding adhesion structure (FC, FA, or FBA) in the plastic control group. Cells were cultured on various ECM for 4 days.
Fibronectin preserved the phenotype of human AF cells
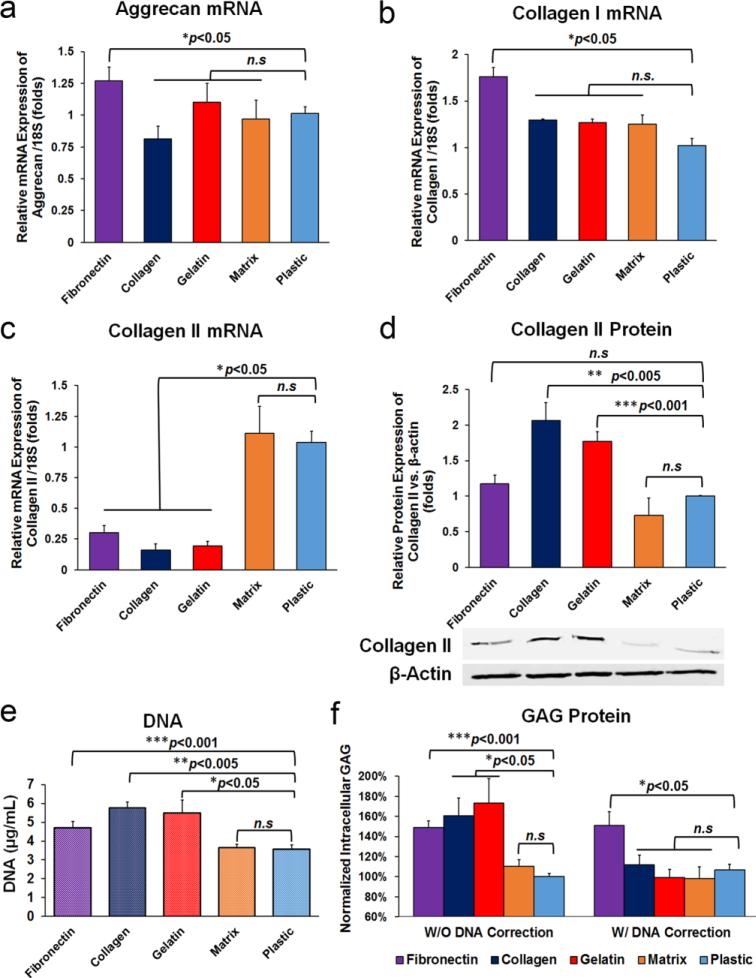
As shown in Fig. 2d-d’’’’, AF cell morphology was not compromised in any of the substrate groups. Fibronectin, collagen and gelatin increased anabolic activity of human AF cells. As shown in Fig. 5a and Fig. 5b, fibronectin increased (*p<0.05) mRNA expression of aggrecan (1.27±0.10,) and collagen I (1.76 ±0.30) compared to the plastic control. To our surprise, the mRNA expression of collagen II was decreased (*p<0.05) in fibronectin (0.30±0.05), collagen (0.16±0.15) and gelatin (0.19±0.04) treated cells and remained at a similar level in the matrix group (1.11±0.22, p>0.05) compared to the plastic control (1.04±0.09) (Fig. 5c). However, the intracellular protein expression of type II collagen (normalized by cytoskeletal β-actin) was significantly elevated in collagen (**p<0.005) and gelatin (***p<0.001) groups compared to plastic control (Fig. 5d). Correspondingly, fibronectin, collagen, and gelatin significantly increased DNA contents to 4.7±0.3 μg/mL, 5.75±0.3 μg/mL, and 5.5±0.7 μg/mL respectively, confirming their beneficial effects in promoting cell proliferation when compared to the plastic control (3.6±0.7 μg/mL) (Fig. 5e). Glycosaminoglycans (GAGs) are linear polysaccharides that are one of the most important extracellular matrix components of IVD. Cellular GAG contents were significantly increased in AF cells treated with fibronectin (149.0±6.3%, **p<0.005), collagen (160.7±17.6%, *p<0.05) and gelatin (173.3±24.3%, *p<0.05) compared to the plastic control. After normalized to DNA content, fibronectin showed ~50% enhancement of GAG/DNA content (151.1±13.8%, *p<0.05) compared to the plastic control (106.9±5.2%) (Fig. 5f). In contrast, NP matrix neither increased cell proliferation nor elevated GAG production (p>0.05 vs. plastic control).
Figure 5.
Anabolic activity of human AF cells affected by various ECM substrates. Real-time RT-PCR suggested that fibronectin elevated the mRNA expression of aggrecan (a) and collagen I (b) compared to other ECM and controls. Although fibronectin, collagen and gelatin decreased mRNA expression of collagen II (c), western blots of total cell lysate suggested increased protein expression of type II collagen (normalized by β-actin) in fibronectin, collage and gelatin coated groups (d). (e) Fibronectin, collagen and gelatin promoted cell proliferation suggested by DNA assay. (f) Fibronectin significantly improved cellular GAG production among all ECM substrates. Target gene expression was normalized to 18S (n=9 per group). Cells were cultured on various ECM for 4 days.
Fibronectin reduced metalloproteinase-2 and metalloproteinase-9 expression of human AF cells
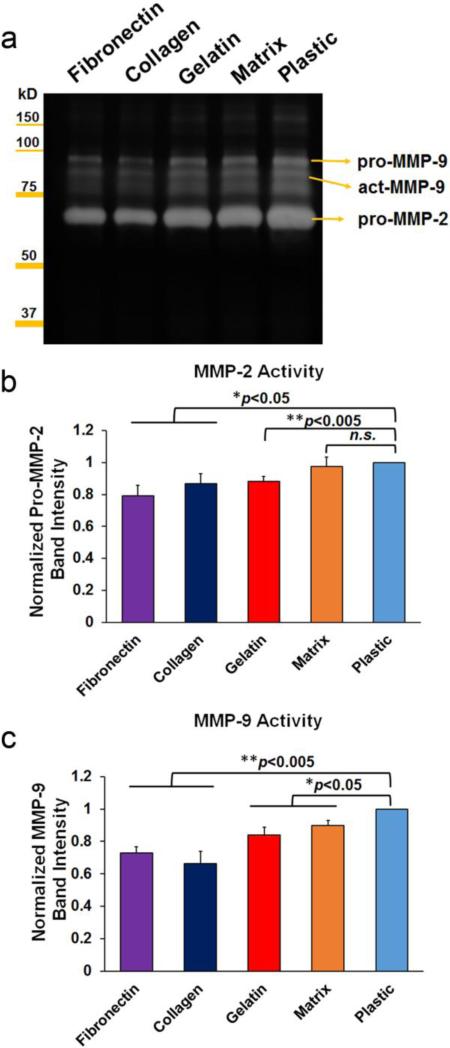
As illustrated in Fig. 6, pro-MMP-2 levels were significantly decreased in media of fibronectin (79.1±6.5%, **p<0.005), gelatin (88.1±3.3%, **p<0.005) and collagen (86.6±6.2%, *p<0.05) coated groups, but were not altered in the matrix (97.6±5.7%, p>0.05) group, compared to the plastic control (100%). In addition, MMP-9 levels were decreased in all ECM groups: fibronectin (73.0±3.5%, **p<0.005), collagen (66.2±7.9%, **p<0.005), gelatin (84.2±4.8%, *p<0.05) and matrix (89.9±3.0%, *p<0.05) compared to the plastic control (100%).
Figure 6.
Gelatinolytic zymography of human AF cell media cultured on ECM substrates. (a) Representative zymography gel image suggested reduced activities of MMPs in fibronectin, collagen and gelatin treated cell media, which was correlated to the quantification of pro-MMP-2 (b) and overall MMP-9 (c). Note, p*<0.05, **0.001<p<0.005 vs. plastic control.
Impact of culture surface on mRNA expression of human AF cells
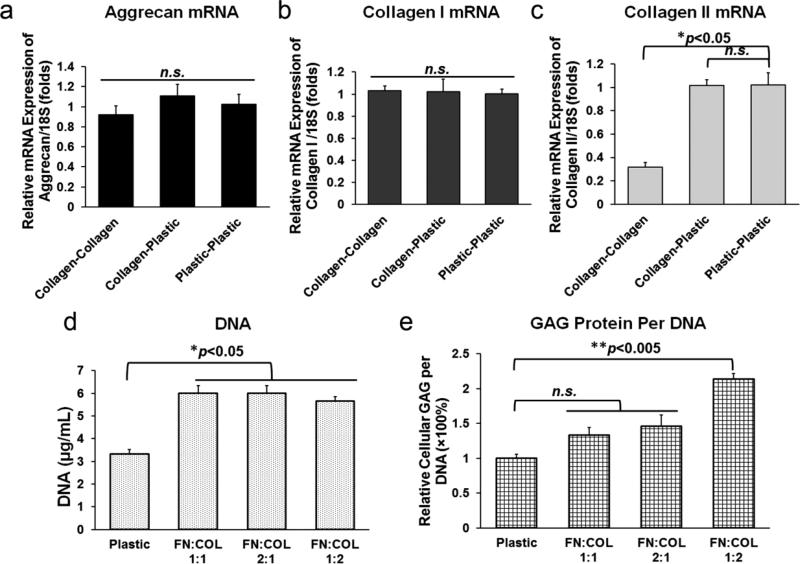
To further examine the impact of culture surface on the mRNA expression profile, human AF cells were initially cultured onto either plastic or collagen coated surfaces for 4 days. Cells in the collagen group were then trypsinized and seeded again onto either plastic or collagen surfaces for another 4 days. As shown in Fig. 7a-c, the mRNA expressions of Aggrecan, Collagen I and Collagen II were either maintained or restored when human AF cells were seeded back onto plastic following culture on collagen coated surface, suggesting these marker genes may have various degree of susceptibility to ECM environmental change.
Figure 7.
Examination of optimized in vitro culture system for human AF cells. The mRNA expressions of aggrecan (a) and collagen I (b) were of no significant difference among three groups Collagen-Collagen, Collagen-Plastic and Plastic-Plastic, while the mRNA level of collagen II (c) was recovered to control. (d) All three fractional ratios of fibronectin (FN) and collagen (COL) mixtures significantly improved cell proliferation suggested by DNA assay, however (e) only when the FN/COL ratio was 1:2 (1.7 and 1.3 μg/mL, respectively) significant elevation of GAG/DNA contents could be observed compared to plastic control. Note, n=6 per group for data shown here. Fibronectin (5 μg/mL) and collagen (2 μg/mL) at varying volumetric ratios of 2:1 (final [FN]: 3.3 μg/mL, [COL]: 0.7 μg/mL), 1:1 (final [FN]: 2.5 μg/mL, [COL]: 1 μg/mL) and 1:2 (final [FN]: 1.7 μg/mL, [COL]: 1.3 μg/mL) were studied.
Combination of collagen and fibronectin served as the optimized substrate system
Substrate mixture of fibronectin (5 μg/mL) and collagen (2 μg/mL) at varying volumetric ratios of 2:1 (final [FN]: 3.3 μg/mL, [COL]: 0.7 μg/mL), 1:1 (final [FN]: 2.5 μg/mL, [COL]: 1 μg/mL) and 1:2 (final [FN]: 1.7 μg/mL, [COL]: 1.3 μg/mL) were assessed regarding their synergistic effects in promoting cell proliferation and GAG production. As shown in Fig. 7d, all three combination groups exhibited significant increase in DNA contents compared to plastic control (*p<0.05), which confirmed the synergistic proliferative effects of mixed fibronectin and collagen. When the volumetric ratio of 1:2 (FN/COL) with resulting final concentrations of fibronectin (1.7 μg/mL) and collagen (1.3 μg/mL) were used, the cellular production of GAG per DNA was significantly enhanced (*p<0.05) compared to plastic and other combination groups (Fig. 7e).
Discussion
ECM proteins play a critical role in IVD development, maintenance and repair. Disc cells are normally dispersed in the matrix. Evoking such native ECM-cell interaction might preserve cell phenotype in an in vitro setting, yet the optimized ECM culture for disc cells is not clear. Ideally, in vitro disc cell culture should mimic the in vivo microenvironment of IVD and replicate disc cell biology (Bedore et al. 2014). To reach this goal, researchers discovered that decellularized ECM deposited by its host tissue or cells was a promising culture system (Zhang et al. 2015; Wang et al. 2014; Pei et al; 2014; Jin et al. 2012). In our study, cell-free ECM deposited by human NP cells was used as a substrate in addition to various ECM proteins. As a result, fibronectin and collagen promoted both proliferation (around 1.7 times faster than the plastic control) (Fig. 2) and adhesion of human AF cells (Fig. 3).
Focal adhesions are large multi-protein complexes that act as transmembrane links between the extracellular matrix and the actin cytoskeleton, in which organized aggregates of specialized proteins are assembled and distributed at the basal surface of cells (Chroev et al. 2014; Case et al. 2015). Focal adhesions play a critical role in many cell functions including cell adhesion and migration. In general, we observed larger, more numerous, and more mature focal adhesion structures in human AF cells cultured on selected ECM substrates compared to plastic surface in Fig. 4, suggesting that these ECM (in particular fibronectin, collagen and gelatin) might contribute to the maturation process of focal complexes. It was reported that cell proliferation activity might be correlated, to a certain extent, with cell flattening on the growth substrate and expression of focal adhesion structures (Musilkova et al. 2015; Mann et al. 2002). Here, we observed improved cell proliferation in fibronectin, collagen and gelatin groups (Fig 2), which might be correlated with enhanced cell spreading and adhesion (Fig. 3) and more numerous and larger focal adhesion structures in these ECM substrates (Fig. 4).
Healthy IVD cells are embedded in the ECM and maintain a balance between anabolism and catabolism. During the aging and degeneration processes, a deficiency in anabolic factors, such as transforming growth factor-β (TGF-β) and insulin-like growth factor-1 (IGF-1), reduce cellular viability and ECM synthesis. Such deteriorated progression may also increase expression of pro-inflammatory cytokines, aggrecanases, and matrix metalloproteinases (MMPs) further depreciating the ECM (Molinos et al. 2015). Our results suggested that fibronectin not only stimulates the anabolic metabolism of human AF cells (elevated mRNA expression of aggrecan and collagen I, increased protein expression of collagen II and proteoglycan) (Fig. 5) but also reduces catabolic MMP activities (Fig. 6). These observations demonstrated that ECM substrates alter the microenvironment of disc cells, which subsequently affects cell phenotype. Although, the mRNA expression of Collagen II was decreased in fibronectin, collagen, and gelatin treated human AF cells (Fig. 5c), the protein levels in those groups were increased (Fig. 5d). Such a discrepancy between the mRNA and protein levels may be caused by the post-transcriptional and post-translational modification, or may result from the time points for these measurements. In fact, only about 40% of cellular protein levels can be predicted from mRNA measurement in human (de Sousa Abreu et al. 2009). Furthermore, mRNA expression of collagen II was restored when seeding back onto the plastic after collagen coated surface (Fig. 7a-c), suggesting collagen II mRNA may be susceptible to change in response to ECM environment. It also turned out that a selective combination of fibronectin and collagen at a ratio of 1:2 provides an optimal condition for enhanced GAG/DNA production (Fig. 7d,e).
Our study has its limitations, which could reasonably be optimized with a more systematic approach where a side-by-side assessment is carried out in comparison between our method and other sophisticated systems such as numerous 3D culture methods. Moreover, we have shown that the formation of focal adhesions potentially plays an important role in mediating human AF cell adhesion which ultimately affects proliferation, however, the specific signaling pathways involved including focal adhesion kinase (FAK), extracellular signal-regulated kinase (ERK), Protein Kinase C (PKC), and phosphatidylinositol-3 kinase (PI3K) require future investigation (Pratsinis et al. 2012; Robles et al. 2006; Fogh et al. 2014). In addition, a few other parameters might cause variability to the proliferation rate of human disc cells, such as donor variability, cell seeding density, and culture condition (hypoxia vs. normoxia). Therefore, the optimized ECM substrate system for human AF cell culture was concluded based on the current experimental setting.
In summary, a simple and cost-effective method for fast proliferation of human AF cells has been established using optimized ECM as in vitro culture substrates. A final combination of fibronectin (1.7 μg/mL) and collagen (1.3 μg/mL) solutions proved to be the most promising substrate system in facilitating cell proliferation, adhesion and maintenance of cell phenotype. Such effects might be attributed to increased formation of focal adhesion complexes under those substrate conditions. Our methodology is highly straightforward and accessible to a broad range of scientific community in their respective niche of disc research.
Acknowledgement and Funding Information
We appreciate the funding supports from National Institute of Arthritis and Musculoskeletal and Skin Diseases R21AR057512 and RO1AR064792.
Footnotes
Conflict of Interest: The authors declared that they have no conflict of interest.
References
- Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354(9178):581–5. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- Bedore J, Leask A, Seguin CA. Targeting the extracellular matrix: matricellular proteins regulate cell-extracellular matrix communication within distinct niches of the intervertebral disc. Matrix Biol. 2014;37:124–30. doi: 10.1016/j.matbio.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Biggs MJP, Richards RG, Gadegaard N, Wilkinson CDW, Dalby MJ. Regulation of implant surface cell adhesion: characterization and quantification of S-phase primary osteoblast adhesions on biomimetic nanoscale substrates. J Orthop Res. 2007;25:273–82. doi: 10.1002/jor.20319. [DOI] [PubMed] [Google Scholar]
- Case LB, Baird MA, Shtengel G, Campbell SL, Hess HF, Davidson MW, Waterman CM. Molecular mechanism of vinculin activation and nanoscale spatial organization in focal adhesions. Nat Cell Biol. 2015;17:880–92. doi: 10.1038/ncb3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorev DS, Moscovitz O, Geiger B, Sharon M. Regulation of focal adhesion formation by a vinculin-Arp2/3 hybrid complex. Nat Commun. 2014;5:3758. doi: 10.1038/ncomms4758. [DOI] [PubMed] [Google Scholar]
- de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol Biosyst. 2009;5:1512–26. doi: 10.1039/b908315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener A, Nebe B, Lüthen F, Becker P, Beck U, Neumann HG, Rychly J. Control of focal adhesion dynamics by material surface characteristics. Biomaterials. 2005;26:383–392. doi: 10.1016/j.biomaterials.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Feng G, Wan Y, Shen FH, Li X. Nucleus pulposus explant culture model. J Orthop Res. 2009;27:814–819. doi: 10.1002/jor.20803. [DOI] [PubMed] [Google Scholar]
- Fogh BS, Multhaupt HAB, Couchman JR. Protein Kinase C, focal adhesions and the regulation of cell migration. J. Histochem Cytochem. 2014;62:172–184. doi: 10.1369/0022155413517701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber HE, Stasky AA, Hanley EN., Jr Characterization and phenotypic stability of human disc cells in vitro. Matrix Biol. 1997;16:285–8. doi: 10.1016/s0945-053x(97)90016-0. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Hanley EN. Human disc cells in monolayer vs 3D culture: cell shape, division and matrix formation. BMC Musculoskelet Disord. 2000;1:1. doi: 10.1186/1471-2474-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley EN, Gruber HE. Method For Producing Human Intervertebral Disc Cells For Implantation. 2008 US Patent 11,151,141.
- Hu X, Beeton C. Detection of Functional Matrix Metalloproteinases by Zymography. J Vis Exp. 2010;(45):e2445. doi: 10.3791/2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Feng G, Reames DL, Shimer AL, Shen FH, Li X. The Effects of Simulated Microgravity on Intervertebral Disc Degeneration. Spine J. 2013;13:235–242. doi: 10.1016/j.spinee.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Liu Q, Scott P, Zhang D, Shen F, Balian G, Li X. Annulus Fibrosus Cell Characteristics Are a Potential Source of Intervertebral Disc Pathogenesis. PLoS ONE. 2014;9:e96519. doi: 10.1371/journal.pone.0096519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Shimmer AL, Li X. The challenge and advancement of annulus fibrosus tissue engineering. Eur Spine J. 2013;22:1090–1100. doi: 10.1007/s00586-013-2663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Wan Y, Shimer AL, Shen FH, Li XJ. Intervertebral disk-like biphasic scaffold—demineralized bone matrix cylinder and poly(polycaprolactone triol malate)—for interbody spine fusion. J Tissue Eng. 2012;3(1):2041731412454420. doi: 10.1177/2041731412454420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto C, Musumeci G, Castorina A, Loreto C, Martinez G. Degenerative disc disease of herniated intervertebral discs is associated with extracellular matrix remodeling, vimentin-positive cells and cell death. Ann Anat. 2011;193:156–62. doi: 10.1016/j.aanat.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Mann BK, West JL. Cell adhesion peptides alter smooth muscle cell adhesion, proliferation, migration, and matrix protein synthesis on modified surfaces and in polymer scaffolds. J Biomed Mater Res. 2002;60(1):86–93. doi: 10.1002/jbm.10042. [DOI] [PubMed] [Google Scholar]
- Molinos M, Almeida CR, Caldeira J, Cunha C, Gonçalves RM, Barbosa MA. Inflammation in intervertebral disc degeneration and regeneration. J R Soc Interface. 2015;12:20150429. doi: 10.1098/rsif.2014.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musilkova J, Kotelnikov I, Novotna K, Pop-Georgievski O, Rypacek F, Bacakova L, Proks V. Cell adhesion and growth enabled by biomimetic oligopeptide modification of a polydopamine-poly(ethylene oxide) protein repulsive surface. J Mater Sci Mater Med. 2015;26:253. doi: 10.1007/s10856-015-5583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Gil ES, Cho H, Mandal BB, Tien LW, Min BH, et al. Intervertebral disk tissue engineering using biphasic silk composite scaffolds. Tissue Eng Part A. 2012;18:447–58. doi: 10.1089/ten.tea.2011.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei M, Li J, Zhang Y, Liu G, Wei L, Zhang Y. Expansion on a matrix deposited by nonchondrogenic urine stem cells strengthens the chondrogenic capacity of repeated-passage bone marrow stromal cells. Cell Tissue Res. 2014;356:391–403. doi: 10.1007/s00441-014-1801-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei M, He F, Kish VL. Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Eng Part A. 2011;17:3067–76. doi: 10.1089/ten.tea.2011.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratsinis H, Constantinou V, Pavlakis K, Sapkas G, Kletsas D. Exogenous and autocrine growth factors stimulate human intervertebral disc cell proliferation via the ERK and Akt pathways. J Orthop Res. 2012;30:958–64. doi: 10.1002/jor.22017. [DOI] [PubMed] [Google Scholar]
- Robles E, Gomez TM. Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding. Nat Neurosci. 2006;9:1274–83. doi: 10.1038/nn1762. [DOI] [PubMed] [Google Scholar]
- Wang X, Li Y, Han R, He C, Wang G, Wang J, Zheng J, Pei M, Wei L. Demineralized bone matrix combined bone marrow mesenchymal stem cells, bone morphogenetic protein-2 and transforming growth factor-beta3 gene promoted pig cartilage defect repair. PLoS One. 2014;9:e116061. doi: 10.1371/journal.pone.0116061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Li X. Nucleus pulposus tissue engineering: a brief review. Eur Spine J. 2009;18:1564–1572. doi: 10.1007/s00586-009-1092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wang D, Hao J, Gong M, Arlet V, Balian G, Shen FH, Li XJ. Enhancement of matrix production and cell proliferation in human annulus cells under bioreactor culture. Tissue Eng Part A. 2011;17:1595–1603. doi: 10.1089/ten.TEA.2010.0449. [DOI] [PubMed] [Google Scholar]
- Yeh C, Jin L, Shen F, Balian G, Li X. miR-221 attenuates the osteogenic differentiation of human annulus fibrosus cells. Spine J. 2016 doi: 10.1016/j.spinee.2016.03.026. doi: 10.1016/j.spinee.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li J, Davis ME, Pei M. Delineation of in vitro chondrogenesis of human synovial stem cells following preconditioning using decellularized matrix. Acta Biomater. 2015;20:39–50. doi: 10.1016/j.actbio.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]