Abstract
Objective
Gene therapy, delivered directly to the blood vessel wall, could potentially prevent atherosclerotic lesion growth and promote atherosclerosis regression. Previously, we reported that a helper-dependent adenoviral vector (HDAd) expressing apolipoprotein (apo) A-I in carotid endothelium of fat-fed rabbits reduced early (4 weeks) atherosclerotic lesion growth. Here we tested whether the same HDAd—delivered to existing carotid atherosclerotic lesions—could promote regression.
Approach and Results
Rabbits (n=26) were fed a high-fat diet for 7 months, then treated with bilateral carotid gene transfer. One carotid was infused with an HDAd expressing apo A-I (HDAdApoAI), the other with a control non-expressing HDAd (HDAdNull). The side with HDAdApoAI was randomized. Rabbits were then switched to regular chow, lowering their plasma cholesterols by over 70%. ApoA-I mRNA and protein were detected in HDAdApoAI-transduced arteries. After 7 weeks of gene therapy, compared to HDAdNull-treated arteries in the same rabbits, HDAdApoAI-treated arteries had significantly less VCAM-1 expression (28%; P=0.04) along with modest but statistically insignificant trends towards decreased intimal lesion volume, lipid and macrophage content, and ICAM-1 expression (9%–21%; P=0.1–0.4). Post-hoc subgroup analysis of rabbits with small-to-moderate-sized lesions (n=20) showed that HDAdApoAI caused large reductions in lesion volume, lipid content, ICAM-1 and VCAM-1 expression (30%–50%; P≤0.04 for all). Macrophage content was reduced by 30% (P=0.06). There was a significant interaction (P=0.02) between lesion size and treatment efficacy.
Conclusion
Even when administered on a background of aggressive lowering of plasma cholesterol, local HDAdApoAI vascular gene therapy may promote rapid regression of small-to-moderate-sized atherosclerotic lesions.
Keywords: apolipoprotein, atherosclerosis, carotid artery, gene therapy, rabbits
Subject Terms: animal models of human diseases, atherosclerosis, gene therapy, translational studies
Atherosclerosis, a chronic disease of medium and large arteries, is characterized by subendothelial accumulation of lipids, lipid-laden macrophages, and lipid-laden smooth muscle cells.1 Current medical therapy aimed at reversing atherosclerosis or halting its progression is essentially limited to drugs (such as statins) that lower plasma low-density lipoprotein (LDL) cholesterol, thereby decreasing lipid entry into the artery wall. LDL-lowering drugs are useful; however, they are only partially effective, require life-long adherence to a daily dosing regimen, and are poorly tolerated by many.2–4 A new therapy for lowering plasma LDL cholesterol, antibodies that inhibit proprotein convertase subtilisin kexin 9, is expensive (> $14,000 per year versus < $50/year for some statins)5, requires biweekly injections for life, and has not yet shown an impact on patient outcomes.6, 7
Drugs that increase plasma high-density lipoprotein (HDL) cholesterol are theoretically a complementary approach to the treatment of atherosclerosis.8, 9 Drug-induced increases in plasma HDL are expected to prevent and reverse atherosclerosis by increasing HDL-mediated removal of cholesterol from cells of the artery wall, a process termed “cholesterol efflux.”10, 11 However, HDL-raising drugs such as niacin and cholesterol ester transfer protein inhibitors have failed repeatedly in clinical trials.12–15 Negative results with these drugs—despite impressively elevated plasma HDL levels in treated patients—might be accounted for by off-target effects or by the failure of these drugs to reliably and robustly accelerate cholesterol efflux in vivo.16–19
Numerous studies establish that apolipoprotein A-I (apo A-I), the most abundant protein in HDL,20 is the component of HDL that is responsible for stimulating cholesterol efflux.9, 21, 22 Apo A-I stimulates cholesterol efflux both as a constituent of HDL particles and as a free protein.23 Accordingly, apo A-I has been tested as a therapy for atherosclerosis both in animals (initially in transgenic mice) and in humans (as a brief protein infusion) and has shown efficacy in both settings.24–27 For example, every-other-day subcutaneous injections of apo A-I in apoE-null mice decreased lesion lipids and macrophages (but not lesion volume) within 1 week28 and weekly infusions of apo A-I-containing reconstituted HDL in humans decreased coronary lesion volume by ~5% within 5 weeks.29 Unfortunately, these experimental apo A-I therapies are not currently translatable into clinical care because transgenesis of humans is neither ethical nor feasible, and lifetime injections or infusions of apo A-I protein (which would likely be required to maintain the effects of acute treatment) are both prohibitively expensive and impractical. Other approaches aimed at exploiting atheroprotective activities of lipid-poor apo A-I include apo A-I-mimetic peptides30 (some of which are orally bioavailable) and reconstituted complexes of human apo A-I and phosphatidylcholine (delivery intravenously).31 These approaches are promising, but are not yet proven clinically. Both would require repeated administration and are likely to be costly.
In contrast, a single dose of gene therapy with an apo A-I-expressing vector could potentially provide durable—if not lifetime—in vivo delivery of apo A-I protein. Moreover, introduction of apo A-I-expressing vectors directly to the artery wall would result in synthesis and release of lipid-poor apo A-I protein precisely where it is most needed: adjacent to a lipid-rich atherosclerotic plaque. To this end, we constructed a helper-dependent adenoviral (HDAd) vector that expresses rabbit apo A-I32 and we showed that HDAdApoAI stimulated cholesterol efflux in vitro and expressed apo A-I for at least 48 weeks in carotid arteries of chow-fed rabbits. HDAdApoAI also significantly retarded development of early carotid atherosclerosis in fat-fed rabbits.33 Here we address the more clinically relevant question of whether HDAdApoAI can promote regression of established atherosclerotic lesions. We treated rabbit carotid atherosclerotic lesions by intraluminal delivery of HDAdApoAI or a control vector (HDAdNull). After 7 weeks of gene therapy, we compared lesion size, lipid content, cellular composition, and adhesion molecule expression in HDAdApoAI- and HDAdNull-treated arteries.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement
Results
Responses to Initiation and Cessation of Atherogenic Diet
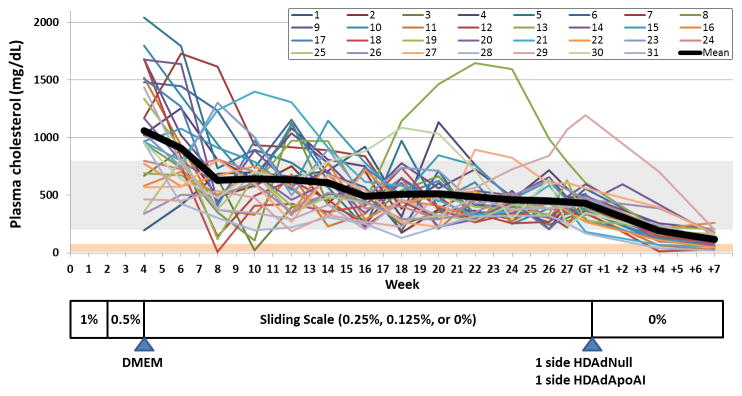
We enrolled 34 rabbits in the study by switching them from regular chow to a high-fat diet (Figure 1). We measured plasma cholesterol after 1 month of this diet and then every two weeks. As expected,34 rabbits had a heterogeneous response to 1 month of fat-feeding, with plasma cholesterols ranging from 200 mg/dL to over 2,000 mg/dL. Subsequent adjustment of dietary cholesterol, according to a sliding scale, narrowed the range of plasma cholesterols substantially, and maintained the majority of rabbits in the targeted 200–800 mg/dL range during the period of lesion progression (4 weeks–28 weeks). We targeted the 200–800 mg/dL range to avoid build-up of hepatic cholesterol stores, which occurs when rabbits are more aggressively fat-fed,35 and which renders them resistant to atherosclerosis regression.36 Of the 34 rabbits, 3 were euthanized due to surgical complications or disease unrelated to this study (see Materials and Methods); all others completed the study. Three rabbits had evidence of dietary toxicity, manifested as particularly high plasma cholesterol levels, low hematocrits, and loss of appetite. These 3 rabbits were switched to a normal chow diet, on which these issues resolved, and they were continued in the study. Cholesterol levels were 1,060±468 mg/dL at the time of lesion initiation (i.e., DMEM infusion at 4 weeks), 431±180 mg/dL at the time of lesion treatment (i.e., vector infusion, typically at 28 weeks), and 117±63 mg/dL at vessel harvest (typically at 35 weeks; Figure I in the online-only Data Supplement). A metabolic panel and CBC were performed on 8 rabbits at 35 weeks: nearly all of the mean values were within reported normal ranges for New Zealand White rabbits (Table I in the online-only Data Supplement).
Figure 1.
Experimental protocol and plasma cholesterol levels. Rabbits were fed a high-fat diet for 4 weeks, containing the indicated percentages of cholesterol. Rabbits then underwent neck surgery with bilateral common carotid artery infusions of DMEM to help initiate atherosclerosis development. Plasma cholesterol was measured at this time and every 2 weeks thereafter. From the end of week 4 through the time of gene transfer (GT), dietary cholesterol was adjusted according to a sliding scale with a goal of maintaining plasma cholesterol in the range of 200–800 mg/dL (gray horizontal bar). Most of the rabbits (~ 80%) underwent gene transfer during week 28. Due to scheduling issues, ~ 20% were treated during week 29. Gene transfer in one rabbit was delayed until week 31 due to an anesthetic complication. Gene transfer included infusion of HDAdNull and HDAdApoAI in contralateral carotids, with the side for each vector randomized. After gene transfer, rabbits were switched to normal chow and carotids removed 3 days or 7 weeks later.
We switched the rabbits to a chow diet at the time of vector delivery for two primary reasons. First, we aimed to rigorously test vascular gene therapy by administering it on a background of aggressive lipid lowering. At present, all humans who are candidates for atherosclerosis regression therapy would first be given a lipid-lowering drug and would continue this as tolerated. Accordingly, for vascular gene therapy to be widely useful, it must be effective on a background of systemic lipid-lowering therapy. Eventually vascular apo A-I gene therapy might replace systemic therapy, but that is a question for the future. Second, we aimed to test whether apo A-I could accelerate lesion regression. Continuing the high-fat diet would likely have caused lesion progression, and our study would have measured effects of apo A-I on progression, not regression.
Persistence of HDAd Genomes and Apo A-I Expression in HDAdApoAI-Transduced Arteries
After 28 weeks of high-fat diet, all rabbits received bilateral common carotid infusions: HDAdApoAI in one carotid and HDAdNull in the other (Figure 1). Our vector-infusion protocol (described in more detail in Materials and Methods) uses a surgical approach to isolate carotid artery segments from the circulation followed by rinsing of the isolated artery lumens and infusion of the vector in the absence of blood. The vector is then withdrawn and blood flow re-established. The side infused with each vector (left or right) was randomized for every rabbit. This aspect of our experimental design eliminates plasma lipid and lipoprotein levels as an independent variable because the left and right arteries in each rabbit are exposed to identical concentrations of plasma lipids and lipoproteins. We—and others—have reported that infusion of adenoviral vectors into isolated carotid artery segments (in both normolipidemic and hyperlipidemic animals) results in gene transfer almost exclusively to luminal endothelial cells in the transduced segments.37–39 Moreover, in rabbits infused with HDAdApoAI according to this protocol we were unable to detect using the HDAdApoAI vector in any tissue other than the transduced artery segments.33 Therefore, this protocol tests the effects of local apo A-I expression in luminal endothelial cells.
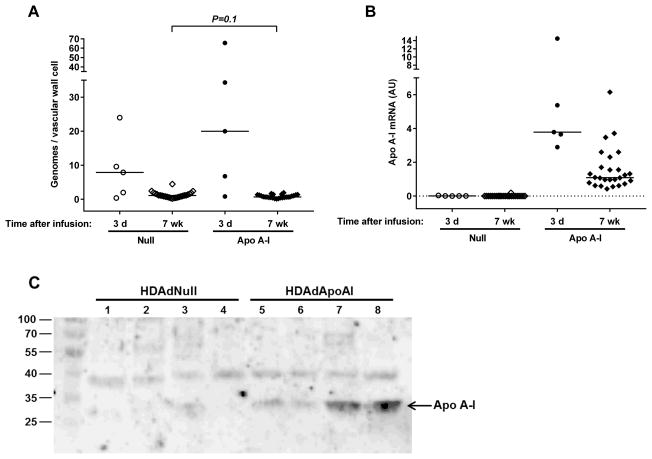
To evaluate gene transfer into existing atherosclerotic lesions, we euthanized 5 rabbits on the third day after vector infusion and measured HDAd genomes and apo A-I mRNA in carotid artery extracts. Vector genomes were detected in all 10 arteries [median (interquartile range) = 9 (3–23) genomes per vascular wall cell; Figure 2A]. This level of genomes is similar to the level of genomes found 3 days after vector infusion into arteries of chow-fed rabbits.40 Apo A-I mRNA was present in all HDAdApoAI-infused arteries removed 3 days after infusion and in none of the HDAdNull-infused arteries (Figure 2B). We also measured vector genomes and apo A-I mRNA in arteries removed at the end of the study (7 weeks after vector infusion). Vector genomes remained far above background in all arteries [0.9 (0.5–1.4) vector genomes per vascular wall cell; n=52; Figure 2A]. Apo A-I mRNA was detected in all 26 HDAdApoAI-infused arteries harvested 7 weeks after vector infusion. Levels of apo A-I mRNA were lower than at 3 days but still well above background. Absence of apo A-I mRNA in HDAdNull-infused arteries confirms: a) no meaningful level of apo A-I gene transfer occurs contralateral to HDAdApoAI infusion; and b) in the unlikely event that any cells in the peripheral blood or bone marrow are transduced by small amounts of systemically released HDAdApoAI vector, none of these cells can be detected in the artery wall. The levels of apo A-I mRNA at 3 days and 7 weeks were similar to levels of apo A-I mRNA that we measured 3 days and 4 weeks, respectively, after infusion of HDAdApoAI in rabbits with developing atherosclerotic lesions (data not shown). This earlier study33 showed significant therapeutic effects of HDAdApoAI on early lesion growth.
Figure 2.
Vector genome persistence and apo A–I expression. Rabbit carotid arteries were transduced with HDAdNull or HDAdApoAI and harvested 3 days or 7 weeks later. (A) Vector genomes in carotid artery extracts were measured by qPCR and normalized to the number of vascular wall cells represented by the total amount of DNA in the extract. (B) Apo A–I mRNA in carotid artery extracts was measured by qRT-PCR, with normalization to GAPDH mRNA in the same extracts. Data points are individual arteries; bars are group medians. Dotted line indicates assay background. (C) Representative western blot of medium conditioned by explanted HDAdNull- or HDAdApoAI-transduced carotid arteries. Paired samples (1 and 5; 2 and 6; 3 and 7; 4 and 8) are from the same rabbits. Size markers are in kilodaltons.
Apo A-I protein was present in medium conditioned by 21 of the 26 HDAdApoAI-infused arteries harvested 7 weeks after vector infusion. In contrast, Apo A-I protein was present in medium conditioned by only 3 of the 26 HDAdNull-infused arteries (P<0.001; Figure 2c and Figure II in the online-only Data Supplement). The low levels of apo A-I protein in the 3 HDAdNull samples presumably are due to ex vivo release of plasma apo A-I that was not completely washed out before placing the arteries in explant culture (see Materials and Methods). Matched plasma samples collected from each rabbit before vector infusion and at harvest 7 weeks later showed no evidence that plasma apo A-I levels were altered by the HDAdApoAI infusion (P=0.7 for comparison of pre- and post-infusion band densities by western blotting; Figure III in the online-only Data Supplement).
Effects of HDAdApoAI on Intimal Area, Lipid, Macrophage and T-Cell Content
We confined our tissue analyses to the two transduced carotid arteries because the experiments were designed to compare local effects of HDAdApoAI to local effects of HDAdNull in different arteries of the same rabbit. We did not examine other vessels because: a) no other vessels were treated with HDAdApoAI; b) we found no evidence that levels of plasma apo A-I protein were altered by local infusion of HDAdApoAI; and c) there was no control group against which vessels other than the infused carotids could be compared.
Intimal lesions were present in all 52 arteries. Median intimal areas in both groups (Null: 150,000 μm2; Apo A-I: 86,000 μm2; Table 1) were far below the median intimal area of recent historical control arteries (median lesion area = 240,000 μm2). These historical control arteries were from rabbits that also underwent bilateral carotid infusions of DMEM after 4 weeks of high-fat diet but were harvested at the 28-week time point instead of receiving infusions of HDAd.34 The smaller intimal areas in the present study suggest that our protocol of diet-induced lowering of plasma cholesterol promoted regression of atherosclerosis in both groups. Consistent with findings of the study in which we developed this model,34 lesion size was highly variable with intimal areas ranging from 355 μm2 to 550,000 μm2 in individual arteries. This variability, along with the high correlation of intimal areas between left and right arteries in the same rabbit (r=0.7; P<0.0002; Figure IV in the online-only Data Supplement), supported our prospective decision to use a paired sample study design for the final data analysis. According to this design, we first calculated and compared overall HDAdNull and HDAdApoAI group medians (Table 1). However, to test for statistical significance of effects of apo A-I expression, we compared HDAdApoAI- and HDAdNull-treated arteries in the same rabbits. We used a ratio-paired test to test the null hypothesis that the median ratio of values in paired arteries (i.e., value in HDAdApoAI-treated artery/value in HDAdNull-treated artery in the same rabbit) was 1.
Table 1.
Histologic and Immunohistochemical Analyses of All Arteries
| HDAdNull* (n = 26) | HDAdApoAI* (n = 26) | Treatment Effect**
|
|||
|---|---|---|---|---|---|
| Median (25%–75%) | Median (25%–75%) | Reduction (−) or Increase (+) in ApoAI versus NullMedian (25%–75%) | # Reduced with ApoAI (of 26 pairs) | P | |
| Intimal Area (μm2 × 103) | 150 (40–230) | 86 (22–250) | −9 (−53 – +44)% | 14 | 0.4 |
| Medial Area (μm2 × 103) | 530 (470–560) | 500 (470–570) | 0 (−7 – +8)% | 13 | 1.0 |
| I/M Ratio | 0.27 (0.07–0.44) | 0.18 (0.04–0.43) | −7 (−52 – +53)% | 15 | 0.3 |
| ORO Area (μm2 × 103) | 48 (12–75) | 32 (6–74) | −14 (−62 – +22)% | 17 | 0.2 |
| ORO area (% of intima) | 34 (23–39) | 32 (21–37) | −12 (−36 – +16)% | 14 | 0.2 |
| HHF-35 Area (μm2 × 103) | 16 (5–32) | 13 (3–31) | −7 (−72 – +100)% | 13 | 0.4 |
| HHF-35 area (% of intima) | 12 (9–16) | 12 (8–18) | −5 (−26 – +30)% | 14 | 0.9 |
| RAM-11 Area (μm2 × 103) | 72 (14–123) | 49 (11–117) | −11 (−42 – +48)% | 16 | 0.3 |
| RAM-11 area (% of intima) | 48 (41–57) | 49 (34–57) | −5 (−24 – +9)% | 16 | 0.3 |
| VCAM-1 Area (μm2 × 103) | 3.1 (1.1–5.1) | 2.0 (0.4–4.2) | −28 (−71 – +5)% | 18 | 0.04 |
| VCAM-1 area (% of intima) | 2.4 (1.5–3.1) | 1.9 (1.0–4.4) | −28 (−59 – +54)% | 16 | 0.2 |
| ICAM-1 Area (μm2 × 103) | 26 (9–56) | 21 (7–41) | −21 (−62 – +23)% | 16 | 0.1 |
| ICAM-1 area (% of intima) | 19 (15–34) | 16 (13–27) | −12 (−41 – +17)% | 17 | 0.1 |
| KEN-5 area (μm2) | 151 (43–299) | 77 (31–196) | −25 (−76 – +81)% | 15 | 0.3 |
| KEN-5 area (% of intima) | 0.12 (0.04–0.32) | 0.08 (0.03–0.30) | −14 (−61 – +45)% | 14 | 0.4 |
Data are from all of the HDAdNull-infused arteries and all of the HDAdApoAI-infused arteries
Data are from paired analyses of HDAdNull- and HDAdApoAI-infused arteries in the same rabbit (26 pairs). A reduction reflects a lower value in the paired HDAdApoAI artery; an increase reflects a higher value in the paired HDAdApoAI artery. P values are for the paired comparisons.
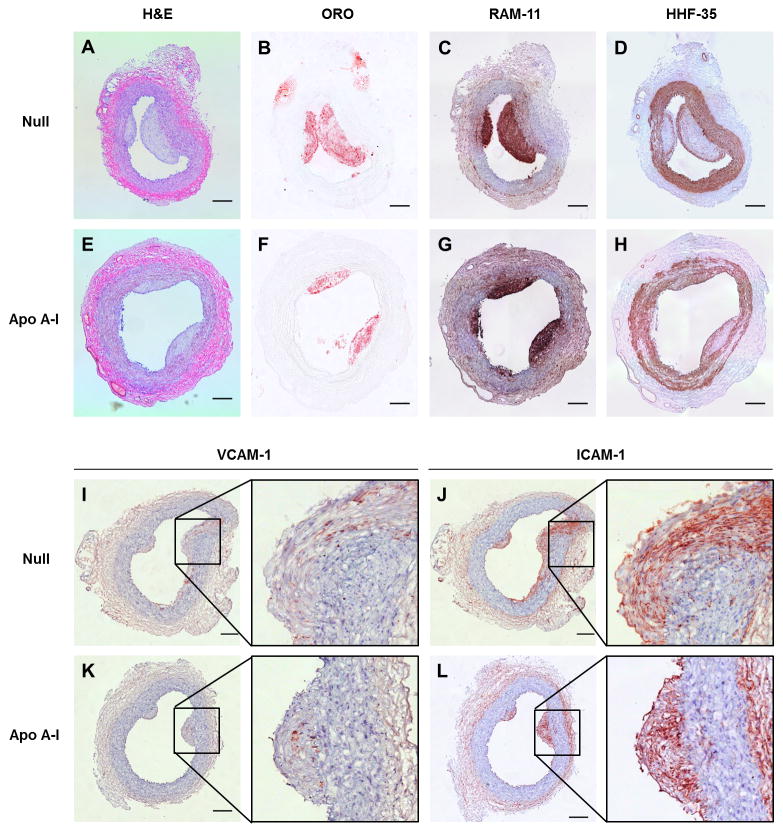
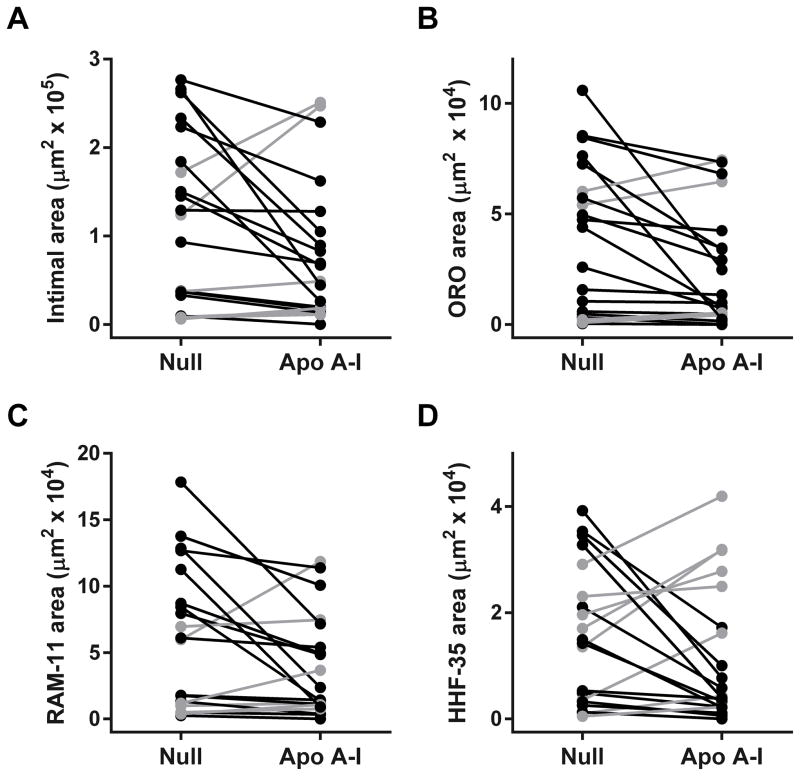
As expected,34 intimal lesions were rich in lipid and macrophages (Figure 3B, 3C, 3F, and 3G). The lesions also contained smooth muscle cells, typically in a lesion cap (Figure 3D and 3H) and rare T cells (Figure V in the online-only Data Supplement). Median intimal area, intimal/medial area ratio, Oil Red O-stained area, and area stained for the macrophage marker RAM-11 or the T cell marker KEN-5 were all nominally lower in the group of 26 HDAdApoAI-treated arteries compared to the group of 26 HDAdNull-treated arteries (32%–49% lower; Table 1; left-most data columns). We also found less intimal area, intimal/medial area ratio, Oil Red O-stained area, RAM-11-stained area and KEN-5-stained area in the 26 HDAdApoAI-treated arteries compared to their paired HDAdNull-treated controls (median reductions of 7%–25%; Table 1, third data column). These data are consistent with reduced atherosclerosis in HDAdApoAI-treated arteries versus paired HDAdNull-treated controls; however, none of the reductions is statistically significant (P=0.2–0.4; Figure 4A–4C, Figure VC in the online-only Data Supplement, and Table 1). The percentages of intimal area staining for Oil Red O, RAM-11, and KEN-5 were similar between the 2 groups (1%–2% absolute differences; Table 1), and were nominally less in HDAdApoAI-treated arteries versus their paired controls (median 5%–14% reductions; P=0.2–0.4; Table 1).
Figure 3.
Carotid atherosclerotic lesions 35 weeks after beginning high-fat diet and 7 weeks after infusion of HDAdNull or HDAdApoAI. Arteries were removed, embedded in OCT, and sectioned at multiple steps. Sections of representative lesions treated either with HDAdNull (Null) or HDAdApoAI (Apo A–I) are shown, stained with: hematoxylin and eosin (H & E) (A, E); Oil Red O (ORO) (B, F); RAM-11 antibody to detect macrophages (C, G); HHF-35 antibody to detect smooth muscle actin (D, H); anti-VCAM-1 (I, K); or anti-ICAM-1 (J, L). Sections A, B, C, and D are from the same artery, as are: sections E, F, G, and H; sections I and J; and sections K and L. All panels except B and F: hematoxylin counterstain. Scale bars = 200 μm.
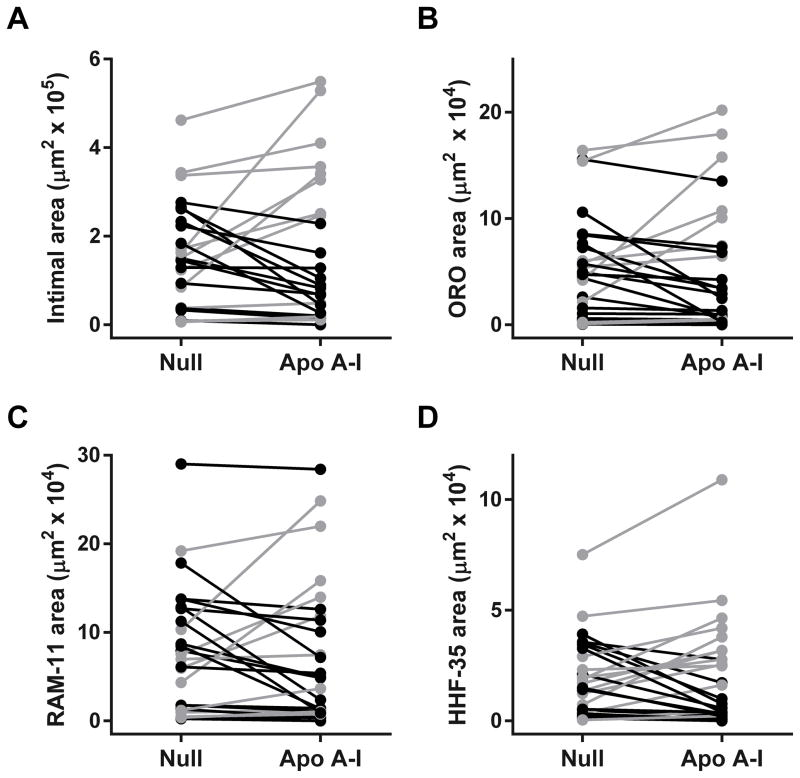
Figure 4.
Intimal lesion size and composition. Arteries were removed 7 weeks after treatment with either HDAdNull (Null) or HDAdApoAI (Apo A–I), sectioned, stained, and analyzed with computer-assisted planimetry (n=26 rabbits; 52 arteries). (A) Intimal areas measured on H&E-stained sections. (B) Intimal areas staining with Oil Red O. (C) Intimal areas staining with the RAM-11 antibody (detects macrophages). (D) Intimal areas staining with the HHF-35 antibody (detects smooth muscle actin). Data points are means for each artery; points from arteries in the same rabbit are connected by bars. Rabbits in which HDAdApoAI-treated arteries had lower values than HDAdNull-treated arteries are indicated in black; rabbits in which HDAdNull-treated arteries had lower values than HDAdApoAI-treated arteries are indicated in grey.
To determine if expression of apo A-I affected lesion macrophage phenotype (as reported after injection of apo A-I protein in mice),28 we measured IL-1β and MRC1 mRNA in lesion extracts (IL-1β and MRC1 are markers of macrophage M1 and M2 phenotype, respectively).41 Median IL-1β mRNA was 34% lower in the group of HDAdApoAI-treated arteries than the group of HDAdNull-infused arteries; median MRC1 mRNA was 7% higher in HDAdApoAI-treated arteries. When compared to their paired HDAdNull-treated controls, HDAdApoAI-treated arteries had 14% lower median IL-1β mRNA expression (P=0.4) and 8% higher median MRC1 mRNA expression (P=0.4). Attempts to measure several other proposed rabbit M1 and M2 markers by qRT-PCR41 were not successful.
Effects of HDAdApoAI on Medial Area and Intimal Smooth Muscle Area
Because atherosclerosis is primarily an intimal disease,1 atheroprotective effects of apo A-I would likely be confined to the intima. Consistent with this, medial areas were nearly identical both between the 2 groups and between HDAdApoAI-treated arteries and their paired HDAdNull-treated controls (6% difference between group medians; 0% median difference between paired arteries; P=1.0; Table 1). The median intimal area staining for smooth muscle actin was 19% less in the group of HDAdApoAI-treated arteries and 7% less in HDAdApoAI-treated arteries versus their paired HDAdNull-treated controls (P=0.4; Table 1 and Figure 4D). The median percentage of intimal area staining for smooth muscle actin was identical between the 2 groups of arteries (Table 1) and only 5% less in HDAdApoAI-treated arteries than their HDAdNull-treated controls (P=0.9; Table 1). The combined percentage of intimal area occupied by macrophages, T cells, and smooth muscle cells was essentially identical in both groups (60% and 61%).
Post-hoc Subgroup Analysis of Small-to-Moderate-Sized Lesions
The full data set (Figure 4) suggested that HDAdApoAI might have a therapeutic effect on small-to-moderate-sized lesions but not on large lesions (note the preponderance of grey data points in the upper part of each panel and black data points in the lower part). To explore this possibility, we performed a post-hoc subgroup analysis that excluded 6 rabbits in which either the left or right carotid artery had an intimal area greater than one standard deviation above the mean of all arteries (Figure 5, Figure VD in the online-only Data Supplement, and Table 2). In this subgroup of 20 rabbits, the group of HDAdApoAI-treated arteries had substantially lower median intimal areas and intimal/medial area ratios than the group of HDAdNull-infused arteries (54% lower for both; Figure 5A–5C and Table 2; left-most data columns). The median reductions in intimal area and intima/media area ratios in HDAdApoAI-treated arteries compared to their paired HDAdNull-treated controls were also large (36% and 34%) and were statistically significant (P=0.04 for both; Table 2). HDAdApoAI-treated arteries in this subgroup also had far less median intimal Oil Red O, RAM-11 (macrophage), and KEN-5 (T cell) staining than HDAdNull arteries (74%, 68%, and 49% less; Table 2). The median reductions in intimal Oil Red O- and RAM-11-stained areas in HDAdApoAI-treated arteries compared to their paired HDAdNull-treated controls were also substantial (30% and 29%) and either met or approached statistical significance (P=0.03 and P=0.06, respectively; Table 2). KEN-5 staining was reduced by 34% in paired HDAdApoAI-treated arteries (P=0.2; Table 2 and Figure VD in the online-only Data Supplement). The percentages of intimal area staining for Oil Red O, RAM-11, and KEN-5 were similar between the 2 groups of arteries. Compared to paired HDAdNull controls, HDAdApoAI-treated arteries had a 15% reduction in percent Oil Red O-stained area (P=0.5), a 12% increase in percent KEN-5-stained area (P=0.8), and no change in percent RAM-11-stained area (Table 2). The combined percentage of intimal area occupied by macrophages, T cells, and smooth muscle cells was similar between the 2 groups (59% for HDAdNull and 64% for HDAdApoAI-treated arteries. Therefore, the smaller intimal areas in HDAdApoAI-treated arteries did not result from a disproportionate decrease in intimal cellularity.
Figure 5.
Intimal lesion size and composition of small-to-moderate lesions. Data are from a subgroup of 20 rabbits (40 arteries), none of which had an intimal lesion area more than one standard deviation above the mean of all lesions. Arteries were removed 7 weeks after treatment with either HDAdNull (Null) or HDAdApoAI (Apo A–I), sectioned, stained, and analyzed with computer-assisted planimetry. Data for these 20 rabbits are the same as in Figure 4, with the y-axis re-scaled to improve readability. (A) Intimal areas measured on H&E-stained sections. (B) Intimal areas staining with Oil Red O (ORO). (C) Intimal areas staining with the RAM-11 antibody (detects macrophages). (D) Intimal areas staining with the HHF-35 antibody (detects smooth muscle actin). Data points are means for each artery; points from arteries in the same rabbit are connected by bars. Rabbits in which HDAdApoAI-treated arteries had lower values than HDAdNull-treated arteries are indicated in black; rabbits in which HDAdNull-treated arteries had lower values than HDAdApoAI-treated arteries are indicated in grey.
Table 2.
Histologic and Immunohistochemical Analyses of Subgroup of Small-to-Moderate Lesions
| HDAdNull* (n = 20) | HDAdApoAI* (n = 20) | Treatment Effect**
|
|||
|---|---|---|---|---|---|
| Median (25%–75%) | Median (25%–75%) | Reduction (−) or Increase (+) in ApoAI versus NullMedian (25% – 75%) | # Reduced with ApoAI (of 20 pairs) | P | |
| Intimal Area (μm2 × 103) | 130 (40–190) | 60 (20–110) | −36 (−59 – +32)% | 14 | 0.04 |
| Medial Area (μm2 × 103) | 530 (480–560) | 480 (470–560) | −5 (−11 – +5)% | 13 | 0.1 |
| I/M Ratio | 0.24 (0.07–0.36) | 0.11 (0.04–0.23) | −34 (−53 – +32)% | 14 | 0.04 |
| ORO Area (μm2 × 103) | 46 (6–63) | 12 (5–37) | −30 (−73 – −9)% | 16 | 0.03 |
| ORO area (% of intima) | 33 (20–38) | 31 (12–37) | −15 (−41 – +21)% | 11 | 0.5 |
| HHF-35 Area (μm2 × 103) | 15 (3–25) | 5 (2–19) | −52 (−78 – +55)% | 12 | 0.1 |
| HHF-35 area (% of intima) | 12 (9–17) | 13 (8–20) | 0 (−39 – +56)% | 10 | 1.0 |
| RAM-11 Area (μm2 × 103) | 60 (11–93) | 19 (10–58) | −29 (−63 – +11)% | 14 | 0.06 |
| RAM-11 area (% of intima) | 47 (39–56) | 51 (29–59) | 0 (−28 – +16)% | 10 | 0.8 |
| VCAM-1 Area (μm2 × 103) | 3.1 (0.9–4.5) | 1.2 (0.2–3.3) | −42 (−78 – −5)% | 15 | 0.03 |
| VCAM-1 area (% of intima) | 2.4 (1.5–3.0) | 1.9 (1.0–4.8) | −16 (−53 – +61)% | 11 | 0.5 |
| ICAM-1 Area (μm2 × 103) | 20 (6–44) | 9 (3–26) | −47 (−80 – −2)% | 15 | 0.02 |
| ICAM-1 area (% of intima) | 20 (14–35) | 17 (13–29) | −12 (−45 – +22)% | 13 | 0.3 |
| KEN-5 area (μm2) | 90 (36–197) | 46 (16–86) | −34 (−84 – +119)% | 12 | 0.2 |
| KEN-5 area (% of intima) | 0.10 (0.03–0.13) | 0.08 (0.04–0.18) | +12 (−58 – +47)% | 9 | 0.8 |
Data are from all of the HDAdNull-infused arteries and all of the HDAdApoAI-infused arteries.
Data are from paired analyses of HDAdNull- and HDAdApoAI-infused arteries in the same rabbit (20 pairs). A reduction reflects a lower value in the paired HDAdApoAI artery; an increase reflects a higher value in the paired HDAdApoAI artery. P values are for the paired comparisons.
Results for the 6 rabbits with large lesions are in Supplemental Figures VI and VII.
Medial areas in this subgroup were similar between the HDAdApoAI-treated and HDAdNull-treated arteries (9% difference in group medians; 5% median difference between paired arteries; P=0.1; Table 2). The median intimal area staining for smooth muscle actin was nominally less in the HDAdApoAI-treated arteries than the HDAdNull-treated arteries (67% decrease in group medians; 52% median decrease in paired HDAdApoAI-treated versus HDAdNull arteries; P=0.1; Figure 5D and Table 2). The median percentage of smooth muscle actin-stained intimal area was nearly identical between the group of HDAdApoAI-treated arteries and the group of HDAdNull-treated arteries (12% and 13%, respectively; Table 2). There was no difference in median percentage of smooth muscle actin-stained intimal area between the HDAdApoAI-treated arteries and their paired HDAdNull-treated controls (0%; P=1.0).
HDAdApoAI Significantly Reduces Intimal VCAM-1 Expression
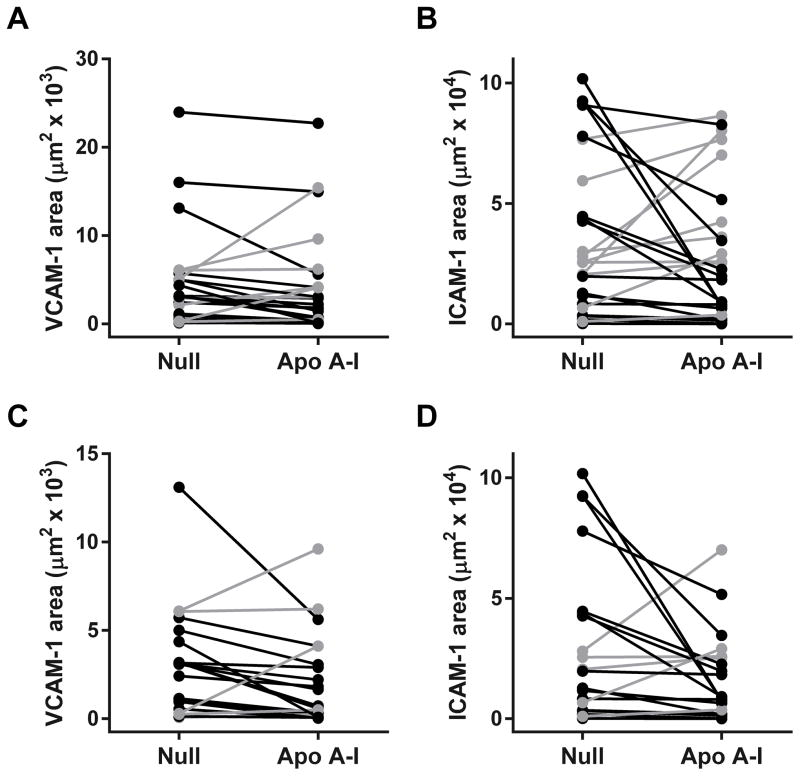
VCAM-1 staining was observed in luminal endothelial cells and deeper within the intima (Figure 3I). ICAM-1 staining was present along the lumen as well as deeper within the intima (Figure 3J), where it colocalized with RAM-11 staining detected on nearby sections (not shown). The group of 26 arteries treated with HDAdApoAI had less median intimal VCAM-1 and ICAM-1 staining than the group of 26 HDAdNull-infused arteries (35% and 19% less, respectively; Figure 6A and 6B and Table 1). The median reductions in intimal VCAM-1 and ICAM-1 staining in HDAdApoAI-treated arteries compared to paired HDAdNull controls were 28% and 21%, respectively. The reduction in VCAM-1 expression was statistically significant (P=0.04; Table 1). In the 20 rabbits with small-moderate lesions, the group of HDAdApoAI-infused arteries had far less median intimal VCAM-1 and ICAM-1 stained areas than the group of HDAdNull arteries (61% and 55% decreases; Table 2). The median reductions in intimal VCAM-1 and ICAM-1 staining in HDAdApoAI-treated arteries compared to their paired HDAdNull-treated controls were also large (42% and 47%, respectively; P=0.03 and 0.02; Table 2).
Figure 6.
Intimal adhesion molecule expression. Arteries were removed 35 weeks after beginning high-fat diet and 7 weeks after treatment with either HDAdNull (Null) or HDAdApoAI (Apo A–I), sectioned, stained, and analyzed with computer-assisted planimetry. (A) and (B) Data for all rabbits (n=26 rabbits; 52 arteries). (A) Intimal areas staining for vascular cell adhesion molecule-1 (VCAM-1). (B) Intimal areas staining for intercellular adhesion molecule 1 (ICAM-1). (C) and (D): data are from a subgroup of 20 rabbits (40 arteries), none of which had an intimal lesion area more than one standard deviation above the mean of all lesions. Data for these 20 rabbits are the same as in (A) and (B), respectively, with the y-axis rescaled in (C) to improve readability. Data points are means for each artery; points from arteries in the same rabbit are connected by bars. Rabbits in which HDAdApoAI-treated arteries had lower values than HDAdNull-treated arteries are indicated in black; rabbits in which HDAdNull-treated arteries had lower values than HDAdApoAI-treated arteries are indicated in grey.
For both the entire set of arteries and the subgroup with small-to-moderate lesions, percentages of intimal area staining for VCAM-1 and ICAM-1 were lower in the group of HDAdApoAI-treated arteries (15%–21%; Tables 1 and 2). There were similar differences between HDAdApoAI-treated arteries and their paired HDAdNull-treated controls (12%–28% median reductions in HDAdApoAI arteries), but none of these differences reached statistical significance (P≥0.1 for all; Tables 1 and 2).
Discussion
We used a rabbit model of carotid artery atherosclerosis to test whether local gene therapy with an apo A-I-expressing vector could regress atherosclerotic lesions. Our major findings were: (1) infusion of HDAdApoAI into atherosclerotic arteries yielded substantial apo A-I expression; (2) 7 weeks of apo A-I expression in atherosclerotic arteries significantly reduced VCAM-1 expression; (3) 7 weeks of apo A-I expression in atherosclerotic arteries did not significantly accelerate atherosclerotic lesion regression, as measured by total intimal area or by lipid or macrophage content of the intima; (4) post-hoc subgroup analysis of small-to-moderate-sized atherosclerotic lesions revealed statistically significant acceleration of lesion regression in HDAdApoAI-treated arteries, including a median ~35% decrease in total intimal area and intimal:medial area ratio, 30% decreases in lesion lipid and macrophage content, and 40%–50% decreases in intimal adhesion molecule expression.
Beginning over 20 years ago, several groups showed that germ line transgenic overexpression of apo A-I retards development of atherosclerosis.25, 42, 43 Subsequent reports established that postnatal apo A-I gene therapy—delivered either by systemic injection of vectors targeting the liver or by infusion of ex vivo transduced bone marrow-derived cells—can both suppress and regress experimental atherosclerosis.44–47 Despite these impressive results, there are several reasons why neither of these approaches has advanced to the clinic: 1) systemic injection of large amounts of viral vectors is toxic;48 2) transgene expression is gradually lost after hepatocyte gene transfer, likely due to cell turnover;49 3) in humans, even the most advanced approaches to hepatocyte gene transfer require immunosuppression to maintain transgene expression;50 and 4) the risks to patient well-being of radiation and bone-marrow transplantation outweigh the potential benefits of slowing atherosclerosis progression. The approach used in the present study has the potential to avoid all of these limitations because local infusion of HDAd to the artery wall is minimally toxic,51 yields transgene expression that appears to last indefinitely (without immunosuppression),33 does not require marrow-ablative chemotherapy, and has a minimal risk of genotoxicity due to use of adenovirus, a non-integrating vector. Moreover, transduction of vascular cells—predominantly endothelial cells in this model37–39—delivers lipid-poor apo A-I directly to the site of atherosclerotic disease. Direct vessel wall delivery of apo A-I should be a particularly effective means of stimulating efflux of cholesterol from atherosclerotic arteries because lipid-poor and minimally lipidated apo A-I are superior acceptors of cholesterol compared to the more lipidated apo A-I forms that circulate in animals with hepatic apo A-I overexpression.23, 52 In addition, continuous synthesis of apo A-I in the artery wall might avoid oxidative damage that impairs the function of liver-derived apo A-I that circulates in plasma and accumulates in the artery wall.53 These theoretical advantages were supported by experiments in our laboratory showing that focal artery wall expression of apo A-I significantly reduces development of early (1 month) atherosclerosis in fat-fed rabbits.33
Here we asked whether vascular wall delivery of apo A-I could promote atherosclerosis regression. This is a clinically relevant question because gene therapy for atherosclerosis will likely be used to treat established atherosclerosis in patients at high risk for clinical events before it will be tried as a preventive measure. To test whether vascular apo A-I gene therapy promotes atherosclerosis regression, we first developed a rabbit model of focal severe carotid atherosclerosis.34 This model yields large intimal lesions that are macrophage and lipid-rich, but also contain smooth muscle cells, often in a fibrous cap. These lesions resemble mature human plaques and are—as noted by others46—a more clinically relevant target than the early fatty streaks present in many mouse models and in our rabbit model of atherosclerosis progression.33 The approach used in the present study relies on efficient gene transfer to these complex lesions. Although others reported low gene-transfer efficiency to atherosclerotic rabbit arteries,54 we found that apo A-I expression in the atherosclerotic arteries was equivalent to the level we reported in arteries of chow-fed rabbits (33 and data not shown).
Arteries infused with HDAdApoAI had significantly less staining for VCAM-1 than control-transduced arteries. All other end points (intimal area, intima/media area ratio, lipid and macrophage accumulation, ICAM-1 and KEN-5 expression) trended towards efficacy; however, the effects were relatively small (~10%–20% decreases) and not statistically significant. We considered several explanations for why the salutary effects of apo A-I were not larger and more consistently significant. First, lesion size in this model is highly variable, making it difficult for small-to-moderate therapeutic effects to achieve statistical significance. Much of the variability derives from rabbit-specific factors, as is evident from the high left-right lesion size correlation in individual rabbits (Figure IV in the online-only Data Supplement). We minimized the contribution of individual rabbit variability by using a left-right paired analysis; however, even with this approach all end points varied widely. Second, we tested apo A-I gene therapy on a background of the highly effective intervention of plasma cholesterol lowering (due to cessation of the high-fat diet). During 7 weeks of gene therapy, mean plasma cholesterols decreased by >70%, with many rabbits entering the normal range. This design is rigorous and clinically relevant because humans with atherosclerosis are routinely treated with cholesterol-lowering medications. However, it may be difficult to detect additive effects of gene therapy on this background. Third, our experimental intervention was brief (7 weeks). Others have detected atherosclerosis regression in as little as 4 weeks, including in rabbits treated systemically with HDL (but maintained on a high-fat diet).55, 56 Longer treatment might have revealed larger effects of apo A-I gene therapy, especially on larger lesions.
After examining our initial results, we considered another possible explanation for the modest overall effects of apo A-I: that robust effects of apo A-I on small-to-moderate lesions were overwhelmed by a lack of any effect on large lesions. Larger lesions are typically more complex and inherently more resistant to regression.36 In addition, our therapeutic approach is based on delivery of apo A-I from a single layer of endothelial cells overlying a plaque. As plaques enlarge, the ratio of endothelial surface to lesion volume decreases in proportion to lesion size. Consequently, in large lesions the intralesional concentration of apo A-I is lower and the distance that apo A-I protein must travel to reach all lesional cells is greater. Both factors would mitigate therapeutic efficacy.
To test the hypothesis that efficacy of our therapy depends on lesion size, we performed a post-hoc subgroup analysis that excluded rabbits (6 of 26) that had at least one lesion with intimal area more than 1 standard deviation above the group mean. In this subgroup of lesions, apo A-I gene therapy positively impacted all end points, with near-uniform statistical significance. To further test this hypothesis,57 we directly compared HDAdApoAI efficacy in large lesions versus small-to-moderate lesions and found a statistically significant interaction between lesion size and response to HDAdApoAI (P=0.02; Figure VI in the online-only Data Supplement). Interactions between lesion size and response to HDAdApoAI were either significant or borderline significant for Oil Red O area, RAM-11 area, and ICAM-1 area (P=0.04–0.08; Figure VII in the online-only Data Supplement). Nevertheless, because we identified the lesion-size subgroups post-hoc, we view these results cautiously and plan to test them prospectively. Prospective testing could be done by repeating the study with exclusion of the largest lesions from the final analysis. Alternatively, we could perform pre-mortem screening with non-invasive means such as transcutaneous ultrasound or MRI and censor rabbits with large lesions. We are aware that gene therapy to promote atherosclerosis regression should be effective on large as well as small lesions. This is our goal; however, it may require higher levels of apo A-I expression, longer duration of treatment, or both.
We carefully considered the possibility that the initial study was truly negative and that HDAdApoAI gene therapy does not promote lesion regression. There are 4 reasons why we believe this is unlikely. First, in the paired analyses of both the entire group of 26 rabbits and the subgroup of 20 rabbits, 100% (7 of 7) measurements of intimal mass, I/M ratio, lipid accumulation, macrophage and T-cell accumulation, and adhesion molecule expression were decreased in HDAdApoAI-treated arteries. Second, VCAM-1 staining, which was significantly decreased by HDAdApoA-I in the full 26-rabbit cohort, is a highly reproducible biochemical marker of apo A-I activity.32, 46, 58–60 Third, treatment with HDAdApoAI had far greater effects on intimal area, lipids, and macrophages than it did on the vascular media, consistent with its known biological effects. Fourth, the subgroup analysis, though post-hoc, has a rational basis: the physical principles that govern surface-to-volume ratio mandate that apo A-I delivery to large lesions is less efficient. Moreover, this was the only subgroup analysis we performed. On the other hand, HDAdApoAI did not significantly alter the relative percentages of intimal cell types (e.g., by decreasing % lesion macrophage area or increasing % lesion SMC area, resulting in a more stable lesion phenotype). This could be viewed as a reason to doubt efficacy; however, absence of such effects could be due to apo A-I-mediated removal of lipid from both macrophages and SMC, decreasing intimal area occupied by both cell types. Alternatively, this 7-week study may have been too brief to detect changes in relative cellular composition of the lesions. Finally, because lesion cellular composition is a calculated quantity that incorporates 2 measurements (and their measurement errors), it is more variable than area measurements and less amenable to detection of significant differences.
In conclusion, our initial test of whether vascular wall-targeted apo A-I gene therapy could promote atherosclerosis regression yielded results that are encouraging but not definitive. Our results are exciting in that they show—for the first time—that vector delivery to established complex atherosclerotic lesions is feasible, safe, durable, and is likely effective. A therapy that complemented the relatively modest effects of current drug therapy on atherosclerosis regression55 would have a major impact on human health. Moving vascular wall-targeted HDAd gene therapy into the clinic will require more convincing proof of efficacy, advances in vector delivery techniques, and viral capsid engineering that circumvents widespread human immunity to type V adenovirus.61 These are daunting challenges; however, progress is being made in both of the latter areas.62, 63 Vascular wall gene therapy that is delivered by a single infusion and reverses human atherosclerosis is beginning to appear feasible.
Supplementary Material
Highlights.
We applied a rabbit model of carotid atherosclerosis to test whether helper-dependent adenoviral delivery of apo A-I to mature atherosclerotic lesions could accelerate lesion regression.
Helper-dependent adenoviral delivery of apo A-I was as efficient in atherosclerotic arteries as we previously reported in normal arteries.
Overexpression of apo A-I significantly reduced intimal expression of VCAM-1 protein.
In the full cohort of 26 rabbits, overexpression of apo A-I resulted in modest trends towards less atherosclerosis that were not statistically significant.
In a subgroup of 20 rabbits with small-to-moderate-sized lesions, overexpression of apo A-I significantly and substantially reduced atherosclerotic lesion size, lipid content, and adhesion molecule expression.
Acknowledgments
We thank AdVec, Inc. for permission to use the HDAd reagents, Lianxiang Bi, Emma Bueren, and Ervin Ham for technical assistance, Julia Feyk for administrative assistance, and the Department of Comparative Medicine veterinary services for surgical advice and support.
Sources of Funding
This work was supported by HL114541 and the John L. Locke, Jr. Charitable Trust.
Abbreviations
- apo A-I
Apolipoprotein A-I
- HDAd
Helper-dependent adenovirus
- ICAM-1
intercellular adhesion molecule-1
- VCAM-1
vascular cell adhesion molecule-1
- ORO
Oil Red O
Footnotes
Disclosures
The authors have no conflicts to disclose.
References
- 1.Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: The gauss-3 randomized clinical trial. JAMA. 2016;315:1580–1590. doi: 10.1001/jama.2016.3608. [DOI] [PubMed] [Google Scholar]
- 3.Waters DD, Hsue PY, Bangalore S. Pcsk9 inhibitors for statin intolerance? JAMA. 2016;315:1571–1572. doi: 10.1001/jama.2016.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mampuya WM, Frid D, Rocco M, Huang J, Brennan DM, Hazen SL, Cho L. Treatment strategies in patients with statin intolerance: The cleveland clinic experience. Am Heart J. 2013;166:597–603. doi: 10.1016/j.ahj.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sible AM, Nawarskas JJ, Anderson JR. Pcsk9 inhibitors: An innovative approach to treating hyperlipidemia. Cardiol Rev. 2016;24:141–152. doi: 10.1097/CRD.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 6.Feinstein MJ, Lloyd-Jones DM. Monoclonal antibodies for lipid management. Curr Atheroscler Rep. 2016;18:39. doi: 10.1007/s11883-016-0593-2. [DOI] [PubMed] [Google Scholar]
- 7.Verbeek R, Stoekenbroek RM, Hovingh GK. Pcsk9 inhibitors: Novel therapeutic agents for the treatment of hypercholesterolemia. Eur J Pharmacol. 2015;763:38–47. doi: 10.1016/j.ejphar.2015.03.099. [DOI] [PubMed] [Google Scholar]
- 8.Barter PJ, Rye KA. Targeting high-density lipoproteins to reduce cardiovascular risk: What is the evidence? Clin Ther. 2015;37:2716–2731. doi: 10.1016/j.clinthera.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Phillips MC. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem. 2014;289:24020–24029. doi: 10.1074/jbc.R114.583658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee-Rueckert M, Escola-Gil JC, Kovanen PT. Hdl functionality in reverse cholesterol transport - challenges in translating data emerging from mouse models to human disease. Biochim Biophys Acta. 2016;1861:566–583. doi: 10.1016/j.bbalip.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The AIM-HIGH Investigators. Niacin in patients with low hdl cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 13.The HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 14.Barter PJ, Rye KA. Cholesteryl ester transfer protein inhibition is not yet dead--pro. Arterioscler Thromb Vasc Biol. 2016;36:439–441. doi: 10.1161/ATVBAHA.115.306879. [DOI] [PubMed] [Google Scholar]
- 15.Kolata G. New York Times. 2016. Dashing hopes, study shows a cholesterol drug had no effect on heart health. [Google Scholar]
- 16.Tchoua U, D’Souza W, Mukhamedova N, Blum D, Niesor E, Mizrahi J, Maugeais C, Sviridov D. The effect of cholesteryl ester transfer protein overexpression and inhibition on reverse cholesterol transport. Cardiovasc Res. 2008;77:732–739. doi: 10.1093/cvr/cvm087. [DOI] [PubMed] [Google Scholar]
- 17.Briand F, Thieblemont Q, Muzotte E, Burr N, Urbain I, Sulpice T, Johns DG. Anacetrapib and dalcetrapib differentially alters hdl metabolism and macrophage-to-feces reverse cholesterol transport at similar levels of cetp inhibition in hamsters. Eur J Pharmacol. 2014;740:135–143. doi: 10.1016/j.ejphar.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Joy T, Hegele RA. Is raising hdl a futile strategy for atheroprotection? Nat Rev Drug Discov. 2008;7:143–155. doi: 10.1038/nrd2489. [DOI] [PubMed] [Google Scholar]
- 19.Brousseau ME, Diffenderfer MR, Millar JS, Nartsupha C, Asztalos BF, Welty FK, Wolfe ML, Rudling M, Bjorkhem I, Angelin B, Mancuso JP, Digenio AG, Rader DJ, Schaefer EJ. Effects of cholesteryl ester transfer protein inhibition on high-density lipoprotein subspecies, apolipoprotein a-i metabolism, and fecal sterol excretion. Arterioscler Thromb Vasc Biol. 2005;25:1057–1064. doi: 10.1161/01.ATV.0000161928.16334.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritter MC, Scanus AM. Role of apolipoprotein a-i in the structure of human serum high density lipoproteins. Reconstitution studies. J Biol Chem. 1977;252:1208–1216. [PubMed] [Google Scholar]
- 21.Fielding CJ, Fielding PE. Evidence for a lipoprotein carrier in human plasma catalyzing sterol efflux from cultured fibroblasts and its relationship to lecithin:Cholesterol acyltransferase. Proc Natl Acad Sci USA. 1981;78:3911–3914. doi: 10.1073/pnas.78.6.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fielding CJ, Moser K. Evidence for the separation of albumin- and apo a-i-dependent mechanisms of cholesterol efflux from cultured fibroblasts into human plasma. J Biol Chem. 1982;257:10955–10960. [PubMed] [Google Scholar]
- 23.Du XM, Kim MJ, Hou L, Le Goff W, Chapman MJ, Van Eck M, Curtiss LK, Burnett JR, Cartland SP, Quinn CM, Kockx M, Kontush A, Rye KA, Kritharides L, Jessup W. Hdl particle size is a critical determinant of abca1-mediated macrophage cellular cholesterol export. Circ Res. 2015;116:1133–1142. doi: 10.1161/CIRCRESAHA.116.305485. [DOI] [PubMed] [Google Scholar]
- 24.Pászty C, Maeda N, Verstuyft J, Rubin EM. Apolipoprotein ai transgene corrects apolipoprotein e deficiency-induced atherosclerosis in mice. J Clin Invest. 1994;94:899–903. doi: 10.1172/JCI117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duverger N, Kruth H, Emmanuel F, Caillaud J-M, Viglietta C, Castro G, Tailleux A, Fievet C, Fruchart JC, Houdebine LM, Denefle P. Inhibition of atherosclerosis development in cholesterol-fed human apolipoprotein a-i–transgenic rabbits. Circulation. 1996;94:713–717. doi: 10.1161/01.cir.94.4.713. [DOI] [PubMed] [Google Scholar]
- 26.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant apoa-i milano on coronary atherosclerosis in patients with acute coronary syndromes: A randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 27.Tardif JC, Heinonen T, Noble S. High-density lipoprotein/apolipoprotein a-i infusion therapy. Curr Atheroscler Rep. 2009;11:58–63. doi: 10.1007/s11883-009-0009-7. [DOI] [PubMed] [Google Scholar]
- 28.Hewing B, Parathath S, Barrett T, et al. Effects of native and myeloperoxidase-modified apolipoprotein a-i on reverse cholesterol transport and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2014;34:779–789. doi: 10.1161/ATVBAHA.113.303044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholls SJ, Tuzcu EM, Sipahi I, Schoenhagen P, Crowe T, Kapadia S, Nissen SE. Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein a-i milano. J Am Coll Cardiol. 2006;47:992–997. doi: 10.1016/j.jacc.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 30.Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Grijalva VR, Yu N, Ansell BJ, Datta G, Garber DW, Fogelman AM. Apolipoprotein a-i mimetic peptides. Arterioscler Thromb Vasc Biol. 2005;25:1325–1331. doi: 10.1161/01.ATV.0000165694.39518.95. [DOI] [PubMed] [Google Scholar]
- 31.Didichenko SA, Navdaev AV, Cukier AM, Gille A, Schuetz P, Spycher MO, Therond P, Chapman MJ, Kontush A, Wright SD. Enhanced hdl functionality in small hdl species produced upon remodeling of hdl by reconstituted hdl, csl112: Effects on cholesterol efflux, anti-inflammatory and antioxidative activity. Circ Res. 2016;119:751–763. doi: 10.1161/CIRCRESAHA.116.308685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flynn R, Buckler JM, Tang C, Kim F, Dichek D. Helper-dependent adenoviral vectors are superior in vitro to first-generation vectors for endothelial cell-targeted gene therapy. Mol Ther. 2010;18:2121–2129. doi: 10.1038/mt.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flynn R, Qian K, Tang C, Dronadula N, Buckler J, Jiang B, Wen S, Dichek H, Dichek D. Expression of apolipoprotein a-i in rabbit carotid endothelium protects against atherosclerosis. Mol Ther. 2011;19:1833–1841. doi: 10.1038/mt.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du L, Zhang J, De Meyer GR, Flynn R, Dichek DA. Improved animal models for testing gene therapy for atherosclerosis. Hum Gene Ther Methods. 2014;25:106–114. doi: 10.1089/hgtb.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daugherty A, Schonfeld G, Sobel BE, Lange LG. Metabolism of very low density lipoproteins after cessation of cholesterol feeding in rabbits. A factor potentially contributing to the slow regression of atheromatous plaques. J Clin Invest. 1986;77:1108–1115. doi: 10.1172/JCI112409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein Y, Stein O. Does therapeutic intervention achieve slowing of progression or bona fide regression of atherosclerotic lesions? Arterioscler Thromb Vasc Biol. 2001;21:183–185. doi: 10.1161/01.atv.21.2.183. [DOI] [PubMed] [Google Scholar]
- 37.Jiang B, Qian K, Du L, Luttrell I, Chitaley K, Dichek DA. Helper-dependent adenovirus is superior to first-generation adenovirus for expressing transgenes in atherosclerosis-prone arteries. Arterioscler Thromb Vasc Biol. 2011;31:1317–1325. doi: 10.1161/ATVBAHA.111.225516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulick AH, Dong G, Newman KD, Virmani R, Dichek DA. Endothelium-specific in vivo gene transfer. Circ Res. 1995;77:475–485. doi: 10.1161/01.res.77.3.475. [DOI] [PubMed] [Google Scholar]
- 39.Gruchala M, Bhardwaj S, Pajusola K, Roy H, Rissanen TT, Kokina I, Kholova I, Markkanen JE, Rutanen J, Heikura T, Alitalo K, Bueler H, Yla-Herttuala S. Gene transfer into rabbit arteries with adeno-associated virus and adenovirus vectors. J Gene Med. 2004;6:545–554. doi: 10.1002/jgm.535. [DOI] [PubMed] [Google Scholar]
- 40.Du L, Dronadula N, Tanaka S, Dichek DA. Helper-dependent adenoviral vector achieves prolonged, stable expression of interleukin-10 in rabbit carotid arteries but does not limit early atherogenesis. Hum Gene Ther. 2011;22:959–968. doi: 10.1089/hum.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamane K, Leung KP. Rabbit m1 and m2 macrophages can be induced by human recombinant gm-csf and m-csf. FEBS Open Bio. 2016;6:945–953. doi: 10.1002/2211-5463.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein ai. Nature. 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 43.Plump AS, Scott CJ, Breslow JL. Human apolipoprotein a-i gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein e-deficient mouse. Proc Natl Acad Sci U S A. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tangirala RK, Tsukamoto K, Chun SH, Usher D, Puré E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein a-i in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 45.Benoit P, Emmanuel F, Caillaud JM, Bassinet L, Castro G, Gallix P, Fruchart JC, Branellec D, Denefle P, Duverger N. Somatic gene transfer of human apoa-i inhibits atherosclerosis progression in mouse models. Circulation. 1999;99:105–110. doi: 10.1161/01.cir.99.1.105. [DOI] [PubMed] [Google Scholar]
- 46.Belalcazar LM, Merched A, Carr B, Oka K, Chen KH, Pastore L, Beaudet A, Chan L. Long-term stable expression of human apolipoprotein a-i mediated by helper-dependent adenovirus gene transfer inhibits atherosclerosis progression and remodels atherosclerotic plaques in a mouse model of familial hypercholesterolemia. Circulation. 2003;107:2726–2732. doi: 10.1161/01.CIR.0000066913.69844.B2. [DOI] [PubMed] [Google Scholar]
- 47.Ishiguro H, Yoshida H, Major AS, Zhu T, Babaev VR, Linton MF, Fazio S. Retrovirus-mediated expression of apolipoprotein a-i in the macrophage protects against atherosclerosis in vivo. J Biol Chem. 2001;276:36742–36748. doi: 10.1074/jbc.M106027200. [DOI] [PubMed] [Google Scholar]
- 48.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM, Batshaw ML. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 49.Brunetti-Pierri N, Ng T, Iannitti D, Cioffi W, Stapleton G, Law M, Breinholt J, Palmer D, Grove N, Rice K, Bauer C, Finegold M, Beaudet A, Mullins C, Ng P. Transgene expression up to 7 years in nonhuman primates following hepatic transduction with helper-dependent adenoviral vectors. Hum Gene Ther. 2013;24:761–765. doi: 10.1089/hum.2013.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.High KA, Anguela XM. Adeno-associated viral vectors for the treatment of hemophilia. Hum Mol Genet. 2016;25:R36–41. doi: 10.1093/hmg/ddv475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wen S, Graf S, Massey PG, Dichek DA. Improved vascular gene transfer with a helper-dependent adenoviral vector. Circulation. 2004;110:1484–1491. doi: 10.1161/01.CIR.0000141574.78032.A9. [DOI] [PubMed] [Google Scholar]
- 52.Rubin EM, Ishida BY, Clift SM, Krauss RM. Expression of human apolipoprotein a-i in transgenic mice results in reduced plasma levels of murine apolipoprotein a-i and the appearance of two new high density lipoprotein size subclasses. Proc Natl Acad Sci U S A. 1991;88:434–438. doi: 10.1073/pnas.88.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang Y, DiDonato JA, Levison BS, et al. An abundant dysfunctional apolipoprotein a1 in human atheroma. Nat Med. 2014;20:193–203. doi: 10.1038/nm.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feldman LJ, Steg PG, Zheng LP, Chen D, Kearney M, McGarr SE, Barry JJ, Dedieu JF, Perricaudet M, Isner JM. Low-efficiency of percutaneous adenovirus-mediated arterial gene transfer in the atherosclerotic rabbit. J Clin Invest. 1995;95:2662–2671. doi: 10.1172/JCI117968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: Insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5:91–102. doi: 10.1038/ncpcardio1086. [DOI] [PubMed] [Google Scholar]
- 56.Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest. 1990;85:1234–1241. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine--reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 58.Cockerill GW, Rye KA, Gamble JR, Vadas MA, Barter PJ. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:1987–1994. doi: 10.1161/01.atv.15.11.1987. [DOI] [PubMed] [Google Scholar]
- 59.Nicholls SJ, Dusting GJ, Cutri B, Bao S, Drummond GR, Rye KA, Barter PJ. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation. 2005;111:1543–1550. doi: 10.1161/01.CIR.0000159351.95399.50. [DOI] [PubMed] [Google Scholar]
- 60.Wu BJ, Chen K, Shrestha S, Ong KL, Barter PJ, Rye KA. High-density lipoproteins inhibit vascular endothelial inflammation by increasing 3beta-hydroxysteroid-delta24 reductase expression and inducing heme oxygenase-1. Circ Res. 2013;112:278–288. doi: 10.1161/CIRCRESAHA.111.300104. [DOI] [PubMed] [Google Scholar]
- 61.Schulick AH, Vassalli G, Dunn PF, Dong G, Rade JJ, Zamarron C, Dichek DA. Established immunity precludes adenovirus-mediated gene transfer in rat carotid arteries. Potential for immunosuppression and vector engineering to overcome barriers of immunity. J Clin Invest. 1997;99:209–219. doi: 10.1172/JCI119149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van-Assche T, Huygelen V, Crabtree MJ, Antoniades C. Gene delivery strategies targeting stable atheromatous plaque. Curr Pharm Des. 2013;19:1626–1637. [PubMed] [Google Scholar]
- 63.Muck-Hausl M, Solanki M, Zhang W, Ruzsics Z, Ehrhardt A. Ad 2.0: A novel recombineering platform for high-throughput generation of tailored adenoviruses. Nucleic Acids Res. 2015;43:e50. doi: 10.1093/nar/gkv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.