Abstract
Excess mortality in persons with severe mental disorders (SMD) is a major public health challenge that warrants action. The number and scope of truly tested interventions in this area remain limited, and strategies for implementation and scaling up of programmes with a strong evidence base are scarce. Furthermore, the majority of available interventions focus on a single or an otherwise limited number of risk factors. Here we present a multilevel model highlighting risk factors for excess mortality in persons with SMD at the individual, health system and socio‐environmental levels. Informed by that model, we describe a comprehensive framework that may be useful for designing, implementing and evaluating interventions and programmes to reduce excess mortality in persons with SMD. This framework includes individual‐focused, health system‐focused, and community level and policy‐focused interventions. Incorporating lessons learned from the multilevel model of risk and the comprehensive intervention framework, we identify priorities for clinical practice, policy and research agendas.
Keywords: Excess mortality, physical health, severe mental disorders, schizophrenia, bipolar disorder, depression, risk factors, individual‐focused interventions, health system‐focused interventions, community level and policy‐focused interventions
Persons with severe mental disorders (SMD) − i.e., schizophrenia and other psychotic disorders, bipolar disorder, and moderate‐to‐severe depression − die 10 to 20 years earlier than the general population. This premature mortality has been well documented in meta‐analyses and systematic reviews1, 2, 3, 4, 5, 6, 7. Additionally, recent studies, commentaries and editorials have brought heightened awareness and attention to the topic8, 9, 10, 11, 12. Despite this, little to no progress has been made – in fact, evidence suggests that the gap may be increasing over time4, and recently published studies show standardized mortality ratios that are higher than those previously reported13.
The majority of deaths in persons with SMD are due to preventable physical diseases, especially cardiovascular disease, respiratory disease, and infections14, 15, 16. These persons have a 2 to 3 times higher risk of dying from cardiovascular diseases than the general population17, 18. Up to 75% of persons with schizophrenia (compared to 33% of the general population) die of coronary heart disease19. Persons with SMD die of respiratory diseases at 2 to 6 times the rate of the general population5, 15, 20, even after controlling for tobacco smoking and substance abuse, and die of infectious diseases at about 2 to 4 times the rate of the general population4. These persons are also more likely to die of diabetes mellitus15 and cancers21. In low‐ and middle‐income countries (LMICs), available studies suggest that excess mortality is similar, if not worse – with the large majority dying of physical diseases, especially infectious diseases16.
The remaining deaths in persons with SMD are due to unnatural causes, including suicide, homicide and accidents. Suicide continues to be an important cause of death, especially in the first year following discharge from an inpatient unit22. Compared with the general population, persons with SMD are about 2‐3 times more likely to die by accidental death, which appears more common than suicide in this population18, 23. Furthermore, persons with SMD appear to be overrepresented among homicide victims and are 2‐4 times more likely to die by homicide or violent deaths than the general population7, 24.
Overall patterns of mortality appear similar across countries, but there are likely differences in which solutions are needed. In the following sections, we present: a) a multilevel model of risk for excess mortality; b) a comprehensive framework, informed by the multilevel model of risk, to guide the development and implementation of effective interventions that offer the promise of reducing excess mortality in persons with SMD25, 26; c) a set of priorities for clinical practice, policy and research agendas in this area. The aims of this paper are in line with the vision statement of the World Health Organization (WHO) Comprehensive Mental Health Action Plan 2013‐202027, which underscores the importance of persons with mental disorders to be able to enjoy the full range of human rights and to access high‐quality, culturally‐appropriate health and social care in a timely way to promote recovery.
MULTILEVEL MODEL OF RISK FOR EXCESS MORTALITY
The multilevel model of risk (Table 1) highlights risk factors for excess mortality in persons with SMD at the individual, health system and socio‐environmental levels.
Table 1.
Multilevel model of risk for excess mortality in persons with severe mental disorders (SMD)
| Individual factors | Health systems | Social determinants of health |
|---|---|---|
|
Disorder‐specific
• Severity of disorder • Family history • Symptoms/pathophysiology • Early age of onset • Recency of diagnosis Behaviour‐specific • Tobacco use • Poor diet • Inadequate physical activity • Sexual and other risk behaviours • Substance use (alcohol and drugs) • Low motivation (e.g., treatment seeking, adherence) |
Leadership
• Absence of relevant policies and guidelines Financing • Low investment in quality care Information • Limited health information systems Service delivery • Verticalization and fragmentation of health services • Lack of care coordination and management • Limited access to services Human resources • Poor quality service provision • Negative beliefs/attitudes of workforce • Poor communication Medications • Antipsychotic medications (no treatment, polypharmacy, higher than recommended dosages) |
Public policies
• Discriminating policies • Low financial protection and limited coverage in health packages Socio‐economic position • Unemployment • Homelessness • Low health literacy Culture and societal values • Stigma and discrimination in society • Negative perceptions about persons with SMD Environmental vulnerabilities • Infections, malnutrition • Access to means of suicide • Impoverished or unsafe neighbourhoods Social support • Limited family, social and community resources |
Risk factors at the individual level include characteristics inherent to SMD or an individual's health‐related behaviours. These can be related to the severity of the SMD (e.g., symptoms, hospitalizations, impulsivity, physiological and emotional dysregulation); affect the engagement or interaction of the person with the health care system (e.g., cognitive deficits, social skills deficits, low motivation or mistrust of providers); or include behaviours that lead to or exacerbate health problems. Importantly, about 50‐60% of persons with SMD smoke, one of the leading preventable causes of death28. Moreover, persons with SMD tend to have poorer diets29 and more sedentary lifestyles30 than the general population.
Health system factors include treatments, delivery of services, and organizational characteristics such as the workforce or information systems infrastructure. These often vary across different settings. As an example, a mainstay of treatment for many persons with SMD is antipsychotic medications, which are associated with well‐known side effects that can contribute to obesity, glucose intolerance and dyslipidemia31, 32. Depending on the setting, both a lack of antipsychotic medication16 and excess dosing of this medication33, 34 appear to be risk factors for elevated mortality. Once antipsychotic medications are prescribed, monitoring for potential side effects is important and requires knowledge and communication between providers10.
Persons with SMD often receive poor quality of physical health care, spanning from health promotion and disease prevention to intervention. Although they have two times as many health care contacts, they receive less physical check‐ups and screenings, less prescriptions and procedures35, 36, and less cardiovascular and cancer diagnoses, even though they have a higher risk of dying from these conditions15, 35, 37. For example, in a study from Western Australia, although persons with SMD had the same cancer incidence as the general population, they were more likely to die from cancer22. Even under universal health care, persons with SMD do not receive adequate treatment for cardiovascular problems, such as a coronary artery by‐pass, prescriptions of beta‐blockers and statins, admissions for stroke, and revascularization procedures36, 37.
When hospitalized for medical care, persons with SMD often have poor outcomes, including more adverse events, more days in an intensive care unit and more complications than those without SMD38, 39. There is also evidence for a time dimension to appropriate care: many studies highlight a peak in excess mortality for both natural and unnatural causes during the first year after discharge from hospital16, 18, suggesting a systematic failure of the health care system to prevent, identify and treat physical diseases during hospitalization for a mental disorder.
Some authors suggest that the poorer health outcomes could be related to providers’ negative beliefs and attitudes towards persons with SMD, including beliefs about the causes of illnesses, ability of persons with SMD to maintain an active and healthy lifestyle, or other beliefs about functioning40. Mental health and primary care providers’ attitudes towards patients with SMD appear related to treatment intentions, including their likelihood of referring patients to a specialist or refilling their prescription41. There is evidence of variation in the quality of care depending on the provider, insurer and type of health care system42.
Fragmented health care systems (e.g., dichotomized physical and mental health care) present a challenge to meeting the complex physical health needs of persons with SMD43. A component cause may be the limited expertise of mental health providers to recognize and address physical health care needs, and of physical health providers to address the full range of health concerns of those with SMD10.
Social determinants of health include, but are not limited to26, public policies, an individual's socio‐economic position, cultural and societal values, environmental vulnerabilities and social support. Persons with SMD often have limited access to health care either due to cost or denial of insurance coverage44. They are also more likely to be poor and at risk for homelessness. In high‐income countries, homelessness and a low socio‐economic status confer additional mortality risk to those with SMD45, 46. Disability associated with the disorder may contribute towards unemployment, which is a strong independent risk factor for increased mortality15, 47.
Persons with SMD also tend to live in less safe neighbourhoods, have less access to healthy foods, and have less opportunities to be involved in healthy activities, which may contribute to poor lifestyle behaviours. They may be perceived as dangerous by others, which may drive the high rates of homicide victimization. A large majority has limited social support, including never being married (e.g., nearly 75%15) or limited family involvement. When family members are involved, they may already be under a heavy caregiver burden, and additional physical health problems may overstretch family support16.
It is important to emphasize that these factors are intertwined, and interrelationships at multiple levels likely contribute towards excess mortality. No single factor alone causes excess mortality: persons with SMD have high rates of adverse health behaviours, including tobacco smoking, substance use, physical inactivity and poor diet; yet, studies clearly demonstrate the role of factors beyond disorder‐specific and lifestyle behaviours in excess mortality. For example, although a large majority of persons with SMD die of cardiovascular diseases, only 25% of them receive a diagnosis for this – after controlling for whether a person had received a diagnosis, the risk due to ischemic heart disease approximates that of the general population15.
Parceling out the effects of clinical factors, health system factors and socio‐economic factors continues to show that factors at each level are involved48. In general, the more factors included in the model, the more variance is accounted for in excess mortality5.
MULTILEVEL INTERVENTION FRAMEWORK TO REDUCE EXCESS MORTALITY
A number of interventions, guidelines and programmes have been developed to address correlates of excess mortality in persons with SMD. These primarily target mental health, lifestyle behavioural risk factors, and screening for and management of physical health conditions. Some interventions have proven to be effective but are not widely disseminated; others have not been rigorously tested; for some the evidence is mixed or inconclusive. For example, care programmes with an emphasis on monitoring and managing the adverse metabolic effects of antipsychotics are being implemented in several contexts, but many have not been well evaluated. Overall, the number and scope of truly tested interventions remain limited, and strategies for implementation and scaling up of programmes with a strong evidence base are scarce. Moreover, the majority of available interventions focus on a single or an otherwise limited number of risk factors.
Informed by the multilevel risk factor model, we describe here a comprehensive framework that may be useful for designing, implementing and evaluating interventions and programmes to reduce excess mortality in persons with SMD (Figure 1).
Figure 1.
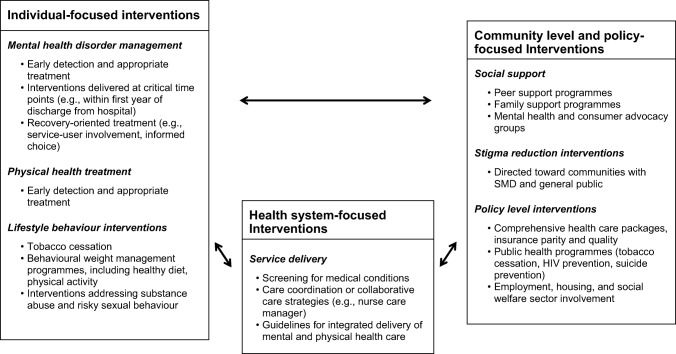
Multilevel model of interventions to reduce excess mortality in persons with severe mental disorders (SMD)
Our first level of interventions is individual‐focused, while the second focuses on health systems. We then incorporate socio‐environmental interventions emphasizing broader social determinants of health, including social support and stigma reduction. Some programmes address components at multiple levels (e.g., simultaneously targeting individual behaviours and health systems through behavioural weight management plus care coordination); we have categorized them based on the main emphasis of the programme. The assumption of our framework is that an effective approach must comprehensively target individual behaviours, health systems and social determinants of health. However, the effective and scalable combinations of these different interventions have yet to be fully evaluated.
Individual‐focused interventions
Interventions at this level include strategies delivered to the individuals with SMD to target their mental health condition, physical health and lifestyle behaviours. Although individual‐focused interventions are described separately, the implementation and impact of these interventions are likely affected by the functioning of the larger health care system.
Mental health disorder management
Persons with SMD first of all require an early detection and appropriate treatment of their mental health condition. Especially in LMICs, no access to treatment or a long interval before mental health treatment is started can increase the risk for mortality16, 49. A comprehensive tool to address most major mental health conditions, the Mental Health Gap Action Programme (mhGAP) intervention guide50, incorporates evidence‐based recommendations for a range of disorders, including SMD. The guide's innovation is in facilitating the delivery of evidence‐based mental health interventions in LMICs through primary health care services, using specific assessments and decision points to reach a comprehensive management plan for each person. Although research on the implementation and impact of the guide is still ongoing, it offers a promising approach to effective and efficient delivery of mental health services.
Appropriate administration of medications can reduce excess mortality in persons with SMD. Recent studies and evidence summaries highlight the beneficial impact on mortality of continuous medication treatment30, 51, proper dosing ranges33 and current and long‐term use compared with no medication, particularly in schizophrenia52. Adherence to medication guidelines − such as the American Schizophrenia Patient Outcomes Research Team (PORT) Treatment Recommendations53 − appear to have an effect on reducing mortality in schizophrenia. Recovery‐oriented programmes, with a focus on psychoeducation and increased awareness of symptoms, coping with stress and problem‐solving skills, are also beneficial54, as well as strategies supporting people with SMD and their families around treatment engagement55.
The risk for suicide is highest within a year following discharge from a psychiatric hospitalization56, with at least one quarter of cases occurring within 30 days of discharge57. Thus, suicide prevention interventions58 need to be an important component in mental health treatment plans for those with SMD, especially early in the course of illness. In addition, persons with SMD are commonly victims of interpersonal violence, with a recent meta‐analysis estimating the prevalence of recent violence at 24%59. Few interventions have addressed victimization in persons with SMD, and more studies are needed in this area.
Physical health treatment
Medical treatment for hypertension, diabetes mellitus and dyslipidemia should be similar for those with SMD as they are for the general population. However, self‐management components (e.g., for diabetes) may require tailoring which accounts for cognitive, functional or motivational deficits.
Available evidence suggests that interventions to improve screening for obesity, hyperlipidemia and hypertension have been effective at improving the detection of these conditions among persons with SMD42, although much more work is needed in this area.
Interventions addressing lifestyle behaviours
Tobacco cessation interventions have proven beneficial in adults with schizophrenia and are recommended at the earliest possible phases of treatment42, 53. Combination treatment with counseling and bupropion with or without nicotine replacement therapy or varenicline is efficacious and has benefits on both point abstinence and continuous abstinence from tobacco, though relapse is common60. Longer‐term studies are needed to better understand optimal treatment duration, and importantly more work is needed to incorporate evidence‐based tobacco cessation treatment into regular health care management for persons with SMD who smoke.
Behavioural weight loss programmes tailored for persons with SMD have been shown in randomized clinical trials to be successful in achieving clinically significant weight loss61, 62, 63. Effective interventions are often built on those shown to be successful for improving diet and increasing exercise in the general population, but with adaptations for cognitive needs of those with SMD, such as tailoring content and delivery to address memory and executive function deficits, and emphasizing environmental supports64, 65, 66.
Some questions remain unanswered for optimal implementation and dissemination of these programmes, especially in LMICs. These include: a) what will be the true needed duration and intensity for long‐term effectiveness of healthy weight interventions, as they are likely relatively labor intense (e.g., more frequent contacts) in persons with SMD; b) who should deliver these interventions in different types of environments; c) how can lay providers be trained to deliver effective weight management and other healthy lifestyle behaviour change interventions to this population.
There is a limited evidence base on effectiveness of interventions addressing substance abuse and risky sexual behaviour. The literature on interventions for reducing substance abuse in persons with SMD is large but inconsistent67. Outcomes for these interventions remain limited, especially due to problems with engagement and retention in programmes68. The impact of interventions for reducing risky sexual behaviours is also limited, even though they might be able to increase other health promoting behaviours, such as immunizations. For example, one comprehensive intervention programme delivered in mental health care settings addressed screening, testing, immunization, reducing risky behaviours and medical referrals for HIV and hepatitis, using a health promotion empowerment model; however, although participants had a higher prevalence of hepatitis B and C testing, higher immunization for hepatitis A and B, increased hepatitis knowledge and decreased substance use than the control group, risky sexual behaviour did not decrease69.
Health system‐focused interventions
The next level in the framework encompasses interventions and programmes within health systems targeting health care providers and service delivery components. These will vary across different settings depending upon many parameters, such as the number of specialists versus primary care providers, the different distribution of health risk factors, the presence or absence of universal health care, and the availability of health technologies and medications. Strengthening of the six building blocks of the health systems – service delivery; health workforce; information; medical products, vaccines and technologies; financing; and leadership and governance (stewardship) – would improve outcomes for persons with SMD70.
Care coordination, collaborative care or integrated care programmes include support to better equip health systems, usually through the provision of additional supportive members who can serve as a liaison between mental health and physical health care systems or through linking of delivery of physical and mental health services. Few randomized trials have tested care coordination programmes for physical health conditions and cardiovascular risk factors in adults with SMD.
One intervention used a nurse care manager at a community mental health center to help participants become more involved in their own health care, communicate with physical and mental health providers, and assist in minimizing system‐level barriers for health care71. At 12 months, nearly 60% of those in the intervention group received recommended preventive services compared to just over 20% in the control group. In addition, the former were more likely to have a primary care provider (71.2% vs. 51.9%) and, among the subset with laboratory data, they had lower (better) Framingham cardiovascular risk scores71.
A recent trial examined a one‐year intervention of care coordination alone, lifestyle coaching plus care coordination, or treatment as usual in adults with schizophrenia‐spectrum disorders and increased waist circumference, with a primary outcome of cardiovascular risk reduction72. A nurse delivered care coordination, including contacting primary care providers and communicating test results and need for physical health care to participants. Lifestyle coaching provided weekly home visits with cardiovascular risk factor counseling based on individual participant preferences. The study did not find differences in outcomes, which may be due in part to the preexisting high quality of health care delivery. Also, while incorporating participant preferences is an important component of behaviour change, the resultant lifestyle coaching may not have been efficacious enough for change in risk behaviours. As suggested by the authors, environmental change may be a next step to investigate for lifestyle modification in that setting72.
Guidelines that incorporate combinations of screening for physical health conditions, care coordination among mental health and primary care providers, metabolic monitoring, and delivery of medical services in mental health settings have been implemented in several countries, including the US, the UK and Australia73, 74, 75, 76, 77, 78.
In the US, the Substance Abuse and Mental Health Services Administration funded 187 grants since 2009 for community‐based agencies to create or increase the capacity to provide primary care services to persons with SMD at settings where they already receive mental health care79. An evaluation of the first years of the program reported that sites provided a range of integrated behavioural health and primary care services to persons with need for care80. Challenges included lower than estimated consumer engagement, financial sustainment, and organizational culture issues. In addition, implementation of lifestyle behavioural interventions for weight management and tobacco smoking was challenging. Several suggestions were put forth for current and future agencies receiving funding, such as incorporating strategies to improve consumer access to services and addressing fidelity to evidence‐based wellness interventions.
In Australia, the Western Australia Department of Health Mental Health Division developed a package of Clinical Guidelines for the Physical Health Screening of Mental Health Consumers and a set of Health Nurse Practitioner protocols81. The package was built up as a preventive, evidence‐based framework for mental health services, to facilitate coordination of care between health providers and with mental health consumers, relevant for hospital, clinic or community care settings. A 2010 report76 showed three key areas of concern: standardization across services, fidelity and frequency of use, and sustainability of the guidelines. Recommendations included management plans modified for each setting and coordination between health professionals to prevent failure to screen or redundant screening.
The set of protocols focuses on nurse practitioners in mental health, and highlights their role as both coordinators and providers, including for: comprehensive physical health evaluation; management and referral; education and support to consumers; enhancing continuity of care for patients; facilitating communication, appropriate access and utilization of hospital services for persons with SMD; collaboration between mental health professionals and primary care, including dieticians and other lifestyle consultants; provision of health promotion; assisting the patient in making appointments or involving the case manager in ensuring the patient is able to attend appointments.
In New South Wales, Australia, a metabolic monitoring programme82 is used to guide public mental health workers to monitor and manage metabolic syndrome and provide education to clinicians and patients. A study showed that this was implemented with about 60% coverage of monitoring of blood glucose and lipids and 54% of weight measurement. The compliance with measurement of waist circumference was lower (7%)83.
In the UK, the National Institute for Health and Care Excellence (NICE) guidelines on psychosis and schizophrenia74 include direction about providers’ assessment and treatment of physical health conditions, and routine monitoring of the physical health side effects of medication, offering behavioural counseling and linking to other guidelines (e.g., obesity or diabetes) when appropriate. Since 2009, NICE has recommended that mental health care providers routinely monitor weight and cardiovascular and metabolic indicators of morbidity in people with SMD and offer interventions for obesity, lipid modification or preventing type 2 diabetes, as appropriate. In 2014, NICE provided updated guidelines about physical health in persons with SMD, specifically new tobacco cessation recommendations. In addition, the guidelines specifically called for data collection on the prevalence of those with schizophrenia who received combined healthy eating and physical activity interventions and tobacco cessation interventions.
Most recently, a multi‐country effort has encouraged the use of the Lester UK Adaptation of the Australian Positive Cardiometabolic Health Resource, which summarizes safe interventions to help frontline staff make assessments of cardiac and metabolic health in persons with SMD78. Several dissemination efforts include a downloadable poster and forms for clinicians and clinics, service user cards for persons with SMD to approach their general practitioner or mental health provider in order to get additional help, and an action planning toolkit to help with the health care delivery system implementation of the resource.
This level of the intervention framework also includes health care leaders implementing national and international guidelines for care of persons with SMD in their organization, and aligning financing policy and information systems for the missions of improving and monitoring quality of care63. An important question for organizational leaders is who will deliver an evidence‐based preventive health or care coordination intervention to decrease premature mortality in SMD. For example, dieticians and exercise leaders may be cost prohibitive, and sustainability may be more likely if mental health employees could deliver a physical health intervention. However, if mental health providers are to implement the intervention, they will likely need specific training and supervision. This is an important area for future research.
While many components of these health system‐focused interventions are evidence based, implementation of these programmes and guidelines on the whole have not been formally evaluated for their success in achieving their intended outcomes. Several doubts remain about sustainability and the most effective and cost‐efficient model of care. Furthermore, these programmes are largely based on high‐income countries; the degree to which they are feasible in LMICs will be an important area of further study. Meanwhile, as the provision of mental health care grows in LMICs in primary care settings49, these settings may provide opportunities to further test and refine effective models of mental health care that can reduce excess mortality.
Interventions focused on socio‐environmental determinants
The broadest level of the framework incorporates socio‐environmental factors and the social determinants of health. This part of the model acknowledges the range of potential interventions originating from the community to address contributors to premature mortality.
Peer support programmes, family support programmes and mental health consumer groups84 are important potential resources that can implement or assist with health interventions, whether focused on health behaviours, chronic disease self‐management, or recovery‐based programmes.
Evidence for peer‐led interventions for chronic disease self‐management appears promising: a 6‐week programme tailors chronic disease self‐management interventions for those in the general population to those with SMD, delivered by peers with SMD85, and addresses tasks common across chronic health conditions such as action planning and feedback, modeling of behaviours and problem‐solving, reinterpretation of symptoms and training in specific disease management techniques. The programme has been shown to improve health status and efficiency of health care utilization. The available evidence shows improvements in quality of life, medication adherence, and a primary care visit86. In a small randomized trial of a different adaptation of the same programme, also using peers with SMD and consisting of 13 weekly group sessions, participants showed improvement in self‐management and better use of health care compared to controls85. Both of these studies had relatively short follow‐up and used self‐report measures for outcomes; however, they support recovery‐oriented illness self‐management interventions for persons with SMD and a chronic medical condition as well as roles for peers with SMD to deliver these interventions. More work is needed to develop the evidence base for peer‐led and peer‐supported interventions to improve physical health in persons with SMD.
Stigma reduction programmes87, 88 also appear important for improving the lives of persons with SMD, within and beyond the health care community. A recent review of effective interventions to reduce mental health related stigma and discrimination reported that, for the general population, interventions can improve short‐term attitudes, and of these, social‐contact based interventions seem to be the most effective. For those with mental disorders, group‐level interventions appear helpful. However, across studies for those with and without SMD, further research is needed with strong designs, longer term follow‐up and a focus on mental health consumers’ perceptions of stigma. In addition, studies should examine behavioural and not only attitudinal change as a result of interventions to decrease stigma and discrimination88, as well as effective stigma reduction strategies in LMICs87.
On a wider scale, policies that have a beneficial effect on all individuals may also be beneficial for those with SMD, or policies may need to be shaped specifically to influence health for persons with SMD. For example, public health policies providing mental health parity could greatly improve lives of those with SMD. Employment programs89 and policies to provide stable housing may impact the ability of persons with SMD to fully integrate into society, which should lead to improved physical health. Policy‐level interventions that affect screening or management of suicide, HIV or tobacco smoking are especially relevant to those with SMD and may have even greater effects on the health and well‐being of this high‐risk population. Knowing how policy‐level interventions need modifications to best improve and lengthen the lives of persons with SMD will be important for future impact. For example, protection legislation may be in force, but individuals may not seek this protection due to not wanting to be identified as having a mental disorder.
In the UK, the Health and Social Care Act 2012 established new legal responsibility for the national health system to deliver parity between mental and physical health, i.e., ensuring that there is as much focus on improving mental health as physical health and that persons with mental health problems receive an equal standard of care. Furthermore, the Commissioning for Quality and Innovation Scheme provides additional income for national health system trusts that meet specific indicators for people with mental health problems under that care, including recording relevant data on patient health, completing yearly physical health checks, and encouraging smoking cessation. Critically, the scheme mandates communication with the patient's general practitioner on discharge from hospital or after review by a community team. Sustainability of such policies will be important in the future.
In the US, a proposed option is the designation of persons with SMD as a health disparity group by the federal government, which would also require the tracking of vital health statistics separately for this population and make them eligible for more technical assistance opportunities63.
Importantly, the factors in this part of the model link across to both health system and individual‐focused interventions. Public health policies affect health systems, and specific environmental or social support programmes are often implemented through health systems (e.g., peer support programmes). Public health policies such as mental health parity or insurance coverage affect the services that the individual mental health consumer can access and will be critical to their sustainability. However, an evidence base for policies that effectively reduce excess mortality in persons with SMD is still needed.
PRIORITIES FOR CLINICAL PRACTICE, POLICY AND RESEARCH AGENDAS
Incorporating lessons learned from the multilevel model of risk for excess mortality and the comprehensive intervention framework, we prioritize the following key action points for clinical practice, policy and research agendas to decrease excess mortality in persons with SMD.
Clinical practice
Evidence from current literature combined with principles of health equity provide sufficient rationale to advance certain practice concepts. Individual practitioners can take steps now to provide guideline‐consistent care. At minimum, the same guidelines for physical health care as the general population can be offered to persons with SMD. Practitioners should be especially attuned not to overlook somatic concerns and to pay attention to the lifestyle behaviours and physical health of persons with SMD.
The evidence base and considerations for health equity support the following practices:
Coordination of outpatient support efforts is recommended in the first year after discharge from psychiatric hospitalization (e.g., following‐up with health care providers, continuity of care) to help with reducing suicides57. This may be especially needed among certain age groups of those with SMD who are at a high risk of suicide22.
Patients with SMD should have providers responsible for their mental health and physical health. If these are different providers (e.g., psychiatrist and primary care physician), there should be communication and coordination between them, so that screening, preventive services, and monitoring for antipsychotic side effects (if applicable) are ensured10, 76, 78.
Patients with SMD should be offered the same basic health screenings90 as the general population (e.g., cardiovascular risk and cancer).
Providers should address tobacco cessation with every patient with SMD. Persons with SMD can quit and many want to quit smoking; however, practitioners often do not address tobacco cessation91, 92, 93.
Lifestyle interventions with an evidence base in SMD to address health behaviours, such as diet and physical activity, should be implemented. Behavioural interventions, if not already tailored, will likely need to be modified to account for motivational and cognitive challenges in this population. These may include social support strategies and environmental supports42.
Persons with SMD should be viewed as a vulnerable population characterized by significant health care disparities. For example, for interventions including smoking cessation, provider training and materials specific to those with SMD may be recommended. Adding environmental supports (i.e., resources or cues in the environment that facilitate functioning, such as smartphone reminders), strategies to adapt for cognitive and motivational deficits (e.g., breaking large tasks or pieces of information into smaller components, repetition, multimodal delivery of information), increased frequency of contact, and social support may help health provider interactions be most effective.
These clinical practice action points are made with an understanding that implementation will vary based on the distribution of specialists, primary care providers and lay health providers in different countries.
Policy
At the international level, reducing excess mortality in persons with SMD should be part of the broader health agenda. The WHO Mental Health Action Plan 2013‐2020 established mental health as a fundamental component of WHO's definition of health, with objectives that include comprehensive and integrated mental health care services27. Mental health is now included as a priority in the United Nations Sustainable Development Goals. Reducing the life expectancy gap in those with SMD would also be a major step towards the goals of achieving universal health care coverage, effective treatment of non‐communicable diseases, tobacco cessation, and suicide reduction58. These policies further promote the rights of persons with SMD to attain the highest level of health possible and full participation in society and at work.
Internationally, top‐level integration in the plans and programmes among various efforts (e.g., mental health and substance abuse, non‐communicable diseases, tobacco cessation, violence prevention, nutrition and physical exercise) would set a precedent for combining efforts and making strides in addressing complex, multifactorial health problems. This might lead to special considerations specifically for those with SMD across health domains that can help with closing the health equity gap in this vulnerable population. For example, the Package of Essential Noncommunicable (PEN) disease interventions for primary health care in low‐resource settings recommends counseling for all health behaviours in the general population94. Persons with SMD may need more resources and more targeted approaches to implement any given guideline than the general population, and special considerations for this population (such as supportive assistance, longer duration and intensity of interventions, and cognitive tailoring) might be included in these documents. Such policies further converge with WHO Mental Health Action Plan's six cross‐cutting principles of universal health care coverage, human rights, evidence‐based practice, a life course approach, a multisectoral approach, and the empowerment of persons with SMD.
At the national level, policies should be geared at strengthening existing health care platforms. These will facilitate the delivery and integration of effective interventions into the health system and the community to improve mental health95.
In addition to specific programmes targeting services for individuals and populations, national policies should enable and provide sufficient resources for routine data collection of key indicators of excess mortality in persons with SMD at local facilities, national and regional databases. Health information and surveillance systems will be needed to monitor mortality records and cite trends. Country‐level data need to be specific to the needs of their populations, examining the impact of excess mortality in persons with SMD on disabilities and deaths, including prevalence of cardiovascular risks, infectious diseases and other relevant conditions. This will be especially important for LMICs, where trends and needs may be different from high‐income countries. Ultimately, this will allow for both intra‐country and international comparisons and provide data to inform efforts to close the mortality gap.
Research
Scientists working to understand causes of excess mortality and design and test interventions and programmes to decrease contributors to premature death in persons with SMD have made progress in recent years, and this is reflected in the evidence supporting the multilevel model of risk presented in this paper. At the same time, there is a need to delineate specific risk factors more clearly, identify which ones are modifiable, and how these may be different across settings, particularly in LMICs.
While evidence for mental health treatments is strong, the evidence for effectiveness of interventions in ordinary settings to prevent and treat physical conditions in those with SMD is limited. Also missing in the literature is the role of resilience and other factors that may be protective, and a parsing out of the roles of factors that are intrinsic to SMD versus those related to socio‐economic and health system variables. This includes the need for a better understanding of attributable risk for excess mortality in those with SMD.
While evidence exists for the effectiveness of specific behavioural and pharmacological interventions for unhealthy dietary habits, sedentary life style and tobacco smoking cessation, behavioural intervention trials for other risk behaviours are needed, especially for comorbid substance abuse. For current evidence‐based interventions, research is needed on optimal length and dose needed to positively affect health, which will also be important for resource allocation. Timing of these behavioural and pharmacological interventions may also instigate health benefits.
Interventions developed for the general population geared at non‐communicable diseases, infectious diseases or other health problems are likely less effective for persons with SMD, given cognitive deficits and special needs of this population. Thus, interventions for SMD require tailoring. However, more work is needed on the degree of tailoring required. Multimodal approaches, which can include behavioural plus pharmacological interventions and include components such as peer support or technology are promising, but have yet to be studied systematically to clarify whether or which multi‐component programs are effective, and which components of the intervention are most beneficial. Recent results suggest that some combined approaches may not be effective or may be dependent on existing health care systems72. We need to consider how structural interventions can facilitate these efforts. Many people with SMD have multiple cardiovascular and other risk behaviours which may be modifiable, and future research studies should test interventions addressing multiple risk factors, as well as those which are directly linked to mortality.
Research is needed to identify and manage barriers to and facilitators of implementing evidence‐based guidance and policy recommendations at all levels (individual, health systems and social determinants) of the intervention framework. We need to understand how to deliver evidence‐based interventions successfully in the real world, taking into account training and workforce issues and often‐limited resources in local community settings. We need to understand to what extent interventions and programmes could or should be disseminated across countries.
Another important area of research will be to assess the effects of health system and policy interventions on excess mortality in SMD. We need to understand why those with SMD have not benefitted from trends in the general population towards reduced mortality in some diseases and smoking cessation. Researchers should take advantage of natural experiments and also design studies in health systems and at the population level to evaluate the impact of these programmes.
Although several guidelines for screening, monitoring and management of mental health and physical conditions have been developed from evidence‐based best practices, the implementation of these guidelines has not been studied systematically in order to support their widespread application and impact on risk factors for excess mortality in persons with SMD. Similarly, integrated care programmes will need to be evaluated for their actual effectiveness on risk factors for excess mortality. Care coordination approaches are often elements of these integrated care programmes and have utilized providers, nurses, peers and others to play key roles in facilitating the adequate provision and connection of mental health and physical health care. Questions remain regarding the appropriate elements of care coordination, including tasks, roles and responsibilities of involved persons. Finally, as these are resource‐intensive programmes, cost‐effectiveness models of different approaches96 in persons with SMD will be important, especially in low‐resource settings. This will be particularly needed as we seek to prioritize understanding risk factors for premature mortality of persons with SMD in LMICs.
CONCLUSIONS
Excess mortality in persons and populations with SMD remains an important global public health problem. Persons with SMD represent a vulnerable group with many and large health care needs. Despite known risk factors for premature mortality, evidence for effective interventions in persons with SMD is limited.
In this paper we proposed and described models to better understand the complex relationships among risk factors and correlates of mortality, and to conceptualize interventions at the individual, health system and socio‐environmental levels. These models guided us to outline key action points for clinical practice, policy and research agendas to move towards health equity for those with SMD.
ACKNOWLEDGEMENTS
All the elements of this paper were discussed during a consultation convened by the Department of Mental Health and Substance Abuse at the World Health Organization (WHO) Headquarters in Geneva in November 2015, and financially supported by Fountain House, New York. S. Saxena and T. Dua are staff members of the WHO. They are responsible for the views expressed in this publication, which do not necessarily represent the decisions, policy or views of the WHO. N.H. Liu and G.L. Daumit are joint first authors of this paper.
REFERENCES
- 1. Wahlbeck K, Westman J, Nordentoft M et al. Outcome of Nordic mental health systems: life expectancy of patients with mental disorders in Denmark, Finland and Sweden 1987‐2006. Br J Psychiatry 2011;199:453‐8. [DOI] [PubMed] [Google Scholar]
- 2. Chesney E, Goodwin GM, Fazel S. Risks of all‐cause and suicide mortality in mental disorders: a meta‐review. World Psychiatry 2014;13:153‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laursen TM, Musliner KL, Benros ME et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord 2016;193:203‐7. [DOI] [PubMed] [Google Scholar]
- 4. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia. Arch Gen Psychiatry 2007;64:1123‐31. [DOI] [PubMed] [Google Scholar]
- 5. Cuijpers P, Vogelzangs N, Twisk J et al. Comprehensive meta‐analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry 2014;171:453‐62. [DOI] [PubMed] [Google Scholar]
- 6. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta‐analysis. JAMA Psychiatry 2015;72:334‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hayes JF, Miles J, Walters K et al. A systematic review and meta‐analysis of premature mortality in bipolar affective disorder. Acta Psychiatr Scand 2015;131:417‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maj M. Physical illness and access to medical services in persons with schizophrenia. Int J Ment Health 2008;37:3‐21. [Google Scholar]
- 9. Thornicroft G. Physical health disparities and mental illness: the scandal of premature mortality. Br J Psychiatry 2011;100:441‐2. [DOI] [PubMed] [Google Scholar]
- 10. De Hert M, Cohen D, Bobes J et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry 2011;10:138‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suetani S, Whiteford HA, McGrath JJ. An urgent call to address the deadly consequences of serious mental disorders. JAMA Psychiatry 2015;72:1166‐7. [DOI] [PubMed] [Google Scholar]
- 12. Charlson FJ, Baxter AJ, Dua L et al. Excess mortality from mental, neurological and substance use disorders in the Global Burden of Disease Study 2010. Epidemiol Psychiatr Sci 2015;24:121‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olfson M, Gerhard T, Huang C et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015;72:1172‐81. [DOI] [PubMed] [Google Scholar]
- 14. Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res 2011;131:101‐4. [DOI] [PubMed] [Google Scholar]
- 15. Crump C, Winkleby MA, Sundquist K et al. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry 2013;170:324‐33. [DOI] [PubMed] [Google Scholar]
- 16. Fekadu A, Medhin G, Kebede D et al. Excess mortality in severe mental illness: 10‐year population‐based cohort study in rural Ethiopia. Br J Psychiatry 2015;206:289‐96. [DOI] [PubMed] [Google Scholar]
- 17. Laursen TM, Mortensen PB, MacCabe JH et al. Cardiovascular drug use and mortality in patients with schizophrenia or bipolar disorder. A Danish population‐based study. Psychol Med 2013;12:1‐13. [DOI] [PubMed] [Google Scholar]
- 18. Nordentoft M, Wahlbeck K, Hallgren J et al. Excess mortality and causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS One 2013;8:e55176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hennekens CH, Hennekens AR, Hollar D et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J 2005;150:1115‐21. [DOI] [PubMed] [Google Scholar]
- 20. Crump C, Sundquist K, Winkleby MA et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry 2013;70:931‐9. [DOI] [PubMed] [Google Scholar]
- 21. Lawrence D, Holman CD, Jablensky AV et al. Excess cancer mortality in Western Australian psychiatric patients due to higher case fatality rates. Acta Psychiatr Scand 2000;101:382‐8. [DOI] [PubMed] [Google Scholar]
- 22. Nordentoft M, Mortensen PB, Pederson CB. Absolute risk of suicide after first hospital contact in mental disorder. Arch Gen Psychiatry 2011;68:1058‐64. [DOI] [PubMed] [Google Scholar]
- 23. Crump C, Sundquist K, Winkleby MA et al. Mental disorders and risk of accidental death. Br J Psychiatry 2013;203:297‐302. [DOI] [PubMed] [Google Scholar]
- 24. Crump C, Sundquist K, Winkleby MA et al. Mental disorders and vulnerability to homicidal death: Swedish nationwide cohort study. BMJ 2013;346:f557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization . A conceptual framework for action on social determinants of health. Geneva: World Health Organization, 2010. [Google Scholar]
- 26. Marmot M, Friel S, Bell R et al. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet 2008;372:1661‐9. [DOI] [PubMed] [Google Scholar]
- 27. World Health Organization . Mental health action plan 2013‐2020. Geneva: World Health Organization, 2013. [Google Scholar]
- 28. Lasser K, Boyd JW, Woolhandler S et al. Smoking and mental illness: a population‐based prevalence study. JAMA 2000;284:2606‐10. [DOI] [PubMed] [Google Scholar]
- 29. Dipasquale S, Pariante CM, Dazzan P et al. The dietary pattern of patients with schizophrenia: a systematic review. J Psychiatry Res 2013;47:197‐207. [DOI] [PubMed] [Google Scholar]
- 30. Janney CA, Ganguli R, Richardson CR et al. Sedentary behaviour and psychiatric symptoms in overweight and obese adults with schizophrenia and schizoaffective disorders (WAIST study). Schizophr Res 2013;145:63‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Correll CU, Detraux J, De Lepeleire J et al. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015;14:119‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Torniainen M, Mittendorfer‐Rutz E, Tanskanen A et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull 2015;41:656‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cullen BA, McGinty EE, Zhang YY et al. Guideline‐concordant antipsychotic use and mortality in schizophrenia. Schizophr Bull 2013;39:1159‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Foley DL, Morley KI. Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch Gen Psychiatry 2011;68:609‐16. [DOI] [PubMed] [Google Scholar]
- 35. Lawrence D, Holman CDJ, Jablensky AV et al. Death rate from ischaemic heart disease in Western Australian psychiatric patients 1980‐1998. Br J Psychiatry 2003;182:31‐6. [DOI] [PubMed] [Google Scholar]
- 36. Kisley S, Campbell LA, Wang Y. Treatment of ischemic heart disease and stroke in individuals with psychosis under universal health care. Br J Psychiatry 2009;195:545‐50. [DOI] [PubMed] [Google Scholar]
- 37. Laursen TM, Munk‐Olsen T, Agerbo E et al. Somatic hospital contacts, invasive cardiac procedures, and mortality from heart disease in patients with severe mental disorder. Arch Gen Psychiatry 2009;66:713‐20. [DOI] [PubMed] [Google Scholar]
- 38. Chen YH, Lin HC, Lin HC. Poor clinical outcomes among pneumonia patients with schizophrenia. Schizophr Bull 2011;37:1088‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Daumit GL, Pronovost PJ, Anthony CB. Adverse events during medical and surgical hospitalizations for persons with schizophrenia. Arch Gen Psychiatry 2006;63:267‐72. [DOI] [PubMed] [Google Scholar]
- 40. Jones S, Howard L, Thornicroft G. ‘Diagnostic overshadowing’: worse physical health care for people with mental illness. Acta Psychiatr Scand 2008;118:169‐71. [DOI] [PubMed] [Google Scholar]
- 41. Corrigan PW, Mittal D, Reaves CM et al. Mental health stigma and primary health care decisions. Psychiatry Res 2014;218:35‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McGinty EE, Baller J, Azrin ST et al. Interventions to address medical conditions and health‐risk behaviors among persons with serious mental illness: a comprehensive review. Schizophr Bull 2016;42:96‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laursen TM, Munk‐Olsen T, Gasse C. Chronic somatic comorbidity and excess mortality due to natural causes in persons with schizophrenia or bipolar affective disorder. PLoS One 2011;6:e24597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Druss BG, Rosenheck RA. Mental disorders and access to medical care in the United States. Am J Psychiatry 1998;155:1775‐7. [DOI] [PubMed] [Google Scholar]
- 45. Morrison DS. Homelessness as an independent risk factor for mortality: results from a retrospective cohort study. Int J Epidemiol 2009;38:877‐83. [DOI] [PubMed] [Google Scholar]
- 46. Nielsen SF, Hjorthoj CR, Erlangsen A et al. Psychiatric disorders and mortality among people in homeless shelters in Denmark: a nationwide register‐based cohort study. Lancet 2011;377:2205‐14. [DOI] [PubMed] [Google Scholar]
- 47. Kiviniemi M, Suvisaari J, Pirkola S et al. Five‐year follow‐up study of disability pension rates in first‐onset schizophrenia with special focus on regional differences and mortality. Gen Hosp Psychiatry 2011;33:1445‐52. [DOI] [PubMed] [Google Scholar]
- 48. Druss BG, Zhao L, von Esenwein et al. Understanding excess mortality in persons with mental illness: 17‐year follow up of a nationally representative US survey. Med Care 2011;49:599‐604. [DOI] [PubMed] [Google Scholar]
- 49. World Health Organization . Mental health gap action programme. Geneva: World Health Organization, 2008. [Google Scholar]
- 50. World Health Organization . Mental health gap action programme intervention guide. Geneva: World Health Organization, 2010. [Google Scholar]
- 51. Sampson S, Mansour M, Maayan N et al. Intermittent drug techniques for schizophrenia. Cochrane Database Syst Rev 2013;7:CD006196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tiihonen J, Lönnqvist J, Wahlbeck K et al. 11‐year follow‐up of mortality in patients with schizophrenia: a population‐based cohort study (FIN11 study). Lancet 2009;374:1‐12. [DOI] [PubMed] [Google Scholar]
- 53. Buchanan RW, Kreyenbuhl J, Kelly DL et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull 2010;36:71‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McGuire AB, Kukla M, Green A et al. Illness management and recovery: a review of the literature. Psychiatr Serv 2014;65:171‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry 2016;15:13‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haklai Z, Goldberger N, Stein N et al. The mortality risk among persons with psychiatric hospitalizations. Israel J Psychiatry Rel Sci 2011;48:230‐9. [PubMed] [Google Scholar]
- 57. Organization for Economic Cooperation and Development . Health at a glance 2015. Geneva: Organization for Economic Cooperation and Development, 2015. [Google Scholar]
- 58. World Health Organization . Preventing suicide: a global imperative. Geneva: World Health Organization, 2014. [Google Scholar]
- 59. Hughes K, Bellis MA, Jones L et al. Prevalence and risk of violence against adults with disabilities: a systematic review and meta‐analysis of observational studies. Lancet 2012;379:1621‐9. [DOI] [PubMed] [Google Scholar]
- 60. Evins AE, Cather C, Pratt SA et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA 2014;311:145‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Daumit GL, Dickerson FB, Wang NY et al. A behavioural weight‐loss intervention in persons with severe mental illness. N Engl J Med 2013;368:1594‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Green CA, Yarborough BJH, Leo MC et al. The STRIDE weight loss and lifestyle intervention for individuals taking antipsychotic medications: a randomized trial. Am J Psychiatry 2015;172:71‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Druss BG, Bornemann TH. Improving health and health care for persons with serious mental illness: the window for US federal policy change. JAMA 2010;303:1972‐3. [DOI] [PubMed] [Google Scholar]
- 64. Appel L, Champagne C, Harsha D et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. Writing Group of the PREMIER Collaborative Research Group. JAMA 2003;289:2083‐93. [DOI] [PubMed] [Google Scholar]
- 65. Whelton PK, Appel LJ, Espeland MA et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA 1998;279:839‐46. [DOI] [PubMed] [Google Scholar]
- 66. World Health Organization . Interventions on diet and physical activity: what works. Geneva: World Health Organization, 2009. [PubMed] [Google Scholar]
- 67. Drake RE, O'Neal E, Wallach MA. A systematic review of psychosocial interventions for people with co‐occurring severe mental and substance use disorders. J Subst Abuse Treat 2008;34:123‐38. [DOI] [PubMed] [Google Scholar]
- 68. Mueser KT, Glynn SM, Cather C et al. Family interventions for co‐occurring substance use and severe psychiatric disorders: participant characteristics and correlates of initial engagement and more extended exposure in a randomized controlled trial. Addict Behav 2012;34:864‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rosenberg SD, Goldberg RW, Dixon LB et al. Assessing the STIRR model of best practices for blood‐borne infections in clients with severe mental illness. Psychiatr Serv 2010;61:885‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. World Health Organization . Strengthening health systems to improve health outcomes. WHO's framework for action. Geneva: World Health Organization, 2007. [Google Scholar]
- 71. Osborn D, Nazareth I, Wright CA et al. Impact of a nurse‐led intervention to improve screening for cardiovascular risk factors in people with severe mental illness. Phase‐two cluster randomized feasibility trial of community mental health teams. BMC Health Serv Res 2010;10:61‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Speyer H, Norgaard HCB, Birk M et al. The CHANGE trial: no superiority of lifestyle coaching plus care coordination plus treatment as usual compared to treatment as usual alone in reducing risk of cardiovascular disease in adults with schizophrenia spectrum disorders and abdominal obesity. World Psychiatry 2016;15:155‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Barnes TR, Paton C, Cavanagh MR et al. UK prescribing observatory for mental health . A UK audit of screening for the metabolic side effects of antipsychotics in community patients. Schizophr Bull 2007;33:1397‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. National Institute for Health and Care Excellence . Lester UK adaptation. Positive cardiometabolic health resource. London: National Institute for Health and Care Excellence, 2014. [Google Scholar]
- 75. Substance Abuse and Mental Health Services Administration . A standard framework for levels of integrated healthcare. Washington: Substance Abuse and Mental Health Services Administration, 2013. [Google Scholar]
- 76. University of Western Australia . Clinical guidelines for the physical care of mental health consumers. Report. Perth: University of Western Australia, 2010. [Google Scholar]
- 77. University of Western Australia . Nurse practitioner protocols for managing the physical health of mental health patients guideline. Perth: University of Western Australia, 2010. [Google Scholar]
- 78. Shiers D, Curtis J. Cardiometabolic health in young people with psychosis. Lancet Psychiatry 2014;1:492‐4. [DOI] [PubMed] [Google Scholar]
- 79. Substance Abuse and Mental Health Services Administration, Center for Mental Health Services . Primary and behavioral health care integration grants (PBHCI). Washington: Substance Abuse and Mental Health Services Administration, 2014. [Google Scholar]
- 80. Scharf DM, Eberhart NK, Schmidt Hackbath N et al. Evaluation of the SAMSHA Primary and Behavioral Health Care Integration (PBHCI) Grant Program: final report. Santa Monica: RAND Corporation, 2014. [PMC free article] [PubMed]
- 81. Stanley SH, Laugharne JDE. Clinical guidelines for the physical care of mental health consumers: a comprehensive assessment and monitoring package for mental health and primary care clinicians. Aust N Z J Psychiatry 2011;45:824‐9. [DOI] [PubMed] [Google Scholar]
- 82. New South Wales Government . Metabolic monitoring, a new mental health clinical documentation module. Sydney: New South Wales Government, 2012. [Google Scholar]
- 83. Organ B, Nicholson E, Castle D. Implementing a physical health strategy in a mental health service. Australas Psychiatry 2010;18:456‐9. [DOI] [PubMed] [Google Scholar]
- 84. Semrau M, Lempp H, Keynejad R et al. Service user and caregiver involvement in mental health system strengthening in low‐ and middle‐income countries: systematic review. BMC Health Serv Res 2016;16:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lorig KR, Sobel DS, Stewart AL et al. Evidence suggesting that a chronic disease self‐management program can improve health status while reducing hospitalization: a randomized trial. Med Care 1999;37:5‐14. [DOI] [PubMed] [Google Scholar]
- 86. Druss BG, Zhao L, von Esenwein SA et al. The Health and Recovery Peer (HARP) Program: a peer‐led intervention to improve medical self‐management for persons with serious mental illness. Schizophr Res 2010;118:264‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Semrau M, Evans‐Lacko S, Koschorke M et al. Stigma and discrimination related to mental illness in low‐ and middle‐income countries. Epidemiol Psychiatr Sci 2015;24:382‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Thornicroft G, Metha N, Clement S et al. Evidence for effective interventions to reduce mental‐health‐related stigma and discrimination. Lancet 2016;387:1123‐32. [DOI] [PubMed] [Google Scholar]
- 89. Burns T, Catty J, Becker T et al. The effectiveness of supported employment for people with severe mental illness: a randomized controlled trial. Lancet 2007;370:1146‐52. [DOI] [PubMed] [Google Scholar]
- 90. Siu AL, US Preventive Services Task Force (USPSTF) , Bibbins‐Domingo K et al. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA 2016;315:380‐7. [DOI] [PubMed] [Google Scholar]
- 91. Schroeder SA. Smoking cessation should be an integral part of serious mental illness treatment. World Psychiatry 2016;15:175‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dixon LB, Dickerson F, Bellack AS et al. The 2009 schizophrenia PORT psychosocial treatment recommendations and summary statements. Schizophr Bull 2010;36:48‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. National Institute for Health and Care Excellence . Quality statement 7: promoting health eating, physical activity and smoking cessation. London: National Institute for Health and Care Excellence, 2015. [Google Scholar]
- 94. World Health Organization . Package of essential NCD interventions for primary health care: cancer, diabetes, heart disease and stroke, chronic respiratory disease. Geneva: World Health Organization, 2010. [Google Scholar]
- 95. Shidhaye R, Lund C, Chisholm D. Closing the treatment gap for mental, neurological and substance use disorders by strengthening existing health care platforms: strategies for delivery and integration of evidence‐based interventions. Int J Ment Health Syst 2015;9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Patel V. Lay health worker led intervention for depression and anxiety disorders in India: impact on clinical and disability outcomes over 12 months. Br J Psychiatry 2011;199:459‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]


