Abstract
The continuing increase in the prevalence of diabetes in the general population is predicted to result in a higher incidence of cardiovascular disease. Although the mechanisms of diabetes associated progression of atherosclerosis are not fully understood, at clinical and pathological level, there is an appreciation of increased disease burden and higher levels of arterial calcification in these subjects. Plaques within the coronary arteries of patients with diabetes generally exhibit larger necrotic cores and significantly greater inflammation consisting mainly of macrophages and T lymphocytes relative to patients without diabetes. Moreover, there is a higher incidence of healed plaque ruptures and positive remodeling in hearts from subjects with type 1 diabetes (T1D) and type 2 diabetes (T2D), suggesting a more active atherogenic process. Lesion calcification in the coronary, carotid and other arterial beds is also more extensive. While the role of coronary artery calcification in identifying cardiovascular disease and predicting its outcome is undeniable, our understanding of how key hormonal and physiological alterations associated with diabetes such as insulin resistance and hyperglycemia influence the process of vascular calcification continues to grow. Important drivers of atherosclerotic calcification in diabetes include oxidative stress, endothelial dysfunction, alterations in mineral metabolism, increased inflammatory cytokine production, and release of osteoprogenitor cells from the marrow into the circulation. Our review will focus on the pathophysiology of T1D- and T2D-associated vascular disease with particular focus on coronary and carotid atherosclerotic calcification.
Keywords: diabetes mellitus, sudden death, coronary artery disease, carotid artery, calcification, atherosclerosis
Introduction
Despite the recent progress in the treatment and diagnosis of cardiovascular disease (CVD) in the past few decades, the risk of diabetes continues to increase.1, 2 Diabetes remains a significant independent cardiovascular risk factor even after adjusting for age, smoking, body mass index (BMI), and hypertension.3 The worldwide estimate of diabetes reported by The International Diabetes Federation in 2014 was 387 million, with an overall prevalence of 8.3% and is expected to rise to 552 million by 2030.4, 5
Vascular calcification can be morphologically classified into two distinct forms depending on the location, i.e., within the intima or the media. Medial calcification is the most common form of vascular calcification in type 2 diabetes (T2D) but it mostly effects the media of peripheral arteries, i.e., smooth muscle cells and the elastic membrane resulting in loss of elasticity. In this review we will focus primarily on the pathologic findings in subjects with (type 1 (T1D) or type 2 (T2D) diabetes with regards to atherosclerotic disease with a focus on vascular calcification, mostly intimal (atherosclerotic) which is the dominant type of calcification seen in coronary and carotid disease. We will review important differences in plaque morphology and patterns of calcification in those with versus those without diabetes and lastly we will briefly discuss the mechanisms by which the metabolic and hormononal abnormalities seen in diabetes influence vascular calcification.
Pathologic Findings in Sudden Coronary Death in Patients With and Without Diabetes
Much of our understanding of the role of diabetes in atherosclerotic disease has been learned through careful autopsy studies of sudden coronary death and in those presenting with unstable coronary syndromes. A recent updated analysis of 438 sudden death victims with diabetes (n=101) as compared to those without diabetes (n=337), failed to show differences in the incidence of acute coronary thrombosis (53%) and stable coronary disease (47%).6 For the entire cohort however, the most frequent etiology of acute thrombosis was plaque rupture (65%), followed by erosion (30%), and eruptive calcified nodule (5%). Coronary lesions from subjects with T1D (n=25) and T2D (n=76) compared with 337 non-diabetic controls showed a lower incidence of acute thrombi (P=0.001 and P=0.02, respectively, Table 1), which is consistent with our previous observations.7 Likewise, ruptures and erosions were numerically lower in subjects with diabetes (both T1 and T2 DM) versus non-diabetic subjects though in some cases this did not reach statistical significance likely because of the small number of cases (Table 1). However, there was a significantly greater plaque burden for T1D (P=0.001) and T2D (P=0.02) compared with non-diabetic subjects (Table 2).
Table 1.
Distribution of culprit plaques in sudden coronary death relative to diabetic status (438 cases)
| Type 1 DM (25 cases) |
Type 2 DM (76 cases) |
Non-DM (337 cases) |
P Value (Type 1 DM vs. Non-DM) |
P Value (Type 2 DM vs. Non-DM) |
|
|---|---|---|---|---|---|
| Acute thrombi | 4 (17) | 35 (45) | 196 (58) | 0.001 | 0.02 |
| Rupture | 3 (13) | 24 (31) | 127 (38) | 0.09 | 0.36 |
| Erosion | 2 (8) | 7 (9) | 61 (18) | 0.28 | 0.06 |
| Calcified nodule | 1 (4) | 2 (3) | 8 (2) | 0.48 | 1.00 |
| Stable CAD | 19 (83) | 43 (55) | 141 (42) | 0.001 | 0.02 |
| CTO | 8 (35) | 14 (18) | 51 (15) | 0.04 | 0.49 |
| No thrombi | 11 (48) | 29 (37) | 90 (27) | 0.07 | 0.05 |
Within each box cases are given first and percentage of total shown in parenthesis. Re-analyzed 438 sudden coronary death cases (from the original 442 as 4 cases did not have information regarding diabetic status) previously reported in Yahagi K et al., Atherosclerosis 2015;239:260–267, and stratified by diabetes mellitus status. CAD = coronary artery disease, CTO = chronic total occlusion, DM = diabetes mellitus. Modified and reproduced with permission from Yahagi K et al., Atherosclerosis 2015;239:260-267
Table 2.
Sudden death registry data of coronary plaque characteristics relative to diabetic status
| Type 1 DM (n=16) |
Type 2 DM (n=50) |
Non-DM (n=66) |
P Value (Type 1 DM vs. non-DM) |
P Value (Type 2 DM vs. non-DM) |
|
|---|---|---|---|---|---|
| Necrotic core area (%) * | 12.0±5.7 | 11.6±8.4 | 9.4±9.3 | 0.05 | 0.004 |
| Macrophage plaque area (mm2) * | 0.15±0.02 | 0.13±0.03 | 0.10±0.02† | 0.03 | 0.03 |
| Calcified matrix area (%) * | 7.8±9.1 | 12.1±11.2 | 11.4±13.5 | 0.9 | 0.05 |
| Fibroatheroma (n) | 7.1±5.0 | 8.8±4.3 | 6.9±4.7 | 0.9 | 0.02 |
| Thin-cap fibroatheroma (n) | 1.0±1.3 | 0.8±0.8 | 0.7±0.8 | 0.5 | 0.8 |
| Healed plaque rupture (n) | 2.6±2.1 | 2.6±1.8 | 1.9±1.8 | 0.2 | 0.04 |
| Total plaque burden (%) | 275±129 | 358±114 | 232±128 | 0.04 | 0.0001 |
| Distal plaque burden (%) | 310±114 | 630±263 | 331±199 | 0.8 | 0.0001 |
Values are expressed as mean ± SD or % normalized to plaque area.
p values calculated using log-normalized data.
p=0.006 vs. type 1 and 2 diabetes combined. DM=diabetes mellitus. Reproduced with permission from Burke AP et al., Arterioscler Thromb Vasc Biol. 2004;24:1266-1271
Overall in males the number of plaque ruptures versus erosions was significantly higher (P<0.05) while erosions were more frequent in females versus ruptures (P<0.05) consistent with our previous observations.8 Similar sex-based trends for diabetic subjects were also noted for ruptures and erosions as well, although differences did not achieve statistical significance likely because of the limited number of cases.
The trend towards a lower incidence of plaque rupture in diabetics corroborates a previous autopsy study by Davies,9 in which coronary thrombi were seen in 84% of men and 59% in women without diabetes as compared to only 34% of diabetic patients of either sex. While high estrogen levels have been suggested as the underlying reason for the differences in plaque morphology between men and women, the underlying reason why diabetics show lower incidence of acute coronary thrombi while having greater plaque burden remains unknown but raises the questions of whether the mechanisms of plaque progression may be fundamentally different in diabetic versus non-diabetic patients and even between T1D and T2D patients. While cardiovascular risk in T1D is driven by hyperglycemia, the etiology of atherosclerosis in T2D is multifactorial, featuring several factors largely absent in T1D such as obesity, dyslipidemia and hypertension.
The lower incidence of luminal thrombi at autopsy in diabetics is somewhat surprising given the increased platelet reactivity seen in patients with versus without diabetes. Factors such as increased blood osmolarity (due to hyperglycemia), hyperglycemia induced activation of protein kinase C (PKC) β, and greater expression of glycoprotein (Gp IIb/IIIa) and GpIb have been implicated in the effect of diabetes on platelet function.10
Coronary Lesion Morphology in Patients With and Without Diabetes
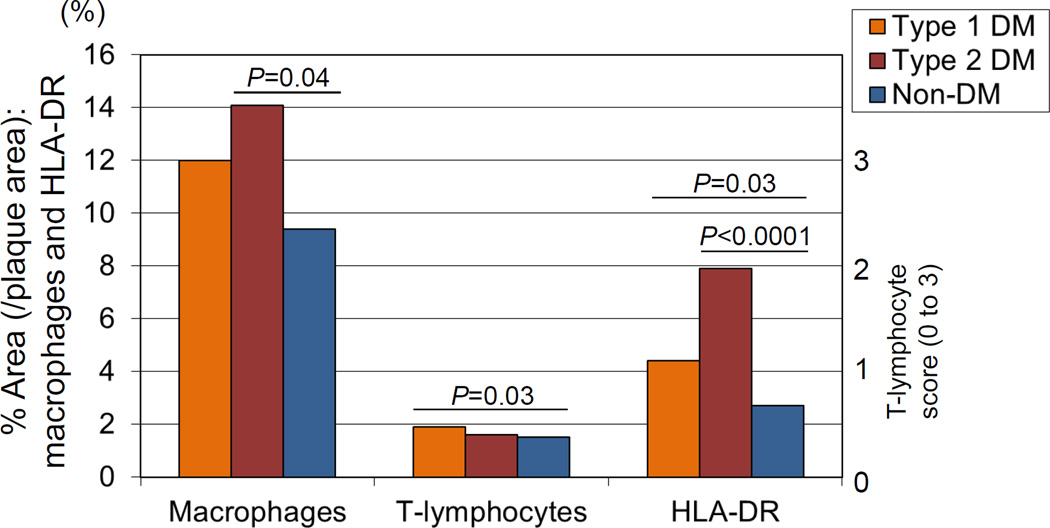
Further analysis of the impact of diabetes on lesion morphology in the same autopsy cohort as mentioned above found that necrotic core size and inflammation characterized by macrophages and T-cells were significantly greater in subjects with T1D and T2D as compared to non-diabetic controls (Table 2). However, calcified matrix area was greater only in T2D (12.1±11.2%), but not in T1D (7.8±9.1%) as compared to non-diabetics (11.4±13.5%, p versus T1D=0.9; p versus T2D=0.05). Moreover, the overall percentage of inflammation relative to total lesion burden was also significantly greater in diabetics with regards to macrophages (T2D, P= 0.04), T-lymphocytes (T1D, P= 0.03), and human leukocyte antigen-DR (HLA-DR) (T1D and T2D, P=0.03 and <0.0001), respectively (Figure 1). In addition, a multivariate analysis determined that HbA1c was an independent predictor of necrotic core size (T=2.8, P=0.005) and macrophage area (T=2.9, P= 0.004) after adjusting for HDL cholesterol, TC/HDL cholesterol ratio, age, smoking and gender.7 Thus, these data suggest differential mechanisms of plaque growth in diabetic versus non-diabetic patients and a link between hyperglycemia, core size, and inflammation.
Figure 1.
Inflammation in diabetic coronary arteries. Coronary fibroatheromas illustrating the extent of macrophages (CD68), T cells (CD45RO), and HLA-DR expression in patients with type 1 and 2 diabetes mellitus (DM) with control, nondiabetic subjects. Reproduced with permission from Burke AP, et al. Arterioscler Thromb Vasc Biol. 2004;24:1266-1271.
A similar histopathologic analysis of 47 coronary atherectomy specimens by Moreno et al. showed that patients with diabetes (13 insulin-dependent and 34 non-insulin-dependent) exhibited a larger percentage of lipid-rich atheroma (7±2%) as compared to non-diabetic controls (2±1%, P=0.01). In addition, there was a greater percentage area occupied by macrophages (diabetes= 22±3%, vs. non diabetics= 12±1%, P= 0.003) and higher prevalence of thrombosis (diabetes= 62% vs. non-diabetics= 40%, P= 0.04).11 Cipollone et al. reported similar pathologic findings in carotid endarterectomy specimens in equal numbers of patients with and without diabetes (n=30 each) with respect to macrophages, T- lymphocytes and the inflammatory cell activation marker human leukocyte antigen-DR (HLA-DR).12 Interestingly the prominent T-cell component in lesions from T1D is not unexpected considering its autoimmune nature and associated genetic susceptibility to similar disorders, such as autoimmune thyroiditis.13
Diabetes and Healed Plaque Ruptures
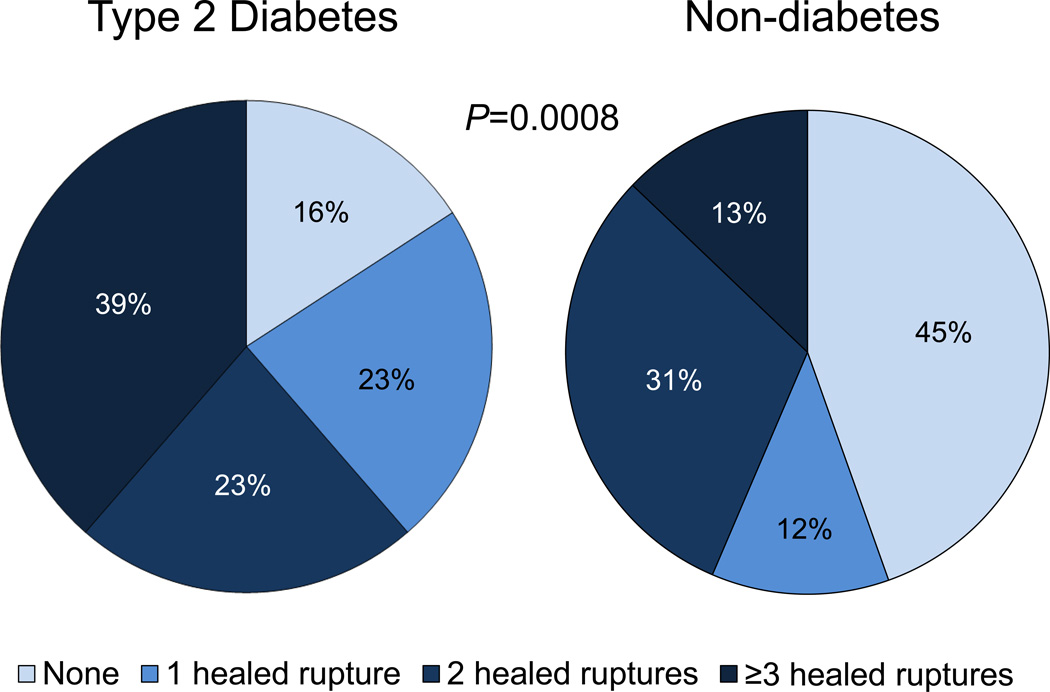
Healed plaque ruptures (HPR) likely result from silent plaque ruptures occurring either in the absence of symptoms or at least occurring undetected at the time of the initial event. In the context of T1D and T2D, there is a significantly higher incidence of asymptomatic ischemic disease, suggesting there should be an increased frequency of HPRs in this population.14,15 This finding is supported by our previous pathologic study of 142 males with sudden coronary death showing a significant increase in the overall number of HPRs per heart and healed myocardial infarctions (HMI) for both T1D and T2D (P=0.02) versus non-diabetic controls.14 An updated analysis using a larger number of patients from our sudden coronary death registry reaffirmed the greater percentage of HPRs in subjects with T2D relative to controls (84% vs. 55%, P= 0.0008) (Figure 2). Interestingly, 39% of sudden coronary deaths in subjects with T2D had ≥3 HPRs per heart as compared to only 13% of subjects without diabetes. In an earlier autopsy study involving 132 sudden death cases, we also reported a higher incidence of HPRs relative to non-diabetic controls (2.6±1.8 vs. 1.9±1.8, P= 0.04) (Table 2), while T1D exhibited a similar trend (2.6±2.1).7 In addition, type 2 diabetic individuals have greater plaque burden, including extensive distal vessel involvement (Table 2) which is consistent with the higher rates and number of HPRs per heart.14 Taken together, these studies point towards more advanced plaques and greater number of previous myocardial infarctions for those with diabetes consistent with greater atherosclerotic burden and progression relative to non-diabetics.
Figure 2.
The pie charts reflect the percentage of healed ruptures (HPR) per heart relative to diabetic status at autopsy. Type 2 diabetes had higher numbers of HPRs compared to non-diabetics (p=0.0008). The data constitutes a re-analysis of 142 sudden coronary death cases, published in Burke AP, et al. Circulation. 2001;103:934-940, and stratified by with or without diabetes mellitus.
Influence of diabetes on positive remodeling
The phenomena of coronary artery expansion referred to as positive remodeling initially described by Glagov concluded that vessel enlargement is linked to increased atherosclerotic plaque burden without luminal compromise up to 40% of cross-sectional area narrowing.16 In an autopsy examination of coronary artery remodeling in sudden coronary death, we have shown that the internal elastic lamina (IEL) area (a histologic indicator of vessel size), when adjusted for the distance from the coronary ostium, is greater in subjects with T1D and T2D relative to those without diabetes (T1D= 18.2±6.6 mm2, T2D= 16.5±4.4 mm2 vs. non-diabetic= 16.0±4.5 mm2, P= 0.001 and P= 0.01, respectively).17 A multivariate analysis also revealed a significant correlation between T1D and IEL area after adjustment for heart weight, plaque area, percent necrotic core and percent plaque calcification (P=0.0004). This same analysis also confirmed a positive correlation with IEL area, percent necrotic core (P=0.05), plaque area (P<0.0001), and heart weight (P= 0.05).17
Historically however, there has been clinical ambiguity regarding the occurrence of positive remodeling of coronary arteries in patients with diabetes.18, 19 Considering the first manifestation of coronary disease in our ongoing autopsy series is sudden death, it is not surprising that we observed positive remodeling. It is likely that coronary plaques from patients with diabetes who survive an acute coronary event may eventually undergo negative remodeling from healed plaque rupture and collagen cross-linking. In this case, however, the overall remodeling processes in patients with diabetes and coronary disease would be best confirmed by serial imaging using either noninvasive multi-slice cardiac computed tomography (MSCT) or intravascular ultrasound (IVUS). On the other hand, necrotic core size and inflammation are strongly associated with IEL expansion, particularly through proteases expressed by resident macrophages which cause extracellular matrix degradation20. Therefore one could expect greater positive remodeling considering these attributes are typically increased in diabetes.
Calcification and Coronary Artery Disease Risk in Diabetics
There is a higher tendency for coronary artery calcification (CAC) in diabetic patients, which correlates with total plaque burden in addition to representing an independent risk factor for adverse outcomes.21,22 Other risk factors for CAC also include age and chronic kidney disease, which are inherently linked to diabetes as well.23 Computed tomography (CT) is the only noninvasive test with a high enough sensitivity and specificity for the detection of calcification, which has made a significant impact on the diagnosis and risk management of coronary and carotid disease.24,25 Conversely, coronary angiography has a low to moderate sensitivity compared to CT for the detection of CAC, but is very specific with a high predictive value. In asymptomatic individuals with low to intermediate risk for cardiovascular events, the absence of CAC on CT is associated with very low risk (<0.5%) of obstructive non-calcified plaques on invasive angiography.26, 27 Despite the association of CAC and future cardiovascular events however, acute coronary syndromes may also occur in the absence of CAC, especially in young individuals who are smokers.28
While the role of CAC in identifying cardiovascular disease and predicting its outcome is undeniable, our understanding of its relationship with important features of diabetes such as insulin resistance and hyperglycemia is slowly evolving especially in relation to atherosclerosis progression.
CAC irrespective of symptomatic or asymptomatic disease is strongly associated with future cardiac or cerebrovascular events,29 and the prevalence of diabetes in these individuals is high.30, 31 Progressive atherosclerotic lesions (i.e. pathological intimal thickening and early fibroatheroma) with micro-calcification cannot be identified by CT,32 whereas late obstructive coronary artery disease is strongly associated with a high CAC score (Agatston score ≥400). Raggi et al. investigated the prognostic value of CAC score for all-cause mortality in asymptomatic individuals including 903 patients with diabetes vs. 9,474 patients without diabetes after 5 years follow-up and found CAC an independent predictor of all-cause mortality independent of diabetic status.22
There is an established link between hyperglycemia, CAC, and diagnostic glycated HbA1c as a predictor of cardiovascular disease.33, 34 In fact, epidemiological evidence supports a greater risk for atherosclerotic coronary vascular disease with increasing dysglycemia, with an estimated 11% to 16% increase in cardiovascular events for every 1% increase in HbA1c.35 In a study of 25,554 Korean adults (41.4±7.0 years) without diabetes, Chang et al. reported greater HbA1c predicted CAC, particularly for women.33 Similarly, the prospective case-cohort study of 1,626 adults with diabetes for the Atherosclerosis Risk in Communities Study by Slevin et al., showed that relative risk (RR) of coronary heart disease (CHD) was 2.37 (95% confidence interval [CI], 1.50–3.72) for the highest quintile of HbA1c level compared with the lowest after adjustment of CHD risk factors.36 The risk of CHD in this study increased throughout the range of HbA1c where in the adjusted model, the RR of CHD for a 1- percentage point increase in HbA1c levels was 1.14 (95 CI, 1.07–1.21). Further evidence for the predictive value of HbA1c comes from a recent five-year follow-up study by Carson et al., which showed that 12.9% of participants without baseline CAC developed CAC. Higher HbA1c was associated with greater incident CAC as well as CAC progression after adjustment for sociodemographic factors.37 The association of HbA1C and CAC progression persisted in multivariable adjusted models.
Pathology of Vascular Calcification
Incidental micro- and macro-calcification is considered a well-known marker of subclinical atherosclerotic burden, especially in the subpopulation of individuals with diabetes. It is well-known that vascular calcification occurs in both the intima and media. However, medial calcification is rare in coronary and carotid vessels, although common in medium and small muscular peripheral arteries.38 Both intimal and medial calcification are increased in patients with T2D, chronic kidney disease, and other less frequent disorders.39 In this regard, over 70% of men and 50% of women with coronary artery disease with a risk factor of T1D will develop CAC by their mid-forties.40
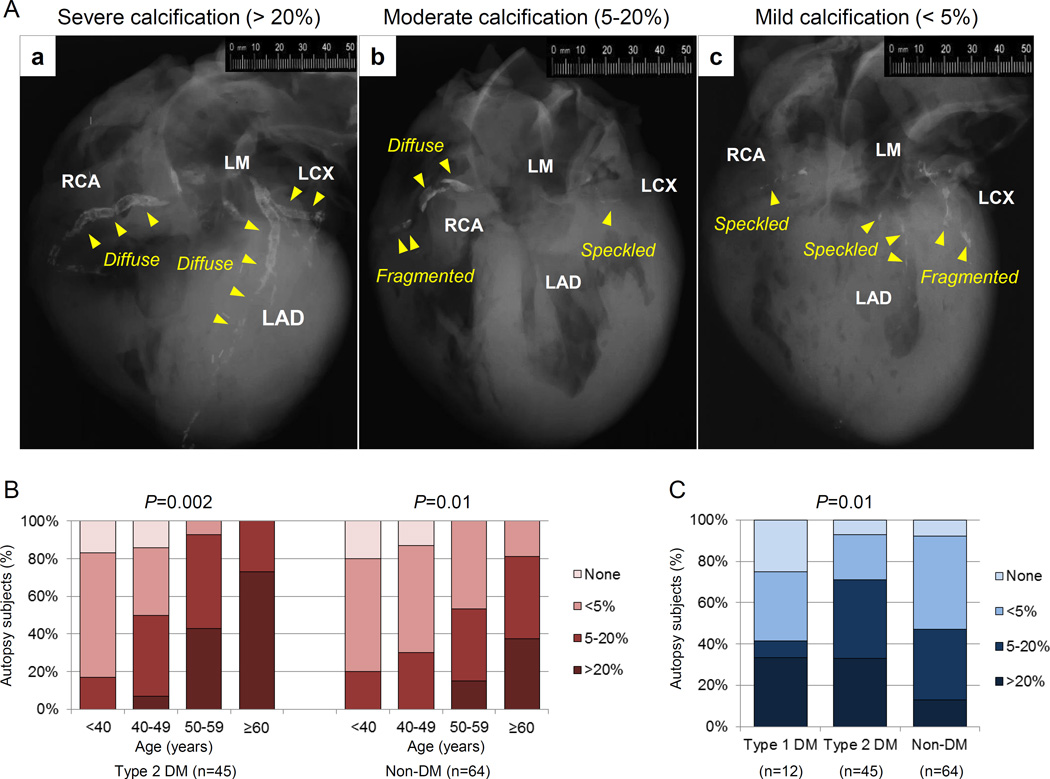
The earliest coronary lesion morphology exhibiting CAC is pathologic intimal thickening41 caused by apoptotic smooth muscle cells and/or matrix vesicles (30 to 300 nm) appearing within cholesterol enriched lipid pools as micro-calcifications in the order of ~0.5 µm to 15 µm in diameter. The extent of plaque calcification is exacerbated with lesion progression, as evidenced by macrophages infiltration of the lipid pool, accompanied by apoptosis/necrosis, with calcification as identified in lesions that transition to the more advanced fibroatheroma. More easily recognized are areas of confluent calcium involving the extracellular matrix and necrotic core by radiography as speckled (<2 mm), fragmented (2 to 5 mm), or diffuse (≥5 mm) (Figure 3) areas. The coalescence and enlargement of calcified fragments result in the formation of calcified plates/sheets, which can be visualized by non-invasive imaging modalities, such as radiography, magnetic resonance imaging (MRI), and electron-beam CT.
Figure 3.
Coronary artery calcification in sudden coronary death evaluated by post-mortem radiography. (A) Representative post-mortem radiographs showing various patterns of calcification. The severity of calcification was assessed based on the percentage of calcification area: (a) severe artery calcification (>20%), (b) moderate calcification (5–20%), and (c) mild calcification (<5%). Arrows indicate type of calcification (speckled [<2mm in length], fragmented [2–5mm] or diffuse [≥5mm] calcification). (B) Percentage of total calcified area, divided into mild, moderate and severe in sudden coronary death patients with type 2 DM and non-DM stratified by decade. (C) Percentage of total calcified area in sudden coronary death comparing type 1 DM, type 2 DM, and non-DM.
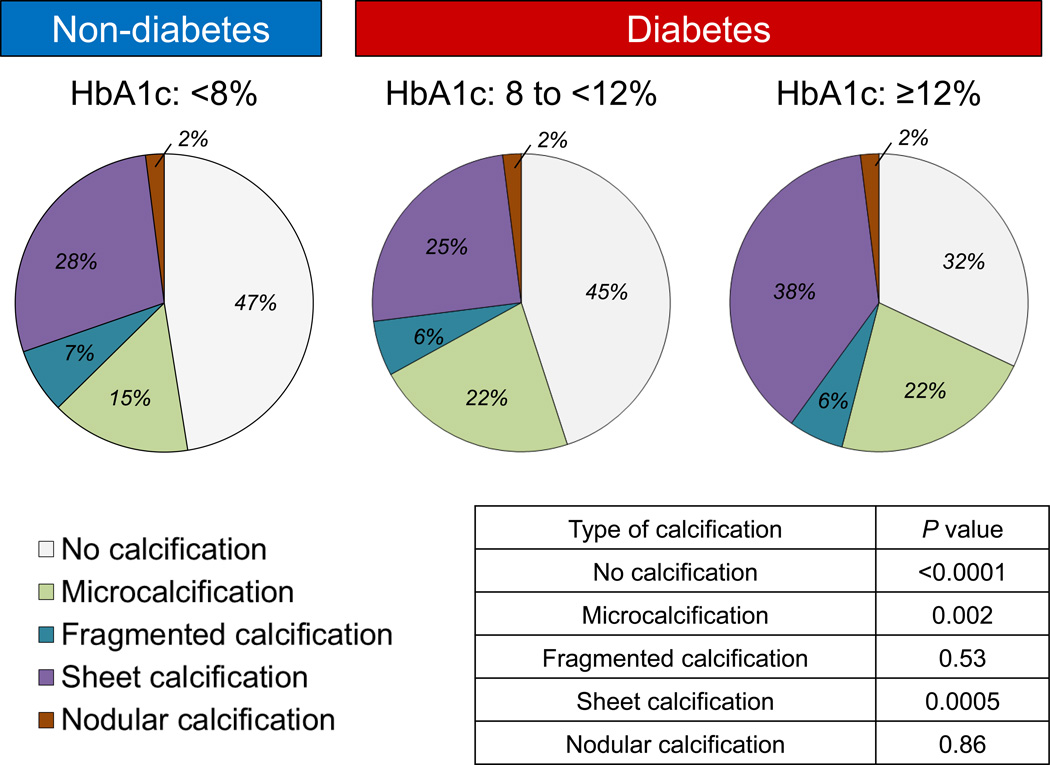
Calcium in advanced plaques exhibits the appearance of sheets integrated within fibrotic tissue consisting of collagen and smooth muscle cells, which may or may not involve the necrotic core. Nodular type of calcification is the least common form existing as small calcified fragments with interspersed fibrin.42 In the context of lesion progression, the extent of CAC has been shown to correlate with plaque burden, but not necessarily with the severity of luminal narrowing. In a review of post-mortem radiographs of sudden death hearts from our registry, the percentage of CAC (based upon percentage of calcification area) was evaluated as absent, mild (<5%), moderate (5% to 20%), or severe (>20%) (Figure 3A). While the percentage of CAC relative to plaque burden increased progressively over decades for T2D (P=0.002) and in controls without diabetes (P=0.01) (Figure 3B), lesions from subjects with either T1D or T2D exhibited an increase in areas of severe CAC (P=0.01) (Figure 3C). When sudden death cases were stratified by HbA1c, there was a decline in the number of lesions without calcification and significant reciprocal increase in sheet calcification with escalating HbA1C levels in patients with diabetes (Figure 4).
Figure 4.
Percentage of sudden deaths based on coronary artery calcification type (none, micro-, fragmented, sheet, and nodular, stratified by HbA1c level (<8%) non-diabetics and diabetic (8% to <12% and ≥12%). 1630 histologic sections (<8%, n=776 sections; 8% to <12%, n=548; and ≥12%, n=306) from 57 patients with stable coronary artery disease were examined. Note the declining shift in the number of lesions without calcification and significant reciprocal increase sheet calcification with escalating HbA1C levels.
Plaque Composition and Risk of Carotid Artery Disease in Diabetics
Diabetes is an important risk factor for ischemic stroke (second only to hypertension) where stroke rates in men and women between 45 and 74 years are reported 2.5 to 3.5 times higher in individuals with diabetes than in those without diabetes.43 The Rotterdam Study involving 1002 patients aged >55 years assessed calcification on MSCT in the coronary, aortic arch, and carotid arteries and showed that diabetes was an independent risk factor for CAC in men and women while in the carotid territory, correlations were selective only for women.44 Patients with T2D undergoing dental panoramic radiography had a 5-fold excess prevalence of calcified carotid arteries, as compared to patient without diabetes.45 Similar to findings in coronary bed, HbA1c has also been shown to be an independent predictor of stroke as well as cardiovascular disease in both individuals with and without diabetes.46–48 Moreover, intensive glucose control in patients with T2D over 5 years who had previous cardiovascular events including stroke had 8.6 times fewer major cardiovascular events per 1000 person-years than those assigned to standard therapy.49 Other studies however, have not shown any benefit on stroke outcomes after intensive glucose lowering in patients with diabetes.50
Carotid plaque composition is an important predictor of cerebrovascular accidents, which is influenced by traditional risk factors.51 Plaques with a thin fibrous cap infiltrated by macrophage and T-cells expressing HLA-DR are more commonly observed in symptomatic as compared to asymptomatic individuals.52 Spagnoli et al. examined 180 carotid endarterectomy (CEA) specimens from patients presenting with transient ischemic attack or stroke and showed that fibrous plaques correlated with both aging and diabetes.53 On the contrary, the presence of thrombosis was significantly less in patients with diabetes compared to those without diabetes (19.6% vs. 42.0%), which is in agreement with our observations in coronary arteries.53 In another study by Scholtes et al. patients with T2D (295 patients) no differences were observed in carotid plaque morphology or expression of inflammatory chemokines, cytokines, or advanced glycation end products between T2D and non-diabetics.54 The study results may be negative because T2D had a higher proportion of previous cardiovascular interventions along with more stringent treatment for hypertension and hypercholesterolemia compared to patients without diabetes (1160 patients).54
Clinical studies have demonstrated the value of carotid plaque calcification for discriminating future cardiovascular events and death in patients with T2D where the risk of MACE progressively increases with plaque calcification.55 The extent of carotid calcification was investigated in our registry of 68 CEA specimens, which included radiographic examination from 14 patients with T2D and 54 controls without diabetes. Total area of calcification by radiography was significantly greater in patients with T2D as compared to controls (T2D = 165±313 mm2 vs. controls = 66±53 mm2, P=0.03) (Figure 5).
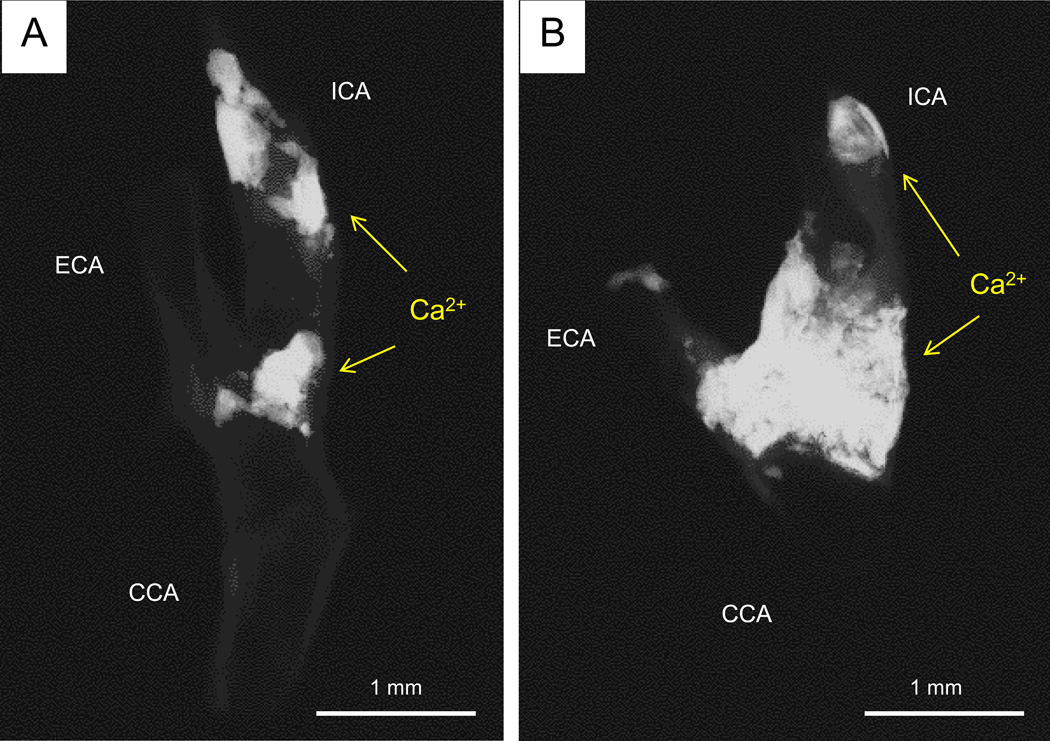
Figure 5.
Representative digital radiography of calcified carotid endarterectomy specimens from diabetic and non-diabetic patients. (A) Focal calcification at the bifurcation and distal internal carotid artery (ICA) in a non-diabetic patient (calcified area 57.6 mm2) (B) The extent of severe calcification in a type 2 diabetic patient involving the ICA and external carotid artery (ECA) (calcified area 196.1 mm2). Total calcification area and percent calcification area in patients with or without diabetes are shown in the table. Abbreviations: CCA= common carotid artery.
In Multi-Ethnic Study of Atherosclerosis (MESA) study, 946 participants were evaluated by MR imaging and ultrasound to determine remodeling index and carotid artery thickness.56 The event rate including myocardial infarction, resuscitated cardiac arrest, angina, stroke and death was significantly greater in diabetics versus non-diabetics. In addition calcification was also greater in diabetics along with remodeling index and carotid thickness.56 In a large MRI plaque imaging study involving 191 patients, moderate to high-grade carotid artery stenoses and advanced lesion phenotypes were over-represented in of T2D patients (57%), and multiple logistic regression analysis revealed association between T2D and MRI-defined high-risk lesion types (OR 2.59; 95% CI [1.15–5.81]), independent of the degree of stenosis.57
Pattterns and Genetics of Vascular Calcification
The link between diabetes and vascular calcification is driven by an active pro-inflammatory and pro-osteogenic program.58 Vascular calcification has been broadly divided into three types by Demer and Tintut: inflammatory, metabolic and genetic, with the latter being mostly medial.59 Contrary to medial calcification which is rarely observed in coronary or carotid vessels, intimal calcification is a systemic process influenced by traditional CVD risk factors in addition to local effects of oxidative stress and inflammation. Despite the association between aging and calcification there is a strong correlation of coronary calcium score with coronary heart disease independent of traditional risk factors. Detrano et al. showed that the adjusted risk of a coronary event was significantly increased by a factor of 7.73 among individuals with CAC score between 101 and 300 and by a factor of 9.67 with CAC above 300 as compared to those with no CAC, with event rates being significantly higher in diabetes.60 Therefore, coronary calcification provides predictive information beyond that provided by standard risk factors.
On the other hand, the dynamics of vascular calcification in the context of coronary disease progression is far more complex. It is well recognized that calcification has a dual function, which either promotes plaque progression towards an unstable, rupture-prone phenotype or favors stabilization, depending on the type and pattern of calcium deposition.61 In general, the patterns of vascular calcification are clinically meaningful such that spotty and/or granular micro-calcification is associated with a pro-inflammatory process and lesion instability while sheet-like or laminated macro-calcification, is often observed in fibrotic lesions, and is thought to support plaque stabilization. Contrary to this view however, sheet-like matrix calcification appears to be prone to fracture forming nodular calcification, which may eventually erupt into the luminal space causing a rare but potentially fatal lesion complicated by thrombosis.42
In addition to biochemical factors, there is also a strong genetic predisposition where genome-wide association studies have identified multiple contributing loci (6p21.3, 6p24, 10q21.3, and 9p21) (Figure 6) linked to atherosclerosis, diabetes, and coronary calcification.62 Methodological limitations however present an issue in assessing types of vascular calcification, i.e., the inability to separate genetic processes underlying intimal from medial calcification although there is strong experimental and clinical evidence that argues for the continued distinction between mechanisms underlying intimal and medial calcification.63 Moreover, because plaque burden and CAC are well correlated, genetic associations could also be confounded by genes underlying atherosclerotic disease rather those controlling calcification.
Figure 6.
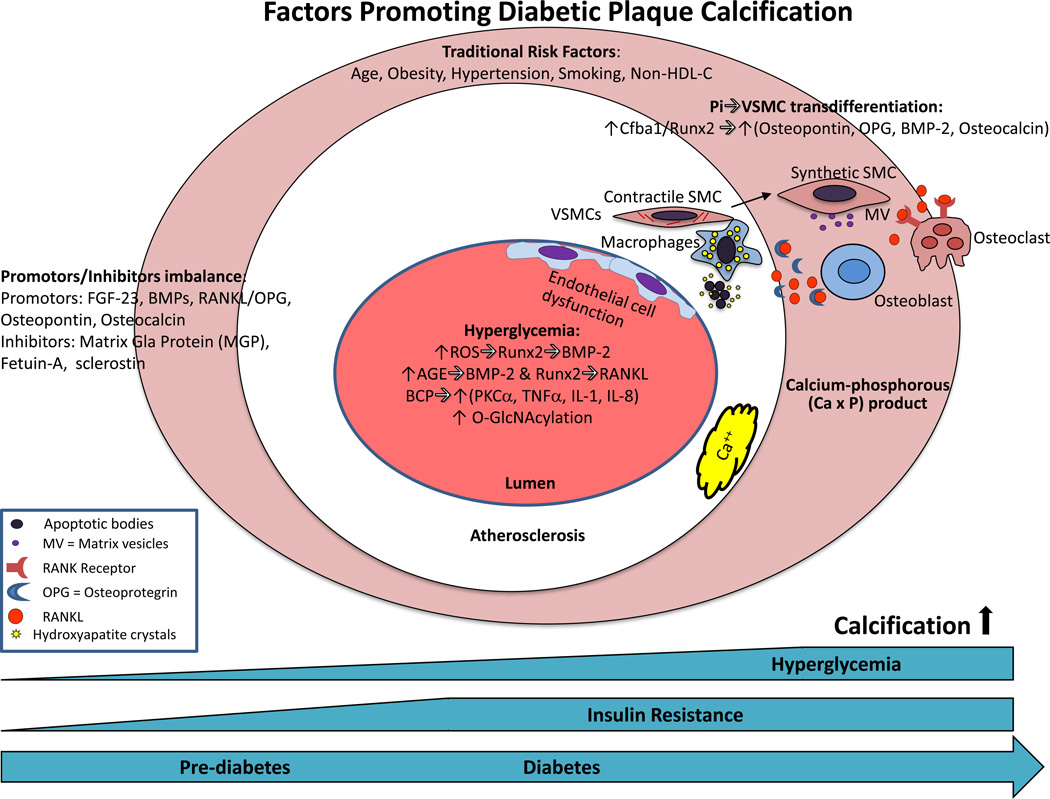
Mechanisms of plaque calcification in diabetes. The earliest form of calcification, microcalcification occurs in apoptotic vascular smooth muscle cells (vSMCs) and macrophages in conjunction with an increase in serum calcium-phosphorous (CaxP) product. Increases in phosphate concentration in vSMCs induce a switch towards an osteoblast-like phenotype, particularly in states of Pi excess. Osteogenic-primed vSMCs express alkaline phosphatase (ALP) and under the control of Cfba-1, secrete bone-associated proteins such as osteopontin, collagen type 1, osteoprotegerin, bone morphogenic protein-2 and osteocalcin, accompanied by the release of mineralization-competent matrix vesicles (MVs). Changes in promoters or inhibitors of mineralization also affect calcification. Hyperglycemia may also affect calcification through multiple mechanisms such as oxidative stress, AGEs, BCP, O-GlcNAcylation, and endothelial dysfunction as discussed in the text. Abbreviations: BMP=bone morphogenic protein; EPCs=endothelial progenitor cells; FGF=fibroblast growth factor; HDL-C=high density lipoprotein-Cholesterol; IL=interleukin; MCCs=myeloid calcifying cells; OPG=osteoprotegrin; ROC=reactive oxygen series; Runx2=Runt-related transcription factor-2;TNF=tumor necrosis factor; TGF=transforming growth factor; BCP=basic calcium phosphate crystals.
Major Mechanisms of Vascular Calcification
Despite its clinical importance, the molecular complexities involved in the regulation of vascular calcification remain incompletely understood.62 It is worth mentioning that our understanding of the genetic basis for vascular calcification has been advanced through mouse models but in general arterial calcification and atherosclerosis do not occur together in these models (unlike in humans). It is rare to see arterial calcification in atherosclerosis-prone mice (i.e. apolipoprotein E or low-density lipoprotein receptor knockouts). Similarly, mice deficient in osteoprotegerin or matrix GLA protein (reviewed below) demonstrate arterial medial calcification in the absence of atherosclerosis. Thus, human lesions are unique because of the co-segregation calcification with atherosclerosis even in the earliest atherosclerotic lesion (i.e. pathologic intimal thickening) which is not completely replicated by any available animal models, with the exception of the monkey.
Although at least four different non-mutually exclusive mechanisms of vascular calcification have been proposed, some are more relevant to the process of medial rather than intimal calcification.64 The process of medial vascular calcification is viewed as a hydroxyapatite mineralization process within the medial layer.39 Loss of inhibitors of mineralization such as matrix Gla protein (MGP) and pyrophosphate promote medial calcification in mice.65, 66 Bone formation inside the vessel wall is also rarely observed in human vascular lesions and suggests osteogenic mechanisms may also play a role in vascular calcification. Bone forming proteins such as such as osteopontin,67 collagen type 1, osteoprotegerin, bone morphogenic protein-2 (BMP-2) and osteocalcin and release of mineralization-competent matrix vesicles (MVs) are critical in this process.67,68 Vascular smooth muscle cells (vSMC) can undergo osteogenic transformation into phenotypically distinct osteoblast-like cells that are capable of expressing and releasing osteochondrogenic proteins.69 Such changes have rarely been observed in vivo in humans. Cell death can also provide phospholipid-rich debris that serve to nucleate apatite. This process starts within lipid pools and progresses with inflammation and the development of necrotic core as lesions process. Lastly, elevated Ca or phosphorus (P) promotes apatite nucleation and crystal growth which further promotes vascular calcification via what are referred to as thermodynamic mechanisms.59 Such mineral imbalances may also exacerbate processes initiated by the other mechanisms mentioned above.
Physiological bone formation and ectopic calcification are often viewed as active processes with the expression of a mineralizing extracellular matrix partially under hormonal control whereas diminished activity and/or loss of calcification inhibitors also leads to arterial calcification but is viewed as a passive process.70 Many of the hormonal and physiological abnormalities associated with diabetes can promote intimal calcification including oxidative stress, endothelial dysfunction, alternations in mineral metabolism, increased inflammatory cytokine production, and release of osteoprogenitor cells from the marrow into the circulation. Below we briefly discuss some of these mechanisms as they relate to the development of vascular calcification in patients with diabetes (Figure 6).
Hyperglycemic mechanisms of diabetic calcification
One of the most significant discoveries in patients with diabetes was unraveling the nature of hyperglycemic damage mainly driven by the accumulation of free radicals, namely superoxide anion, which is capable of activating an array of cellular pathways including polyol and hexosamine flux, advanced glycation end products (AGEs), protein kinase C (PKC), and NF-κB-mediated vascular inflammation.71 Increased levels of glucose and other reducing sugars such as galactose and fructose react with amino groups of proteins to form Schiff bases to yield AGEs. The interaction of AGEs with receptors for advanced glycation end production (RAGEs) activates PKC-ζ to trigger downstream activation of signaling through p38 mitogen activated protein kinase, transforming growth factor beta and NF-κB.72 Significant data support that AGE treatment of VSMC promotes calcification through multiple mechanisms including increasing levels of alkaline phosphatase, a bone matrix protein, decreased expression of VSMCs markers, and increased expression of Runx2, suggesting RAGE promotes transformation of VSMCs into osteoblast-like phenotype.72
AGE/RAGE signaling also exacerbates oxidative stress though a feed-forward loop. AGE activation results in the increased production of ROS by stimulating specific signaling cascades such as TGF-β, NF-κB, and Nox-1.72 SMC expression of S100A12, a human RAGE ligand, increased medial calcification in proximal aorta and innominate arteries of ApoE −/− mice which was associated with increases in bone morphogenetic protein-2 (BMP2) and Runx2.73 The actions of S100A12 were dependent upon RAGE and oxidative stress signaling since both recombinant soluble RAGE (decoy receptor for RAGE) and NAD(P)H oxidase (Nox) inhibition reduced osteogenic programming and calcification.73 Ligands for RAGE are quenched by the soluble form of the receptor, and serum levels of this decoy receptor in hemodialysis patients are inversely associated with vascular calcification.74 In each of these experiments, while AGEs have been shown to induce vascular calcification the specific links to diabetes remain somewhat elusive.
Hyperglycemia itself also increases oxidative stress by increasing glucose oxidation in the citric acid cycle.71 Generation of mitochondrial reactive oxygen species (ROS) is an important contributing factor, as treatment with a mitochondrial uncoupler prevents upregulation of ROS. When glucose levels are elevated the polyol pathway converts glucose to sorbitol using NADPH as a cofactor. As a result, the anti-oxidant glutathione, which also uses NADPH as a cofactor, becomes dysfunctional decreasing cellular resistance to oxidative stress.75 A number of groups have shown oxidative stress powerfully upregulates Runx2 and promotes vascular smooth muscle cell calcification.76,77 Oxidative stress as well as oxidized lipids also induce receptor activator of nuclear factor-κB ligand (RANKL) in mouse VSMCs via Runx2.78, 79 Mice deficient in a decoy receptor for RANKL, osteoprotegerin (OPG), develop extensive vascular calcification which is reduced by OPG treatment.69
Hyperglycemia also can activate the PKC pathway by increasing synthesis of diacylglycerol which plays a critical role in activating protein kinase-C, -β, -δ, and -α.80 Globally genes involved in vessel dilation such as nitric oxide are decreased while those involved in vessel constriction such as endothelin-1 are increased.81 Basic calcium phosphate (BCP) crystals deposit in atherosclerotic lesions and co-localize with inflammatory macrophages. Nadra et al. showed that ingestion of BCP in macrophages triggers a proinflammatory response, including secretion of the inflammatory cytokines tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-8. PKCα was a key mediator of these effects.82 Others have suggested TNF-induced NF-κB can promote inorganic phosphate-induced calcification of human aortic smooth muscle cells while suppressing pyrophosphate (inhibitor of calcification).83 Thus activation of PKC by hyperglycemia might produce a vicious cycle whereby ingestion of BCPs by macrophages not only induces inflammation but also promotes calcification.
Glucose metabolism through the hexosamine biosynthetic pathway produces UDP-β-D-N-acetylglucosamine (UDP-GlcNAc), an active sugar donor for O-linked β-N- acetylglucosamine modification (O-GlcNAcylation).84 O-GlcNAcylation is the glycosylation process through which N-acetylglucosamine (O-GlcNAc) gets added to serine and threonine residues of proteins. O-GlcNAcylation has been shown to stimulate chondrogenesis and osteogenesis and correlates with the transcriptional activity of the osteogenesis regulator, Runx2.85 86 Previous studies have demonstrated elevation of O-GlcNAcylation in human diabetic carotid plaques.87 Heath et al. identified O-GlcNAcylation of AKT on T430 and T479 amino acids as a potential regulator of diabetes mellitus–induced calcification.88 In streptozotocin-treated mice, they found a strong increase in vascular O-GlcNAcylation along with increases in vascular calcification. Blocking the removal of O-GlcNAc further enhanced calcium levels both in vitro in cultured vascular smooth muscle cells and in vivo in mice.
Diabetic patients are also at increased risk for endothelial cell dysfunction (ECD). Major risk factors for ECD in type 1 diabetes are poor glycemic control and diabetes duration while in type 2 diabetes insulin resistance is an important risk factor. The mechanisms by which hyperglycemia induces activation of various pathways (DAG, PKC, hexosamine) of glucose metabolism and production of oxidative stress, formation of AGEs etc. also apply to endothelial cells. Driven by hyperglycemia and oxidative stress, apoptosis of endothelial cells and their overall dysfunction may promote endothelial permeability, exposing VSMCs to hyperglycemia and other pro-inflammatory circulating factors known to promote calcification such as alkaline phosphatase (ALP) and RANKL. Moreover, production of TNFα from both endothelial and smooth muscle cells induces production of bone morphogenic protein −2 (BMP-2), a potent osteoblastic differentiation factor, which promotes osteogenenesis by activating the homeobox homolog (Msx2) and Wnt signaling pathways.89
Dysregulation of phosphate homeostasis and vascular calcification: The bone-kidney-vascular axis
Several observations link serum phosphate levels with a tendency toward vascular calcification, as high serum phosphate levels (hyperphosphatemia, i.e. phosphate levels higher than the normal adult range of 1.0 to 1.5 mmol/L) highly correlate with extent of vascular calcification and vascular disease.90, 91 One of the most common causes of hyperphosphatemia is chronic renal failure treated with hemodialysis, in which serum inorganic phosphate (Pi) levels can typically exceed 2 mmol/L and are commonly associated with widespread vascular calcification.92, 93 Vascular calcification observed in these patients is routinely referred to as metastatic calcification because it occurs in the presence of a systemic mineral imbalance.
High levels of phosphate may exacerbate mechanisms of vascular calcification mediated by osteogenic transcription factors. Vascular smooth muscle cells (vSMC) can undergo osteogenic transformation into phenotypically distinct osteoblast-like cells that are capable of expressing and releasing osteochondrogenic proteins. Increases in phosphate concentration in vSMCs induce a switch towards an osteoblast-like phenotype mainly driven by Cfba1/Runx2 (core-binding factor subunit 1α/runt-related transcription factor 2), particularly in states of Pi excess. Osteogenic-primed vSMCs express alkaline phosphatase (ALP) and under the control of Cfba-1, secrete bone-associated proteins such as osteopontin,67 collagen type 1, osteoprotegerin, bone morphogenic protein-2 and osteocalcin94, 95 accompanied by the release of mineralization-competent matrix vesicles (MVs) (Figure 6).96
Approximately 50% of diabetic subjects develop microalbuminuria, which progresses towards established diabetic nephropathy in 1/3 of patients and elevated serum phosphate (sPi).97 Phosphate homeostasis is maintained by the gut, bone, and kidney and is regulated by many hormones such as parathyroid hormone (PTH), and 1α,25-dihydroxyvitamin D3 (1α,25-(OH)2D3), and the more recently described FGF23 and its required cofactor Klotho.98, 99 Regarding the latter, FGF23 is a circulating phosphaturic hormone that is elevated in patients with chronic kidney disease and strongly associated with cardiovascular mortality.100, 101 High plasma FGF23 concentrations independent from traditional risk factors have also been observed in African Americans and patients with type 2 diabetes mellitus and high CAC scores.102 The specific link between FGF23 and vascular calcification however, is unclear considering FGF23 or its co-receptor, klotho, does not appear to be present in human or mouse vascular SMCs or normal or calcified mouse aorta.103 Moreover, quantified coronary artery and thoracic aortic calcification by computed tomography in 1501 patients from the Chronic Renal Insufficiency Cohort (CRIC) study showed that baseline plasma FGF23 was not associated with the prevalence or severity of calcification even after multivariable adjustment.103 The absence of FGF23/klotho expression in both mouse and human SMCs combined with the failure to shows a direct association with vascular calcification possibly argues this pathway may not have a direct effect on vascular calcification.
The initiation of calcium phosphate deposits may start as MVs,104 which are then released by mineralization-competent cells into extracellular matrix. MVs are spherical bodies in 30 to 300 nm in diameter, enriched in tissue-nonspecific alkaline phosphatase (TNAP), which is indispensable for mineralization.105 It has long been established that inorganic pyrophosphate or polyphosphate must be removed from the sites of mineralization, before calcification can occur.106 vSMCs release MVs under normal physiological conditions and these MVs are protected from mineralization by the presence of calcification inhibitors. Under pathological conditions however, a combination of factors, including the influence of TNAP, makes the MVs “mineralization competent”.107
Circulating cell theory of vascular calcification
An emerging theory suggests that vSMCs may also transdifferentiate to form osteochondrogenic precursors.108 This process is characterized by a phenotypic change in the expression of the osteochondrogenic markers osteopontin, osteocalcin, and ALP in addition to the osteochondrogenic transcription factors core binding factor a1 (Cbfα1) and Runx2 with a reciprocal loss in the vSMC marker α-smooth muscle actin (α-SMA).109 Two recent studies found that the proportion of circulating progenitor cells with osteogenic markers is significantly increased in patients with diabetes mellitus. The exact role of these cells in the setting of diabetic calcification is still being investigated (Figure 6).
In addition, a subpopulation of circulating myeloid-derived calcifying cells has been identified and suggested to be involved in vascular calcification, especially in subjects with T2D, which is characterized by increased circulating levels of these cells, which express ALP and Runx2.110 The transdifferentiation of vSMCs towards an osteochondrogenic phenotype may participate in the initial phase of calcification, it especially characterizes the subsequent, eventual formation of large plates of organized calcium deposits (i.e. “macro-calcification”) within the vessel wall, which may even recapitulate mature bone tissue.111
Clinical studies of CVD and bone matrix regulator proteins
Several clinical studies have reported the association between coronary artery disease and bone matrix regulatory proteins.112, 113 For instance, plasma BMP-2 level was significantly higher in T2D compared to non-diabetes patients. In a multivariable linear regression analysis, plasma BMP-2 was significantly and positively associated with HbA1c and Syntax score. Interestingly, plasma BMP-2 level was positively correlated with plaque burden and CAC assessed by IVUS, whereas negatively correlated with lumen volume.114 Similarly, Chen et al. showed high serum glucose levels were associated with an increased expression of Cbfα1 and BMP-2, which enhanced the calcification of vascular smooth muscle cells.115
Summary
Coronary artery disease in diabetics is associated with larger necrotic core size and greater inflammatory infiltrates of macrophages and T-lymphocytes, resulting in more diffuse atherosclerosis. A higher prevalence of recurrent plaque ruptures with subsequent healing likely also leads to enhanced development of obstructive lesions in diabetes, which further suggests a natural history of disease progression attributed to more active lesions. Calcification is a well-recognized complication of atherosclerotic lesions in diabetic patients, which correlates with increased plaque burden. The diabetic milieu contributes to the development of vessel calcification through multiple mechanisms including hyperglycemia induced increases in oxidative stress, endothelial dysfunction, renal function-induced alterations in mineral metabolism, increased inflammatory cytokine production, and release of osteoprogenitor cells from the marrow into the circulation. Continued growth in our understanding of diabetic vascular disease should eventually lead to the development of better therapeutic strategies to retard its progression and improve clinical outcomes for these patients.
Highlights.
-
➢
Diabetes in the general population is likely to result in a higher incidence of cardiovascular disease, due to the increase in obesity in the general population.
-
➢
Inflammatory (macrophage and T-cell) infiltrate and necrotic core is greater in type 1 and type 2 diabetes versus non-diabetics
-
➢
Type 1 and 2 diabetes is associated with increased plaque burden, healed plaque ruptures, and positive remodeling, along with greater calcification in type 2 diabetes.
-
➢
Vascular calcification is likely driven by specific diabetes-associated mechanisms such as oxidative stress, PKC activation, polylol and hexosamine pathways which are dictated by hyperglycemia.
-
➢
Vascular smooth muscle cells undergo osteogenic transformation promoting vascular calcification
Acknowledgments
Sources of Funding
CVPath Institute Inc., a private non-profit research organization, provided major support for this work, which was also partially supported by National Institutes of Health grant R01 DK094434-01A1.
Footnotes
Disclosure
None
References
- 1.Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Budaj A. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation. 2000;102:1014–1019. doi: 10.1161/01.cir.102.9.1014. [DOI] [PubMed] [Google Scholar]
- 2.Mukamal KJ, Nesto RW, Cohen MC, Muller JE, Maclure M, Sherwood JB, Mittleman MA. Impact of diabetes on long-term survival after acute myocardial infarction: comparability of risk with prior myocardial infarction. Diabetes Care. 2001;24:1422–1427. doi: 10.2337/diacare.24.8.1422. [DOI] [PubMed] [Google Scholar]
- 3.Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam DW, LeRoith D. The worldwide diabetes epidemic. Curr Opin Endocrinol Diabetes Obes. 2012;19:93–96. doi: 10.1097/MED.0b013e328350583a. [DOI] [PubMed] [Google Scholar]
- 5.Group IDFDA. Update of mortality attributable to diabetes for the IDF Diabetes Atlas: Estimates for the year 2013. Diabetes Res Clin Pract. 2015;109:461–465. doi: 10.1016/j.diabres.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 6.Yahagi K, Davis HR, Arbustini E, Virmani R. Sex differences in coronary artery disease: Pathological observations. Atherosclerosis. 2015;239:260–267. doi: 10.1016/j.atherosclerosis.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 8.Farb A, Burke AP, Tang AL, Liang TY, Mannan P, Smialek J, Virmani R. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354–1363. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 9.Davies MJ. The composition of coronary-artery plaques. N Engl J Med. 1997;336:1312–1314. doi: 10.1056/NEJM199705013361809. [DOI] [PubMed] [Google Scholar]
- 10.Schneider DJ. Factors contributing to increased platelet reactivity in people with diabetes. Diabetes Care. 2009;32:525–527. doi: 10.2337/dc08-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreno PR, Murcia AM, Palacios IF, Leon MN, Bernardi VH, Fuster V, Fallon JT. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation. 2000;102:2180–2184. doi: 10.1161/01.cir.102.18.2180. [DOI] [PubMed] [Google Scholar]
- 12.Cipollone F, Iezzi A, Fazia M, Zucchelli M, Pini B, Cuccurullo C, De Cesare D, De Blasis G, Muraro R, Bei R, Chiarelli F, Schmidt AM, Cuccurullo F, Mezzetti A. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation. 2003;108:1070–1077. doi: 10.1161/01.CIR.0000086014.80477.0D. [DOI] [PubMed] [Google Scholar]
- 13.Levin L, Tomer Y. The etiology of autoimmune diabetes and thyroiditis: evidence for common genetic susceptibility. Autoimmun Rev. 2003;2:377–386. doi: 10.1016/s1568-9972(03)00080-6. [DOI] [PubMed] [Google Scholar]
- 14.Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J, Virmani R. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934–940. doi: 10.1161/01.cir.103.7.934. [DOI] [PubMed] [Google Scholar]
- 15.Arenja N, Mueller C, Ehl NF, Brinkert M, Roost K, Reichlin T, Sou SM, Hochgruber T, Osswald S, Zellweger MJ. Prevalence, extent, and independent predictors of silent myocardial infarction. Am J Med. 2013;126:515–522. doi: 10.1016/j.amjmed.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 16.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 17.Virmani R, Burke AP, Kolodgie F. Morphological characteristics of coronary atherosclerosis in diabetes mellitus. Can J Cardiol. 2006;(22 Suppl B):81b–84b. doi: 10.1016/s0828-282x(06)70991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gyongyosi M, Yang P, Hassan A, Weidinger F, Domanovits H, Laggner A, Glogar D. Coronary risk factors influence plaque morphology in patients with unstable angina. Coron Artery Dis. 1999;10:211–219. doi: 10.1097/00019501-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Weissman NJ, Sheris SJ, Chari R, Mendelsohn FO, Anderson WD, Breall JA, Tanguay JF, Diver DJ. Intravascular ultrasonic analysis of plaque characteristics associated with coronary artery remodeling. Am J Cardiol. 1999;84:37–40. doi: 10.1016/s0002-9149(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 20.Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002;105:297–303. doi: 10.1161/hc0302.102610. [DOI] [PubMed] [Google Scholar]
- 21.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 22.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43:1663–1669. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 23.Alexopoulos N, Raggi P. Calcification in atherosclerosis. Nat Rev Cardiol. 2009;6:681–688. doi: 10.1038/nrcardio.2009.165. [DOI] [PubMed] [Google Scholar]
- 24.Tanenbaum SR, Kondos GT, Veselik KE, Prendergast MR, Brundage BH, Chomka EV. Detection of calcific deposits in coronary arteries by ultrafast computed tomography and correlation with angiography. Am J Cardiol. 1989;63:870–872. doi: 10.1016/0002-9149(89)90060-x. [DOI] [PubMed] [Google Scholar]
- 25.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, Delago A, Min JK. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 26.Cheng VY, Lepor NE, Madyoon H, Eshaghian S, Naraghi AL, Shah PK. Presence and severity of noncalcified coronary plaque on 64-slice computed tomographic coronary angiography in patients with zero and low coronary artery calcium. Am J Cardiol. 2007;99:1183–1186. doi: 10.1016/j.amjcard.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 27.Blaha MJ, Cainzos-Achirica M, Greenland P, McEvoy JW, Blankstein R, Budoff MJ, Dardari Z, Sibley CT, Burke GL, Kronmal RA, Szklo M, Blumenthal RS, Nasir K. Role of Coronary Artery Calcium Score of Zero and Other Negative Risk Markers for Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2016;133:849–858. doi: 10.1161/CIRCULATIONAHA.115.018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmermund A, Baumgart D, Gorge G, Seibel R, Gronemeyer D, Ge J, Haude M, Rumberger J, Erbel R. Coronary artery calcium in acute coronary syndromes: a comparative study of electron-beam computed tomography, coronary angiography, and intracoronary ultrasound in survivors of acute myocardial infarction and unstable angina. Circulation. 1997;96:1461–1469. doi: 10.1161/01.cir.96.5.1461. [DOI] [PubMed] [Google Scholar]
- 29.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46:158–165. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 30.Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JA, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–1791. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 31.Khazai B, Luo Y, Rosenberg S, Wingrove J, Budoff MJ. Coronary Atherosclerotic Plaque Detected by Computed Tomographic Angiography in Subjects with Diabetes Compared to Those without Diabetes. PLoS One. 2015;10:e0143187. doi: 10.1371/journal.pone.0143187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otsuka F, Sakakura K, Yahagi K, Joner M, Virmani R. Has our understanding of calcification in human coronary atherosclerosis progressed? Arterioscler Thromb Vasc Biol. 2014;34:724–736. doi: 10.1161/ATVBAHA.113.302642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang Y, Yun KE, Jung HS, Kim CW, Kwon MJ, Sung E, Ryu S. A1C and coronary artery calcification in nondiabetic men and women. Arterioscler Thromb Vasc Biol. 2013;33:2026–2031. doi: 10.1161/ATVBAHA.113.301587. [DOI] [PubMed] [Google Scholar]
- 34.Jung CH, Rhee EJ, Kim KJ, Kim BY, Park SE, Chang Y, Ryu S, Park CY, Mok JO, Oh KW, Kim CH, Park SW, Kang SK, Lee WY. Relationship of glycated hemoglobin A1c, coronary artery calcification and insulin resistance in males without diabetes. Arch Med Res. 2015;46:71–77. doi: 10.1016/j.arcmed.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 36.Selvin E, Coresh J, Golden SH, Brancati FL, Folsom AR, Steffes MW. Glycemic control and coronary heart disease risk in persons with and without diabetes: the atherosclerosis risk in communities study. Arch Intern Med. 2005;165:1910–1916. doi: 10.1001/archinte.165.16.1910. [DOI] [PubMed] [Google Scholar]
- 37.Carson AP, Steffes MW, Carr JJ, Kim Y, Gross MD, Carnethon MR, Reis JP, Loria CM, Jacobs DR, Jr, Lewis CE. Hemoglobin a1c and the progression of coronary artery calcification among adults without diabetes. Diabetes Care. 2015;38:66–71. doi: 10.2337/dc14-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho CY, Shanahan CM. Medial Arterial Calcification: An Overlooked Player in Peripheral Arterial Disease. Arterioscler Thromb Vasc Biol. 2016 doi: 10.1161/ATVBAHA.116.306717. [DOI] [PubMed] [Google Scholar]
- 39.Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, St Hilaire C, Shanahan C. Medial vascular calcification revisited: review and perspectives. Eur Heart J. 2014;35:1515–1525. doi: 10.1093/eurheartj/ehu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olson JC, Edmundowicz D, Becker DJ, Kuller LH, Orchard TJ. Coronary calcium in adults with type 1 diabetes: a stronger correlate of clinical coronary artery disease in men than in women. Diabetes. 2000;49:1571–1578. doi: 10.2337/diabetes.49.9.1571. [DOI] [PubMed] [Google Scholar]
- 41.Burke AP, Kolodgie FD, Farb A, Virmani R. Pathogenesis and significance of calcification in coronary atherosclerosis. In: Virmani R, Narula J, Leon MB, Willerson JT, editors. The Vulnerable Atherosclerotic Plaque: Strategies for Diagnosis and Management. 1. Wiley-Blackwell; 2007. pp. 77–94. [Google Scholar]
- 42.Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, Virmani R. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol. 2016;13:79–98. doi: 10.1038/nrcardio.2015.164. [DOI] [PubMed] [Google Scholar]
- 43.De Angelis M, Scrucca L, Leandri M, et al. Prevalence of carotid stenosis in type 2 diabetic patients asymptomatic for cerebrovascular disease. Diabetes Nutr Metab. 2003;16:48–55. [PubMed] [Google Scholar]
- 44.Odink AE, van der Lugt A, Hofman A, Hunink MG, Breteler MM, Krestin GP, Witteman JC. Risk factors for coronary, aortic arch and carotid calcification; The Rotterdam Study. J Hum Hypertens. 2010;24:86–92. doi: 10.1038/jhh.2009.42. [DOI] [PubMed] [Google Scholar]
- 45.Friedlander AH, Maeder LA. The prevalence of calcified carotid artery atheromas on the panoramic radiographs of patients with type 2 diabetes mellitus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:420–424. doi: 10.1016/s1079-2104(00)70122-3. [DOI] [PubMed] [Google Scholar]
- 46.Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004;164:2147–2155. doi: 10.1001/archinte.164.19.2147. [DOI] [PubMed] [Google Scholar]
- 47.van ‘t Riet E, Rijkelijkhuizen JM, Alssema M, Nijpels G, Stehouwer CD, Heine RJ, Dekker JM. HbA1c is an independent predictor of non-fatal cardiovascular disease in a Caucasian population without diabetes: a 10-year follow-up of the Hoorn Study. Eur J Prev Cardiol. 2012;19:23–31. doi: 10.1097/HJR.0b013e32833b0932. [DOI] [PubMed] [Google Scholar]
- 48.Roquer J, Rodriguez-Campello A, Cuadrado-Godia E, Giralt-Steinhauer E, Jimenez-Conde J, Soriano C, Ois A. The role of HbA1c determination in detecting unknown glucose disturbances in ischemic stroke. PLoS One. 2014;9:e109960. doi: 10.1371/journal.pone.0109960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, McCarren M, Duckworth WC, Emanuele NV. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197–2206. doi: 10.1056/NEJMoa1414266. [DOI] [PubMed] [Google Scholar]
- 50.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mauriello A, Sangiorgi G, Palmieri G, Virmani R, Holmes DR, Jr, Schwartz RS, Pistolese R, Ippoliti A, Spagnoli LG. Hyperfibrinogenemia is associated with specific histocytological composition and complications of atherosclerotic carotid plaques in patients affected by transient ischemic attacks. Circulation. 2000;101:744–750. doi: 10.1161/01.cir.101.7.744. [DOI] [PubMed] [Google Scholar]
- 52.Carr S, Farb A, Pearce WH, Virmani R, Yao JS. Atherosclerotic plaque rupture in symptomatic carotid artery stenosis. J Vasc Surg. 1996;23:755–765. doi: 10.1016/s0741-5214(96)70237-9. discussion 765-6. [DOI] [PubMed] [Google Scholar]
- 53.Spagnoli LG, Mauriello A, Palmieri G, Santeusanio G, Amante A, Taurino M. Relationships between risk factors and morphological patterns of human carotid atherosclerotic plaques. A multivariate discriminant analysis. Atherosclerosis. 1994;108:39–60. doi: 10.1016/0021-9150(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 54.Scholtes VP, Peeters W, van Lammeren GW, Howard DP, de Vries JP, de Borst GJ, Redgrave JN, Kemperman H, Schalkwijk CG, den Ruijter HM, de Kleijn DP, Moll FL, Rothwell PM, Pasterkamp G. Type 2 diabetes is not associated with an altered plaque phenotype among patients undergoing carotid revascularization. A histological analysis of 1455 carotid plaques. Atherosclerosis. 2014;235:418–423. doi: 10.1016/j.atherosclerosis.2014.05.941. [DOI] [PubMed] [Google Scholar]
- 55.Vigili de Kreutzenberg S, Fadini GP, Guzzinati S, Mazzucato M, Volpi A, Coracina A, Avogaro A. Carotid plaque calcification predicts future cardiovascular events in type 2 diabetes. Diabetes Care. 2015;38:1937–1944. doi: 10.2337/dc15-0327. [DOI] [PubMed] [Google Scholar]
- 56.Zavodni AE, Wasserman BA, McClelland RL, Gomes AS, Folsom AR, Polak JF, Lima JA, Bluemke DA. Carotid artery plaque morphology and composition in relation to incident cardiovascular events: the Multi-Ethnic Study of Atherosclerosis (MESA) Radiology. 2014;271:381–389. doi: 10.1148/radiol.14131020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Esposito L, Saam T, Heider P, Bockelbrink A, Pelisek J, Sepp D, Feurer R, Winkler C, Liebig T, Holzer K, Pauly O, Sadikovic S, Hemmer B, Poppert H. MRI plaque imaging reveals high-risk carotid plaques especially in diabetic patients irrespective of the degree of stenosis. BMC Med Imaging. 2010;10:27. doi: 10.1186/1471-2342-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shemesh J, Tenenbaum A, Fisman EZ, Koren-Morag N, Grossman E. Coronary calcium in patients with and without diabetes: first manifestation of acute or chronic coronary events is characterized by different calcification patterns. Cardiovasc Diabetol. 2013;12:161. doi: 10.1186/1475-2840-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Demer LL, Tintut Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler Thromb Vasc Biol. 2014;34:715–723. doi: 10.1161/ATVBAHA.113.302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 61.Pugliese G, Iacobini C, Blasetti Fantauzzi C, Menini S. The dark and bright side of atherosclerotic calcification. Atherosclerosis. 2015;238:220–230. doi: 10.1016/j.atherosclerosis.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 62.Evrard S, Delanaye P, Kamel S, Cristol JP, Cavalier E. Vascular calcification: from pathophysiology to biomarkers. Clin Chim Acta. 2015;438:401–414. doi: 10.1016/j.cca.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 63.Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1599–1605. doi: 10.2215/CJN.02120508. [DOI] [PubMed] [Google Scholar]
- 64.Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol. 2004;15:2959–2964. doi: 10.1097/01.ASN.0000145894.57533.C4. [DOI] [PubMed] [Google Scholar]
- 65.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 66.Zhou X, Cui Y, Zhou X, Han J. Phosphate/pyrophosphate and MV-related proteins in mineralisation: discoveries from mouse models. Int J Biol Sci. 2012;8:778–790. doi: 10.7150/ijbs.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giachelli CM, Bae N, Almeida M, Denhardt DT, Alpers CE, Schwartz SM. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993;92:1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 69.Harper E, Forde H, Davenport C, Rochfort KD, Smith D, Cummins PM. Vascular calcification in type-2 diabetes and cardiovascular disease: Integrative roles for OPG, RANKL and TRAIL. Vascul Pharmacol. 2016;82:30–40. doi: 10.1016/j.vph.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 70.Schinke T, Amling M. Mineralization of bone: an active or passive process? Weinheim (Germany): Wiley-VCH Verlag; 2007. [Google Scholar]
- 71.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 72.Kay AM, Simpson CL, Stewart JA., Jr The Role of AGE/RAGE Signaling in Diabetes-Mediated Vascular Calcification. J Diabetes Res. 2016;2016:6809703. doi: 10.1155/2016/6809703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hofmann Bowman MA, Gawdzik J, Bukhari U, Husain AN, Toth PT, Kim G, Earley J, McNally EM. S100A12 in vascular smooth muscle accelerates vascular calcification in apolipoprotein E-null mice by activating an osteogenic gene regulatory program. Arterioscler Thromb Vasc Biol. 2011;31:337–344. doi: 10.1161/ATVBAHA.110.217745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim HS, Chung W, Kim AJ, Ro H, Chang JH, Lee HH, Jung JY. Circulating levels of soluble receptor for advanced glycation end product are inversely associated with vascular calcification in patients on haemodialysis independent of S100A12 (EN-RAGE) levels. Nephrology (Carlton) 2013;18:777–782. doi: 10.1111/nep.12166. [DOI] [PubMed] [Google Scholar]
- 75.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 76.Sun Y, Byon CH, Yuan K, Chen J, Mao X, Heath JM, Javed A, Zhang K, Anderson PG, Chen Y. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ Res. 2012;111:543–552. doi: 10.1161/CIRCRESAHA.112.267237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Byon CH, Sun Y, Chen J, Yuan K, Mao X, Heath JM, Anderson PG, Tintut Y, Demer LL, Wang D, Chen Y. Runx2-upregulated receptor activator of nuclear factor kappaB ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arterioscler Thromb Vasc Biol. 2011;31:1387–1396. doi: 10.1161/ATVBAHA.110.222547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, Lopez-Ongil S, Coll B, Fernandez E, Valdivielso JM. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res. 2009;104:1041–1048. doi: 10.1161/CIRCRESAHA.108.189001. [DOI] [PubMed] [Google Scholar]
- 80.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–866. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- 81.Derubertis FR, Craven PA. Activation of protein kinase C in glomerular cells in diabetes. Mechanisms and potential links to the pathogenesis of diabetic glomerulopathy. Diabetes. 1994;43:1–8. doi: 10.2337/diab.43.1.1. [DOI] [PubMed] [Google Scholar]
- 82.Nadra I, Mason JC, Philippidis P, Florey O, Smythe CD, McCarthy GM, Landis RC, Haskard DO. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: a vicious cycle of inflammation and arterial calcification? Circ Res. 2005;96:1248–1256. doi: 10.1161/01.RES.0000171451.88616.c2. [DOI] [PubMed] [Google Scholar]
- 83.Zhao G, Xu MJ, Zhao MM, Dai XY, Kong W, Wilson GM, Guan Y, Wang CY, Wang X. Activation of nuclear factor-kappa B accelerates vascular calcification by inhibiting ankylosis protein homolog expression. Kidney Int. 2012;82:34–44. doi: 10.1038/ki.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393:684–688. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]
- 85.Nagel AK, Schilling M, Comte-Walters S, Berkaw MN, Ball LE. Identification of O-linked N-acetylglucosamine (O-GlcNAc)-modified osteoblast proteins by electron transfer dissociation tandem mass spectrometry reveals proteins critical for bone formation. Mol Cell Proteomics. 2013;12:945–955. doi: 10.1074/mcp.M112.026633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andres-Bergos J, Tardio L, Larranaga-Vera A, Gomez R, Herrero-Beaumont G, Largo R. The increase in O-linked N-acetylglucosamine protein modification stimulates chondrogenic differentiation both in vitro and in vivo. J Biol Chem. 2012;287:33615–33628. doi: 10.1074/jbc.M112.354241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–472. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 88.Heath JM, Sun Y, Yuan K, Bradley WE, Litovsky S, Dell’Italia LJ, Chatham JC, Wu H, Chen Y. Activation of AKT by O-linked N-acetylglucosamine induces vascular calcification in diabetes mellitus. Circ Res. 2014;114:1094–1102. doi: 10.1161/CIRCRESAHA.114.302968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alfrey AC, Ibels LS. Role of phosphate and pyrophosphate in soft tissue calcification. Adv Exp Med Biol. 1978;103:187–193. doi: 10.1007/978-1-4684-7758-0_20. [DOI] [PubMed] [Google Scholar]
- 91.Block GA, Port FK. Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: recommendations for a change in management. Am J Kidney Dis. 2000;35:1226–1237. doi: 10.1016/s0272-6386(00)70064-3. [DOI] [PubMed] [Google Scholar]
- 92.Winchester JF, Rotellar C, Goggins M, Robino D, Rakowski TA, Argy WP. Calcium and phosphate balance in dialysis patients. Kidney Int Suppl. 1993;41:S174–S178. [PubMed] [Google Scholar]
- 93.Sarkozi L, Szabo A. Effect of hemodialysis on the distribution of phosphates in blood. Clin Physiol Biochem. 1989;7:184–188. [PubMed] [Google Scholar]
- 94.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 95.Levy RJ, Schoen FJ, Levy JT, Nelson AC, Howard SL, Oshry LJ. Biologic determinants of dystrophic calcification and osteocalcin deposition in glutaraldehyde-preserved porcine aortic valve leaflets implanted subcutaneously in rats. Am J Pathol. 1983;113:143–155. [PMC free article] [PubMed] [Google Scholar]
- 96.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 97.Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol. 2009;20:381–387. doi: 10.1681/ASN.2008040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. 2011;109:697–711. doi: 10.1161/CIRCRESAHA.110.234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Degirolamo C, Sabba C, Moschetta A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov. 2016;15:51–69. doi: 10.1038/nrd.2015.9. [DOI] [PubMed] [Google Scholar]
- 100.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. Jama. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Freedman BI, Divers J, Russell GB, Palmer ND, Bowden DW, Carr JJ, Wagenknecht LE, Hightower RC, Xu J, Smith SC, Langefeld CD, Hruska KA, Register TC. Plasma FGF23 and Calcified Atherosclerotic Plaque in African Americans with Type 2 Diabetes Mellitus. Am J Nephrol. 2015;42:391–401. doi: 10.1159/000443241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scialla JJ, Lau WL, Reilly MP, et al. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int. 2013;83:1159–1168. doi: 10.1038/ki.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anderson HC. Matrix vesicles and calcification. Curr Rheumatol Rep. 2003;5:222–226. doi: 10.1007/s11926-003-0071-z. [DOI] [PubMed] [Google Scholar]
- 105.Whyte MP. Physiological role of alkaline phosphatase explored in hypophosphatasia. Ann N Y Acad Sci. 2010;1192:190–200. doi: 10.1111/j.1749-6632.2010.05387.x. [DOI] [PubMed] [Google Scholar]
- 106.Fleish H, Neuman WF. Mechanisms of calcification: role of collagen, polyphosphates, and phosphatase. Am J Physiol. 1961;200:1296–1300. doi: 10.1152/ajplegacy.1961.200.6.1296. [DOI] [PubMed] [Google Scholar]
- 107.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 110.Fadini GP, Albiero M, Menegazzo L, et al. Widespread increase in myeloid calcifying cells contributes to ectopic vascular calcification in type 2 diabetes. Circ Res. 2011;108:1112–1121. doi: 10.1161/CIRCRESAHA.110.234088. [DOI] [PubMed] [Google Scholar]
- 111.Shanahan CM. Inflammation ushers in calcification: a cycle of damage and protection? Circulation. 2007;116:2782–2785. doi: 10.1161/CIRCULATIONAHA.107.749655. [DOI] [PubMed] [Google Scholar]
- 112.Snell-Bergeon JK, Budoff MJ, Hokanson JE. Vascular calcification in diabetes: mechanisms and implications. Curr Diab Rep. 2013;13:391–402. doi: 10.1007/s11892-013-0379-7. [DOI] [PubMed] [Google Scholar]
- 113.Bostrom KI, Jumabay M, Matveyenko A, Nicholas SB, Yao Y. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ Res. 2011;108:446–457. doi: 10.1161/CIRCRESAHA.110.236596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang M, Sara JD, Wang FL, Liu LP, Su LX, Zhe J, Wu X, Liu JH. Increased plasma BMP-2 levels are associated with atherosclerosis burden and coronary calcification in type 2 diabetic patients. Cardiovasc Diabetol. 2015;14:64. doi: 10.1186/s12933-015-0214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen NX, Duan D, O’Neill KD, Moe SM. High glucose increases the expression of Cbfa1 and BMP-2 and enhances the calcification of vascular smooth muscle cells. Nephrol Dial Transplant. 2006;21:3435–3442. doi: 10.1093/ndt/gfl429. [DOI] [PubMed] [Google Scholar]