Abstract
The paper presents a description of essential equipment requirements for scrotal ultrasonography, including current ultrasound techniques, as well as a review of the most common scrotal pathologies. Patient preparation for the examination as well as ultrasound methodology for the assessment of scrotal and inguinal canal structures are discussed. The standard for scrotal ultrasound examination includes a precise B-mode evaluation, including testicular volumetric assessment performed using automatic measurement options based on the formula of a rotating ellipsoid or three measurements perpendicular to one another. Also, criteria for morphological assessment of abnormalities within testicular or epididymal parenchyma, including a precise evaluation of lesion size, delineation, shape and vascular pattern obtained with Doppler US, have been proposed. Standard assessment further includes epididymal evaluation, including epididymal size in the case of enlargement. The paper additionally discusses the method of ultrasonographic examination and describes the most common pathologies occurring within scrotal structures, including a quantitative analysis of hydrocele and other abnormal fluid reservoirs. We have also presented criteria for the assessment of varicocele as well as color and spectral Doppler flows in scrotal pathologies. Furthermore, we have proposed key components of scrotal ultrasound documentation, so that the contained data could be used to establish appropriate diagnosis, allowing for both adequate clinical management and the reproducibility of subsequent US evaluations performed by either the same or a different examiner. The most common causes of diagnostic errors have also been discussed.
Keywords: scrotal ultrasonography, testicular and epididymal ultrasonography, scrotum
Abstract
Praca przedstawia opis niezbędnych wymagań aparaturowych w badaniu moszny, uwzględniający obecnie stosowane techniki ultrasonograficzne, oraz przegląd najczęściej występujących nieprawidłowości w worku mosznowym. Omówiono sposób przygotowania pacjenta do badania oraz metodykę wykonania badania ultrasonograficznego narządów worka mosznowego i kanałów pachwinowych. Standard badania narządów moszny obejmuje dokładną ocenę B-mode, uwzględniającą wolumetrię jąder uzyskaną przy pomocy automatycznych opcji pomiarowych, wykorzystujących wzory na elipsoidę obrotową bądź trzy prostopadłe do siebie pomiary. Zaproponowano kryteria oceny morfologicznej nieprawidłowości występujących w miąższu jąder i najądrzy, uwzględniające dokładną analizę wielkości zmian, ich odgraniczenia, kształtu oraz wzorca unaczynienia uzyskanego w badaniach dopplerowskich. Standardowe badanie zawiera również ocenę najądrzy, łącznie z podaniem pomiarów w przypadku ich powiększenia. W artykule omówiono także sposób badania i opisu najczęściej występujących patologii w strukturach worka mosznowego, w tym przeprowadzenia ilościowej oceny wodniaków i innych nieprawidłowych zbiorników płynowych. Przedstawiono również kryteria oceny żylaków powrózków nasiennych i przepływów naczyniowych uzyskiwanych w badaniach kolorowego i spektralnego dopplera w przypadkach nieprawidłowości w narządach worka mosznowego. Zaproponowano niezbędne elementy opisu badania ultrasonograficznego worka mosznowego, tak by zawarte dane posłużyły do ustalenia odpowiedniego rozpoznania i umożliwiły prawidłowe postępowanie kliniczne oraz stanowiły o powtarzalności dalszych badań wykonywanych zarówno przez tego samego badającego, jak i przez różnych badających. Omówiono także najczęstsze przyczyny popełnianych błędów diagnostycznych.
Introduction
The development of testicular tumors or hydrocele is one of the oldest indications for scrotal ultrasound. However, the number of indications has increased significantly as a result of technological advances in ultrasonography as well as its growing popularity. These indications depend on patient’s age, the clinical picture and medical history. Determining the presence of testes in the case of cryptorchidism is the main indication for ultrasound in newborns and infants. Testicular pain, suspected orchitis and epididymitis, testicular and epididymal asymmetry, as well as trauma, pedicle torsion or endocrine disorders, such as precocious puberty, gynecomastia or feminization and abnormal laboratory and tumor marker findings, e.g. alpha-fetoprotein and human chorionic gonadotropin (β-HCG), are indications for scrotal ultrasound in older children. Indications for scrotal ultrasound in adult men include abnormal testicular consistency, suspected testicular tumors, scrotal or inguinal hernia, hydrocele, spermatic cord hydrocele, as well as extraperitoneal and inguinal lymphadenopathy, hematospermia and reproductive failure. The wide range of indications and the high polymorphism of the clinical picture require an ultrasound examiner to have knowledge on the spectrum of the above mentioned pathologies and the applied ultrasound standards, which allows for an assessment of all scrotal structures. Ultrasound standardization is aimed to create appropriate conditions for repeatability and comparability of scrotal ultrasound scans preformed by the same or other examiners(1–4).
Equipment
Scrotal ultrasound is performed using linear high-frequency transducers (above 7 MHz). Broadband transducers with a frequency range of 6 up to 12 MHz or higher are preferred. The frequency range depends on the testicle size. The linear transducer should have the trapezoidal imaging feature to expand the ultrasound field, thus allowing for an assessment of the maximum longitudinal section of the parenchyma of the entire testis and epidydymis. The penetration depth of the ultrasound beam should be set at about 1–5 cm, so that it would be focused on the entire scrotal and inguinal canal content. Scrotal ultrasound equipment should feature a wide dynamic range (dB) as well as Power or Color and Spectral Doppler options with the possibility to analyze slow blood flow when assessing testicular and spermatic cord perfusion. These options are particularly important in the case of suspected pedicle torsion and for the diagnosis of varicocele. Elastography should be optionally considered, particularly in reference centers, to qualify patients for surgical treatment in the case of testicular, epididymal or extratesticular focal lesions as well as to monitor their treatment.
An ultrasound device should feature a calculation option to measure testicular volume in order to assess three measurement sections perpendicular to one another, as well as a rotating ellipsoid calculation option. An ultrasound device should feature multiple zoom function. The obtained images should be registered in the form of videoprinter or using other carriers, such as CD or USB(5–7).
Technique
Scrotal ultrasound should be preceded by a thorough medical history and palpation. Palpation is performed to assess testicular and epididymal size, symmetry, tenderness as well as to examine the spermatic cords. In the case of suspected pedicle torsion, palpation should be performed in a standing position. The scrotum (together with its contents) should be elevated with a hand, which alleviates pain accompanying orchitis and epididymitis, and increases pain associated with testicular torsion (Prehn’s sign).
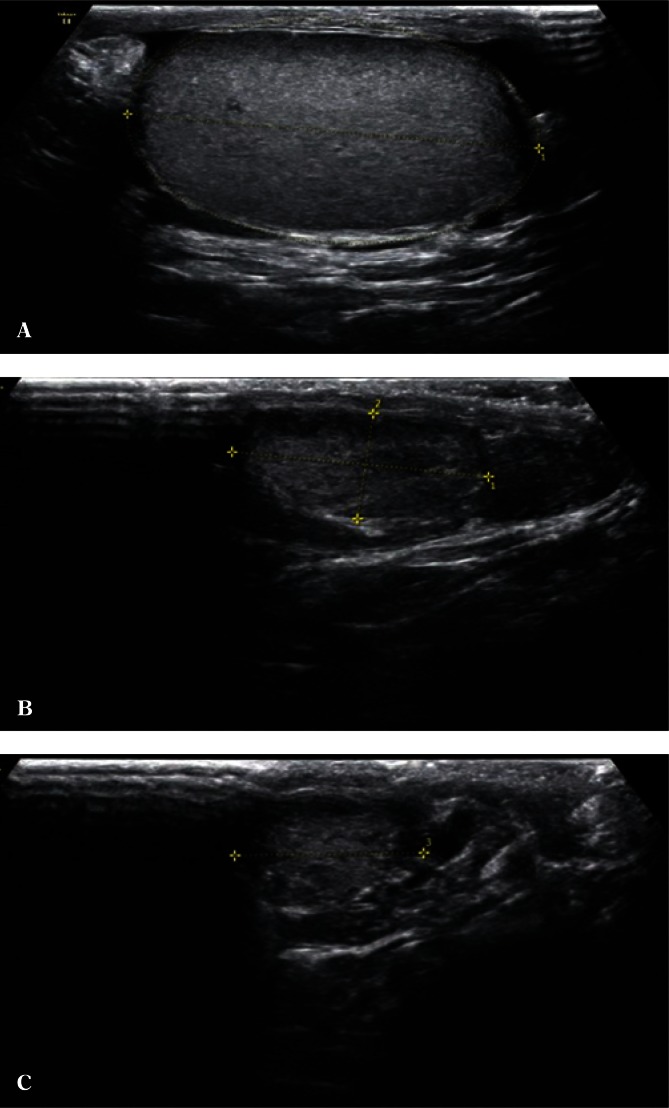
Scrotal ultrasound is performed in a patient in a supine or standing position. A lignin roll placed on patient’s thighs to support and elevate the scrotum can be used to allow for an assessment of all scrotal structures. A similar effect can be achieved with the patient crossing his legs. This prevents scrotal movement between the thighs and, consequently, its unreliable evaluation. Ultrasound examination begins with an assessment of testicular parenchyma, symmetry, size and echogenicity. Rotating ellipsoid measurement with a precise determination of testicular borders in a maximum longitudinal section can be used for testicular size assessment. Three perpendicular measurements, i.e. top-to-bottom, sagittal and frontal dimensions, can be also used for testicular measurement (Fig. 1).
Fig. 1.
A. A measurement of testicular volume based on a rotating ellipsoid. B. A measurement of testicular volume based on three perpendicular measurements (a × b × c – the maximum longitudinal section). C. A measurement of testicular volume based on three perpendicular measurements (a × b × c – the maximum cross-section)
Testicular volume measurement is a very important component of ultrasound assessment, which allows to determine the effects of detected clinical abnormalities on the size of the gonads(8, 9). These measurements are performed using the available ultrasound calculation software. An assessment of testicular volume in young children, particularly in cryptorchidism and migratory testis, is a sensitive parameter of growth or growth inhibition. In the case of testicular size or volume asymmetry, an accurate morphometry allows to determine testicular atrophy index (TAI), which describes the difference in the testicular size expressed as percentage, or testicular volume ratio (TVR), which describes the volume ratio of the inappropriately located testis to that of testis located in the scrotum(10, 11). The repeatability of the obtained dimensions is achieved by performing measurements in maximum sections, with the axial section always showing the mediastinum testis. Ultrasound transducer compression on the testes changes their shape and can lead to false measurements. Preferably, the maximum longitudinal epididymal section should be visualized with the parenchyma of the entire testis. Testicular volume measurement is important in adult patients receiving hormonal therapy following scrotal surgeries.
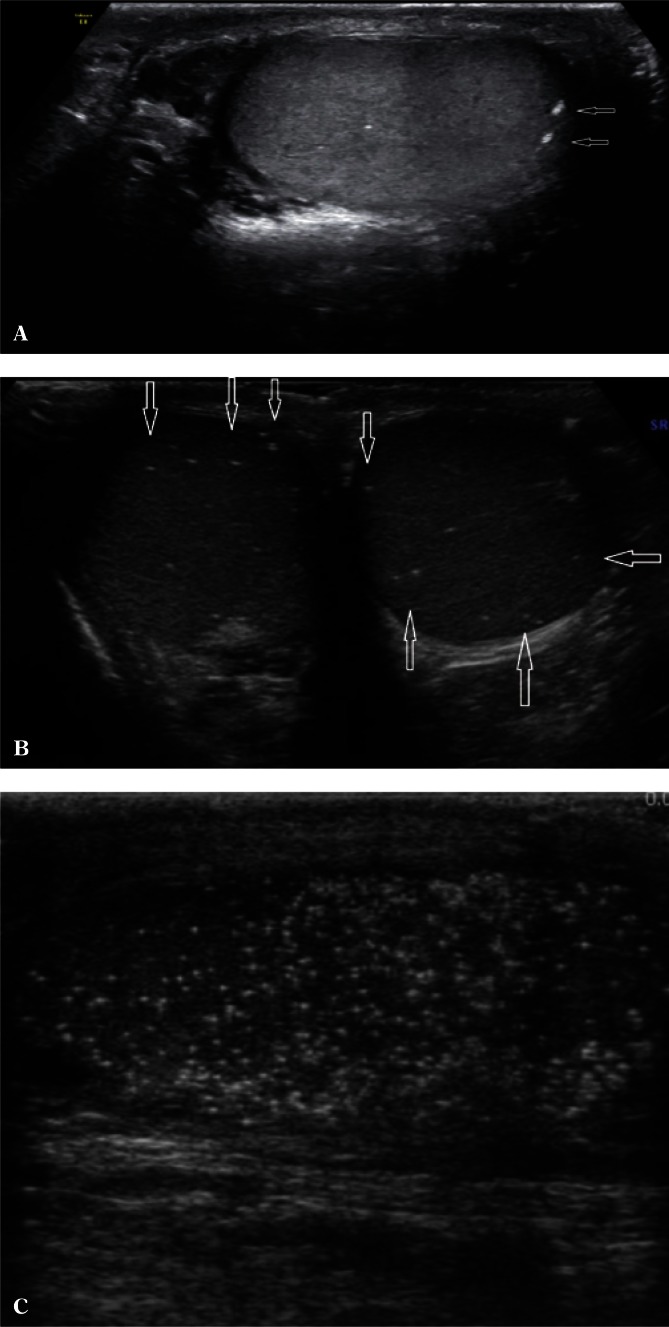
The evaluation of testicular parenchyma should be performed accurately, starting from the longitudinal sections, through cross-sections and intermediate sections. A comparison between the two testes in terms of echogenicity allows to assess the symmetry of abnormalities, e.g. in post-inflammatory or post-traumatic lesions, lesions secondary to chronic, uncontrolled diabetes and other diseases. Furthermore, attention should be paid to the appearance of stromal connective tissue within the so-called testicular septa. If micro- or macrocalcifications are found in testicular parenchyma, it is necessary to determine whether they are single, have the maximum dimension in long axes, or they are multiple/very multiple and show a uniform uni- or bilateral distribution (Fig. 2). Microcalcifications are calcium deposits present in the lumen of the seminiferous tubules or within the basement membrane in a number higher than 5 in the entire testis or, according to another definition, in a number higher than 5 in the field of vision(12).
Fig. 2.
A. Occasional macrocalcifications (arrows). B. Few microcalcifications in both testes (cross-section). C. Numerous microcalcifications (longitudinal section)
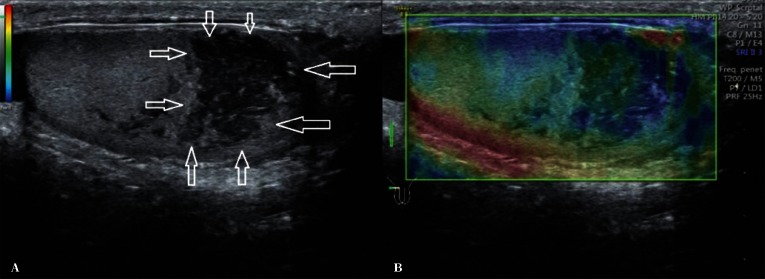
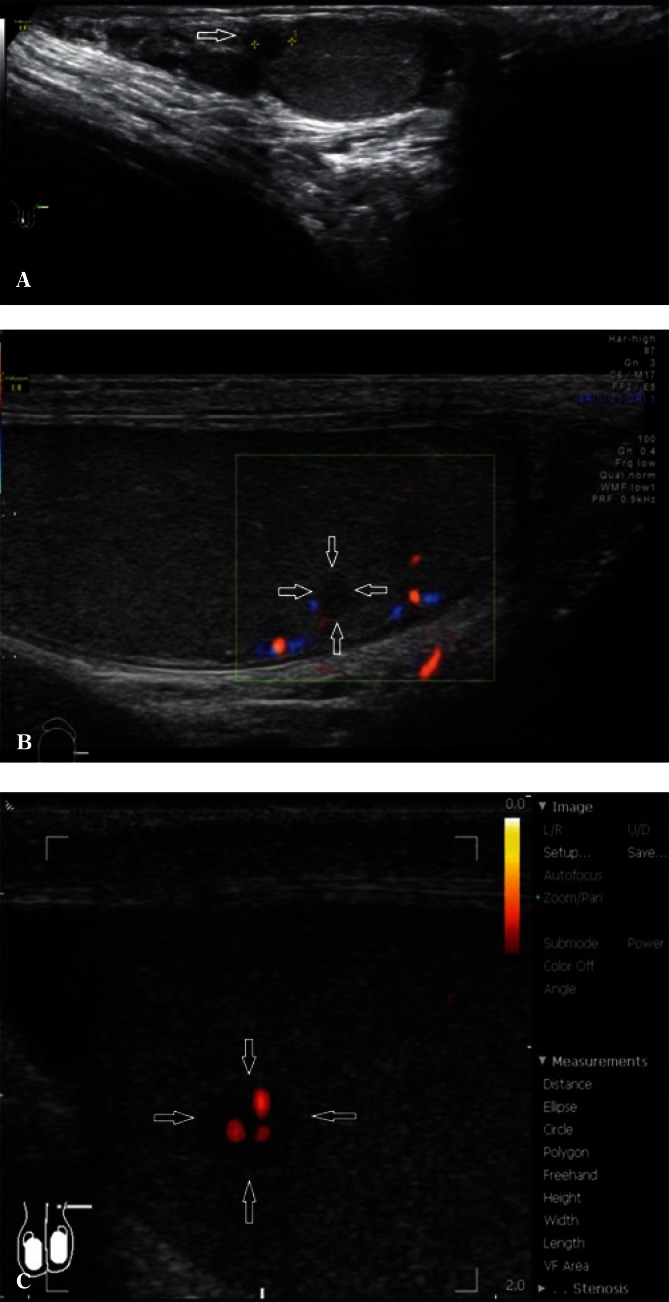
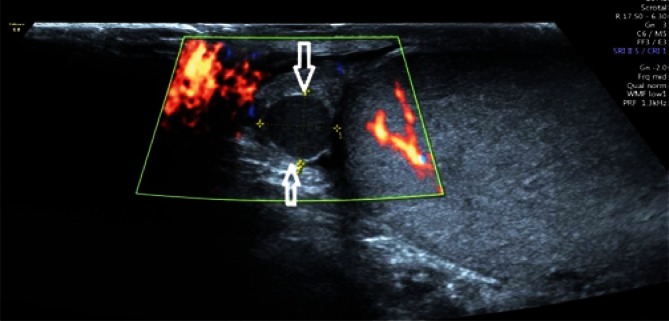
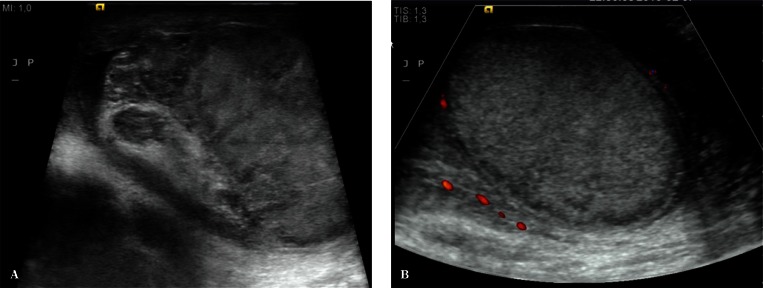
If abnormal testicular parenchymal structure is found, it is necessary to assess testicular location (upper pole, lower pole, medial border, outer border). An assessment of the shape of the lesion, i.e. regular/irregular, polycyclic outlines, with/without clear demarcation, hypoechoic/isoechoic/hyperechoic, is a very important component of evaluation. Identification of multiple lesions and determining their location as well as accurate biometry in the case of suspected benign lesions increases the probability of achieving high repeatability of the examination as well as a correct assessment of the size dynamics of the lesion (Fig. 3). Information on the presence or absence of internal calcifications as well as the vascular pattern (peripheral vasculature of the lesion, the presence of internal vascular segments in the lesion) is also important. In the case of enlarged mediastinum testis with the signs of retention, with cystic, tubular contents of the seminiferous tubules, it is necessary to document the size of the mediastinum testis at maximum sections(13) (Fig. 4). Identification of an occasional, non-palpable or clearly palpable hypoechoic lesion with an increased consistency is an indication for an urgent urology consultation (Fig. 5).
Fig. 3.
A hypoechoic lesion (arrows) with polycyclic outlines in the lower testicular pole – longitudinal B-mode section and elastography
Fig. 4.
Seminiferous tubule retention in the region of the mediastinum testis with a coexisting cyst (arrows)
Fig. 5.
A. A 36-year-old patient with hypogonadism (3 mm in diameter, hypoechoic focal lesion – an arrow). B. A hypoechoic lesion with a diameter of 4 mm, with single marginal vascular segments in color Doppler (arrows). C. A hypoechoic lesion with a diameter of 5 mm in power Doppler; a wide range of power Doppler (arrows)
The obtained ultrasound image of the tumor can indicate the need to determine tumor markers, such as alpha-feto-protein and β-HCG(14). If a focal lesion is found, the conclusions of the examination should contain a suggestion on the need for other imaging modalities of the abdomen, retroperitoneal lymph nodes and parenchymal organs.
Scrotal trauma
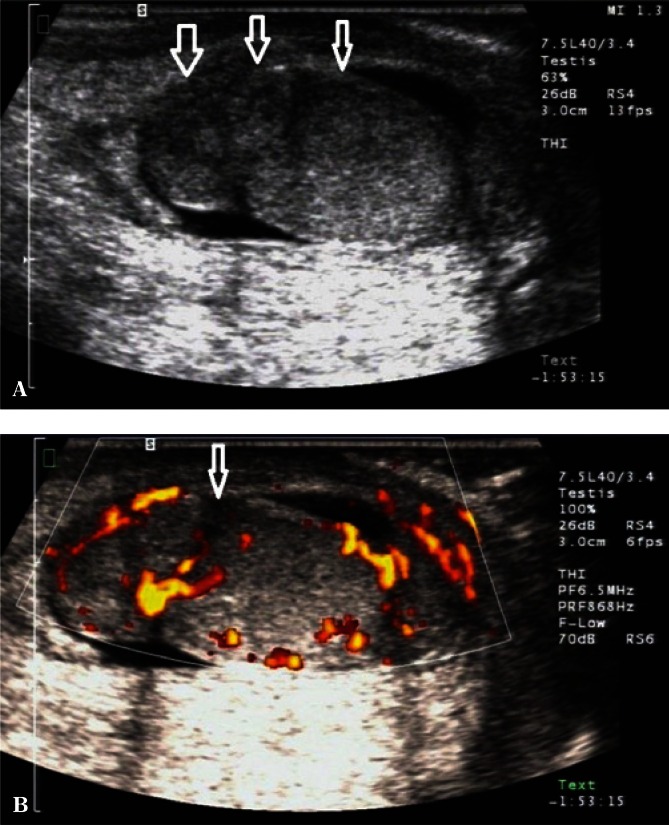
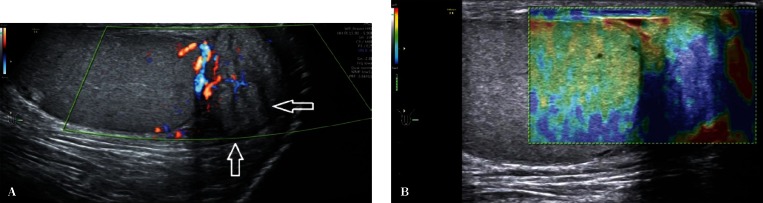
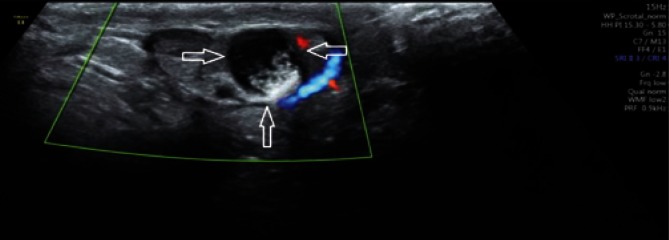
An assessment of the continuity of the tunica albuginea, testicular and epididymal size and structure, as well as Doppler evaluation of the testicular parenchyma are important components of scrotal ultrasound in patients with post-traumatic pain and lesions (Fig. 6). If hematoma is identified, it is necessary to evaluate its size and location. The high sensitivity and specificity of the examination provide an urologist with a wide range of information necessary for treatment qualification(15).
Fig. 6.
A. B-mode images. Scrotal injury in the region of the upper testicular and epididymal pole with a disrupted tunica albuginea (an arrow). B. Power Doppler images. An assessment of the distribution of flow velocities in the patient from Fig. 6 A (an arrow)
Abnormal scrotal fluid reservoirs
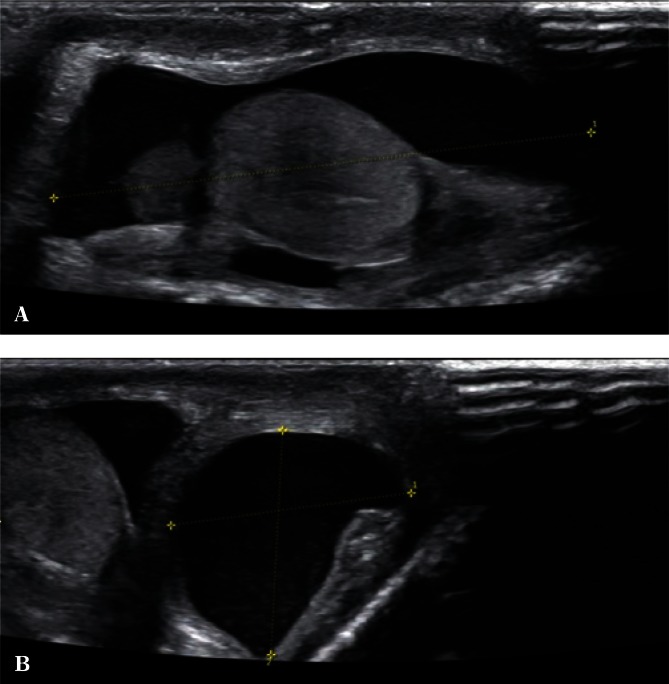
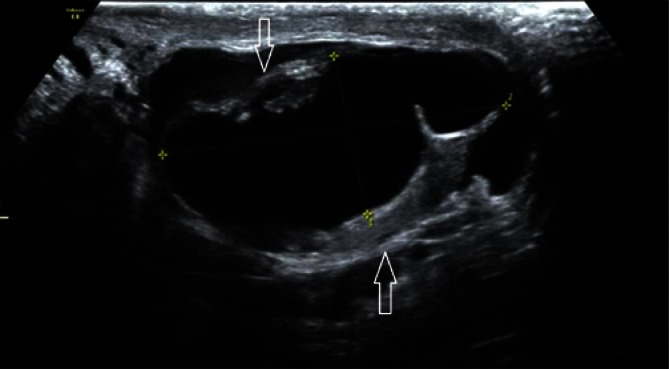
A small, physiological amount of liquid between testicular sheaths in the scrotum is necessary for normal testicular, epididymal and spermatic cord mobility. The evaluation of an increased amount of liquid is complex. The borderline between the term “hydrocele” and a physiological amount of fluid still remains unclear. Apparently benign hydrocele can be etiologically associated with embryonal testicular tumors or precede their development(16) in the case of mesothelioma of the tunica vaginalis testis(17), metastases, e.g. adenocarcinomas(18), granulosa cell tumor(19), as well as rhabdomyosarcoma(20) and other(21). A hydrocele can sometimes indicate the presence of post-traumatic lesions or partial pedicle torsion(22). Therefore, it is important to determine the amount of liquid, preferably by measuring its volume (Fig. 7).
Fig. 7.
A. A testicular hydrocele – a longitudinal section. B. A testicular hydrocele – a cross-section, hydrocele volume measurement
In the case of clinically suspected malignancy, volume measurement as well as the growth of hydrocele accompanied by an increase in its consistency may be an indication for fluid diagnostic cytopathology(23). It should be noted that the amount of abnormal fluid in children can be associated with rapid growth and reduction due to the so-called hydrocele in communication with the abdominal cavity. An assessment of the volume and the dynamics of hydrocele is significant in the case of postoperative complications in inguinal hernia, varicocelectomy and other surgical procedures, indicating the need for a revision surgery (24).
Epididymis
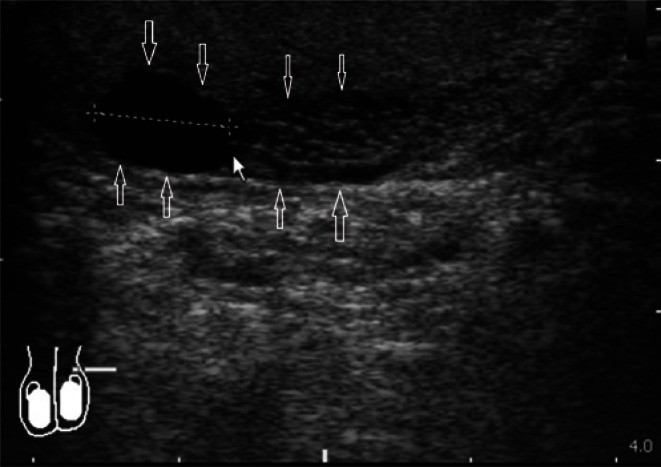
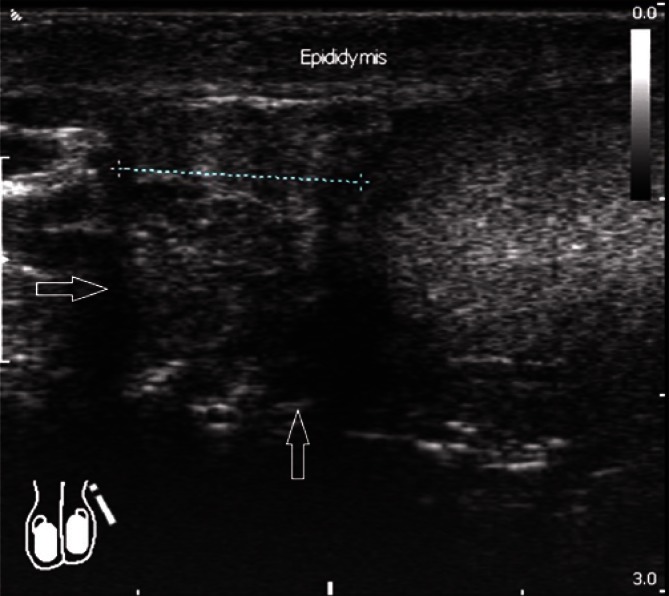
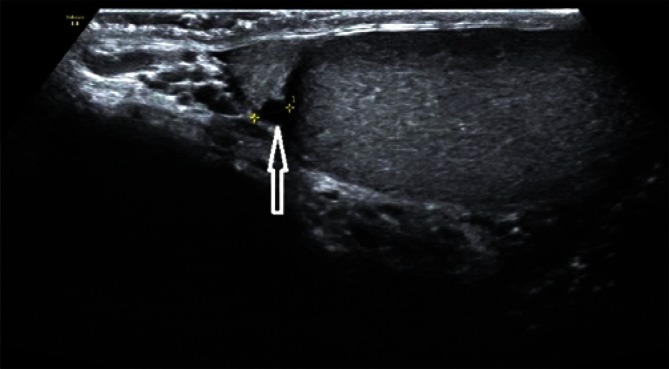
In the case of normal, non-enlarged epididymis, usually mainly the head and the body are visualized. It is usually difficult to separate the epididymal tail from the initial part of the spermatic cord. Determining the size of epididymis seems unnecessary in the absence of enlargement. In the case of epididymal enlargement with clearly visible body and tail, it is recommended to document the size of the head in the sagittal plane as well as the size of the body and the tail, also in the sagittal plane (Fig. 8).
Fig. 8.
Epididymal enlargement in the head region with a sagittal dimension of 12 mm, with the signs of epididymal duct retention (arrows)
Normal epididymis is characterized by a homogenous echogenicity. Sometimes, a few-millimeter epididymal or testicular appendices can be seen at the junction between the epididymal head and the testis. Their presence and structure should be evaluated in the case of pain symptoms, particularly in boys, as these structures may be subject to torsion.
Cystic degeneration can be also seen in these appendices. If abnormal fluid spaces are visualized within the epididymis, this is an indication for shape and size determination. Multiple, small linear fluid structures seen within the epididymis indicate epididymal duct retention and may result from vas deferens obstruction or, at the level of prostate, ejaculatory duct obstruction (Fig. 9).
Fig. 9.
Non-inflammatory dilatation of epididymal head and body in Power Doppler; single vascular segments present in the testicular parenchyma – a picture of vas deferans obstruction (arrows)
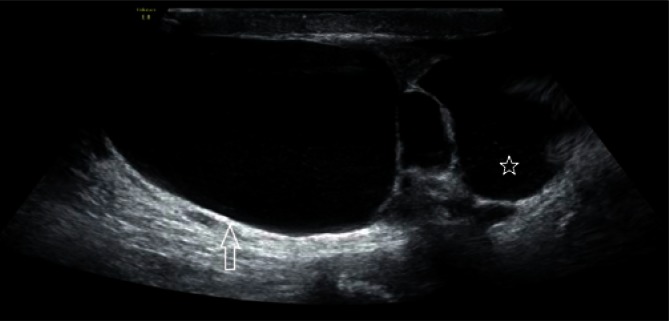
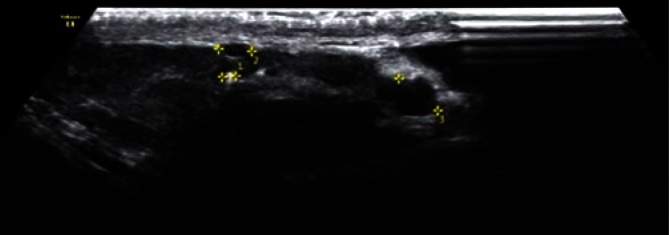
Epididymal cysts, which usually take a regular shape and are up to a few millimeters in diameter, are another common pathology (Fig. 10). Cysts with polycyclic, irregular borders require a three-plane measurement (Fig. 11).
Fig. 10.
An epididymal head cyst with smooth walls and a regular shape, with a diameter of 3 mm (an arrow)
Fig. 11.
Epididymal head destruction due to a multi-chambered cyst with polycyclic outlines (arrows)
An assessment of the dynamics of cystic size as well as a spermogram, particularly in men with reproductive failures, provide important information for the decision on surgical treatment. Therefore, it is important to determine the number and the distribution of abnormal fluid reservoirs. Epididymal cysts can be multiple. Abnormal fluid reservoirs can also occur in the epididymis as well as in the spermatic cord, forming an obstructive barrier preventing sperm passage and causing pain or discomfort (Fig. 12).
Fig. 12.
Abnormal fluid reservoirs within the spermatic cord (an arrow) and the epididymal head (an asterisk)
Abnormal changes in the epididymal tissue require shape, size and vascular pattern determination (Figs. 13 and 14).
Fig. 13.
A well-delineated hypoechoic lesion within the epididymal head, with a diameter of 7 mm in Power Doppler ultrasound: no flow velocity (arrows)
Fig. 14.
A. An isoechoic lesion with polycyclic outlines in Power Doppler ultrasound, with rich flow velocities (arrows). B. Elastography – compressibility distribution in elastography evaluation: the lesion presented in Fig. 14 A
Spermatic cord assessment
The spermatic cord is a structure mostly formed by a network of arterial blood vessels supplying blood to scrotal organs with blood as well as veins, which form the so-called pampiniform venous plexus, lymphatic vessels and the vas deferens (whose lumen is usually invisible).
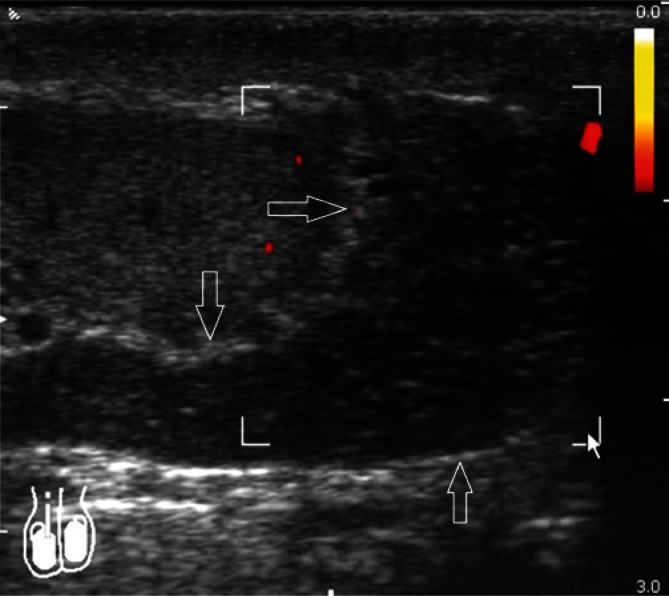
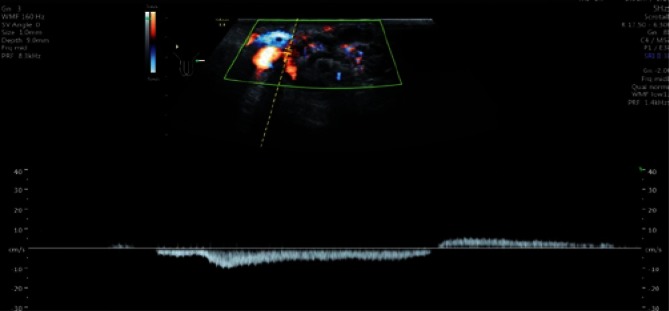
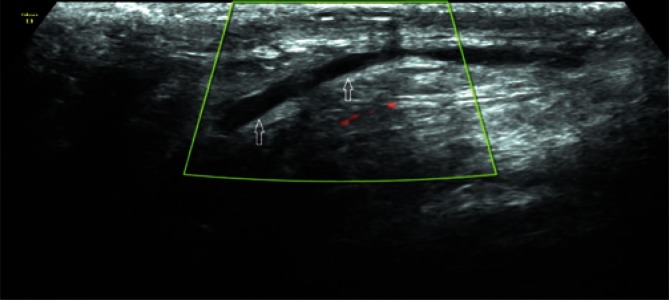
Due to the slow, a few-millimeter venous blood flow, these vessels can show no Doppler signal. The number of veins is subject to intersubject variability (between one and a few vessels). Differentiation between venous and arterial vessels in grey-scale ultrasound is very difficult, and even impossible in some cases. The so-called Valsalva maneuver, which increases the intra-abdominal pressure and, in the case of varicocele, elicits an increased Doppler signal (Fig. 15) and blood flow reversal with venous dilatation (Fig. 16) can help differentiate the nature of vascular blood flows. Spectral Doppler can be used to confirm the reversed blood flow.
Fig. 15.
An increased venous flow in Color Doppler US
Fig. 16.
Reversed flow direction in left-sided varicocele in Spectral Doppler US
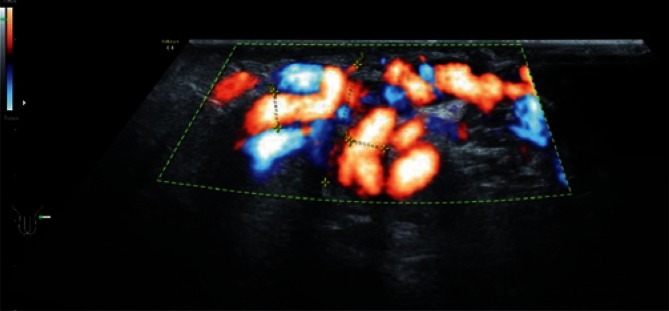
Various scales, depending on varicocele location (spermatic cord, scrotum), or Dubin and Amelar’s classification can be used for a reliable quantitative assessment of varicocele(25). Varicocele diagnosis belongs to more controversial ultrasound examinations in urology. The Valsalva maneuver results in an excessive mobility of the vessels of the spermatic cord, which leads to a false-positive diagnosis, while poor quality of an ultrasound device can account for a false-negative diagnosis. The measurement of spermatic cord diameter is particularly significant in the monitoring of testicular and epididymal inflammation, when vascular perfusion impairment as well as a common increase in echogenicity of spermatic cord structures and spermatic cord thickening occur. An assessment of vascular flow within the spermatic cord is particularly important in the case of suspected pedicle torsion. An evaluation of vascular flow impairment or absence can be of high importance in the diagnosis of the height and type of testicular (Fig. 17) or epididymal torsion. Doppler ultrasound as well as the evaluation of testicular and epididymal blood flow provide important information for the differentiation between pedicle torsion and testicular/epididymal inflammation.
Fig. 17.
A. B-mode images. Heterogeneously reduced testicular and epididymal echogenicity in a patient with suspected testicular torsion. B. The absence of Doppler signal in a patient with pedicle torsion
If solid focal lesions are detected in the spermatic cord, thorough measurements in three perpendicular planes should be performed.
It is important to determine the vascular pattern (Fig. 18).
Fig. 18.
A mixed-echogenicity lesion of the spermatic cord in Power Doppler US; trace peripheral flow velocity (arrows)
Neoplastic lesions of the spermatic cord are very rare. These are usually adenomas or reservoirs containing hypercellular fluid that imitate tissue lesions. Visualization of the vas deferens (Fig. 19) and the fluid it contains is very rare. It is most often seen in obstructive defects, vas deferens obstruction, prostatitis or neoplastic invasion (Fig. 20).
Fig. 19.
The vas deferens lumen seen in the spermatic cord
Fig. 20.
Vas deferens obstruction with coexisting cystic lesions – CFTR gene mutation (arrows)
The inguinal canals
Since a number of abnormalities affecting the ultrasound image and causing testicular and epididymal symptoms can occur within the inguinal canals, these structures also require assessment together with the scrotum. The most common lesions include easily distinguished spermatic cord hydrocele, hernias(26), and, sometimes, tumors(27). Due to the location of the spermatic cord in the inguinal canal and the possible development of the above mentioned abnormalities, they should be thoroughly assessed in men with reproductive failures. Any compression or pathology within the spermatic cord can be an etiology of male infertility(28).
Documentation
The documentation of ultrasound examination should include (in addition to the type of an ultrasound apparatus, the type of an ultrasound transducer and patient’s data): a description of testicular and epididymal morphology, echogenicity and size as well as testicular volume; a description of all pathological lesions with their exact location and size; an assessment of the vasculature of testicular and epididymal pathological lesions; a measurement of venous width and Valsalva maneuver findings in the diagnosis of varicocele; an assessment of the contents of the inguinal canal in the case of cryptorchidism or hernias.
In the case of diagnosed testicular tumor or varicocele, the patient should be informed on the need for abdominal and retroperitoneal ultrasound (optionally para-aortic lymph nodes, liver metastases, kidney cancer).
In the case of scrotal trauma, ultrasound findings should include a description of morphological changes in the skin and the septum of the scrotum; a description of morphological changes in the tunica albuginea, testicular/epididymal parenchyma and spermatic cord; an assessment of testicular and epididymal vasculature; as well as an assessment of the presence of fluid or blood clots in the scrotum.
The description of morphological changes should end with diagnostic conclusions and, optionally, proposals of further diagnostic or follow-up ultrasonographic evaluation.
The description should also contain photographic documentation, showing all the identified and described pathological lesions.
Conflict of interest
Authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
References
- 1.Jakubowski W, Szopiński T. Moszna. In: Sudoł-Szopińska I, Szopiński T, editors. Diagnostyka ultrasonograficzna w urologii. Warszawa–Zamość: Praktyczna Ultrasonografia; 2007. pp. 129–153. [Google Scholar]
- 2.Pajk A, Jakubowski W. Diagnostyka ultrasonograficzna narządów moszny. Gdańsk: Wydawnictwo Medyczne MAKmed; 2002. [Google Scholar]
- 3.American Institute of Ultrasound in Medicine. AIUM Practice Guideline – Scrotal Ultrasound. 2015. AIUM Practice Guideline for the Performance of Scrotal Ultrasound Examinations. Available from: www.aium.org. [DOI] [PubMed] [Google Scholar]
- 4.Dogra V, Bhatts S. Ultrasonografia moszny. In: Dogra V, Rubens DJ, editors. Sekrety ultrasonografii. Wrocław: Urban & Partner; 2005. [Google Scholar]
- 5.Jędrzejewski G, Wieczorek AP. Wieloparametryczna ultrasonografia w diagnostyce worka mosznowego i jąder niezstąpionych u chłopców. J Ultrason. 2013;13:425–430. doi: 10.15557/JoU.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyloch J. Diagnostyka usg chorób zapalnych nerek, pęcherza moczowego, stercza i moszny – punkt widzenia urologa. Część 4: Choroby zapalne moszny. Ultrasonografia. 2009;9:96–99. [Google Scholar]
- 7.Martino P, Galosi AB, Bitelli M, Consonni P, Fiorini F, Granata A, et al. Imaging Working Goup – Societa Italiana Urologia (SIU); Societa Italiana Ecografia Urologica Andrologica Nefrologica (SIEUN): Practical recommendations for performing ultrasound scanning the urological and andrological fields. Arch Ital Urol Androl. 2014;86:56–78. doi: 10.4081/aiua.2014.1.56. [DOI] [PubMed] [Google Scholar]
- 8.Jędrzejewski G, Woźniak MM, Madej T, Kryza R, Zielonka-Lamparska E, Wieczorek AP. The differences in testicular volumes in boys 8-36 months old with undescended retractile and hydrocele testis – usefulness of scrotal screening ultrasound. Early Hum Dev. 2012;88:185–189. doi: 10.1016/j.earlhumdev.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Kim So, Na SW, Yu HS, Kwon D. Epididymal anomalies in boys with undescended testis or hydrocele: Significance of testicular location. BMC Urol. 2015;15:108. doi: 10.1186/s12894-015-0099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zvizdic Z, Milisic E, Halimic A, Zvizdic D, Zubovic SV. Testicular volume and testicular atrophy index as predictors of functionality of unilaterally cryptorchid testis. Med Arch. 2014;68:79–82. doi: 10.5455/medarh.2014.68.79-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jędrzejewski G, Wieczorek AP, Osemlak P, Nachulewicz P. The role of ultrasound in the management of undescended testes before and after orchidopexy – an update. Medicine (Baltimore) doi: 10.1097/MD.0000000000005731. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richenberg J, Belfield J, Ramchandani P, Rocher L, Freeman S, Tsili AC, et al. Testicular microlithiasis imaging and follow-up: guidelines of the ESUR scrotal imaging subcommittee. Eur Radiol. 2015;25:323–330. doi: 10.1007/s00330-014-3437-x. [DOI] [PubMed] [Google Scholar]
- 13.Kara T, Durmaz MS, Ceken K. Ultrasonography of tubular ectasia of the rete testis with associated scrotal findings. J Med Ultrason. 2013;40:27–32. doi: 10.1007/s10396-012-0390-7. [DOI] [PubMed] [Google Scholar]
- 14.Moona MS, Fatima D, Turezbek A. Primary testicular leiomyosarcoma. J Pak Med Assoc. 2011;61:1014–1016. [PubMed] [Google Scholar]
- 15.Rizvi SA, Ahmad I, Siddqui MA, Zaheer S, Ahmad K. Role of color Doppler ultrasonography in evaluation of scrotal swelling: pattern of disease in 120 patients with review of literature. Urol J. 2011;8:60–65. [PubMed] [Google Scholar]
- 16.Huang SS, Wu DL, Gui YP, Zhao X, Xie H. Diagnosis and treatment of yolk sac tumor of the testis with hydrocele in children: report of 7 cases. Zhonghua Nan Ke Xue. 2013;19:1007–1010. [PubMed] [Google Scholar]
- 17.Tei H, Kurahasi T, Maruyama S, Tanaka H, Tashiro T. Malignant mesotheliomas of the tunica vaginalis testis. Hinoyokika Kiyo. 2013;59:603–606. [PubMed] [Google Scholar]
- 18.Kim YW, Kim JW, Kim JH, Lee J, Lee E, Kim MY, et al. Metastatic testicular tumor presentung as a scrotal hydrocele: An initial manifestation of pancreatic adenocarcinonoma. Oncol Lett. 2014;7:1793–1795. doi: 10.3892/ol.2014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallonthaiel AG, Kakkar A, Singh A, Dogra PN, Ray R. Adult granulosa cell tumor of the testis masquerading as hydrocele. Int Braz J Urol. 2015;41:1226–1231. doi: 10.1590/S1677-5538.IBJU.2014.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto F, Onitake Y, Shimada K. Paratesticular Rhabdomyosarcoma Presenting With a Giant Abdominoscrotal Gydrocele in a Toddler. Urology. 2016;87:200–201. doi: 10.1016/j.urology.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Gerscovich EO, Nederi S, Gandour-Edward RF. Serous papillary carcinoma of the tunica vaginalis testis. J Ultrasound Med. 2011;30:418–420. doi: 10.7863/jum.2011.30.3.418. [DOI] [PubMed] [Google Scholar]
- 22.Dagrosa LM, McMenaman KS, Pais VM., Jr Tension hydrocele: an unusual cause of acute scrotal pain. Pediatr Emerg Care. 2015;31:584–585. doi: 10.1097/PEC.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 23.Jain A, Khadwai A, Prakash G, Gupta N, Varma S, Malhotra P. Cytopathological diagnosis of an unusual cause of malignant hydrocele. Clin Med Insights Pathol. 2016;9:29–31. doi: 10.4137/CPath.S40517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nees SN, Glasberrg KI. Observations on hydroceles following adolescent varicocelectomy. J Urol. 2011;186:2402–2407. doi: 10.1016/j.juro.2011.07.116. [DOI] [PubMed] [Google Scholar]
- 25.Pauroso S, Di Leo N, Fulle I, Di Segni M, Alessi S, Maggini E. Varicocele: Ultrasonographic assessment in daily clinical practice. J Ultrasound. 2011;14:199–204. doi: 10.1016/j.jus.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer LS. Hernias and hydroceles. Pediatr Rev. 2013;34:457–464. doi: 10.1542/pir.34-10-457. [DOI] [PubMed] [Google Scholar]
- 27.Londeree W, Kerns T. Liposarcoma of the spermatic cord masqueradung as an inguinal hernia. Care Rep Med. 2014;2014:735380. doi: 10.1155/2014/735380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update. 2015;21:56–83. doi: 10.1093/humupd/dmu042. [DOI] [PubMed] [Google Scholar]