Abstract
Background
TP53 gene mutations predict for poor prognosis in acute myeloid leukemia (AML).
Methods
Peripheral blood or bone marrow samples from 293 newly diagnosed AML patients were analysed with targeted amplicon-based next-generation sequencing based mutation analysis.
Results
We found TP53 mutations in 53 (18%; 45 were missense mutations; the most common pattern of amino acid substitution, in 13 of the 53 patients, was a substitution of arginine to histidine on different codons). The clinical characteristics, pattern of mutations, response to different therapies, and outcomes of patients with AML -TP53-mutated (n=53) vs. TP53-wildtype (n=240) were compared. TP53-mutations were significantly more likely in patients with complex karyotype, abnormalities of chromosome 5, 7, and 17, and therapy-related AML. TP53-mutated AMLs have significantly lower incidence of mutations in FLT3, RAS, and NPM1 and higher incidence of coexisting MPL mutations compared to wild type. Distribution of TP53-mutations was equal in both age groups(<60 years vs ≥ 60 years). TP53 mutated AML was associated with a lower response rate(CR 41% vs. 57%; p=0.04), significantly inferior complete remission duration (CRD) (at 2 yrs 30% vs. 55%; p=0.001) and overall survival (OS) (at 2 yrs 9% vs. 24%; p= <0.0001) irrespective of the age or the type of treatment used - high vs low intensity chemotherapies.
Conclusions
The type of treatment did not improve the outcome in younger or older patients with TP53 mutated AML. These data suggest that novel therapies are needed to improve the outcome of patients with TP53 mutations.
Keywords: TP53, AML, Adverse prognosis AML, complex karyotype, biomarkers of resistance
Introduction
TP53 is a tumor suppressor protein encoded by the TP53 gene, located on the short arm of chromosome 17. TP53 plays a pivotal role in maintaining genomic stability in response to DNA damage; it activates DNA repair programs, and triggers cell-cycle arrest.TP53 is mutated in over half of human cancers.1 TP53 mutations represent an important mechanism of resistance to DNA-damaging chemotherapeutic agents.1, 2 The transcription factor encoded by the TP53 gene is composed of a transcription activation domain, DNA binding domain, proline rich domain and a tetramerization domain.3 Most TP53 mutations in cancers involve the DNA binding domain in covering exons 5-8. An alteration in the amino acid residues (specifically involving the arginine) can induce profound changes in the activity of TP53 gene.3 The majority (70-80%) of TP53 mutations are represented by missense substitutions leading to amino acid changes; the remaining mutations are truncating lesions. A significant proportion of mutations recurrently target ‘hot-spot’ codons. These mutations either directly disrupt the DNA-binding domain of TP53 or cause conformational changes of the TP53 protein, thus leading to severely impaired TP53 function.
In patients with acute myeloid leukemia (AML), mutated TP53 is predominantly observed in therapy related AML (t-AML)4, 5 and/or in patients with complex karyotype (70-80%)6. The reported frequency pf TP53 mutations is 5-10% in de-novo AML.7-9 Studies from mouse models of AML have shown that gain of function mutations in hot spot regions can promote a more aggressive AML.3, 10-12 One study showed that prior chemotherapy does not directly induce TP53 mutations; rather very small clones of TP53 mutations (<0.7%) exist in the hematopoietic stem cells from healthy individuals which expand after genotoxic stress induced by chemotherapy.13 The precise reason for the decreased frequency of FLT3, and NPM1 mutations 14 in TP53 mutated AML is unclear.15 Previous studies 9, 16, 17 have demonstrated that presence of TP53 mutations correlates with poor response to chemotherapy and poor survival in patients with or without complex karyotype.18 In multivariate analysis, the presence of TP53 mutations without aberrant cytogenetic abnormality predicted for poor overall survival and inferior response to treatment.16, 19 Moreover, 17p-loss of heterozygosity (LOH)20, chromosome 17 aberrations 21 or overexpression of full length protein isoform 22 also predict for adverse prognosis in AML.
In this study, we describe the characteristics and the impact of TP53 mutations on outcomes of patients with AML after treatment with different frontline modalities, and evaluated whether the type of chemotherapy influences outcome in patients in different age groups.
Patients and Methods
This retrospective analysis evaluated patients with newly diagnosed AML treated at MD Anderson Cancer Center (MDACC) between 2012 and 2015 who had available cytogenetic and molecular data. Patients with core-binding factor (CBF) and acute promyelocytic leukemia (APL) were excluded. Initial therapies were selected based on age, fitness and comorbidities and irrespective of the status of TP53 mutation. The treatment regimens were divided into three groups: (1) high-dose cytarabine-based (500 – 2000 mg/m2/day for 3 to 5 days) combination chemotherapy, (2) hypomethylating agent-based chemotherapy (5-azacytidine, decitabine, guadecitabine), or (3) low-intensity therapy other than hypomethylating agents (cladribine, low-dose cytarabine, clofarabine, omacetaxine, etc.). Post remission therapy for younger, fit patients was based on a risk-adapted strategy: patients with favorable risk received further consolidation cycles with high-dose cytarabine (HiDAC)-based therapy; patients with intermediate and adverse risk cytogenetics received further consolidation with HiDAC-based therapy and were referred for stem cell transplant (SCT). Patients treated on the lower-intensity approaches continued on consolidation/maintenance strategies inherent to the lower-intensity programs for up to 18-24 months. All treatment protocols were approved by the Institutional Review Board (IRB); patients signed a written informed consent prior to enrollment in accordance with the Declaration of Helsinki.
Response Criteria
Responses were assessed using standard criteria and based on morphological evaluation. Complete remission (CR) is defined by a bone marrow differential showing < 5% blasts, neutrophil count ≥ 1.0 × 109/L and platelet count ≥ 100 × 109/L and no evidence of extramedullary leukemia. CR with incomplete recovery of blood counts (CRi) meets all criteria for CR, except for either residual neutropenia (ANC < 1.0 × 109/L) or thrombocytopenia (platelet count < 100 × 109/L). CR with incomplete recovery of platelets (CRp) meets all criteria for CR, except for persistent thrombocytopenia (platelet count < 100 × 109/L).
Cytogenetics and Molecular Analysis
Cytogenetic analysis was performed on bone marrow at diagnosis, prior to initiation of therapy as described previously.23 Complex karyotype was defined as the presence of ≥ 3 chromosomal abnormalities. Molecular testing to detect known recurrent point mutations was performed by the institutional Molecular Diagnostics Laboratory. Peripheral blood and/or bone marrow cells from 293 patients with AML were analyzed prospectively with use of an amplicon-based next-generation sequencing HiSeq system (Illumina, Inc., San Diego, CA) designed to detect somatic mutations in hotspot regions of 28 genes. The exons (codons) covered on the TP53 gene were 2 (1-25), 4 (33-45), 4 (41-80), 4 (72-112), 4-6 (107-214), 6 (210-224), 7-10 (234-367). Adequate coverage was defined as a total coverage depth of at least 250X.
Statistical Analysis
Overall survival (OS) was defined as the time from diagnosis until death or last follow-up. Complete remission (CR) duration was defined as the time from achieving a CR, or CR with incomplete recovery of blood counts (CRi), or CR with incomplete platelet recovery (CRp) until relapse or death from any cause. Fisher's exact test and the Mann-Whitney U test were used for categorical variables and for continuous variables, respectively. Survival distributions was estimated by Kaplan and Meier curves and survival differences were evaluated using the log-rank test. All differences with p < 0.05 were considered to be statistically significant (two tailed). SPSS V22 (Armonk, NY, USA) was used for statistical analyses.
Results
Patient characteristics
A total of 293 patients with newly-diagnosed (excluding CBF and APL) AML were evaluated. Their median age was 67 years (range, 20-92 years). Ninety-seven(33%) patients were <60 years old and 196 (67%) patients were ≥ 60 years. TP53 mutations were detected in 53/293 (18%) patients. TP53 mutations were equally distributed in younger and older patients - 18% (17/97) and 18% (36/196) respectively. Table-1 (A-B) summarizes the characteristics of patients with wild type vs. mutated TP53, according to age. Among younger patients, TP53-mutated AML (mut-TP53) was associated with significantly lower peripheral blood and bone marrow (BM) blasts (P=0.03 and 0.008 respectively) and a trend for lower fibrinogen levels as compared to wildtype-TP53 (wt-TP53). In older patients, those with mut-TP53 AML tended to be older, have lower WBC counts, lower platelet count, and a significantly lower bone marrow blast % compared to those with wt-TP53.
Table 1.
(A-B) - Comparison of baseline patient characteristics according to TP53 mutation status in patients with AML - age groups (<60 and > 60 years).
| A - Age <60 (N=97) | |||
|---|---|---|---|
| Characteristic, Median (range) | TP53 – Mutated | TP53 – Wild Type | P-value |
| Age (Years) | 49 (20-59) | 51 (22-59) | 0.8 |
| WBC (103/μL) | 2.7 (0.9 - 30) | 4.9 (0.5 - 103) | 0.46 |
| Platelet (103/μL) | 32 (4 - 153) | 39 (1 - 708) | 0.35 |
| Peripheral blasts (%) | 6 (0 - 63) | 27 (0 - 97) | 0.03 |
| LDH (IU/L) | 937 (392 - 10298) | 717 (231 - 11952) | 0.25 |
| Fibrinogen (mg/dL) | 330 (43 - 558) | 413 (67 - 1117) | 0.06 |
| BM Blasts (%) | 27 (12 - 91) | 54 (1 - 96) | 0.008 |
| B - Age >60 (N=196) | |||
| Characteristic, Median (range) | TP53 – Mutated | TP53 – Wild Type | P-value |
| Age (Years) | 74 (62-90) | 71 (60-92) | 0.02 |
| WBC (103/μL) | 2.3 (0.7-17.5) | 2.9 (0.2-164.5) | 0.09 |
| Platelet (103/μL) | 34 (8-321) | 45 (1-1069) | 0.07 |
| Peripheral blasts (%) | 10 (0-86) | 8 (0-96) | 0.8 |
| LDH (IU/L) | 535 (286-3616) | 616 (284-17486) | 0.4 |
| Fibrinogen (mg/dL) | 407 (213-753) | 388 (102-923) | 0.5 |
| BM Blasts (%) | 32 (3-97) | 47 (4-93) | 0.009 |
Compared to wt-TP53 AML, patients with mut-TP53 AML were significantly more likely to exhibit a complex karyotype (35/240 [15%] vs. 43/53 [81%]; P<0.0001) and therapy-related AML (31/240 [13%] vs 16/53 [30%]; P<0.0035). TP53 mutated AML were more likely to have abnormalities of chromosomes 5 and 7 (38/53 [72%] vs 38/240 [16%]; P<0.0001). In addition, a higher proportion of patients with chromosome 17 abnormalities had mut-TP53: 68% vs. 10% in those without chromosome 17 abnormalities (p<0.0001).
Pattern of TP53 mutations
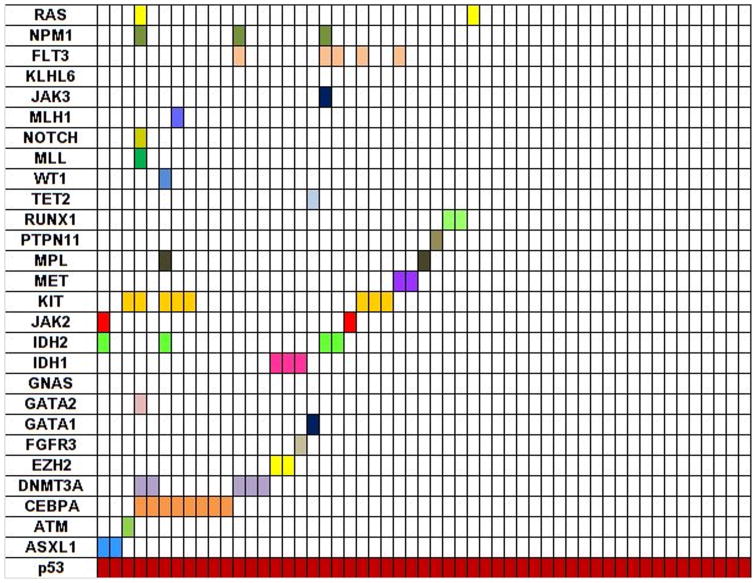
Most mutations in TP53 gene were found in exons 5 through 8, most commonly being in exon 7 (18 patients) (Supplemental Figure 1). These exons span the DNA-binding domain and are also found in other cancers with TP53 mutations. Missense mutations were observed in 45/53 (85%) patients, frameshift in 4, nonsense in 3, and deletion in 1. Substitution of arginine with other amino acids was most common (14/53;26%); codon 248 was also involved (6/14;43%). Figure-1 illustrates the mutations coexisting in mut-TP53 AML. Sole TP53 mutations were detected in 22/53 (42%) patients. The frequencies of gene mutations among patients with and without mutated TP53 are summarized in Table-2 Compared to wt-TP53 AML, mut-TP53 AML was associated with a trend for lower incidence of DNMT3a (9% vs 15%; p=0.4), IDH1/2 (14% vs. 23%; p=0.3), and RUNX1 (0% vs. 7%; p=0.09) mutations. There was also a trend for higher incidence of mutations in EZH2 (4% vs. 1%; p=0.15), KIT (15% vs. 9%; p=0.21), and MPL (4% vs. 0%; p=0.03) as compared to wt-TP53 AML. There was a significant under-representation of mutations in FLT3 (6% vs. 19%; p=0.02), RAS (4% vs. 14%; p=0.04), and NPM1 (8% vs. 20%; p=0.03) in mut-TP53 AML compared with wt-TP53.
Figure 1. Distribution of mutations in patients with TP53 mutated AML (n=53).
The left-hand column lists the 28 genes that were tested in the panel. Each column represents a single patient and each colored bar indicates the presence of a mutation in the indicated gene. This illustrates the spectrum of coexistent mutations in patients with TP53 mutated AML.
Table 2. Pattern of distribution of co-existing mutations in patients with/without TP53 mutations.
| TP53-Mutated (N=53) | % | TP53-Wild Type (N=240) | % | P-value | |
|---|---|---|---|---|---|
| ASXL1 | 2 | 4 | 9 | 4 | 1 |
| ATM | 1 | 2 | 3 | 1 | 0.6 |
| CEBPA | 8 | 15 | 28 | 12 | 0.5 |
| DNMT3A | 5 | 9 | 35 | 15 | 0.4 |
| EZH2 | 2 | 4 | 2 | 1 | 0.15 |
| FBXW7 | 0 | 0 | 1 | 0.4 | 1 |
| FGFR3 | 1 | 2 | 1 | 0.4 | 0.33 |
| GATA1 | 1 | 2 | 0 | 0 | 0.18 |
| GATA2 | 1 | 2 | 5 | 2 | 1 |
| GNAS | 0 | 0 | 1 | 0.4 | 1 |
| IDH1 | 3 | 6 | 21 | 9 | 0.6 |
| IDH2 | 4 | 8 | 34 | 14 | 0.26 |
| JAK2 | 1 | 2 | 8 | 3 | 1 |
| KIT | 8 | 15 | 22 | 9 | 0.21 |
| MET | 2 | 4 | 4 | 2 | 0.3 |
| MPL | 2 | 4 | 0 | 0 | 0.03 |
| PTPN11 | 1 | 2 | 10 | 4 | 0.7 |
| RUNX1 | 0 | 0 | 16 | 7 | 0.09 |
| TET2 | 3 | 6 | 17 | 7 | 1 |
| WT1 | 1 | 2 | 4 | 2 | 1 |
| MLL | 1 | 2 | 0 | 0 | 0.18 |
| NOTCH1 | 1 | 2 | 0 | 0 | 0.18 |
| MLH1 | 0 | 0 | 1 | 0.4 | 1 |
| JAK3 | 1 | 2 | 1 | 0.4 | 0.33 |
| KLHL6 | 0 | 0 | 1 | 0.4 | 1 |
| FLT3 | 3 | 6 | 45 | 19 | 0.02 |
| RAS | 2 | 4 | 34 | 14.2 | 0.04 |
| NPM1 | 4 | 8 | 49 | 20 | 0.03 |
Response to treatments
Table 3 summarizes the complete remission (CR) rates according to age and the type of treatments administered in mut-TP53 vs wt-TP53 AML patients. Patients with mut-TP53 AML had lower CR rates irrespective of age (p= not significant). Selection of treatment was based on age, performance status, comorbidities, and pretreatment disease characteristics. The number of patients with mut-TP53 vs wt-TP53, respectively who received the three type of treatment modalities were: high-dose cytarabine-based chemotherapy (11 vs. 89), hypomethylating agents (24 vs. 76) and low intensity therapy (18 vs. 75). Within the hypomethylating agents group, there was no significant difference between mut-TP53 and wt-TP53 patients, when CR rates were compared according to treatment with azacytidine or decitabine. Patients with mut-TP53 AML had lower CR rates irrespective of type of treatment modality. However, there was a trend towards better response with high-dose cytarabine among patients with wt-TP53 AML (55% vs. 72%; p=0.3).
Table 3. Complete remission (CR) rates in patients with/without TP53 mutations – Overall and according to the type of frontline therapy - High dose Ara-C, hypomethylating agents (azacytidine and decitabine) and low intensity chemotherapy.
| TP53 Mutated | TP53 Wild Type | P-Value | |
|---|---|---|---|
| Age Group | Responder/Total | Responder/Total | |
| < 60 years | 9/17 (53%) | 56/80 (70%) | 0.25 |
| > 60 years | 13/36 (36%) | 80/160 (50%) | 0.19 |
| Type of Treatment regimen | CR% | CR% | P-Value |
| High dose Ara-C | 55 | 72 | 0.3 |
| Hypomethylating agents | 29 | 34 | 0.8 |
| Azacytidine | 40 | 37 | 0.98 |
| Decitabine | 62 | 37 | 0.21 |
| Low intensity chemotherapy | 50 | 60 | 0.6 |
Time to event outcomes
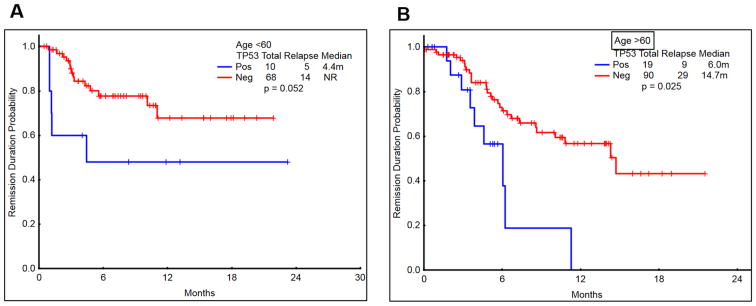
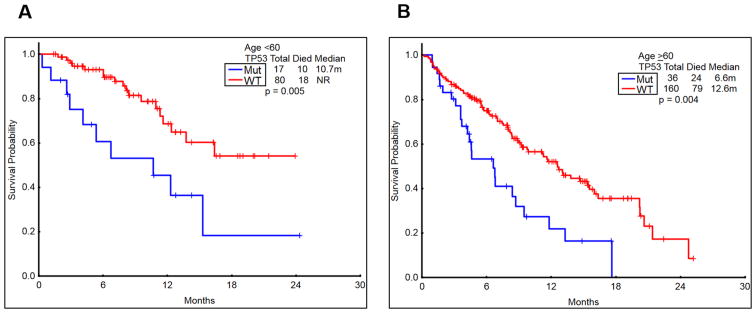
The CRD was significantly shorter in patients with mut-TP53 AML, irrespective of age (P=0.05 in younger patients and P=0.025 in older patients; Figure 2 A-B). The overall survival (OS) was also significantly shorter in patients with mut-TP53 AML, irrespective of age (P=0.005 in younger patients and P=0.004 in older patients; Figure-3 A-B). Fourteen patients had substitution of arginine; arginine residues in p53 gene are considered as mutational hotspots 3. However, the survival of patients who had arginine vs. non arginine substitution in mut-TP53 AML was similar (not shown).
Figure 2. Comparison of complete remission duration (CRD) in patients with/without TP53 mutation according to age (<60 vs >60 years).
A-B) The CR duration among patients with TP53-mutated AML was significantly inferior to those with wild type TP53 in both younger (<60 years; P=0.052) and older patients (>60 years; P=0.025).
Figure 3. Comparison of overall survival in patients with/without TP53 mutation regardless of therapy, by age (<60 vs >60 years).
A-B) The survival among patients with TP53-mutated AML was significantly inferior to those with wild type TP53 in both younger (<60 years; P=0.005) and older patients (>60 years; P=0.004).
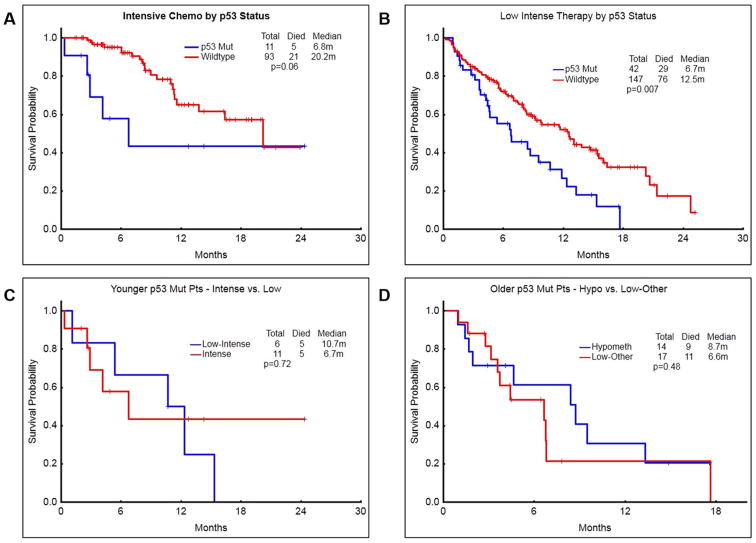
Patients with mut-TP53 had inferior survival compared to wt-TP53 AML, irrespective of the intensity of treatment modality (P=0.06 and 0.007 in high and low intensity chemotherapy regimens; Figure-4 A-B). Finally, we analysed whether mut-TP53 AML patients in different age groups respond differentially to various treatments. Figure-4 C-D shows that the intensity of chemotherapy does not impact the survival outcomes significantly in patients with mut-TP53 AML with any age group.
Figure 4. Survival outcomes according to the type of treatment – intensive chemotherapy vs low intensity treatments.
A) Patients with TP53-mutated AML who received intensive chemotherapy had a median survival of 6.8 months compared to 20.2 months in those with wild type-TP53 (P=0.06). B) Patients with TP53-mutated AML who received low intensity chemotherapy had a significantly inferior median survival of 6.7 months, compared to 12.5 months in those with wild type TP53 (P=0.007). C) Among the younger patients (<60 years age), survival was not significantly different when treated with intensive chemotherapy or low intensity chemotherapy. D) Among the older patients (>60 years age), survival was not significantly different after treatment with hypomethylating agents or with other low intensity therapies.
Discussion
Novel molecular techniques such as next-generation sequencing have unraveled the molecular heterogeneity of AML patients.24 These advances have helped in identifying newer therapeutic targets and have refined risk stratification in patients with AML. Several groups have reported that mutations in TP53 gene confer an adverse prognosis in AML.7, 9, 14, 16, 19, 25 In this study, we have made some important observations. First, the incidence of TP53 mutations in our cohort was 18% - higher than the 5-10% incidence previously reported 6,8,13,15,18. This likely reflects the referral pattern to a tertiary referral cancer center. Second, the analysis confirmed the inferior outcome associated with mut-TP53 AML and showed this adverse prognosis to be irrespective of age or type of treatment modality used. Third, we summarized the pattern of coexisting mutations in our patients and noted interesting associations between TP53 and other molecular aberrations. These patterns may indicate potential interactions which might be relevant in the pathobiology of mut-TP53 AML.
Patients were treated heterogeneously on different protocols based on the age, comorbidities, and pretreatment karyotype. This heterogeneity provided an opportunity to analyse the outcomes of mut-TP53 AML according to the age and the type of therapy that has not been previously reported. Overall survival rates among younger and older patients with mut-TP53 AML were clearly inferior to their wild-type counterparts. Rates of CR in younger and older patients with mut-TP53 AML were lower than those with wt-TP53, but these, interestingly, did not reach statistical significance. When we examined the CR rates by type of therapy, we noted no significant difference in CR rates among patients receiving low-intensity therapy or HMA, but a trend towards inferior responses to HiDAC-based chemotherapy among mut-TP53 AML compared to wt-TP53 AML. This highlights a critical need to develop newer therapies with a TP53-independent mechanism of action. Also, patients deemed to have increased risks of mortality and morbidity with intensive chemotherapy may be offered lower-intensity approaches, particularly since the intensity of therapy did not predict for improved survival (Figure 4C).
The risk of relapse after achieving a remission is the second major issue impacting poor outcomes in patients with mut-TP53 AML. In both older and younger cohorts, patients with mut-TP53 AML had a significantly shorter CR duration compared to those with wt-TP53 AML. Post-remission therapy including prolonged consolidation and allogeneic stem cell transplant (SCT) plays an important role25, but may not be suitable for a significant proportion of the patients. In our cohort, 4 (24%) patients < 60 years with mut-TP53 AML underwent allogeneic SCT, while only 2 (6%) patients ≥ 60 years received the procedure. While noting that selection bias does play a role in this retrospective analysis, SCT was associated with an improved outcome (1-yr OS 75% vs. 29%; p=0.012, data not shown) in the younger cohort. Unfortunately, the majority of patients, including almost all of the patients ≥ 60 years did not obtain the benefit of an allogeneic SCT. A second priority, therefore, must be to design prolonged consolidation/maintenance strategies for these patients. Immunotherapeutic approaches such as vaccines, immune checkpoint blockade, or chimeric antigen receptor T-cells are currently being investigated and may be more feasible than SCT for most patients 26.
Another approach to better understand this biology is by studying the associated genetic aberrations in these patients. At the cytogenetic level, the frequency of complex karyotype was significantly higher in patients with mut-TP53 AML compared to wt-AML (81% vs 15%), consistent with previously published reports.7, 16 A phenotype of genomic instability, ineffective DNA-repair, and impaired apoptosis may define this entity. At the molecular level, we found that the majority of mutations in TP53 occurred in the exons involved in the DNA-binding domain. Furthermore, as a result of missense mutations, we observed that arginine was the most common amino acid substituted in mut-TP53 AML patients, occurring in 26% of the patients. In this small subset, we did not observe significant differences in outcome of patients with arginine vs non-arginine amino acid substitutions (not shown). Arginine residues in the P53 protein are considered mutational hotspots that affect DNA binding and significantly alter the transcriptional activity of the mutant protein, to a much greater extent compared to other non-arginine amino acid substitutions.27-29 It is possible, therefore, that these TP53 missense mutations are not only loss of function, but a neoplastic gain-of-function. This has been recently suggested 1.
Although the numbers were small, we observed specific patterns of coexisting mutations that will need to be confirmed in larger studies. Compared to wt-TP53, patients with mut-TP53, demonstrated a trend for lower incidence of DNMT3a, IDH1/2, and RUNX1 mutations and a trend for higher incidence of mutations in EZH2 and KIT. Patients with mut-TP53 had a statistically significant higher frequency of MPL mutations and significantly lower frequency of mutations in FLT3, RAS, and NPM1 compared to wt-TP53 AML. Previous studies have observed a similar finding with a lower frequency of NPM1 and FLT3 mutations in mut-TP53 AML.14, 16 the mechanistic relevance of these possible interaction/associations and its potential impact on the biology of AML blasts needs to be studied further.
In summary, we identified that TP53 mutated AML is characterized by complex cytogenetics, low blast counts, and underrepresentation of concomitant mutations in FLT3, RAS, NPM1, and RUNX1. These patients exhibit lower response rates to intensive chemotherapy and shorter CR durations, translating into inferior survival in both younger and older patients. Better understanding of the biology of TP53 mutations and the mechanism of chemoresistance in AML along with newer approaches to improve the CR rates, and prolong survival are critically needed.
Supplementary Material
Acknowledgments
Our funding was from the following sources - Supported in part by the MD Anderson Cancer Center Support Grant CA016672 (PI: Dr. Ronald DePinho) and Award Number P01 CA049639 (PI: Dr. Richard Champlin) from the National Cancer Institute. None of the authors are employed by NIH. JC is recipient of grant from NCI (PI of Project 1 of P01 CA049639).
Footnotes
Conflicts of Interests - The authors report no competing conflicts of interest.
Authorship Contributions – T.K., J.C. and H.K. designed the study.
T.K., P.J, H.K., and J.C. analyzed results.
T.K., P.J., and H.K. wrote the paper.
T.K., P.J., S.P., H.K., and J.C. did clinical correlation and R.K.S. and K.P. provided molecular data
T.K., M.A., F.R., G.N.G., K.T., G.B., N.D., E.J., C.D., Z.E., J.C., T.K. and H.K. contributed patient samples.
All authors reviewed and gave the final approval for the paper.
References
- 1.Kim MP, Zhang Y, Lozano G. Mutant p53: Multiple Mechanisms Define Biologic Activity in Cancer. Front Oncol. 2015;5:249. doi: 10.3389/fonc.2015.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li R, Sutphin PD, Schwartz D, et al. Mutant p53 protein expression interferes with p53-independent apoptotic pathways. Oncogene. 1998;16:3269–3277. doi: 10.1038/sj.onc.1201867. [DOI] [PubMed] [Google Scholar]
- 3.Cho Y, Gorina S, Jeffrey PD, Pavletich NP. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 1994;265:346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- 4.Ok CY, Patel KP, Garcia-Manero G, et al. Mutational profiling of therapy-related myelodysplastic syndromes and acute myeloid leukemia by next generation sequencing, a comparison with de novo diseases. Leuk Res. 2015;39:348–354. doi: 10.1016/j.leukres.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen-Bjergaard J, Andersen MK, Andersen MT, Christiansen DH. Genetics of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2008;22:240–248. doi: 10.1038/sj.leu.2405078. [DOI] [PubMed] [Google Scholar]
- 6.Schoch C, Kern W, Kohlmann A, Hiddemann W, Schnittger S, Haferlach T. Acute myeloid leukemia with a complex aberrant karyotype is a distinct biological entity characterized by genomic imbalances and a specific gene expression profile. Genes Chromosomes Cancer. 2005;43:227–238. doi: 10.1002/gcc.20193. [DOI] [PubMed] [Google Scholar]
- 7.Rucker FG, Schlenk RF, Bullinger L, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. 2012;119:2114–2121. doi: 10.1182/blood-2011-08-375758. [DOI] [PubMed] [Google Scholar]
- 8.Hou HA, Chou WC, Kuo YY, et al. TP53 mutations in de novo acute myeloid leukemia patients: longitudinal follow-ups show the mutation is stable during disease evolution. Blood Cancer J. 2015;5:e331. doi: 10.1038/bcj.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowen D, Groves MJ, Burnett AK, et al. TP53 gene mutation is frequent in patients with acute myeloid leukemia and complex karyotype, and is associated with very poor prognosis. Leukemia. 2009;23:203–206. doi: 10.1038/leu.2008.173. [DOI] [PubMed] [Google Scholar]
- 10.Song H, Hollstein M, Xu Y. p53 gain-of-function cancer mutants induce genetic instability by inactivating ATM. Nat Cell Biol. 2007;9:573–580. doi: 10.1038/ncb1571. [DOI] [PubMed] [Google Scholar]
- 11.Zuber J, Radtke I, Pardee TS, et al. Mouse models of human AML accurately predict chemotherapy response. Genes Dev. 2009;23:877–889. doi: 10.1101/gad.1771409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigal A, Rotter V. Oncogenic mutations of the p53 tumor suppressor: the demons of the guardian of the genome. Cancer Res. 2000;60:6788–6793. [PubMed] [Google Scholar]
- 13.Wong TN, Ramsingh G, Young AL, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 2015;518:552–555. doi: 10.1038/nature13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haferlach C, Dicker F, Herholz H, Schnittger S, Kern W, Haferlach T. Mutations of the TP53 gene in acute myeloid leukemia are strongly associated with a complex aberrant karyotype. Leukemia. 2008;22:1539–1541. doi: 10.1038/leu.2008.143. [DOI] [PubMed] [Google Scholar]
- 15.Stirewalt DL, Kopecky KJ, Meshinchi S, et al. FLT3, RAS, and TP53 mutations in elderly patients with acute myeloid leukemia. Blood. 2001;97:3589–3595. doi: 10.1182/blood.v97.11.3589. [DOI] [PubMed] [Google Scholar]
- 16.Seifert H, Mohr B, Thiede C, et al. The prognostic impact of 17p (p53) deletion in 2272 adults with acute myeloid leukemia. Leukemia. 2009;23:656–663. doi: 10.1038/leu.2008.375. [DOI] [PubMed] [Google Scholar]
- 17.Parkin B, Erba H, Ouillette P, et al. Acquired genomic copy number aberrations and survival in adult acute myelogenous leukemia. Blood. 2010;116:4958–4967. doi: 10.1182/blood-2010-01-266999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakano Y, Naoe T, Kiyoi H, et al. Prognostic value of p53 gene mutations and the product expression in de novo acute myeloid leukemia. Eur J Haematol. 2000;65:23–31. doi: 10.1034/j.1600-0609.2000.90138.x. [DOI] [PubMed] [Google Scholar]
- 19.Yanada M, Yamamoto Y, Iba S, et al. TP53 mutations in older adults with acute myeloid leukemia. Int J Hematol. 2016 doi: 10.1007/s12185-016-1942-1. [DOI] [PubMed] [Google Scholar]
- 20.Christiansen DH, Andersen MK, Pedersen-Bjergaard J. Mutations with loss of heterozygosity of p53 are common in therapy-related myelodysplasia and acute myeloid leukemia after exposure to alkylating agents and significantly associated with deletion or loss of 5q, a complex karyotype, and a poor prognosis. J Clin Oncol. 2001;19:1405–1413. doi: 10.1200/JCO.2001.19.5.1405. [DOI] [PubMed] [Google Scholar]
- 21.Nahi H, Lehmann S, Bengtzen S, et al. Chromosomal aberrations in 17p predict in vitro drug resistance and short overall survival in acute myeloid leukemia. Leuk Lymphoma. 2008;49:508–516. doi: 10.1080/10428190701861645. [DOI] [PubMed] [Google Scholar]
- 22.Anensen N, Hjelle SM, Van Belle W, et al. Correlation analysis of p53 protein isoforms with NPM1/FLT3 mutations and therapy response in acute myeloid leukemia. Oncogene. 2012;31:1533–1545. doi: 10.1038/onc.2011.348. [DOI] [PubMed] [Google Scholar]
- 23.Khoury JD, Sen F, Abruzzo LV, Hayes K, Glassman A, Medeiros LJ. Cytogenetic findings in blastoid mantle cell lymphoma. Hum Pathol. 2003;34:1022–1029. doi: 10.1053/s0046-8177(03)00412-x. [DOI] [PubMed] [Google Scholar]
- 24.Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–1089. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Middeke JM, Herold S, Rucker-Braun E, et al. TP53 mutation in patients with high-risk acute myeloid leukaemia treated with allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2016;172:914–922. doi: 10.1111/bjh.13912. [DOI] [PubMed] [Google Scholar]
- 26.Martner A, Thoren FB, Aurelius J, Hellstrand K. Immunotherapeutic strategies for relapse control in acute myeloid leukemia. Blood Rev. 2013;27:209–216. doi: 10.1016/j.blre.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008. doi: 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soussi T, Lozano G. p53 mutation heterogeneity in cancer. Biochem Biophys Res Commun. 2005;331:834–842. doi: 10.1016/j.bbrc.2005.03.190. [DOI] [PubMed] [Google Scholar]
- 29.Goh AM, Xue Y, Leushacke M, et al. Mutant p53 accumulates in cycling and proliferating cells in the normal tissues of p53 R172H mutant mice. Oncotarget. 2015;6:17968–17980. doi: 10.18632/oncotarget.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.