Abstract
Millions of children have been born worldwide though assisted reproductive technologies (ART). Consistent with the Developmental Origins of Health and Disease hypothesis, there is concern that ART can induce adverse effects, especially because procedures coincide with epigenetic reprogramming events. Although the majority of studies investigating the effects of ART have focused on perinatal outcomes, more recent studies demonstrate that ART-conceived children may be at increased risk for postnatal effects. Here, we present the current epidemiological evidence that ART-conceived children have detectable differences in blood pressure, body composition, and glucose homeostasis. Similar effects are observed in the ART mouse model, which have no underlying infertility, suggesting that cardiometabolic effects are likely caused by ART procedures and not due to reasons related to infertility. We propose that the mouse system can, consequently, be used to adequately study, modify, and improve outcomes for ART children.
Keywords: Assisted Reproductive Technologies, In vitro fertilization, Chronic disease, Hypertension, Diabetes, Epigenetics, Mouse
1. Introduction
The Developmental Origins of Health and Disease (DOHaD) hypothesis posits that environmental stresses or exposures during development can increase the risk of disease later in life. The first compelling evidence for the existence of DOHaD in humans comes from epidemiological studies showing that nutritional state during prenatal development increases the risk for metabolic syndromes and cardiovascular disease [1]. At the center of the DOHaD hypothesis is the concept of developmental plasticity, in which the biological pathways that govern prenatal development are not fixed. This developmental plasticity, which allows for phenotypic changes in the fetus in response to environmental stress, may be beneficial if the stress is within normal range [2]. Adaptive response mechanisms during development may allow offspring to be better suited to the environment in which they will be born. In contrast, phenotypic changes that result from stresses outside the normal range or from exposures that humans have not evolved defenses against are likely non-adaptive and may lead to adverse effects. With respect to human development, assisted reproductive technologies (ART) are an example of extreme ‘exposures’, requiring the in vitro handling of gametes and embryos in a synthetic culture environment.
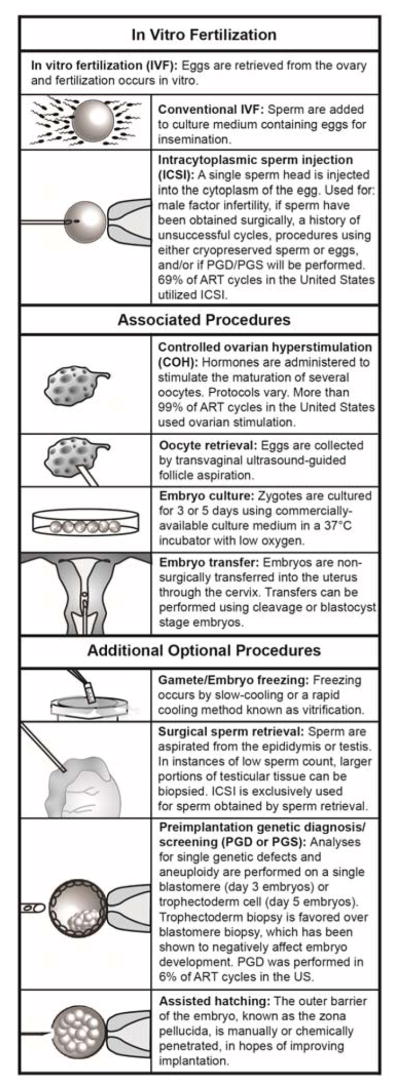
Well-over 5,000,000 babies have been born worldwide via ART [3]. ART and its many associated procedures are constantly changing to fit the needs of patients [4] (Box 1). The two most common types of ART are conventional in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), which are accompanied by controlled ovarian hyperstimulation (COH), oocyte retrieval, embryo culture, and embryo transfer procedures. Recent technological advancements have led to the availability of several additional procedures, including the cryopreservation of gametes/embryos, preimplantation genetic diagnosis/screening (PGD/PGS), and others. Even with the successful implementation of these technologies, ART is associated with a number of complications, including a higher risk of congenital anomalies, hypertensive disorders of pregnancy, disorders of the placenta, preterm birth, low birth weight, perinatal mortality and small size for gestational age, imprinting disorders, among other problems presenting at birth, as well as later onset problems [5–8]. Animal models of ART with no underlying infertility, can exhibit analogous complications that have been described in ART children, suggesting that ART procedures are the source of these complications rather than reasons associated with infertility [9]. In this review, we discuss how ART procedures may impact epigenetic reprogramming coinciding with embryonic development and summarize the current epidemiological and experimental evidence suggesting ART procedures result in adverse postnatal cardiometabolic outcomes. It is important to consider how ART procedures may affect long-term health in offspring given the number of babies that are born using ART each year.
2. Periconception as a critical window
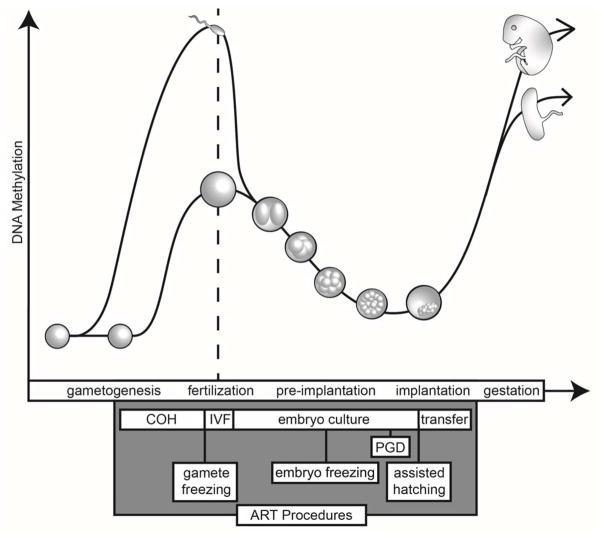
Notably, the procedures used in ART take place at times when maximal epigenetic reprogramming is occurring, including during female gametogenesis and immediately after fertilization. Reprogramming of the epigenome is critical to gametogenesis and early embryonic development, involving the erasure and reestablishment of DNA methylation and alterations of histone posttranslational modifications, which are essential to reset the gene expression patterns necessary for germ cell maturation. Following fertilization, the epigenome introduced by the gametes must again be reset to establish the pluripotency that is required for development of embryonic lineages. Figure 1 depicts the changes in DNA methylation that occur during reprogramming of the germline and early embryo. Although it is well known that histone posttranslational modifications are also dynamically reprogrammed at this time [10], much more information exists regarding DNA methylation changes because single nucleotide resolution on small numbers of cells has, until recently, been more robust for DNA methylation profiling. Thus, for the sake of this review, we will focus on DNA methylation. In this section, we briefly describe the vast DNA methylation changes coinciding with ART procedures, as DNA methylation changes during gametogenesis and early embryonic development have been discussed in detail elsewhere [10–12].
Figure 1. ART and its associated procedures.
A brief description of the different modes of ART (IVF and ICSI) and associated procedures.
The progenitors of the mouse and human germline, primordial germ cells (PGCs), undergo vast demethylation of the genome, with the exception of some (largely younger) repetitive elements [13]. Notably, some loci associated with metabolic and neurological disease are resistant to DNA methylation in human PGCs, which renders them tantalizing candidates for conferring phenotypes across generations [14]. Subsequent to the periods of demethylation, male germ cells initiate and complete the majority of remethylation during prenatal development before entering mitotic arrest until puberty [10–12]. In contrast, oocytes in the female remain hypomethylated, arresting at prophase of meiosis I [10–12]. DNA methylation is then acquired in the oocyte after recruitment as part of a cohort driven by hormonal regulation in the postpubertal cycling female [15]. COH leads to the development of multiple immature oocytes, thus, the COH occurs simultaneously with the reprogramming of the oocyte genome (Figure 1). In the United States, COH protocols are utilized in 99% of ART cycles, as its use dramatically increases the number of eggs, and therefore the number of chances for a successful pregnancy [4].
The embryonic genome is again extensively reprogrammed immediately following fertilization. At this time, a significant proportion of the genome is demethylated (Figure 1), with the paternal genome (sperm) actively demethylated, likely through a combination of oxidation of 5-methylcytosine to 5-hydromethylcytosine, base excision repair, elongator complex activity, and DNA replication [16]. The maternal genome (egg) is passively demethylated through the absence of maintenance methylation during replication [16]. In contrast to the demethylated genomic unique sequences, the imprinting control regions (ICRs) of imprinted genes escape demethylation [17]. Parental-specific DNA methylation of ICRs is acquired in the germline and must remain after fertilization so that this unique class of genes is expressed in a monoallelic, parent-of-origin specific manner; the failure to do so results in aberrant expression of imprinted genes and adverse developmental outcomes. Although it is incompletely understood how the few ICRs maintain differential methylation, a series of proteins that are candidates for protecting ICRs from DNA demethylation have been identified, including ZFP57 and PGC7/Stella [18,19]. Finally, remethylation of the genome by the de novo DNA methyltransferases initially occurs in the inner cell mass of the blastocyst and subsequently in the extraembryonic cells [16]. These postfertilization reprogramming events occur simultaneously with IVF/ICSI, embryo culture, PGS (if used), and embryo transfer. Thus the postfertilization reprogramming period coincides with most of the techniques used in the generation of IVF/ICSI embryos, raising the possibility that the suboptimal ex vivo environment could render the IVF/ICSI embryo susceptible to environmental perturbations that may only be realized much later in life.
3. Evidence that ART can cause epigenetic perturbations
Adverse epigenetic changes that occur in the conceptus as the direct result of ART manipulations can have one of four generalized outcomes. The first are those that impact the embryo and the extraembryonic tissues so severely that there is pregnancy loss. Although early pregnancy loss has been associated with ART, it is impossible to determine if the etiology of pregnancy loss is due to ART procedures or problems associated with underlying infertility [20]. Evidence from experimental models using fertile animals as oocyte and sperm donors shows that ART procedures are indeed associated with an increased embryo loss and reduced litter size [21,22]. Most early pregnancy loss after ART is attributed to chromosomal abnormalities, and to our knowledge, there is no existing evidence or current attempts to link pregnancy loss to epigenetic perturbations. The second possible outcome is that epigenetic changes that occur in the embryo as the direct result of ART manipulations lead to a live birth but result in detectable congenital disorders or malformations. For example, the imprinting disorders Beckwith-Wiedemann Syndrome and Angelman Syndrome occur in a higher than expected frequency in children conceived through ART and these cases typically occur though loss of DNA methylation in ICRs [23]. Although there are reports that infertility/subfertility status of the parents may play a factor in increasing the incidence of these disorders [24], it is hypothesized that ART procedures disrupt the mechanism that maintains DNA methylation of ICRs in the preimplantation embryo. In support of this contention, we and others have investigated procedures used in ART, including superovulation, embryo culture and embryo transfer, using a mouse model system and have found that blastocysts and fetuses are susceptible to loss of DNA methylation at ICRs and disruption of imprinted gene expression [25–30].
The remaining possible outcomes are more relevant to the DOHaD hypothesis. Mild epigenetic changes that occur in the embryo as the direct result of ART manipulations with no detectable adverse effects at birth may still result in increased risk of chronic diseases later in life. Conversely, less severe epigenetic changes that impact the placenta, but do not originate in the embryo, may trigger compensatory mechanisms in line with adaptive responses resulting in indirect epigenetic changes in the embryo that lead to long-term health problems. In the next section we summarize the literature characterizing ART and increased risk of cardiometabolic effects. Currently, there is a vast gap in understanding how ART leads to these chronic diseases and how epigenetic mechanisms mediate adaptive and adverse physiological changes in ART offspring.
4. Evidence that ART can increase the risk of cardiometabolic diseases
4.1. Cardiovascular differences in humans
Several studies have reported subclinical but significant cardiovascular changes in ART children (Table 1) [31–43]. In some of the first reports, systemic blood pressure levels were higher in IVF and ICSI children by comparison with children that were conceived spontaneously [31,33]. Importantly, blood pressure changes were still significant after correcting for birth weight, gestational age, and body size of the children [33]. Similar results were observed in another study in Greece. Systemic blood pressure was higher in IVF children after correcting for low birth weight and maternal factors, including body mass index (BMI), maternal age, and maternal reproductive diseases [39]. Subsequent studies have shown that subtle blood pressure differences may only become apparent after a stress challenge or under certain conditions. For example, an increase in systolic blood pressure was observed in young adults conceived by IVF but only after three days of dietary overfeeding, i.e., increased fat and total caloric intake [35]. Contradictory to these studies, Belva et al. did not detect any differences in resting blood pressure or blood pressure after mild psychological testing in adolescent children conceived by ICSI [32]. One attempt to understand the role of different infertility treatments underlying subfertility on blood pressure in offspring was undertaken in a study from the Netherlands. Blood pressure was assessed in five to six year-old children, conceived by IVF/ICSI, ovulation induction, artificial insemination, or conceived after more than 12 months (subfertile) or conceived naturally within 12 months (fertile). Children in the ovulation induction or subfertile group had significantly higher systemic blood pressure than children from the fertile group, while IVF/ICSI and artificial insemination groups were not different from the fertile group [37]. Subgroups were small in this study-- 28, 51, 34, and 220 children in the IVF/ICSI, ovulation induction, artificial insemination, or subfertile group, respectively, by comparison to over 2,000 children assessed for blood pressure in the fertile group. Thus larger sample sizes may be required to fully assess the impact of individual procedures in human studies.
Table 1.
Human studies of ART and cardiovascular effects
| Reference | Cohort | Groups | Number of participants | Age (years) | Inclusion criteria | Findings |
|---|---|---|---|---|---|---|
| Belva et al. 2007 | ICSI group born at Vrije Universiteit Brussel Centre of Reproductive Medicine, 8yrs old between 02/2001–12/2003. | ICSI-only Controls: Age, sex, and maternal education level-matched children from naturally conceived pregnancies |
ICSI: 150 (15 with major congenital malformations) Controls: 147 (5 with major congenital malformations) |
8 | Singleton, caucasian, ≥32 gestation | ICSI children had significantly increased systemic BP compared to controls. |
| Belva et al. 2012a | ICSI group conceived at the UZ Brussel, 14yrs old between 01/2008–03/2011. Controls recruited from local schools. | ICSI-only Controls: Age and sex-matched children from naturally conceived pregnancies |
ICSI: 217 Controls: 223 |
14 | Singleton, caucasian, ≥32 gestation | No differences in resting BP were detected in ICSI children. No differences in BP in response to mild psychological stress was detected in a small subset of ICSI children, after correcting for current characteristics (height, weight, and pubertal stage). |
| Ceelen et al. 2008 | OMEGA Dutch cohort + VU medical center participants born 1986–1995, overlapping with Ceelen et al. 2007. | IVF (ICSI not specified) Controls: Age and sex-matched children conceived by subfertile couples. |
IVF: 225 Controls: 225 |
8–18 | None stated | Systemic BP was higher in IVF children than in control children. |
| Ceelen et al. 2009 | VU medical center participants born 1986–1995 overlapping with Ceelen et al. 2007. | IVF (ICSI not specified) Controls: Age and sex-matched children conceived by subfertile couples. |
IVF: 233 Controls: 233 |
8–18 | Singleton | Increases in systemic BP in IVF children were linked to the amount of weight gain during the 1–3 year-old period. |
| Chen et al. 2014 | Recruited through IVF birth records in South Australia and newspaper advertisements. | IVF Controls: Sex and BMI-matched. |
IVF: 14 Controls: 20 |
Not specified. Mean age±SEM was 21.5±0.6 for IVF and 20.6±0.6 for controls. | Normal birth weight, no medical conditions, no medications interfering with glucose metabolism, no family history of type 2 diabetes or CVD, no smoking, limited alcohol consumption. | Systolic BP was increased in response to three days of overfeeding (increase of 1,250 kcal/day; nutrient composition increased 15% in fat and decreased 15% in carbohydrates, no change in protein composition). |
| Liu et al. 2015 | Recruited from from First Affiliated Hospital of Nanjing Medical University | IVF (ICSI not specified) Controls: Age and sex-matched children from low risk pregnancies |
IVF: 100 Controls: 100 |
5 | None stated. | Alterations in multiple cardiac parameters and increases in left and right MPI in IVF children. No differences in cardiac morphometry. |
| Pontesilli et al. 2015 | Amsterdam ABCD study. Pregnant women were recruited between 01/2003–03/2004. | IVF/ICSI Artificial insemination Ovulation induction Spontaneously conceived after more than 12 months (subfertile) Controls: Spontaneously conceived within 12 months (fertile) |
IVF/ICSI: 28 Artificial insemination: 51 Ovulation induction: 34 Subfertile: 220 Controls: (fertile): 2244 |
5–6 | Singleton, no severe medical conditions. | Systemic BP was higher in ovulation induction and subfertile groups than fertile controls. No differences in BP in IVF/ICSI or artificial insemination groups compared with fertile controls. |
| Rimoldi et al. 2015 | Subset of Scherrer et al. 2012 | IVF/ICSI Controls: Age and sex-matched children from low risk pregnancies |
IVF/ICSI: 21 Controls: 21 |
Not specified. Mean age±SD was 12.0±2.5 for IVF/ICSI and 12.5±2.5 for controls. | Singleton, >37 weeks gestation, normal birth weight >2.5 kg, no pregnancy complications, no acute illness, no medications, no high altitude exposure, no antioxidant supplements. | IVF/ICSI children displayed reduced FMD, increased carotid femoral PWV, and hypoxia-induced pulmonary hypertension, FMD and pulmonary hypertension was improved after 4 weeks of antioxidant treatment. |
| Sakka et al. 2010 | Same as Sakka et al. 2009 | IVF-only Controls: Age and sex-matched children |
IVF: 106 Controls: 68 |
4–14 | Healthy and no medications. | IVF children had higher systemic BP than controls. |
| Scherrer et al. 2012 | Swiss children born 10/2007–04/2010. Control children were recruited by the families of ART children. | IVF/ICSI Naturally-conceived, hormone stimulation Controls: Naturally-conceived age and sex-matched children from low risk pregnancies. |
IVF/ICSI: 65 Naturally-conceived, hormone stimulation: 16 Controls: 57 |
Not specified. Mean age±SD was 11.9±2.3 for IVF/ICSI and 11.1±2.4 for controls. | Singleton, >37 weeks gestation, normal birth weight >2.5 kg, no pregnancy complications, no medications. | FMD of the brachial artery was reduced in IVF/ICSI children. Carotid-femoral PWV and carotid intima-media thickness was increased in IVF/ICSI children. Pulmonary hypertension was induced in IVF/ICSI children at high altitude. No difference in systemic BP. |
| Seggers et al. 2014 | Groningen cohort, born 03/2005–12/2006 | IVF/ICSI IVF/ICSI with no hormone stimulation Controls: children naturally-conceived by subfertile couples |
IVF/ICSI: 63 IVF/ICSI with no hormone stimulation: 52 Controls: 79 |
4 | Singleton | IVF/ICSI children conceived using hormone stimulation had higher systemic BP than IVF/ICSI children without hormone stimulation. No differences in either IVF group when compared to controls. |
| von Arx et al. 2015 | Children examined at the Universities of Bern and Lausanne. Control children were recruited by the families of ART children. | IVF/ICSI Controls: Naturally-conceived age and sex-matched children from low risk pregnancies |
IVF/ICSI: 54 Controls: 54 |
7–18 | Singleton, >37 weeks gestation, normal birth weight >2.5 kg, no pregnancy complications, no medications, no high altitude exposure. | Increased right ventricle end-diastolic area and diastolic dysfunction in IVF/ICSI children. No differences were observed in IVF/ICSI children at low altitude. |
| Xu et al. 2014 | Women’s Hospital, School of Medicine, Zhejiang University born 2003–2007. | Mother with OHSS IVF (ICSI not specified) Mother no OHSS, matched for gestational age and birth weight to IVF-OHSS group Controls: naturally-conceived children matched for gestational age and birth weight to IVF-OHSS group |
Mother with OHSS: 42 Mother no OHSS: 34 Controls: 48 |
3–7 | No maternal history of CVD. | IVF children from mothers with and without OHSS display alterations in heart rate, diastolic function, and FMD compared to controls. Children from mothers with OHSS had severely reduced FMD. No differences in systemic BP. |
BP=blood pressure; BMI=body mass index; CVD=cardiovascular disease; FMD=flow-mediated dilation; ICSI=intracytoplasmic sperm injection; IVF=in vitro fertilization; MPI=myocardial performance index; OHSS=ovarian hyperstimulation syndrome; PWV=pulse wave velocity; SD=standard deviation; SEM=standard error mean.
Because low birth weight, prematurity, and multiple births are overrepresented in the ART population and known to independently influence cardiac dysfunction, a study by Scherrer and colleagues only compared singletons born at term with no signs of perinatal complications [40]. Although they did not observe any differences in systemic blood pressure, they did observe differences in flow mediated dilation (FMD), arterial stiffness, and carotid intima-media thickness (IMT) [40,44]. These factors were investigated because they are involved in the development of atherosclerosis [45–47]. Indicative of endothelial dysfunction, FMD of the brachial artery was reduced by 25% in IVF/ICSI children by comparison with spontaneously conceived children. IVF/ICSI children also display increased arterial stiffness as assessed by pulse-wave velocity (PWV) and increased carotid IMT. Higher PWV and IMT values are associated with increased cardiovascular risk [45,46]. Scherrer and colleagues also found that high altitude conditions induced a 30% increase in systolic pulmonary artery pressure in IVF/ICSI children by comparison with naturally conceived children [40]. In a separate report, the same research group observed increased right ventricle end-diastolic area and diastolic dysfunction in IVF/ICSI children at high altitude, but not at low altitude [42]. In both cases, pulmonary hypertension differences were observed only under hypoxic stress. The authors note that this response strongly suggests that IVF/ICSI children have underlying endothelial dysfunction because high altitude conditions are known to amplify pulmonary hypertension in individuals with endothelial dysfunction [48]. Because antioxidants have already been shown to improve endothelial dysfunction, the authors tested if the ART-induced pulmonary hypertension in IVF/ICSI children could be alleviated with antioxidant treatment. Indeed, a daily regimen of 1g Vitamin C and 400IU of Vitamin E for four weeks was sufficient to improve FMD and reduce pulmonary hypertension in IVF/ICSI children [38].
In a different study conducted in China, researchers did not observe any differences in systemic blood pressure or carotid IMT [43]. However, they did observe significant differences in FMD, carotid systolic and diastolic diameter, diastolic function parameters, and heart rate. The authors noted that FMD was severely reduced in IVF/ISCI children whose mothers experienced ovarian hyperstimulation syndrome, suggesting that the pathological changes associated with this complication may increase the severity of cardiovascular impairments in offspring. Using conventional echocardiography and two-dimensional speckle tracking imaging, another group detected cardiac alterations in a number of different parameters in five year-old children conceived via IVF/ICSI [36]. Although cardiac morphology parameters in IVF/ICSI children were normal, researchers detected alterations in both systolic and diastolic function. Global cardiac function as measured by left and right myocardial performance indexes (MPI) were also significantly different in IVF/ICSI children by comparison to naturally conceived children. The relevance of the MPI differences is unknown as the MPI values for IVF/ICSI children still fall within normal range [49].
Children with increased blood pressure have a high likelihood to have high blood pressure as adults, making it important to understand how ART could be affecting blood pressure so early in life [50]. Because the reported blood pressure differences in ART children are subclinical and the increases appear small, it is essential to determine if offspring conceived by ART have greater incidence of prehypertension and hypertension as adults. Even small increases in blood pressure above normal (120/80 mmHg) are associated with increased risk for developing cardiovascular disease and permanent kidney damage [51,52]. To our knowledge, there are currently no published studies that have investigated the effect of ART on blood pressure levels in adults.
In a study by Ceelen and colleagues, researchers found that the high systemic blood pressure in IVF children was associated with the amount of catch-up growth during early childhood (1–3 years-old), but not catch-up growth during infancy (3 months-1 year-old) [34]. A causative link between catch-up growth in IVF children and high blood pressure has yet to be elucidated, but if it exists, close monitoring of this developmental stage may be critical for possible lifestyle interventions.
4.2. Cardiovascular differences from experimental studies in mouse
As predicted by observations in ART children, adult male mice produced using IVF exhibit cardiovascular alterations (Table 2). Impaired endothelial-dependent artery vasodilation and increased carotid artery stiffness in vitro, and higher arterial blood pressure in vivo were observed in IVF male mice by comparison with mice that were conceived naturally [53]. Blood pressure differences could not be attributed to superovulation alone or extended embryo culture. Furthermore, epigenetic changes in select imprinted genes were detected and the promoter of the endothelial nitric oxide synthase 3 gene (Nos3) was hypermethylated in the aorta of ART mice. Hypermethylation of Nos3 was associated with reduced Nos3 expression and nitrous oxide synthesis. Nitric oxide is a well-known regulator of cardiac function, with vascular-dependent and independent effects. The authors hypothesized that cardiovascular dysfunction is due to this affected pathway, mediated through hypermethylation of Nos3. Indeed, administration of butyrate, a deacetylase inhibitor, during adulthood corrected Nos3 promoter methylation and improved vascular function. These results are consistent with the claim that ART-induced vascular dysfunction is linked to epigenetic changes in the aorta, and that dysfunction can be reversed with treatment.
Table 2.
Mouse studies of ART and cardiovascular effects
| Reference | Experimental groups | Genetic background | Superovulation | Embryo Culture | Transfer | Findings |
|---|---|---|---|---|---|---|
| Donjacour et al. 2014 | IVF Whitten’s medium Controls: SO+ET |
CF-1xB6D2F1 | 5 IU PMSG 5 IU hCG |
Whitten’s medium under 20% oxygen | surgical transfer to CF-1 females | Whitten’s medium males had reduced systolic BP and enlarged left heart. Diastolic BP and heart weight were normal. |
| Ramirez-Perez et al. 2014 | SO+IVC+ET SO+IVC+ET high fat diet Control: natural Control: natural high fat diet |
CF-1xB6D2F1 | 5 IU PMSG 5 IU hCG |
two-cell to blastocyst stage in Whitten’s medium | surgical transfer to CF-1 females | SO+IVC+ET high fat diet mice display mesenteric artery endothelial dysfunction and signs of vascular remodeling. |
| Rexhaj et al. 2015 | IVF Control: natural |
FVB | 5 IU PMSG 5 IU hCG |
to blastocyst stage in sequential G1 and G2 media | surgical transfer to NMRI females | IVF mice exhibited mesenteric artery endothelial dysfunction and increased vascular stiffness in vitro, and increased arterial BP in vivo. Effects were normalized when adult males were administered butyrate. |
| Schenewerk et al. 2013 | SO+IVC+ET Control: natural |
CF-1xB6D2F1 | 5 IU PMSG 5 IU hCG |
two-cell to blastocyst stage in Whitten’s medium | surgical transfer to CF-1 females | No differences in mean arterial BP under normal diet conditions or high fat diet. ART mice display increased oxidative stress in mesenteric resistance arteries of juvenile offspring regardless of diet. |
| Watkins et al. 2007 | SO+IVC+ET SO+ET Control: natural Control: natural, litter culled to 6 pups |
CBA/B6xMF1 | 5 IU PMSG 5 IU hCG |
two-cell to blastocyst stage in T6 medium with or without BSA | surgical transfer to CBA/B6 females | In both males and females, SO+IVC+ET had higher systolic BP than SO+ET or both natural groups. |
BP=blood pressure; BSA=bovine serum albumin; ET=embryo transfer; hCG=human chorionic gonadotropin; ICSI=intracytoplasmic sperm injection; IU=international unit; IVC=in vivo fertilization but embryos were cultured; IVF=in vitro fertilization; PMSG=pregnant mare gonadotropin; SO=superovulation.
In a subsequent study, the authors demonstrated that the addition of melatonin in the culture media prevented artery vasodilation, arterial blood pressure, and associated Nos3 promoter methylation effects in IVF mice [54]. While the exact mechanism of melatonin rescue has yet to be elucidated, importantly, this work highlights the importance of embryo culture conditions in the prevention of adverse effects. In support of this, we determined that optimized embryo culture could alleviate some of the epigenetic defects observed in blastocysts and fetuses that were derived from the culture of 2-cell embryos to blastocysts [25,26].
Other studies have directly tested the in vitro culture as the source of cardiovascular defects. Watkins et al. showed that both male and female mice produced from superovulation and subsequent culture of embryos that were fertilized in vivo had significantly higher systolic blood pressure than those that were not cultured, regardless of whether ovarian stimulation was used [55]. Donjacour et al. demonstrated reduced systolic blood pressure and enlarged left heart in male mice produced after IVF and suboptimal embryo culture conditions [56]. In contrast, blood pressure changes were not observed in a different study that also targeted embryo culture procedures [57,58]. However, under high fat diet conditions, mice produced from superovulation and subsequent culture of embryos that were fertilized in vivo exhibited impaired endothelial-dependent artery vasodilation and vasculature structural changes [58]. Taken together, these studies demonstrate that ART in the mouse appropriately models the postnatal cardiovascular dysfunction observed in humans and has provided experimental evidence that culture conditions may be the source of adverse effects contributing to cardiac dysfunction in offspring.
4.3. Body composition and metabolic differences in humans
Body composition and other signs of metabolic dysregulation have been noted in IVF children and adolescents (Table 3). IVF children and adolescents exhibit increased peripheral adipose tissue mass and sum of skinfolds, with decreased lean tissue by comparison with those that were conceived spontaneously in subfertile couples, after controlling for differences in birth weight and gestational age [59,60]. Similar differences were observed in 14 year-olds conceived by ICSI. ICSI girls had increased peripheral, central, and total adiposity, while an increase in peripheral adiposity was also observed in ICSI boys, but only when comparing those of the more advanced pubertal stages [61]. In both studies, the IVF and ICSI cohorts had significant differences in birth weight, gestational age, and parity in comparison with naturally conceived children, supporting the notion that body composition phenotypes are associated with reduced weight at birth. In contrast, no differences in fat mass or BMI were detected in other cohorts of children or adults [35,39,62], and in one study, IVF/ICSI children were reported to have lower BMI than naturally conceived children [63]. Interestingly, Sakka et al. found that IVF children had increased thyroid stimulating hormone (TSH) levels by comparison with naturally conceived children, indicating subclinical hypothyroidism [64]. Although no children presented with a clinical thyroid condition, this implores further investigation given that thyroid function plays important roles in metabolism and lipid profiles. In these children, aged 4–14, there were no detectable differences in adiposity, BMI, or glucose homeostasis [39], suggesting that subtle hormonal changes in TSH may precede other signs of metabolic dysregulation.
Table 3.
Human studies of ART and metabolic effects
| Reference | Cohort | Groups | Number of participants | Age (years) | Inclusion criteria | Findings |
|---|---|---|---|---|---|---|
| Belva et al. 2012b | ICSI group conceived at the UZ Brussel, 14yrs old between 01/2008–03/2011. Controls recruited from local schools. | ICSI-only Controls: Age and sex-matched children from naturally conceived pregnancies |
ICSI: 217 Controls: 223 |
14 | Singleton, caucasian, ≥32 gestation | ICSI girls had increased peripheral, central, and total adiposity compared to controls after controlling for gestational age, parity, current age, pubertal stage, schooling, and frequency of activity. In boys, peripheral adiposity was different only in ICSI boys of advanced pubertal stages. |
| Ceelen et al. 2007 | OMEGA Dutch cohort + VU medical center participants born 1986–1995. | IVF (ICSI not specified) Controls: Age and sex-matched children conceived by subfertile couples |
IVF: 233 Controls: 233 |
8–18 | None stated. | Increased peripheral adipose tissue mass and decreased lean tissue in IVF children than controls. No differences in height, weight, BMI, or BMD. |
| Ceelen et al. 2008 | OMEGA Dutch cohort + VU medical center participants born 1986–1995, overlapping with Ceelen et al. 2007. | IVF (ICSI not specified) Controls: Age and sex-matched children conceived by subfertile couples. |
IVF: 225 Controls: 225 |
8–18 | None stated | Increased sum of skinfolds and fasting glucose levels in IVF children. No differences in insulin concentrations, insulin resistance, height, weight, or BMI. |
| Chen et al. 2014 | Recruited through IVF birth records in South Australia and newspaper advertisements | IVF Controls: Sex and BMI-matched. |
IVF: 14 Controls: 20 |
Not specified. Mean age±SE M was 21.5±0.6 for IVF and 20.6±0.6 for controls. | Normal birth weight, no medical conditions, no medications interfering with glucose metabolism, no family history of type 2 diabetes or CVD, no smoking, limited alcohol consumption. | IVF adults were more insulin resistant than control individuals, but no differences in BMI, fat mass, cholesterol levels, fasting glucose levels, insulin levels, or triglyceride levels. |
| Green et al. 2013 | IVF/ICSI children recruited by Fertility Associates (New Zealand) born between 09/1993–07/2005. Controls were classmates of IVF/ICSI children. | IVF/ICSI fresh IVF/ICSI frozen Controls: Age, sex, ethnicity, and socio-economic background-matched. |
IVF/ICSI fresh: 72 IVF/ICSI frozen: 43 Controls: 94 |
3.5–11 | Singleton, >37 weeks gestation, no medical conditions, SGA, or mothers with medical conditions. IVF/ICSI cycles using donor gametes were excluded. | IVF/ICSI girls using fresh embryos were taller than those using frozen embryos or conceived naturally. No differences in bone age, BMI, percent body fat, glucose, insulin, total cholesterol, and LDL. Triglyceride levels were significantly lower in IVF/ICSI fresh vs. frozen or naturally conceived children. HDL levels were highest in IVF/ICSI fresh children and lowest in IVF/ICSI frozen children. |
| Miles et al. 2007 | IVF/ICSI children recruited by Fertility Associates (New Zealand) born between 01/1995–12/2000. Controls were classmates or siblings of IVF/ICSI children. | IVF/ICSI fresh Controls: Age, sex, ethnicity, and socio-economic background-matched. |
IVF/ICSI fresh: 69 Controls: 71 |
4–10 | Singleton, >37 weeks gestation, no medical conditions, | IVF/ICSI children had lower BMI and triglyceride levels, but HDL levels were higher. No difference in resting glucose or insulin levels, total cholesterol, or LDL levels. |
| Pontesilli et al. 2015 | Amsterdam ABCD study. Pregnant women were recruited between 01/2003–03/2004. | IVF/ICSI Artificial insemination Ovulation induction Spontaneousl y conceived after more than 12 months (subfertile) Controls: Spontaneousl y conceived within 12 months (fertile) |
IVF/ICSI: 28 Artificial insemination : 51 Ovulation induction: 34 Subfertile: 220 Controls: (fertile): 2244 |
5–6 | Singleton, no severe medical conditions. | No difference in BMI, HDL, LDL, total cholesterol, among any groups. Ovulation induction children had higher triglyceride levels than fertile controls. Fasting glucose levels were higher in IVF/ICSI and ovulation induction groups when compared to fertile controls. |
| Sakka et al. 2009 | IVF Section of the First Department of Obstetrics and Gynocology of the University of Athens. Controls were naturally conceived healthy children routinely examined at Aghia Sophia Children’s Hospital. | IVF-only Controls: Age and sex and pubertal stage-matched children |
IVF: 106 Controls: 68 |
4–14 | Caucasian, no medications, living in iodine-replete areas, no history of congenital hypothyroidism. | IVF children had increased thyroid stimulating hormone (TSH) levels, indicative of mild TSH resistance/subclinica l hypothyroid or other thyroid dysfunction. |
| Sakka et al. 2010 | Same as Sakka et al. 2009 | IVF-only Controls: Age and sex-matched children |
IVF: 106 Controls: 68 |
4–14 | Healthy and no medications. | IVF children had significantly higher triglyceride levels than control children. No difference in adiposity, BMI, fasting glucose, insulin, total cholesterol, or inflammation markers. |
BMD=bone mineral density; BMI=body mass index; CVD=cardiovascular disease; HDL=high-density lipoprotein cholesterol; ICSI=intracytoplasmic sperm injection; IVF=in vitro fertilization; LDL=low density lipoprotein cholesterol; SGA=small for gestational age
Higher fasting glucose levels have been reported in at least two different studies. Young children conceived through IVF/ICSI or by ovulation induction had significantly higher fasting glucose levels by comparison with fertile controls [37]. Similarly, pubertal children conceived through IVF also had higher fasting glucose levels than their control counterparts. These differences were still significant after controlling for birth weight, gestational age, and current body size of the children [60]. However, differences in glucose homeostasis have not been observed in other cohorts [39,62,63,65]. Similarly, insulin resistance has only been observed in one study. When examined as young adults, Chen et al. detected a significant change in insulin resistance in IVF versus spontaneously conceived individuals [35]. Many other studies consisting of only children, have not detected differences in total insulin levels or insulin resistance [39,60,62,63]. Given that these glucose homeostasis changes were only detected in pubertal and adult cohorts, it is possible metabolic dysregulation may take longer to manifest, thus not detected in younger or mixed age cohorts. This highlights the importance of long-term follow-up of IVF-conceived individuals.
Other metabolic parameters have been detected in some studies but not others. Higher triglyceride levels were observed in IVF children by comparison with naturally conceived children [39]. In contrast, no differences in triglyceride levels were detected by Chen et al. [35], and in one study, IVF/ICSI children derived from fresh cycles had significantly lower triglyceride levels compared to children conceived using frozen embryos or that were naturally conceived [62]. IVF/ICSI children from fresh cycles also had the highest HDL cholesterol levels while IVF/ICSI children from frozen cycles had the lowest HDL levels [62]. No differences were detected with respect to total cholesterol levels or LDL cholesterol levels in other studies [35,39,62,63].
4.4. Metabolic differences from experimental studies in mouse
Experimental studies to determine if ART procedures result in adverse metabolic phenotypes so far have conflicting findings and show sex-dependent differences in offspring (Table 4). In some studies, only female mice exhibited metabolic effects in response to ART procedures [66,67]. IVF and ICSI female offspring displayed impaired glucose tolerance compared to naturally conceived controls, while no effects were detected in IVF or ICSI males [66]. In Feuer et al., IVF females derived from embryos cultured under suboptimal conditions displayed impaired glucose tolerance. IVF females from optimal culture conditions had significantly increased fat deposition and fasting glucose levels, even though glucose tolerance was normal [67].
Table 4.
Mouse studies of ART and metabolic effects
| Reference | Experimental groups | Genetic background | Superovulation | Embryo Culture | Transfer | Sex | Findings |
|---|---|---|---|---|---|---|---|
| Calle et al. 2012 | SO+IVC+ET Control: Not explicitly described. |
B6xCBA | 7.5 IU PMSG 7.5 IU hCG |
KSOM medium with 10% FCS | surgical transfer to CD-1 females | Males only | Mice produced after IVC had normal fasting glucose levels but impaired glucose tolerance and insulin resistance. No differences in mean body weight but differences in variance. |
| Chen et al. 2014a | IVF SO+ET Control: ET IVF+ET high fat diet SO+ET high fat diet Control: ET high fat diet |
C57BL/6 | 7.5 IU PMSG 7.5 IU hCG |
Research Vitro Cleave medium to the blastocyst stage under 5% oxygen | surgical transfer to CBAxB6 females | Males only | Birth weight of SO+ET and IVF mice was reduced compared to controls. IVF mice had increased fasting glucose levels and impaired glucose tolerance compared to ET or SO+ET mice on regular or high fat diet. Insulin levels were reduced in IVF and SO+ET mice on high fat diet only. |
| Chen et al. 2014b | IVF SO+ET Control: ET IVF+ET high fat diet SO+ET high fat diet Control: ET high fat diet |
C57BL/6 | 7.5 IU PMSG 7.5 IU hCG |
Research Vitro Cleave medium to the blastocyst stage under 5% oxygen | surgical transfer to CBAxB6 females | Females only | Birth weight of SO+ET and IVF mice was reduced compared to controls. Body weight reduced in IVF mice until weaning. SO+ET groups displayed catch-up growth. Both IVF and SO+ET mice had increased fasting glucose and impaired glucose tolerance in response to high fat diet in comparison with controls. |
| Donjacour et al. 2014 | IVF Whitten’s medium Control: SO+ET |
CF-1xB6D2F1 | 5 IU PMSG 5 IU hCG |
Whitten’s medium under 20% oxygen | surgical transfer to CF-1 females | Males and females | IVF mice cultured in Whitten’s medium had impaired glucose tolerance compared to IVF males under more optimal conditions or controls. No differences among any groups in percent body fat, fasting insulin levels, or insulin resistance. |
| Feuer et al. 2014 | IVF Whitten’s medium IVF KSOM+AA medium Control: SO+ET |
C57BL/6 | 5 IU PMSG 5 IU hCG |
Whitten’s medium under 20% oxygen or KSOM+AA under 5% oxygen | surgical transfer to CF-1 females | Males and females | IVF Whitten’s males and females were smaller at birth than controls. IVF Whitten’s females had impaired glucose tolerance males trended, but were not significant. BMI was reduced in Whitten’s males and females. IVF KSOM females were smaller at birth than controls, exhibited catch-up growth and increased fat deposition as adults. IVF KSOM females had increased fasting glucose levels but glucose tolerance was normal. No metabolic differences were observed in IVF KSOM males. |
| Rexhaj et al. 2013 | IVF Control: natural |
FVB | 5 IU PMSG 5 IU hCG |
to blastocyst stage in sequential G1 and G2 media | Surgical transfer to NMRI females | Males only | IVF mice had 25% reduced lifespan than controls on high fat diet. |
| Scott et al. 2010 | IVF ICSI Control: natural with unilateral tubal ligation to reduce litter size |
B6C3F1 | 5 IU PMSG 5 IU hCG |
CZB medium culture to two-cell stage | Surgical transfer to CD-1 females | Males and females | IVF and ICSI males and females increased birth weight, but were normal by 1 week. IVF and ICSI females had impaired glucose tolerance but fasting glucose levels were normal. No differences were observed in males. |
BP=blood pressure; ET=embryo transfer; FCS=fetal calf serum; hCG=human chorionic gonadotropin; ICSI=intracytoplasmic sperm injection; IU=international unit; IVC=in vivo fertilization but embryos were cultured; PMSG=pregnant mare serum gonadotropin; SO=superovulation.
Other studies report nearly the opposite pattern of response, where males are more susceptible. Males that were derived from embryos cultured under suboptimal culture conditions displayed impaired glucose homeostasis, but not females, while mice of both sexes that were cultured under optimal conditions had normal glucose homeostasis [56]. Similarly, Calle and colleagues, who only examined males, observed impaired glucose homeostasis after in vitro culture [68]. Yet, in another study, both male and female mice produced via IVF exhibited signs of metabolic dysregulation. Chen et al. examined the effects of IVF on glucose homeostasis in offspring compared to embryo transfer (ET) control group to control for litter size and additionally looking at the effect of superovulation alone [35,65]. Mice were produced by IVF, or by transfer of in vivo fertilized embryos with ovarian stimulation (SO+ET), or without ovarian stimulation (ET). IVF males and females on regular and high fat chow had significantly increased fasting glucose levels and impaired glucose tolerance in comparison to the other groups, suggesting that IVF can induce metabolic effects on metabolism under regular dietary conditions. High fat diet further affected fasting insulin levels in both SO+ET and IVF males by comparison with controls [35]. In female offspring, the SO+ET group also displayed increased fasting glucose levels and impaired glucose tolerance with high fat diet [65]. This suggests that, in females, impaired glucose homeostasis may not only be influenced by embryo culture but also by ovarian stimulation protocols.
Low birth weight is a known independent risk factor for diabetes in mice [69]. In the study by Scott et al., both IVF males and females were larger at birth than natural controls [66]. This effect may be due to larger litter size in controls, as supported by experiments by Donjacour et al. [56]. Regardless of the reason, offspring in the Scott study were not smaller than controls. IVF offspring in the Donjacour study had similar birth weight to ET controls of comparable litter sizes. In Chen et al., both IVF and SO+ET males and females had significantly reduced birth weight compared to ET controls, but only IVF males displayed impaired glucose homeostasis on either diet [35,65]. Thus, in all three cases, low birth weight was not associated with nor a predictor of impaired glucose homeostasis. Notably, IVF male mice had an approximately 25% shorter lifespan compared with controls when challenged with high fat diet [53]. Thus, elucidating the effects of ART and how it predisposes offspring to poor cardiometabolic outcomes is critical.
5. Conclusions
There are many questions that need to be addressed concerning the potential effects on long-term health in humans with ART. Longitudinal long-term follow up of individuals is clearly warranted. Subclinical signs of cardiometabolic alterations are detectable in children, but because cardiovascular disease and metabolic syndrome are chronic, adult-onset diseases, more prominent signs of disease may take years to develop. Improvements need to be made to ART procedures, and unfortunately, investigating the mechanism of disease development through epidemiological studies is difficult. ART pregnancies are at high risk for low birth weight and pregnancy complications [70], factors that independently affect the risk of cardiometabolic diseases. Regardless of whether these effects are caused by ART procedures or related to the patient’s underlying infertility, interpreting the data from such studies becomes more complex. The power of epidemiological studies is dramatically reduced if ART offspring are sorted based on the types of ART procedures performed, by the origin of infertility, or by age and sex of the ART individuals. Moreover, although there are recommended guidelines, patient care is highly individualized. Procedure protocols can vary greatly from patient to patient and trends vary among the different clinics, making it difficult to make comparisons between different studies/cohorts. Notably, no one has addressed the interaction between genetics and ART, which could most certainly contribute to the variable effects observed in human studies.
Importantly, ART cardiometabolic phenotypes in humans can be phenocopied in fertile mice strongly suggesting the procedures themselves promote adverse effects. The mouse allows for controlled investigation of the different procedures individually and allows access to tissues for molecular analyses at various developmental time points. This access is key for elucidating the mechanism of the initial changes as well as the adaptive changes, which may be advantageous early in development, but may contribute to disease later in life. The mouse also allows the longitudinal study of animals to advanced age as well as multigenerational effects in a timely manner that is not feasible in human studies.
ART will continue to be used to help couples conceive children and further work is necessary to assure that ART is not only effective, but also safe for the future health of the offspring. We also advocate that the mouse is an excellent model to test procedures used in ART due to (1) its relatively short pregnancy and juvenile periods, (2) research costs feasibility, (3) the ability to study the technology without underlying infertility issues and (4) the extensive evidence demonstrating that mouse appropriately phenocopies the complications observed in humans.
Figure 2. The timing of ART procedures coincides with dynamic DNA methylation changes in gametes and embryos.
Controlled ovarian hyperstimulation (COH) allows the maturation of several immature oocytes. DNA methylation is acquired during this period of oocyte maturation. Fertilization and pre-implantation embryo development occur in vitro with commercially available culture media at 37°C and low oxygen conditions. Following fertilization, there is both active and passive demethylation of the paternal and maternal genomes, respectively, in the embryo. Embryo culture and transfer coincide with this demethylation. DNA methylation acquisition occurs promptly in the postimplantation embryo. DNA methylation levels in the extraembryonic tissues are relatively hypomethylated compared to levels in the embryo. IVF=in vitro fertilization; PGD=pre-implantation genetic diagnosis.
Acknowledgments
We thank Joanne Thorvaldsen for helpful comments during the preparation of this manuscript and Olivia Chao for assistance in table preparation. This work was supported by the National Institutes of Health [grant HD06817 (M.S.B.)] as well as a postdoctoral fellowship from The Lalor Foundation (L.A.V.). Funding sources had no role in the opinions expressed in this review.
Glossary
- ART
Assisted Reproductive Technologies
- DOHaD
Developmental Origins of Health and Disease
- IVF
In vitro fertilization
- ICSI
Intracytoplasmic sperm injection
- COH
Controlled ovarian hyperstimulation
- PGD/PGS
Preimplantation genetic diagnosis or screening
- ICRs
Imprinting control regions
- BMI
Body mass index
- FMD
Flow mediated dilation
- IMT
Carotid intima-media thickness
- MPI
Myocardial performance index
- TSH
Thyroid stimulating hormone
- HDL
High density lipoprotein cholesterol
- LDL
Low density lipoprotein cholesterol
- SO
Superovulation
- ET
Embryo transfer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker DJP, Bagby SP. Developmental antecedents of cardiovascular disease: a historical perspective. J Am Soc Nephrol. 2005;16:2537–2544. doi: 10.1681/ASN.2005020160. [DOI] [PubMed] [Google Scholar]
- 2.Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev. 2014;94:1027–1076. doi: 10.1152/physrev.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamson GD, Zegers-Hochschild F, Ishihara O, Sullivan EA, Mansour R, Nygren KG, et al. ICMART World Report: Preliminary 2008 Data. Eur. Soc. Hum. Reprod. Embryol. Annu. Meet; London, UK. 2012. [Google Scholar]
- 4.Centers for Disease Control and Prevention, S. for A.R.T. American Society for Reproductive Medicine. 2013 Assisted Reproductive Technology National Summary Report. Atlanta, GA: 2015. [Google Scholar]
- 5.Daniel Y, Schreiber L, Geva E, Amit a, Pausner D, Kupferminc MJ, et al. Do placentae of term singleton pregnancies obtained by assisted reproductive technologies differ from those of spontaneously conceived pregnancies? Hum Reprod. 1999;14:1107–1110. doi: 10.1093/humrep/14.4.1107. [DOI] [PubMed] [Google Scholar]
- 6.Schieve LA, Cohen B, Nannini A, Ferre C, Reynolds MA, Zhang Z, et al. A population-based study of maternal and perinatal outcomes associated with assisted reproductive technology in Massachusetts. Matern Child Health J. 2007;11:517–525. doi: 10.1007/s10995-007-0202-7. [DOI] [PubMed] [Google Scholar]
- 7.Wisborg K, Ingerslev HJ, Henriksen TB. In vitro fertilization and preterm delivery, low birth weight, and admission to the neonatal intensive care unit: a prospective follow-up study. Fertil Steril. 2010;94:2102–2106. doi: 10.1016/j.fertnstert.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:485–503. doi: 10.1093/humupd/dms018. [DOI] [PubMed] [Google Scholar]
- 9.Feuer SK, Camarano L, Rinaudo PF. ART and health: Clinical outcomes and insights on molecular mechanisms from rodent studies. Mol Hum Reprod. 2013;19:189–204. doi: 10.1093/molehr/gas066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantone I, Fisher AG. Epigenetic programming and reprogramming during development. Nat Struct Mol Biol. 2013;20:282–289. doi: 10.1038/nsmb.2489. [DOI] [PubMed] [Google Scholar]
- 11.Saitou M, Kagiwada S, Kurimoto K. Epigenetic reprogramming in mouse pre-implantation development and primordial germ cells. Development. 2012;139:15–31. doi: 10.1242/dev.050849. [DOI] [PubMed] [Google Scholar]
- 12.Smallwood SA, Kelsey G. De novo DNA methylation: A germ cell perspective. Trends Genet. 2012;28:33–42. doi: 10.1016/j.tig.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 13.von Meyenn F, Reik W. Forget the Parents: Epigenetic Reprogramming in Human Germ Cells. Cell. 2015;161:1248–51. doi: 10.1016/j.cell.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 14.Tang WWC, Dietmann S, Irie N, Leitch HG, Floros VI, Bradshaw CR, et al. A unique gene regulatory network resets the human germline epigenome for development. Cell. 2015;161:1453–1467. doi: 10.1016/j.cell.2015.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomizawa SI, Nowacka-Woszuk J, Kelsey G. DNA methylation establishment during oocyte growth: Mechanisms and significance. Int J Dev Biol. 2012;56:867–875. doi: 10.1387/ijdb.120152gk. [DOI] [PubMed] [Google Scholar]
- 16.Reik W, Surani MA. Germline and Pluripotent Stem Cells. 2015:1–24. doi: 10.1101/cshperspect.a019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barlow DP, Bartolomei MS. Genomic imprinting in mammals. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Ito M, Zhou F, Youngson N, Zuo X, Leder P, et al. A maternal-zygotic effect gene, Zfp57, maintains both maternal and paternal imprints. Dev Cell. 2008;15:547–57. doi: 10.1016/j.devcel.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura T, Liu YJ, Nakashima H, Umehara H, Inoue K, Matoba S, et al. PGC7 binds histone H3K9me2 to protect against conversion of 5mC to 5hmC in early embryos. Nature. 2012;486:415–9. doi: 10.1038/nature11093. [DOI] [PubMed] [Google Scholar]
- 20.Torrealday S. Is the fertility treatment itself a risk factor for early pregnancy loss? Curr Opin Obstet Gynecol. 2014;26:174–180. doi: 10.1097/GCO.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 21.Delle Piane L, Lin W, Liu X, Donjacour A, Minasi P, Revelli A, et al. Effect of the method of conception and embryo transfer procedure on mid-gestation placenta and fetal development in an IVF mouse model. Hum Reprod. 2010;25:2039–2046. doi: 10.1093/humrep/deq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S, Sun FZ, Huang X, Wang X, Tang N, Zhu B, et al. Assisted reproduction causes placental maldevelopment and dysfunction linked to reduced fetal weight in mice. Sci Rep. 2015;5:10596. doi: 10.1038/srep10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manipalviratn S, DeCherney A, Segars J. Imprinting disorders and assisted reproductive technology. Fertil Steril. 2009;91:305–315. doi: 10.1016/j.fertnstert.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doornbos ME, Maas SM, McDonnell J, Vermeiden JPW, Hennekam RCM. Infertility, assisted reproduction technologies and imprinting disturbances: a Dutch study. Hum Reprod. 2007;22:2476–80. doi: 10.1093/humrep/dem172. [DOI] [PubMed] [Google Scholar]
- 25.Doherty AS, Mann MR, Tremblay KD, Bartolomei MS, Schultz RM. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod. 2000;62:1526–1535. doi: 10.1095/biolreprod62.6.1526. [DOI] [PubMed] [Google Scholar]
- 26.Mann MRW, Lee SS, Doherty AS, Verona RI, Nolen LD, Schultz RM, et al. Selective loss of imprinting in the placenta following preimplantation development in culture. Development. 2004;131:3727–3735. doi: 10.1242/dev.01241. [DOI] [PubMed] [Google Scholar]
- 27.Rivera RM, Stein P, Weaver JR, Mager J, Schultz RM, Bartolomei MS. Manipulations of mouse embryos prior to implantation result in aberrant expression of imprinted genes on day 9.5 of development. Hum Mol Genet. 2008;17:1–14. doi: 10.1093/hmg/ddm280. [DOI] [PubMed] [Google Scholar]
- 28.Fortier AL, Lopes FL, Darricarrère N, Martel J, Trasler JM. Superovulation alters the expression of imprinted genes in the midgestation mouse placenta. Hum Mol Genet. 2008;17:1653–1665. doi: 10.1093/hmg/ddn055. [DOI] [PubMed] [Google Scholar]
- 29.De Waal E, Mak W, Calhoun S, Stein P, Ord T, Krapp C, et al. In Vitro Culture Increases the Frequency of Stochastic Epigenetic Errors at Imprinted Genes in Placental Tissues from Mouse Concepti Produced Through Assisted Reproductive Technologies 1. 2014;90:1–12. doi: 10.1095/biolreprod.113.114785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Waal E, Vrooman La, Fischer E, Ord T, Mainigi Ma, Coutifaris C, et al. The cumulative effect of assisted reproduction procedures on placental development and epigenetic perturbations in a mouse model. Hum Mol Genet. 2015;24:6975–6985. doi: 10.1093/hmg/ddv400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belva F, Henriet S, Liebaers I, Van Steirteghem A, Celestin-Westreich S, Bonduelle M. Medical outcome of 8-year-old singleton ICSI children (born 32 weeks’ gestation) and a spontaneously conceived comparison group. Hum Reprod. 2007;22:506–515. doi: 10.1093/humrep/del372. [DOI] [PubMed] [Google Scholar]
- 32.Belva F, Roelants M, De Schepper J, Roseboom TJ, Bonduelle M, Devroey P, et al. Blood pressure in ICSI-conceived adolescents. Hum Reprod. 2012;27:3100–3108. doi: 10.1093/humrep/des259. [DOI] [PubMed] [Google Scholar]
- 33.Ceelen M, Van Weissenbruch MM, Vermeiden JPW, Van Leeuwen FE, Delemarre-Van De Waal HA. Cardiometabolic differences in children born after in vitro fertilization: Follow-up study. J Clin Endocrinol Metab. 2008;93:1682–1688. doi: 10.1210/jc.2007-2432. [DOI] [PubMed] [Google Scholar]
- 34.Ceelen M, Van Weissenbruch MM, Prein J, Smit JJ, Vermeiden JPW, Spreeuwenberg M, et al. Growth during infancy and early childhood in relation to blood pressure and body fat measures at age 8–18 years of IVF children and spontaneously conceived controls born to subfertile parents. Hum Reprod. 2009;24:2788–2795. doi: 10.1093/humrep/dep273. [DOI] [PubMed] [Google Scholar]
- 35.Chen M, Wu L, Zhao J, Wu F, Davies MJ, Wittert GA, et al. Altered glucose metabolism in mouse and humans conceived by IVF. Diabetes. 2014;63:3189–3198. doi: 10.2337/db14-0103. [DOI] [PubMed] [Google Scholar]
- 36.Liu H, Zhang Y, Gu HT, Feng QL, Liu JY, Zhou J, et al. Association between assisted reproductive technology and cardiac alteration at age 5 years. JAMA Pediatr. 2015;169:603–5. doi: 10.1001/jamapediatrics.2015.0214. [DOI] [PubMed] [Google Scholar]
- 37.Pontesilli M, Painter RC, Grooten IJ, van der Post JA, Mol BW, Vrijkotte TGM, et al. Subfertility and assisted reproduction techniques are associated with poorer cardiometabolic profiles in childhood. Reprod Biomed Online. 2015;30:258–267. doi: 10.1016/j.rbmo.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Rimoldi SF, Sartori C, Rexhaj E, Bailey DM, De Marchi SF, Mceneny J, et al. Antioxidants improve vascular function in children conceived by assisted reproductive technologies: A randomized double-blind placebo-controlled trial. Eur J Prev Cardiol December. 2015;22:1399–1407. doi: 10.1177/2047487314535117. [DOI] [PubMed] [Google Scholar]
- 39.Sakka SD, Loutradis D, Kanaka-Gantenbein C, Margeli A, Papastamataki M, Papassotiriou I, et al. Absence of insulin resistance and low-grade inflammation despite early metabolic syndrome manifestations in children born after in vitro fertilization. Fertil Steril. 2010;94:1693–1699. doi: 10.1016/j.fertnstert.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 40.Scherrer U, Rimoldi SF, Rexhaj E, Stuber T, Duplain H, Garcin S, et al. Systemic and pulmonary vascular dysfunction in children conceived by assisted reproductive technologies. Circulation. 2012;125:1890–1896. doi: 10.1161/CIRCULATIONAHA.111.071183. [DOI] [PubMed] [Google Scholar]
- 41.Seggers J, Haadsma ML, La Bastide-Van Gemert S, Heineman MJ, Middelburg KJ, Roseboom TJ, et al. Is ovarian hyperstimulation associated with higher blood pressure in 4-year-old IVF offspring? Part I: multivariable regression analysis. Hum Reprod. 2014;29:502–509. doi: 10.1093/humrep/det396. [DOI] [PubMed] [Google Scholar]
- 42.von Arx R, Allemann Y, Sartori C, Rexhaj E, Cerny D, de Marchi SF, et al. Right ventricular dysfunction in children and adolescents conceived by assisted reproductive technologies. J Appl Physiol. 2015;118:1200–1206. doi: 10.1152/japplphysiol.00533.2014. [DOI] [PubMed] [Google Scholar]
- 43.Xu GF, Zhang JY, Pan HT, Tian S, Liu ME, Yu TT, et al. Cardiovascular dysfunction in offspring of ovarian-hyperstimulated women and effects of estradiol and progesterone: A retrospective cohort study and proteomics analysis. J Clin Endocrinol Metab. 2014;99:E2494–E2503. doi: 10.1210/jc.2014-2349. [DOI] [PubMed] [Google Scholar]
- 44.Rimoldi SF, Sartori C, Rexhaj E, Cerny D, Von Arx R, Soria R, et al. Vascular dysfunction in children conceived by assisted reproductive technologies: Underlying mechanisms and future implications. Swiss Med Wkly. 2014;144:1–11. doi: 10.4414/smw.2014.13973. [DOI] [PubMed] [Google Scholar]
- 45.Järvisalo MJ, Jartti L, Näntö-Salonen K, Irjala K, Rönnemaa T, Hartiala JJ, et al. Increased aortic intima-media thickness: a marker of preclinical atherosclerosis in high-risk children. Circulation. 2001;104:2943–2947. doi: 10.1161/hc4901.100522. [DOI] [PubMed] [Google Scholar]
- 46.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 47.Charakida M, Masi S, Lüscher TF, Kastelein JJP, Deanfield JE. Assessment of atherosclerosis: the role of flow-mediated dilatation. Eur Heart J. 2010;31:2854–61. doi: 10.1093/eurheartj/ehq340. [DOI] [PubMed] [Google Scholar]
- 48.Allemann Y, Stuber T, de Marchi SF, Rexhaj E, Sartori C, Scherrer U, et al. Pulmonary artery pressure and cardiac function in children and adolescents after rapid ascent to 3,450 m. Am J Physiol Heart Circ Physiol. 2012;302:H2646–53. doi: 10.1152/ajpheart.00053.2012. [DOI] [PubMed] [Google Scholar]
- 49.Dyer KL, Pauliks LB, Das B, Shandas R, Ivy D, Shaffer EM, et al. Use of myocardial performance index in pediatric patients with idiopathic pulmonary arterial hypertension. J Am Soc Echocardiogr. 2006;19:21–27. doi: 10.1016/j.echo.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171–3180. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whelton PK, He J, Perneger TV, Klag MJ. Kidney damage in “benign” essential hypertension. Curr Opin Nephrol Hypertens. 1997;6:177–83. doi: 10.1097/00041552-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 52.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 53.Rexhaj E, Paoloni-Giacobino A, Rimoldi SF, Fuster DG, Anderegg M, Somm E, et al. Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span. J Clin Invest. 2013;123:5052–5060. doi: 10.1172/JCI68943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rexhaj E, Pireva A, Paoloni-Giacobino A, Allemann Y, Cerny D, Dessen P, et al. Prevention of vascular dysfunction and arterial hypertension in mice generated by assisted reproductive technologies by addition of melatonin to culture media. Am J Physiol Heart Circ Physiol. 2015;309:H1151–H1156. doi: 10.1152/ajpheart.00621.2014. [DOI] [PubMed] [Google Scholar]
- 55.Watkins AJ, Platt D, Papenbrock T, Wilkins A, Eckert JJ, Kwong WY, et al. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc Natl Acad Sci U S A. 2007;104:5449–5454. doi: 10.1073/pnas.0610317104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donjacour A, Liu X, Lin W, Simbulan R, Rinaudo PF. In vitro fertilization affects growth and glucose metabolism in a sex-specific manner in an outbred mouse model. Biol Reprod. 2014;90:1–10. doi: 10.1095/biolreprod.113.113134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schenewerk AL, Ramirez F, Foote C, Ji T, Martinez-Lemus LA, Rivera RM. Effects of the use of assisted reproduction and high caloric diet consumption on body weight and cardiovascular health of juvenile mouse offspring. Reproduction. 2013;147:111–123. doi: 10.1530/REP-13-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramirez-Perez FI, Schenewerk AL, Coffman KL, Foote C, Ji T, Rivera RM, et al. Effects of the use of assisted reproductive technologies and an obesogenic environment on resistance artery function and diabetes biomarkers in mice offspring. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ceelen M, Van Weissenbruch MM, Roos JC, Vermeiden JPW, Van Leeuwen FE, Delemarre-van De Waal HA. Body composition in children and adolescents born after in vitro fertilization or spontaneous conception. J Clin Endocrinol Metab. 2007;92:3417–3423. doi: 10.1210/jc.2006-2896. [DOI] [PubMed] [Google Scholar]
- 60.Ceelen M, van Weissenbruch MM, Vermeiden JPW, van Leeuwen FE, Delemarre-van de Waal HA. Cardiometabolic differences in children born after in vitro fertilization: follow-up study. J Clin Endocrinol Metab. 2008;93:1682–1688. doi: 10.1210/jc.2007-2432. [DOI] [PubMed] [Google Scholar]
- 61.Belva F, Painter R, Bonduelle M, Roelants M, Devroey P, De Schepper J. Are ICSI adolescents at risk for increased adiposity? Hum Reprod. 2012;27:257–264. doi: 10.1093/humrep/der375. [DOI] [PubMed] [Google Scholar]
- 62.Green MP, Mouat F, Miles HL, Hopkins SA, Derraik JGB, Hofman PL, et al. Phenotypic differences in children conceived from fresh and thawed embryos in in vitro fertilization compared with naturally conceived children. Fertil Steril. 2013;99:1898–1904. doi: 10.1016/j.fertnstert.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Miles HL, Hofman PL, Peek J, Harris M, Wilson D, Robinson EM, et al. In vitro fertilization improves childhood growth and metabolism. J Clin Endocrinol Metab. 2007;92:3441–3445. doi: 10.1210/jc.2006-2465. [DOI] [PubMed] [Google Scholar]
- 64.Sakka SD, Malamitsi-Puchner A, Loutradis D, Chrousos GP, Kanaka-Gantenbein C. Euthyroid hyperthyrotropinemia in children born after in vitro fertilization. J Clin Endocrinol Metab. 2009;94:1338–1341. doi: 10.1210/jc.2008-1624. [DOI] [PubMed] [Google Scholar]
- 65.Chen M, Wu L, Wu F, Wittert GA, Norman RJ, Robker RL, et al. Impaired glucose metabolism in response to high fat diet in female mice conceived by in vitro fertilization (IVF) or ovarian stimulation alone. PLoS One. 2014;9:e113155. doi: 10.1371/journal.pone.0113155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scott KA, Yamazaki Y, Yamamoto M, Lin Y, Melhorn SJ, Krause EG, et al. Glucose Parameters Are Altered in Mouse Offspring Produced by Assisted Reproductive Technologies and Somatic Cell Nuclear Transfer. Biol Reprod. 2010;83:220–227. doi: 10.1095/biolreprod.109.082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feuer SK, Liu X, Donjacour A, Lin W, Simbulan RK, Giritharan G, et al. Use of a mouse in vitro fertilization model to understand the developmental origins of health and disease hypothesis. Endocrinology. 2014;155:1956–1969. doi: 10.1210/en.2013-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Calle A, Miranda A, Fernandez-Gonzalez R, Pericuesta E, Laguna R, Gutierrez-Adan A. Male Mice Produced by In Vitro Culture Have Reduced Fertility and Transmit Organomegaly and Glucose Intolerance to Their Male Offspring. Biol Reprod. 2012;87:1–9. doi: 10.1095/biolreprod.112.100743. [DOI] [PubMed] [Google Scholar]
- 69.Jimenez-Chillaron JC, Hernandez-Valencia M, Reamer C, Fisher S, Joszi A, Hirshman M, et al. Beta-cell secretory dysfunction in the pathogenesis of low birth weight-associated diabetes: a murine model. Diabetes. 2005;54:702–711. doi: 10.2337/diabetes.54.3.702. [DOI] [PubMed] [Google Scholar]
- 70.Barker DJP. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49:270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]