Abstract
Ethnopharmacological Relevance
Bacopa monnieri (L) Wettst (common name, bacopa) is a medicinal plant used in Ayurveda, the traditional system of medicine of India, as a nootropic. It is considered to be a “medhya rasayana”, an herb that sharpens the mind and the intellect. Bacopa is an important ingredient in many Ayurvedic herbal formulations designed to treat conditions such as memory loss, anxiety, poor cognition and loss of concentration. It has also been used in Ayurveda to treat inflammatory conditions such as arthritis. In modern biomedical studies, bacopa has been shown in animal models to inhibit the release of the pro-inflammatory cytokines TNF-α and IL-6. However, less is known regarding the anti-inflammatory activity of Bacopa in the brain.
Aim Of The Study
The current study examines the ability of Bacopa to inhibit the release of pro-inflammatory cytokines from microglial cells, the immune cells of the brain that participate in inflammation in the CNS. The effect of Bacopa on signaling enzymes associated with CNS inflammatory pathways was also studied.
Materials And Methods
Various extracts of Bacopa were prepared and examined in the N9 microglial cell line in order to determine if they inhibited the release of the proinflammatory cytokines TNF-α and IL-6. Extracts were also tested in cell free assays as inhibitors of caspase-1 and matrix metalloproteinase-3 (enzymes associated with inflammation) and caspase-3, which has been shown to cleave protein Tau, an early event in the development of Alzheimer's disease.
Results
The tea, infusion, and alkaloid extracts of bacopa, as well as Bacoside A significantly inhibited the release of TNF-α and IL-6 from activated N9 microglial cells in vitro. In addition, the tea, infusion, and alkaloid extracts of Bacopa effectively inhibited caspase 1 and 3, and matrix metalloproteinase-3 in the cell free assay.
Conclusions
Bacopa inhibits the release of inflammatory cytokines from microglial cells and inhibits enzymes associated with inflammation in the brain. Thus, Bacopa can limit inflammation in the CNS, and offers a promising source of novel therapeutics for the treatment of many CNS disorders.
Keywords: 2.104-Anxiolytic, 2.132—Ayurveda, 2.348—Inflammation, 2.476—phytochemistry, 2.548—signal transduction, Bacopa, inflammation, cytokines, caspases, microglia
1. Introduction
Bacopa monniera (L) Wettst, also known as water hyssop, Brahmi, Bramabhi, and nirabarhmi, is a creeping plant found in warm, marshy wetland areas, including those of the Indian subcontinent, East Asia, Australia, and the United States. Bacopa has white to light purple flowers and small leaves, and the genus Bacopa contains over 100 species of the plant. (Lurie DI 2015b; Russo and Borrelli, 2005; Shinomol and Muralidhara, 2011; Williamson, 2002). Bacopa has been used medicinally for thousands of years by Ayurvedic physicians, the practitioners of the traditional system of medicine of India. Bacopa was first chronicled in several ancient Ayurvedic texts including the Caraka Samhita (2500 B.C.) and the Susrata Samhita (2300 B.C.) where clear reference was made to its action on the central nervous system (CNS) (P.V., 2011; Rai et al., 2003). It has been described as a brain tonic and recommended for the management anxiety, poor cognition, and lack of concentration (Russo and Borrelli, 2005). Bacopa has also been used to treat numerous inflammatory conditions such as asthma, bronchitis, dropsy, and rheumatism (Channa et al., 2006).
Bacopa is used in Ayurveda as a nootropic to improve intellect and memory and is an important component of many Ayurvedic herbal formulations that target the CNS and manage conditions such as memory, lack of concentration, and anxiety (Aguiar and Borowski, 2013). Bacopa is also considered to be a very powerful cardiotonic, nervine and diuretic. The effect of Bacopa on memory and cognition has been extensively studied and many excellent review articles describe the nootropic functions of bacopa (Kongkeaw et al., 2014; Pase et al., 2012; Stough et al., 2013). However, bacopa is also used in Ayurvedic medicine to treat inflammatory conditions such as asthma and arthritis and several studies have documented the anti-inflammatory properties of bacopa in animal models of arthritis (Viji and Helen, 2008, 2011; Viji et al., 2010a; Viji et al., 2010b). These studies demonstrate that bacopa is able to modulate systemic inflammation.
However, less is known regarding the ability of bacopa to modulate inflammation in the CNS (neuroinflammation). Neuroinflammation is thought to play a role in many CNS disorders including neurodegenerative diseases such as Alzheimer's disease, and psychiatric diseases such as anxiety, depression, bipolar disorder, and Schizophrenia. Short-term neuroinflammation occurs when the CNS is injured or during disease, and is a means of clearing cellular debris or destroying pathogens. In contrast, long term neuroinflammation is detrimental and can lead to neurodegeneration, as seen in diseases such as Alzheimer's Disease, Parkinson's Disease, and Multiple Sclerosis.
Neuroinflammation is mediated by microglial cells, which are the resident macrophages in the CNS. When pathogens or other inflammatory signals threaten the CNS, microglia migrate to the site of injury or disease and assume an activated phenotype. Activated microglia can either transform into their neurotoxic phenotype (called M1) or a neuroprotective phenotype (called M2). Microglia exist in two distinct functional states; the M1 phenotype that produces proinflammatory cytokines such as Tumor Necrosis Factor alpha (TNF-α) and Interleukin 6 (IL-6), and the M2 phenotype that produces the anti-inflammatory cytokine IL-10 and downregulates the M1 response(Nakagawa and Chiba, 2015) (Gonzalez et al., 2014) (Ganguly and Brenhouse, 2014; Heneka et al., 2014). M1 microglia are a defense against invading pathogens and can clear cellular waste in preparation for tissue repair. Chronic inflammation can result from an imbalance between the M1 and M2 subsets, and under certain circumstances such as major injury or disease, microglia remain in the M1 phenotype and perpetuate the inflammatory response. This leads to upregulation of proinflammatory cytokines, and ultimately neuronal cell death. This activation and upregulation is not only seen in neurodegenerative diseases; recent studies have also shown the involvement of neuroinflammation in psychiatric diseases such as anxiety, depression, and schizophrenia. For both neurodegenerative and psychiatric diseases, the regulation of microglial activation is a potential therapeutic target(Nakagawa and Chiba, 2015).
Bacopa has been shown to have anti-inflammatory effects on macrophages and inhibits the release of IL-6 and TNF-α from monocytes that have been stimulated with lipopolysaccharide (LPS) (Lurie DI 2015a; Viji and Helen, 2011; Viji et al., 2010b; Williams et al., 2014). Microglia are the resident mononuclear phagocytes within the CNS, but no study to date has examined whether bacopa is able to modulate the release of proinflammatory cytokines such as IL-6 and TNF-α from LPS activated microglia. If bacopa can modulate inflammatory mediators such as cytokines and caspases that link systemic inflammation and neuroinflammatory conditions, then bacopa or its various constituents could be excellent candidates for treating CNS disorders including neurodegenerative diseases and psychiatric disorders.
The current study examines the ability of a tea, infusion, and alkaloid extract of bacopa to inhibit inflammation. The goal for this study is to identify those extracts of bacopa that have anti-inflammatory properties as measured in microglial and cell free assays. To that end, crude water extracts (tea and infusion) and an alkaloid extract will be used to determine whether these extracts have anti-inflammatory properties and compared to Bacoside A, a mixture whose properties have been well investigated. The objective is to determine if these crude mixtures have anti-inflammatory properties. Extracts that are found to inhibit inflammation in this study will be used in future studies to isolate the various constituents that may be responsible for the anti-inflammatory activity.
In the current study, we examined whether these crude extracts inhibited the release of IL-6 and TNF-α from LPS-activated microglia in vitro. In addition, the extracts were tested for inhibitory activity against enzymes involved in inflammation and cell death including 1) Matrix Metalloproteinase-3 (MMP-3): upregulated in many inflammatory disorders (Warner et al., 2004) and cancers (Lee et al., 2010; Roy et al., 2009), 2) caspase 1: involved in the activation of pro-inflammatory cytokines and chronic inflammation (Franchi et al., 2009), and 3) caspase 3: an executioner caspase involved in apoptosis and the development of Alzheimer's diseases and other neurodegenerative disorders (Gamblin et al., 2003). Bacopa was found to significantly inhibit the release of IL-6 and TNF-α from LPS activated microglia and also significantly inhibited the enzyme activity of MMP-3, and caspase 1 and 3. Thus, we demonstrate that bacopa has the therapeutic potential for treating a wide range of CNS disorders that have a major neuroinflammatory component, including neurodegenerative diseases and psychiatric disorders such as depression, anxiety, and schizophrenia.
2. Materials and Methods
2.1 Plant material
Bacopa monnieri (powdered plant certified organic) was obtained from Banyan Botanicals (Albuquerque, NM; product lot number 641615, batch number BM468P). The plant name was verified at www.plantlist.org. Banyan Botanicals provided a certificate of analysis with the plant material certifying its characteristics and that it is free from contaminating microorganisms. The powdered material obtained from Banyan Botanicals was verified independently by a commercial laboratory, Covance Laboratories (Madison, WI USA), to be bacopa, using a phytochemical reference standard of bacopa from ChromaDex (Irvine, CA, USA). ChromaDex provided a photograph of the voucher specimen that was used as the reference standard (voucher number LQV BM5287) and provided a Certificate of Analysis for this voucher specimen that included photographs of the plant material, a macroscopic and microscopic analysis, and HPTLC chromatograms. Covance laboratories used this voucher specimen from ChromaDex and provided a certificate of Analysis that stated that the powdered material obtained from Banyan Botanicals was the dried powder of the whole plant Bacopa monnieri as authenticated by High Performance Thin Layer Chromatography (HPTLC) (Supplemental Figure 1).
2.2 Preparation of Extracts
2.2.1 Tea
10.0 g of powdered bacopa was added to 100 ml of boiling deionized water, which was then removed from the heat source. The suspension was stirred and allowed to steep for 30 minutes. The suspension was cooled, then vacuum filtered with Whatman quantitative filter paper (Grade 40, 8 μm) to remove the plant material. The resulting filtrate was lyophilized to remove the water, yielding the final tea extract (0.461 g).
2.2.2 Infusion
10.0 g of powdered bacopa was added to 100 ml of room temperature deionized water and brought to a boil. The suspension was stirred and allowed to boil gently for 30 minutes. It was cooled, then vacuum filtered with Whatman quantitative filter paper (Grade 40, 8 μm) to remove the plant material. The resulting filtrate was lyophilized to remove the water, yielding the final infusion extract (0.516 g).
2.2.3 Alkaloid
10.0 g of powdered bacopa was added to 100 mL of boiling deionized water and the suspension was steeped for 15 minutes. After the suspension cooled to room temperature, it was vacuum-filtered as described above, and then extracted with chloroform to remove any nonpolar, neutral compounds. After the chloroform extract was removed, sodium carbonate was added to the remaining aqueous phase to raise the pH to 9. This basic solution was then re-extracted with chloroform, and the resulting alkaloid extract was dried on a rotary evaporator (0.082 g).
2.2.4 Bacoside A
Bacoside A (L12040387 )was derived from an ethanolic extract of bacopa (purity 97.03%) and was kindly provided Laila Pharmaceuticals, Vijayawada India.
For the tissue culture experiments, the bacopa extracts and Lipopolysaccharide (LPS) from E. coli (O55:B5, 1 μg/ml, Sigma), were dissolved in complete RPMI 1640 medium (see below). The alkaloid extract and Bacoside A was first dissolved in 25 μl of DMSO (Sigma) and then diluted in RPMI so that the DMSO was less than 2% of the final working solution volume.
2.3 Cell Culture and microglial stimulation
The murine N9 microglial cell line, originally derived from CBA mice, was provided by Dr. P Ricciardi-Castagnoli (University of Milano-Bicocca, Milan Italy). The cells were grown and maintained in complete RPMI 1640 medium supplemented with 5% heat-inactivated FBS with 2mM glutamine, 25mM HEPES, and 1% Penicillin-Streptomycin (Sigma). Cultures were passaged every 3 days and then seeded at passage 5-7 at a density of 5.7 × 105 cells/ml/well in twelve-well plates. The plates were placed in the incubator for 15-25 minutes to allow the cells to attach. The cultures were then exposed to either the bacopa extract alone (tea (12.5-50 μg/ml), infusion (12.5-50 μg/ml), alkaloid (0.125-1.0 μg/ml), Bacoside A (0.25-1 μg/ml) or bacopa extract +LPS(1 μg/ml) for 24 hours at 37°C. The cell free supernatant was then collected for analysis and the CellTiter 96® Aqueous One Solution Cell Proliferation Assay (MTS) (Promega, USA) was performed on selected cultures to assess cell death.
2.4 Cytokine ELISAs
The cell supernatants were collected and analyzed for IL-6 and TNF-α release using murine cytokine Enzyme-Linked Immunosorbent Assay (ELISA) kits according to the manufacturer's protocol (R&D Systems, Minneapolis, MN). Plates at A450nm for the ELISA assays on a Spectra Max 190 (Molecular Devices, USA) plate reader.
2.4 MTS Assay
Cell death was quantified in selected cultures following 24 h of treatment with the various extracts using the CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (MTS assay) according to the manufacturer's instruction (Promega, USA). Controls of RPMI media and the surfactant Triton X were used to quantify no cell death and complete cell death respectively. Plates were read at A490nm for the MTS assay on a Spectra Max 190 (Molecular Devices, USA) plate reader. Extract concentrations that did not result in any cell death were used for the microglial assays.
2.5 Enzyme Inhibition assays
Three cell-free commercial enzyme inhibition assay kits were used to assess the activity of the bacopa extracts: 1) Caspase-1 Fluorogenic Assay Kit for Drug Discovery – Enzo Life Sciences Inc., BML-AK-701; 2) MMP-3 Colorimetric Drug Discovery Kit – Enzo Life Sciences Inc., BML-AK-400; and 3) Caspase-3 Fluorometric Drug Discovery Kit – Enzo Life Sciences Inc., BML-AK700-0001. Each of the crude bacopa extracts (tea, infusion, and alkaloid) was assayed against each of the three enzymes as per the manufacturer's instructions. The three extracts were tested at 100 μg/well. Two controls were included for each assay: Control 1 (assay buffer, substrate and enzyme) and control 2 (assay buffer, substrate, enzyme, and enzyme specific inhibitor). The tests were run in triplicate using the Spectra Max 190 microplate reader at A412nm, and the mean of the replicates was calculated.
For the MMP-3 inhibition assay a colorimetric thiopeptide substrate, Acetyl-Pro-Leu-Gly-[2-mercapto-4-methyl-pentanoyl]-Leu-Gly- OC2H5 (100 μM), was used to produce a yellow product, 2-nitro-5-thiobenzoic acid, which was measured at 405 nm. N-isobutyl-N-(4-methoxyphenylsulfonyl) glycyl hydroxamic acid (NNGH) (1.3 μM) was used as the control inhibitor for MMP-3. The enzyme activity was 0.02 U/μL, where one U=100 pmol/min@ 37°C, 100 μM thiopeptolide P125. This assay utilizes Escherichia coli recombinant human MMP-3 catalytic domain (calculated MW 19.5 kDa).
For the caspase-1 inhibition assay the fluorogenic substrate Ac-YVAD-AMC (Acetyl-Tyr-Val-Ala-Asp-amino-methyl coumarin, 50 μM) was used to yield the fluorescent product AMC (7-amino-4-methyl coumarin, Ex:360 nm/Em:460 nm). The control inhibitor was Ac-YVADCHO (Acetyl-Tyr-Val-Ala-Asp-aldehyde, 0.1μM). For the fluorimetric assay, the enzyme activity was 0.5 U/μl where one U=1 pmol/min @ 30°C, 200 μM YVAD-pNA. The enzyme was recombinant human caspase-1 with >90% purity by SDS-PAGE.
For the caspase-3 inhibition assay the fluorogenic substrate Ac-DEVD-AMC (Acetyl-Asp-Glu-Val-Asp-amino-methyl coumarin, 30 μM) was used to yield the fluorescent product AMC (7-amino-4-methyl coumarin, Ex. 360 nm/Em. 460 nm). The control inhibitor was Ac-DEVDCHO (Acetyl-Asp-Glu-Val-Asp-aldehyde, 0.1μM). For the fluorimetric assay human recombinant caspase-3 was used. The enzyme activity was 0.5 U/μl where one U=1 pmol/min@30°C, 200 μM DEVD-pNA, with >95% purity by SDS-PAGE.
2.6 Data Analysis
Each extract was tested in two to three separate experiments. Supernatants from all the replicate experiments were pooled and the ELISA was run in triplicate. An average value was obtained for each parameter (extract and dose) and the standard deviation and standard error were calculated. Significance between the groups was determined by a one-way ANOVA in conjunction with a Tukey's post-hoc analysis for variance. All ANOVA models were performed with Prism software version 6 (Graphpad Software, USA). A P value of <0.05 was considered significant compared to either the media or LPS controls.
3. Results
3.1 Concentrations of bacopa extracts
In order to determine the appropriate concentration of the various bacopa extracts to be used in the microglial cell culture experiments, an MTS assay for cell toxicity was performed at various concentrations of the tea, infusion, and Alkaloid extracts and Bacoside A. No cell death was seen in any of the selected concentrations for the tea, infusion, and alkaloid fractions (Figure 1A, B, C). The Bacoside A showed significant cell death at 6 μg/ml (Figure 1D). In addition, there were small but significant increases in cell survival compared to media controls in the 12.5 μg/ml tea, the 0.125 and 025 μg/ml alkaloid, and the 1.5 μg/ml Bacoside A suggesting that these concentrations are beneficial for cell survival. Concentrations of bacopa extracts that did not result in cell death were selected for the cytokine release studies.
Figure 1.

MTS assay of concentrations of bacopa extracts (μg/ml) in N9 microglial cell cultures following 24h of treatment. None of the concentrations of the infusion (A), tea (B), or alkaloid (C) fraction resulted in cell death. The double asterisks indicate that cell death occurred at 6 μg/ml of the Bacoside A (D). There were small but significant increases in cell survival compared to media controls in the 12.5 μg/ml tea, the 0.125 and 025 μg/ml alkaloid, and the 1.5 μg/ml Bacoside A suggesting that these concentrations are beneficial for cell survival. Graphs represent mean ± SEM. *p<0.05; ANOVA with Dunnett's post hoc test compared to media control.
3.2 Cytokine release from non-activated N9 microglia
In order to determine if the bacopa extracts themselves modulate cytokine release from non-activated N9 microglial cells, the extracts were added to the microglial cultures, and cytokine release was assayed after 24 hours of treatment. None of the concentrations of extracts used in this study resulted in cell death as measured by the MTS assay (data not shown). Figure 2 illustrates that the media control cells release an extremely small amount of IL-6 and neither the alkaloid nor the Bacoside A fractions induce any additional release of IL-6 from N9 cells (Fig 2C-D). Both the tea and the infusion extracts induce a small but significant increase in the release of IL-6 at 25 and 50 ug/ml compared to the media control (Fig. 2A-B).
Figure 2.
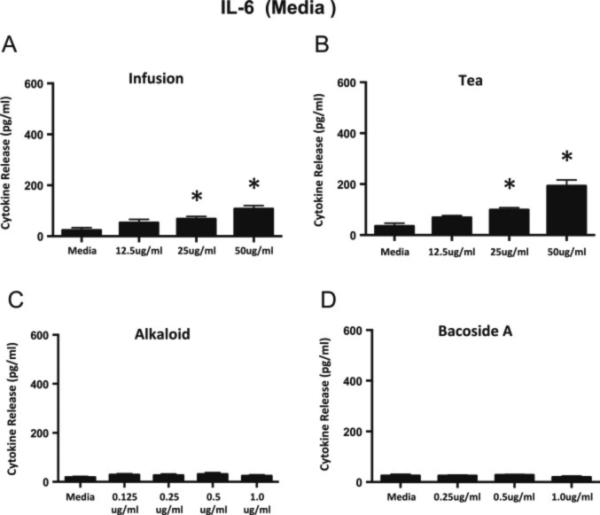
IL-6 release from non-activated N9 microglial cells following a 24 h incubation with the bacopa extracts. The infusion (A) and tea (B) extracts induce a very small but significant release in IL-6 at the two highest concentrations of the extracts. Neither the alkaloid fraction (C) nor the Bacoside A (D) results in increased release of IL-6 compared to media controls. Graphs represent mean ± SEM. *p<0.05; ANOVA with Dunnett's post hoc test compared to media control.
Very small amounts of TNF-α are released from media controls of non-activated microglia and the addition of the infusion, alkaloid, or Bacoside A fractions does not significantly increase this small release (Figure 3A, C-D). A very small but significant amount of TNF-α is released at the highest dose of the tea fraction compared to the media control (Figure 3B). We found that the highest doses of the tea and infusion do result in a small amount of cytokine release, particularly IL-6. This is interesting, because low concentrations of IL-6 are thought to result in neuronal survival and outgrowth, while high concentrations are considered to lead to neuronal cell death (Spooren et al, 2011). However, in general, the bacopa extracts do not induce the release of substantial amounts of IL-6 and TNF-α from the non-activated microglia.
Figure 3.
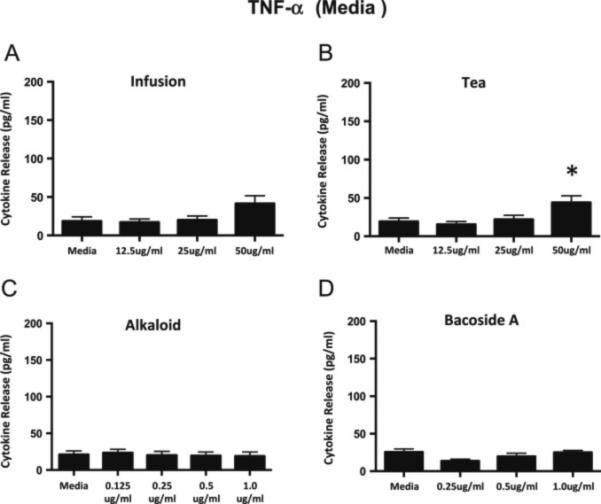
TNF-α release from non-activated N9 microglial cells following a 24 h incubation with the bacopa extracts. Neither the infusion (A), or alkaloid (C) fractions or the Bacoside A (D) induces an increase in the release of TNF-α from the microglial cells compared to media controls. The highest concentration of the tea extract (B) results in a small but significant release of TNF-α compared to the media control. Graphs represent mean ± SEM. *p<0.05; ANOVA with Dunnett's post hoc test compared to media control.
3.3 Bacopa inhibits cytokine release in LPS-activated N9 microglia
There was some variability from experiment to experiment in terms of the amount of cytokine release in response to LPS alone. LPS alone resulted in the release of IL-6 in the range of 2,000-5,000 μg/ml depending on the experiment. LPS alone resulted in the release of TNF-α in the range of 500-800 μg/ml depending on the experiment. In order to compile data from replicate experiments, all data for the extracts + LPS were normalized to the LPS only values for each individual experiment. Therefore, Figures 4 and 5 show the LPS normalized to one, and the values for the LPS + extracts are then normalized to the LPS control.
Figure 4.

IL-6 release from LPS-activated N9 microglial following a 24 h incubation with LPS plus the bacopa extracts. The infusion extract significantly inhibited the release of IL-6 from Fi LPS activated microglial at 25 and 50 μg compared to LPS controls (A). The highest concentration of the alkaloid fraction (1 μg) also inhibited the release of IL-6 from activated microglia (C). Neither the tea nor the Bacoside A significantly inhibited IL-6 release compared to LPS controls (B and D). Data is normalized to IL-6 release from LPS activation only. Graphs represent mean ± SEM. *p<0.05; ANOVA with Dunnett's post hoc test compared to LPS control.
Figure 5.
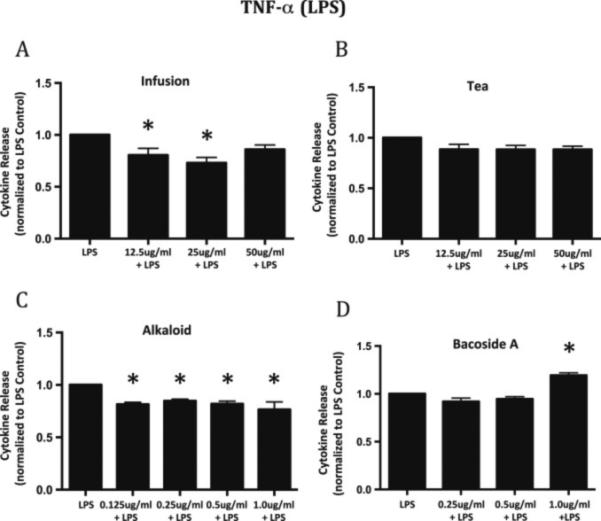
TNF-α release from LPS activated N9 microglial cells following a 24 h incubation with bacopa plus the extracts. Both the infusion (A) and the alkaloid (C) fraction showed significant inhibition of the release of TNF-α from LPS activated microglia of approximately 20%-27% compared to LPS alone. The tea extract (B) did not inhibit the release of TNF-α. The Bacoside A (D) showed a small but significant increase in TNF-α release at the highest concentration. Data is normalized to TNF-α release from LPS activation only. Graphs represent mean ± SEM. *p<0.05; ANOVA with Dunnett's post hoc test compared to LPS control.
The infusion extract significantly inhibits the release of IL-6 from activated microglia at 25 and 50 μg/ml following 24 h. of treatment compared to the LPS controls (Figure 4A). The highest concentration of the alkaloid extract (1 μg/ml) also significantly inhibits the release of IL-6 compared to LPS controls (Figure 4C). Neither the tea, extract, or Bacoside A alters the release of IL-6 from the activated microglial (Figure 4 B&D). The infusion extract inhibits IL-6 release by 25-30%, and the alkaloid fraction inhibits IL-6 release by approximately 17% compared to LPS controls.
Similarly, both the infusion and the alkaloid extract inhibit the release of TNF-α from the activated microglia compared to LPS controls. Figure 5A illustrates that there is a 20-27% decrease in TNF-α from activated microglia that have been incubated with 12.5 and 25 μg/ml respectively of the infusion extract compared to LPS alone. All concentrations of the alkaloid extract significantly decrease the release of TNF-a from the activated microglia by approximately 20% compared to LPS alone (Figure 5C). In contrast, neither the tea extract nor Bacoside A result in decreased release of TNF-μ. In fact, the highest concentration of Bacoside A leads to a small but significant increase in TNF-μ release (Figure 5D).
These results show that the infusion and alkaloid bacopa fractions reduce the release of both TNF-α and IL-6 by approximately 20% from activated microglia compared to LPS alone. Thus, bacopa can significantly suppress the release of pro-inflammatory cytokines from activated microglia. It is interesting that in our hands, Bacoside A does not significantly reduce the release of these inflammatory cytokines. Bacoside A is generally considered to be one of the active constituents of bacopa and is thought to be responsible, at least in part, for its biological activity.
3.4 Bacopa extracts inhibit caspase 1, MMP3, and caspase 3
Each bacopa extract was evaluated for its ability to inhibit caspase-1, caspase-3, and MMP-3 in 96 well assays. These data are expressed as percent enzyme inhibition and were determined as a function of the amount of product formed. All three extracts inhibited all three enzymes (Figure 6). Efforts are now underway to determine which compounds contribute to the inhibition of each of these enzymes.
Figure 6.
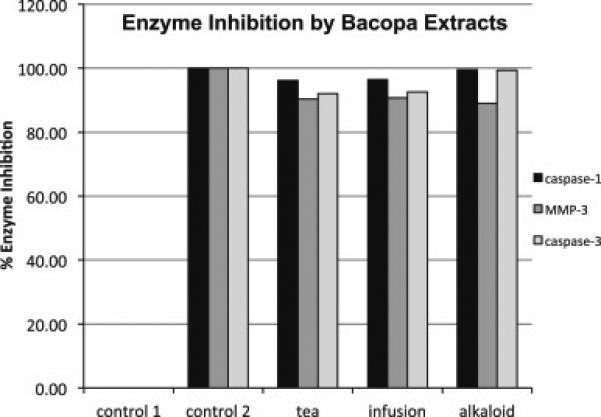
Each bacopa extract was evaluated for its ability to inhibit caspase-1, caspase-3, and MMP-3 in 96 well assays. These data are expressed in terms of % inhibition of enzyme activity. Control 1 included the substrate and enzyme (no inhibitor) and Control 2 included the substrate, enzyme and a specific inhibitor (as described).
Taken together, these results show that bacopa can significantly inhibit the release of proinflammatory cytokines from LPS-activated microglia, and can also inhibit the activity of enzymes that play a key role in neuroinflammation and neurodegeneration.
4. Discussion
The current study demonstrates that extracts of bacopa significantly inhibit the release of TNF-α and IL-6 from LPS-activated microglia, and almost completely inhibit the activity of caspase 1 and 3 and MMP3 in a cell free assay system. It is important to note that in the microglial culture model, not all of the extracts were able to modulate the release of the proinflammatory cytokines. The infusion extract and the highest concentration of the alkaloid extract significantly inhibited IL-6 while the infusion and the alkaloid extract significantly inhibited the release of TNF-α. Few alkaloids have been reported from bacopa, and no biological activity has been associated with these compounds (Murthy et al., 2006). The results of this study suggest that the alkaloid fraction warrants further investigation
Interestingly, Bacoside A did not reduce IL-6 or TNF-α levels following LPS activation, and in fact, the highest concentration of Bacoside A actually increased TNF-α concentrations in the tissue culture media of the activated microglia. This was surprising, because Bacoside A, a dammarane saponin triterpenoid, is thought to be responsible, at least in part, for the biological activity and health benefits of bacopa. Bacoside A and Bacoside B were named as the original organic extracts that were derived from bacopa, and each are made up of a mixture of four bacopasides (Lurie DI 2015a). Our data suggests that the alkaloid and the infusion fractions contain the greatest anti-inflammatory activity at least in terms of microglial release of pro-inflammatory cytokines.
Microglial cells express are considered to be the resident mononuclear phagocytes of the CNS. There are two distinct functional states of activated microglia, a neurotoxic phenotype (termed M1) and a neuroprotective phenotype (termed M2). The M1 phenotype produce proinflammatory mediators such as TNF-α, IL-6 and IL-1β and function in the host defense against infection (Nakagawa and Chiba, 2015). The M2 phenotype is considered to be neuroprotective, and produces the anti-inflammatory cytokine IL-10 that can downregulate the M1 mediated inflammation. The M1 polarization of microglia results in the chronic inflammation that has been shown to be associated with neurodegenerative diseases such as Alzheimer's disease, and more recently, in psychiatric diseases such as depression, anxiety, and schizophrenia.
Our results suggest that bacopa can significantly ameliorate the M1 microglial response by reducing the release of TNF-α and IL-6, leading to a decrease in neuroinflammation. Preliminary studies in our laboratory have found that the LPS-activated microglial cultures release only small amounts of IL-10, and bacopa does not have an effect on this release. Thus, our initial studies suggest that bacopa appears to ameliorate the M1 phenotype rather than facilitating the M2 or neuroprotective phenotype.
The present study demonstrates that the infusion and alkaloid extracts of bacopa are able to significantly inhibit the release of IL-6 from activated microglia. The cytokine IL-6 is a small signaling glycoprotein that is produced in the CNS by astrocytes and microglia (Gruol, 2015) and is involved in many cellular functions including cell proliferation and differentiation, survival, and apoptosis (Guzmán, 2010). It is found in systems including the hematopoietic system and the nervous system and can effect many different tissues and organ systems (Guzman et al, 2010) (Guzmán, 2010). While IL-6 is generally considered to be a proinflammatory cytokine whose overexpression has a negative effect, a number of studies suggest that lower levels of IL-6 might be anti-inflammatory and regulate neuronal survival (Guzman et al, 2010) (Guzmán, 2010). Overexpression of IL-6 is associated with a number of CNS disorders (Trapero and Cauli, 2014) including Alzheimer's disease and is associated with psychiatric disorders such as depression and schizophrenia (Gruol, 2015). Bacopa, or its constituents, could therefore represent novel therapeutics for the treatment of these disorders.
The alkaloid and infusion extracts of bacopa significantly decrease the amount of TNF-α that is released by activated microglia. TNF-α is a proinflammatory cytokine that is primarily involved in the innate immune defense system but it is also important for maintaining homeostasis in cells and tissues (Probert, 2015). It is produced very rapidly following a stimulus, mostly by activated monocytes and macrophages, and coordinates the inflammatory response that is necessary for clearing pathogens and healing damaged tissue. There are many checkpoints in place to limit the effect of TNF-α to an acute response, and when these checkpoints fail, the result is chronic inflammation. In pathological conditions within the CNS, microglia and to some extent astrocytes, release TNF-α and this contributes to the neuroinflammation associated with various neurological diseases including Alzheimer's disease, Parkinson's disease, traumatic brain injury, and ischemia. (Olmos and Llado, 2014). Thus, bacopa could act to limit TNF-α mediated neuroinflammation in the CNS.
All of the bacopa extracts inhibited caspases 1 and 3, and MMP3. The activity of caspase 1 showed the greatest inhibition by the bacopa extracts. Up-regulation of caspase-1 and concomitant chronic inflammation has been associated with a number of different pathologies. Caspase-1 is activated upon binding to the NLRP3 inflammasome, a multiprotein complex that plays a key role in innate immunity by activating the proinflammatory cytokines interleukin 1-β, IL-18, and IL-33 (Franchi et al., 2009). Specific caspase-1 inhibitors have been proposed as potential therapies for many neurodegenerative disorders including Parkinson's (Jha et al., 2010) and Alzheimer's diseases (Gamblin et al., 2003), as well as certain cancers (Granot et al., 2006; Lewis et al., 2006; Schlosser et al., 2001).
Caspase-3 is an executioner caspase and is important in apoptosis. In activated microglia and in the presence of excitotoxicity, upregulated caspase-3 can lead to cell death. It is upregulated following ischemic events including stroke and is associated with tissue damage following reperfusion (Rosell et al., 2008). It is also involved in the development of Alzheimer's disease (Gamblin et al., 2003) and other neurodegenerative disorders and bacopa could act in vivo to limit cell death.
MMP-3 is upregulated in many inflammatory disorders (Warner et al., 2004) and cancers (Lee et al., 2010; Roy et al., 2009). It leads to activation of microglia and concomitant inflammatory cascades. One important hallmark of neuroinflammation is the opening of the blood–brain barrier (BBB) and subsequent neutrophil influx. Upregulation of MMP-3 is an early event in LPS-stimulated neuroinflammation, preceding and contributing to activation of TNF-α and MMP-9. Elevated levels of MMP-3 can also lead to activated microglia, with the concomitant production of reactive oxygen species (ROS), damage to the blood brain barrier, and epithelialmesenchymal transitions. Activated microglia can induce assemblage of the NLRP3 inflammasome, which in turn generates proinflammatory cytokines, including TNF-α and IL-6.
Thus, bacopa can effectively inhibit many of the inflammatory enzyme cascades that utilize caspase 1 and 3, and MMP-3, resulting in a decreased release of pro-inflammatory cytokines such as TNF-α and IL-6 from activated CNS cells. This is exactly what we see in the microglial cultures, where bacopa significantly inhibits the release of TNF-α and IL-6.
5. Conclusions
To the best of our knowledge, the present study is the first to document that bacopa has an anti-inflammatory effect on brain microglia. Williams et al 2015 recently demonstrated that various extracts of bacopa had anti-inflammatory effects in cultured macrophages and human blood cells that included an inhibition of TNF-α release and Interferon gamma, and suppression of nitric oxide. Interestingly, this group found that the soluble ethyl acetate extract of bacopa was more active than the bacoside-enriched fraction, which is similar to our results that found no anti-inflammatory activity with the Bacoside A fraction. Many studies have documented the anti-inflammatory effect of bacopa on peripheral macrophages (Vijayan et al., 2011; Viji and Helen, 2008, 2011; Viji et al., 2010a; Viji et al., 2010b; Williams et al., 2014), and we have now extended these findings to include CNS microglia. Because bacopa is able to ameliorate inflammation, a potential link between its systemic anti-inflammatory properties and its effect on the mind could well lie in its ability to modulate neuroinflammation in the CNS. Indeed, our data supports this hypothesis, with bacopa targeting inflammatory mediators such as IL-6 and TNF-α that are found both in the periphery and in the CNS, and are links between systemic inflammation such as arthritis and neuroinflammation. Our results strongly suggest that bacopa and its constituents are promising candidates for the development of novel therapeutics that target neuroinflammation, and have the potential for treating a wide range of CNS disorders including Alzheimer's disease, depression, and schizophrenia.
Supplementary Material
Acknowledgements
This work was funded by NIH P20GM103546 (A.A.S., D.B.S.), the University of Montana (D.I.L.), and The University of Montana Honors College Research Award (M.D.N.).
Glossary
- TNF-α
Tumor Necrosis Factor alpha
- IL-6
Interleukin 6
- MMP-3
Matrix Metalloproteinase-3
- LPS
lipopolysaccharide
- Microglia
Resident macrophages of the central nervous system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguiar S, Borowski T. Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation research. 2013;16:313–326. doi: 10.1089/rej.2013.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channa S, Dar A, Anjum S, Yaqoob M, Atta Ur R. Anti-inflammatory activity of Bacopa monniera in rodents. J Ethnopharmacol. 2006;104:286–289. doi: 10.1016/j.jep.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nature immunology. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly P, Brenhouse HC. Broken or maladaptive? Altered trajectories in neuroinflammation and behavior after early life adversity. Developmental cognitive neuroscience. 2014 doi: 10.1016/j.dcn.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H, Elgueta D, Montoya A, Pacheco R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. Journal of neuroimmunology. 2014;274:1–13. doi: 10.1016/j.jneuroim.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Granot T, Milhas D, Carpentier S, Dagan A, Segui B, Gatt S, Levade T. Caspase-dependent and -independent cell death of Jurkat human leukemia cells induced by novel synthetic ceramide analogs. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2006;20:392–399. doi: 10.1038/sj.leu.2404084. [DOI] [PubMed] [Google Scholar]
- Gruol DL. IL-6 regulation of synaptic function in the CNS. Neuropharmacology. 2015;96:42–54. doi: 10.1016/j.neuropharm.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán CH-C,C, López-Griego L, Morales-Montor J. Interleukin-6: A Cytokine with a Pleiotropic Role in the Neuroimmunoendocrine Network. The Open Neuroendocrinology Journal. 2010;3:152–160. [Google Scholar]
- Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nature reviews. Immunology. 2014;14:463–477. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- Jha S, Srivastava SY, Brickey WJ, Iocca H, Toews A, Morrison JP, Chen VS, Gris D, Matsushima GK, Ting JP. The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:15811–15820. doi: 10.1523/JNEUROSCI.4088-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongkeaw C, Dilokthornsakul P, Thanarangsarit P, Limpeanchob N, Norman Scholfield C. Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract. Journal of ethnopharmacology. 2014;151:528–535. doi: 10.1016/j.jep.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Kim SY, Hyun JW, Min SW, Kim DH, Kim HS. Glycitein inhibits glioma cell invasion through down-regulation of MMP-3 and MMP-9 gene expression. Chemicobiological interactions. 2010;185:18–24. doi: 10.1016/j.cbi.2010.02.037. [DOI] [PubMed] [Google Scholar]
- Lewis AM, Varghese S, Xu H, Alexander HR. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. Journal of translational medicine. 2006;4:48. doi: 10.1186/1479-5876-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie DI CJ. The role of bacopa monnieri in inflammatory and neurodegenerative diseases. In: Motohashi N, editor. Occurrences, Structure, Biosynthesis, and Health Benefits Based on Their Evidences of Medicinal Phytochemicals in Vegetables and Fruits. Nova Science Publishers; New York, USA: 2015a. pp. 27–61. [Google Scholar]
- Lurie DI CJ. The role of bacopa monnieri in inflammatory and neurodegenerative diseases. In: Motohashi N, editor. Occurrences, Structure, Biosynthesis, and Health Benefits Based on Their Evidences of Medicinal Phytochemicals in Vegetables and Fruits. Nova Science Publishers; New York, USA: 2015b. pp. 219–238. [Google Scholar]
- Murthy PB, Raju VR, Ramakrisana T, Chakravarthy MS, Kumar KV, Kannababu S, Subbaraju GV. Estimation of twelve bacopa saponins in Bacopa monnieri extracts and formulations by high-performance liquid chromatography. Chemical & pharmaceutical bulletin. 2006;54:907–911. doi: 10.1248/cpb.54.907. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Chiba K. Diversity and plasticity of microglial cells in psychiatric and neurological disorders. Pharmacology & therapeutics. 2015;154:21–35. doi: 10.1016/j.pharmthera.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Olmos G, Llado J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators of inflammation. 2014;2014:861231. doi: 10.1155/2014/861231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- P.V. S. Caraka Samhita. Chaukhambha Orientalia; Varanasi, India: 2011. [Google Scholar]
- Pase MP, Kean J, Sarris J, Neale C, Scholey AB, Stough C. The cognitive-enhancing effects of Bacopa monnieri: a systematic review of randomized, controlled human clinical trials. Journal of alternative and complementary medicine. 2012;18:647–652. doi: 10.1089/acm.2011.0367. [DOI] [PubMed] [Google Scholar]
- Probert L. TNF and its receptors in the CNS: The essential, the desirable and the deleterious effects. Neuroscience. 2015;302:2–22. doi: 10.1016/j.neuroscience.2015.06.038. [DOI] [PubMed] [Google Scholar]
- Rai D, Bhatia G, Palit G, Pal R, Singh S, Singh HK. Adaptogenic effect of Bacopa monniera (Brahmi). Pharmacol Biochem Behav. 2003;75:823–830. doi: 10.1016/s0091-3057(03)00156-4. [DOI] [PubMed] [Google Scholar]
- Rosell A, Cuadrado E, Alvarez-Sabin J, Hernandez-Guillamon M, Delgado P, Penalba A, Mendioroz M, Rovira A, Fernandez-Cadenas I, Ribo M, Molina CA, Montaner J. Caspase-3 is related to infarct growth after human ischemic stroke. Neuroscience letters. 2008;430:1–6. doi: 10.1016/j.neulet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A, Borrelli F. Bacopa monniera, a reputed nootropic plant: an overview. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2005;12:305–317. doi: 10.1016/j.phymed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Schlosser S, Gansauge F, Ramadani M, Beger HG, Gansauge S. Inhibition of caspase-1 induces cell death in pancreatic carcinoma cells and potentially modulates expression levels of bcl-2 family proteins. FEBS letters. 2001;491:104–108. doi: 10.1016/s0014-5793(01)02144-5. [DOI] [PubMed] [Google Scholar]
- Shinomol GK, Muralidhara Bacopa monnieri modulates endogenous cytoplasmic and mitochondrial oxidative markers in prepubertal mice brain. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2011;18:317–326. doi: 10.1016/j.phymed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Stough C, Scholey A, Cropley V, Wesnes K, Zangara A, Pase M, Savage K, Nolidin K, Lomas J, Downey L. Examining the cognitive effects of a special extract of Bacopa monniera (CDRI08: Keenmnd): a review of ten years of research at Swinburne University. Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques. 2013;16:254–258. doi: 10.18433/j35g6m. [DOI] [PubMed] [Google Scholar]
- Trapero I, Cauli O. Interleukin 6 and cognitive dysfunction. Metabolic brain disease. 2014;29:593–608. doi: 10.1007/s11011-014-9551-2. [DOI] [PubMed] [Google Scholar]
- Vijayan V, Shyni GL, Helen A. Efficacy of Bacopa monniera (L.) Wettst in alleviating lysosomal instability in adjuvant-induced arthritis in rats. Inflammation. 2011;34:630–638. doi: 10.1007/s10753-010-9272-6. [DOI] [PubMed] [Google Scholar]
- Viji V, Helen A. Inhibition of lipoxygenases and cyclooxygenase-2 enzymes by extracts isolated from Bacopa monniera (L.) Wettst. Journal of ethnopharmacology. 2008;118:305–311. doi: 10.1016/j.jep.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Viji V, Helen A. Inhibition of pro-inflammatory mediators: role of Bacopa monniera (L.) Wettst. Inflammopharmacology. 2011;19:283–291. doi: 10.1007/s10787-010-0046-4. [DOI] [PubMed] [Google Scholar]
- Viji V, Kavitha SK, Helen A. Bacopa monniera (L.) wettst inhibits type II collagen-induced arthritis in rats. Phytotherapy research : PTR. 2010a;24:1377–1383. doi: 10.1002/ptr.3135. [DOI] [PubMed] [Google Scholar]
- Viji V, Shobha B, Kavitha SK, Ratheesh M, Kripa K, Helen A. Betulinic acid isolated from Bacopa monniera (L.) Wettst suppresses lipopolysaccharide stimulated interleukin-6 production through modulation of nuclear factor-kappaB in peripheral blood mononuclear cells. International immunopharmacology. 2010b;10:843–849. doi: 10.1016/j.intimp.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Warner RL, Bhagavathula N, Nerusu KC, Lateef H, Younkin E, Johnson KJ, Varani J. Matrix metalloproteinases in acute inflammation: induction of MMP-3 and MMP-9 in fibroblasts and epithelial cells following exposure to pro-inflammatory mediators in vitro. Experimental and molecular pathology. 2004;76:189–195. doi: 10.1016/j.yexmp.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Williams R, Munch G, Gyengesi E, Bennett L. Bacopa monnieri (L.) exerts anti-inflammatory effects on cells of the innate immune system in vitro. Food & function. 2014;5:517–520. doi: 10.1039/c3fo60467e. [DOI] [PubMed] [Google Scholar]
- Williamson EM. Major Herbs of Ayurveda. Churchill LIvingston, Elsevier Limited; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


