Abstract
Graphene oxide (GO) can be potentially used in many medical and industrial fields. Using assay system of Caenorhabditis elegans, we identified the NLG-1/Neuroligin-mediated neuronal signaling dysregulated by GO exposure. In nematodes, GO exposure significantly decreased the expression of NLG-1, a postsynaptic cell adhesion protein. Loss-of-function mutation of nlg-1 gene resulted in a susceptible property of nematodes to GO toxicity. Rescue experiments suggested that NLG-1 could act in AIY interneurons to regulate the response to GO exposure. In the AIY interneurons, PKC-1, a serine/threonine protein kinase C (PKC) protein, was identified as the downstream target for NLG-1 in the regulation of response to GO exposure. LIN-45, a Raf protein in ERK signaling pathway, was further identified as the downstream target for PKC-1 in the regulation of response to GO exposure. Therefore, GO may dysregulate NLG-1-mediated molecular signaling in the interneurons, and a neuronal signaling cascade of NLG-1-PKC-1-LIN-45 was raised to be required for the control of response to GO exposure. More importantly, intestinal RNAi knockdown of daf-16 gene encoding a FOXO transcriptional factor in insulin signaling pathway suppressed the resistant property of nematodes overexpressing NLG-1 to GO toxicity, suggesting the possible link between neuronal NLG-1 signaling and intestinal insulin signaling in the regulation of response to GO exposure.
Graphene family, 2D carbon engineered nanomaterials (ENMs), have attracted massive attention due to its unique mechanical, electronic, and thermal properties1. Graphene family, including graphene oxide (GO), can be potentially used in many fields, especially in the catalysis, the biosensor, and the biomedicine2. Meanwhile, so far, the evidence from both in vitro and in vivo studies has demonstrated the possible toxicity of some members in the graphene family, such as GO, on organisms3,4,5,6,7. Moreover, the mechanisms of both genetic and epigenetic control of response to GO exposure have been examined in human cell lines, such as HepG2 cell and GLC-82 cell, and animals, such as mice4,8,9.
Caenorhabditis elegans, a classic model animal, has been widely used as an in vivo assay system for toxicological study of environmental toxicants10,11,12. C. elegans has the typical properties of model animals, and the properties at least include the short life-cycle, short lifespan, transparent body, self-fertilization, and ease of culture13. Using a series of sublethal endpoints, C. elegans has been successfully used in the toxicity assessment of many environmental toxicants including the ENMs14,15. Exposure to GO could potentially cause toxic effects on the functions of both primary targeted organs such as intestinal cells and secondary targeted organs such as neurons and reproductive organs in nematodes16,17,18,19,20,21. Some important signaling pathways, such as c-Jun N-terminal kinase (JNK), insulin, p38 mitogen-activated protein kinase (MAPK), Wnt, oxidative stress associated, apoptosis, and DNA damage signaling pathways, have been further identified to be involved in the regulation of GO toxicity in nematodes22,23,24,25,26,27,28,29,30. Besides these signaling pathways, some microRNAs (miRNAs), such as mir-231 and mir-360, were also shown to participate in the control of GO toxicity in nematodes23,26,31.
In animals, besides the behaviors, the nervous system also regulates some other biological processes, such as longevity, fat storage, and stress response32,33. However, it is still largely unclear for the role of neuronal signals in the regulation of response to ENMs in animals. In C. elegans, nlg-1 gene encodes a Neuroligin, a postsynaptic cell adhesion protein. Neuroligins can bind to presynaptic proteins like neurexins and act as central organizing molecules that connect pre- and post-synaptic neurons34. NLG-1 has been shown to be required for the control of synaptic function and oxidative stress in nematodes35,36,37. In the present study, using the in vivo assay system of C. elegans, we investigated the effect of GO exposure on NLG-1-mediated signaling in neurons in animals. Moreover, we examined the possible underlying molecular mechanism for the function of neuronal NLG-1 in the regulation of response to GO exposure. Our results provide the important molecular basis for NLG-1-mediated neuronal signaling in the regulation of response to GO exposure in organisms.
Results
Effects of GO exposure on expression of nlg-1 gene
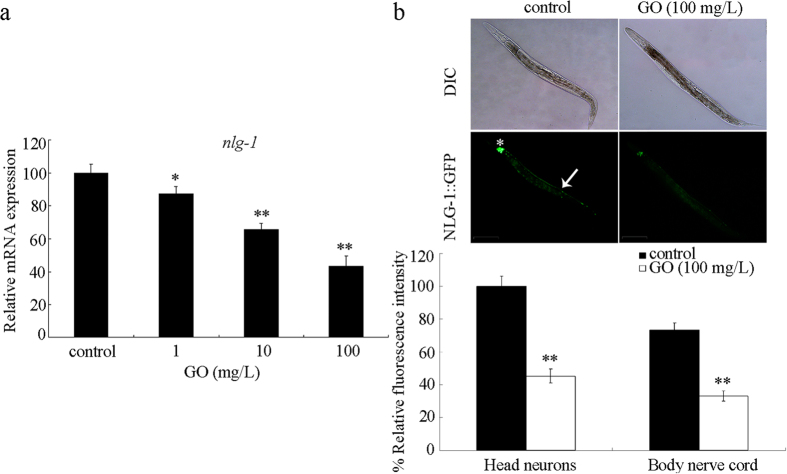
It was reported that GO at the concentration of 1 mg/L could cause the decrease in locomotion behavior, and the significant induction of ROS production in nematodes17. We selected the 1, 10, and 100 mg/L as the working concentrations for GO. After prolonged exposure from L1-larvae to young adults, GO at the concentrations of 10 and 100 mg/L significantly decreased the transcriptional expression of nlg-1 gene in wild-type N2 nematodes (Fig. 1a). GO at the concentration of 1 mg/L moderately but significantly decreased the transcriptional expression of nlg-1 gene in wild-type N2 nematodes (Fig. 1a). Moreover, GO at the concentration of 100 mg/L significantly reduced the expression of NLG-1::GFP in both the head neurons and the body nerve cord in wild-type N2 nematodes (Fig. 1b). Therefore, GO exposure may potentially decrease the NLG-1 expression in wild-type N2 nematodes.
Figure 1. Effects of GO exposure on expression of nlg-1 gene in wild-type nematodes.
(a) Effects of GO exposure at different concentrations on transcriptional expression of nlg-1 gene. (b) Effects of GO exposure on expression of NLG-1::GFP in neurons. Asterisk indicates the head neurons, and arrowhead indicates the body nerve cord. GO exposure concentration was 100 mg/L. Prolonged exposure was performed from L1-larvae to young adults. Bars represent means ± SD. **P < 0.01 vs control.
Mutation of nlg-1 gene induced a susceptible property of nematodes to GO toxicity
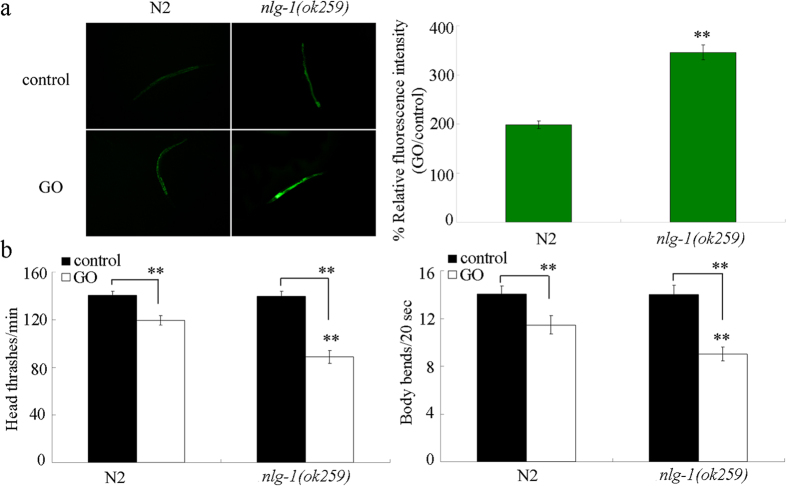
We next used the loss-of-function mutant of nlg-1(ok259) to investigate the effects of nlg-1 mutation on the formation of GO toxicity. Under the normal condition, we detected an obvious induction of intestinal reactive oxygen species (ROS) production in nlg-1(ok259) mutant (Fig. 2a). After GO (1 mg/L) exposure, a more significant induction of intestinal ROS production was observed in nlg-1(ok259) mutant compared with that in wild-type N2 nematodes (Fig. 2a). Under the normal condition, we observed the normal locomotion behaviors of head thrash and body bend in nlg-1(ok259) mutant (Fig. 2b). After GO (1 mg/L) exposure, a significantly decreased head thrash or body bend was found in nlg-1(ok259) mutant compared with that in wild-type nematodes (Fig. 2b). Therefore, a susceptible property to GO toxicity may be formed in loss-of-function nlg-1 mutant nematodes.
Figure 2. Mutation of nlg-1 gene induced a susceptible property to GO toxicity.
(a) Mutation of nlg-1 gene induced a susceptible property to GO toxicity in inducing intestinal ROS production. (b) Mutation of nlg-1 gene induced a susceptible property to GO toxicity in decreasing locomotion behavior. Prolonged exposure was performed from L1-larvae to young adults. GO exposure concentration was 1 mg/L. Bars represent means ± SD. **P < 0.01 vs N2 (if not specially indicated).
Neuron-specific activities of NLG-1 in the regulation of response to GO exposure
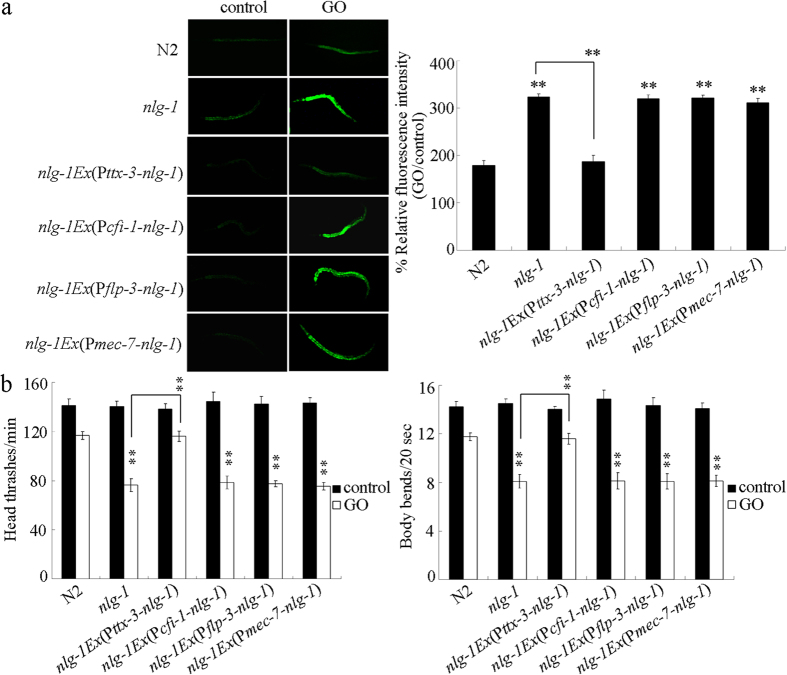
In C. elegans, NLG-1 is observed to be expressed in neurons, including PVD sensory neurons, URB and AIY interneurons, and URA motor neurons28. Using the corresponding promoter for AIY, URA, URB, or PVD neurons, we investigated the neuron-specific activities of NLG-1 in the regulation of response to GO exposure. After GO (1 mg/L) exposure, we found that expression of the NLG-1 in the AIY interneurons could suppress the susceptible property to GO toxicity in inducing intestinal ROS production and in decreasing locomotion behavior in nlg-1(ok259) mutant nematodes (Fig. 3). In contrast, after GO (1 mg/L) exposure, exposure of the NLG-1 in the PVD sensory neurons, the URB interneurons, or the URA motor neurons could not obviously affect the susceptible property to GO toxicity in inducing intestinal ROS production and in decreasing locomotion behavior in nlg-1(ok259) mutant nematodes (Fig. 3). These results suggest that NLG-1 can act in the AIY interneurons to regulate the response to GO exposure.
Figure 3. Neuron-specific activities of NLG-1 in regulating response to GO exposure.
(a) Neuron-specific activities of NLG-1 in regulating GO toxicity in inducing intestinal ROS production. (b) Neuron-specific activities of NLG-1 in regulating GO toxicity in decreasing locomotion behavior. Prolonged exposure was performed from L1-larvae to young adults. GO exposure concentration was 1 mg/L. Bars represent means ± SD. **P < 0.01 vs N2 (if not specially indicated).
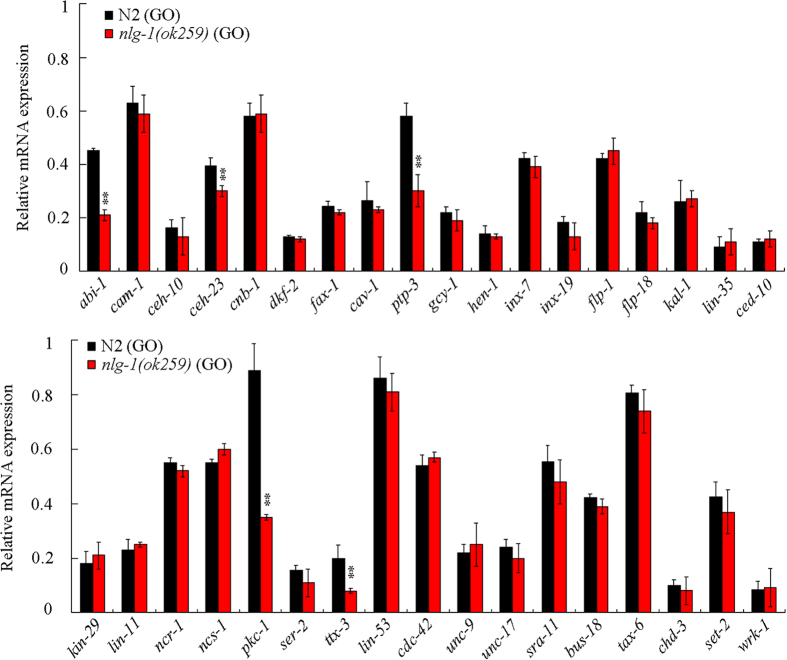
Mutation of nlg-1 gene dysregulated the expression of some genes expressed in the AIY interneurons in GO exposed nematodes
To determine the molecular targets for NLG-1 in AIY interneurons in the regulation of response to GO exposure, we investigated the expression patterns of genes expressed in the AIY interneurons (http://legacy.wormbase.org/db/searches/expr_search#results) in GO exposed wild-type and GO exposed nlg-1(ok259) mutant nematodes. After GO (1 mg/L) exposure, among the examined 35 genes expressed in the AIY interneurons, we found that mutation of nlg-1 gene significantly decreased the expression levels of abi-1, ceh-23, ptp-3, pkc-1, and ttx-3 (Fig. 4). In C. elegans, abi-1 encodes an Abl interactor, ceh-23 encodes a homeodomain protein, ptp-3 encodes a receptor-like tyrosine phosphatase, pkc-1 encodes a serine/threonine protein kinase C (PKC) protein, and ttx-3 encodes a LIM homeodomain protein.
Figure 4. Effects of nlg-1 mutation on expression patterns of genes expressed in AIY interneurons in GO exposed nematodes.
Prolonged exposure was performed from L1-larvae to young adults. GO exposure concentration was 1 mg/L. Bars represent means ± SD. **P < 0.01 vs N2 (GO).
PKC-1 was involved in the control of response to GO exposure
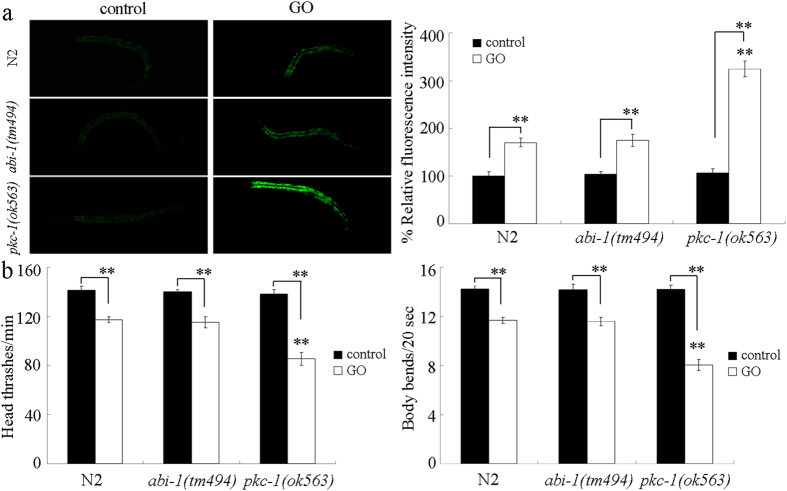
Considering the fact that ttx-3, ceh-23, and ptp-3 genes are required for the cell fate specification of AIY interneurons or the presynaptic differentiation in C. elegans38,39, we next examined whether ABI-1 and PKC-1 are involved in the control of response to GO exposure. PKC family plays critical roles in cell signaling in organisms40. In C. elegans, PKC-1 plays crucial roles in regulating the cell signaling and function of nervous system41,42. abi-1 gene is required for the proper cell migration and apoptotic cell engulfment43,44. After GO (1 mg/L) exposure, we found that mutation of pkc-1 gene induced a susceptible property to GO toxicity in inducing intestinal ROS production and in decreasing locomotion behavior (Fig. 5). In contrast, after GO (1 mg/L) exposure, mutation of abi-1 gene did not obviously affect the GO toxicity in inducing intestinal ROS production and in decreasing locomotion behavior (Fig. 5). Therefore, our results suggest that PKC-1 may act a downstream target for NLG-1 in AIY interneurons to regulate the response to GO exposure.
Figure 5. Effects of abi-1 or pkc-1 mutation on GO toxicity.
(a) Effects of abi-1 or pkc-1 mutation on GO toxicity in inducing intestinal ROS production. (b) Effects of abi-1 or pkc-1 mutation on GO toxicity in decreasing locomotion behavior. Prolonged exposure was performed from L1-larvae to young adults. GO exposure concentration was 1 mg/L. Bars represent means ± SD. **P < 0.01 vs N2 (if not specially indicated).
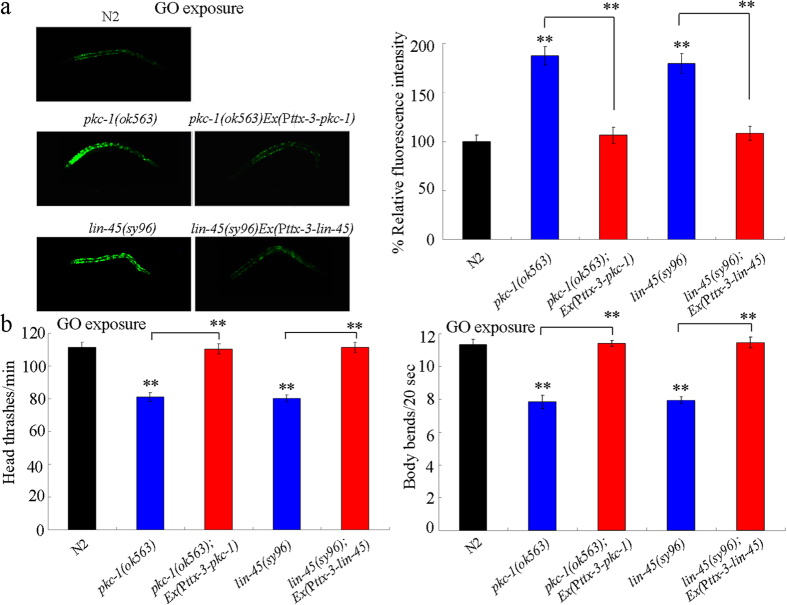
PKC-1 acted downstream of NLG-1 in the AIY interneurons to regulate the response to GO exposure
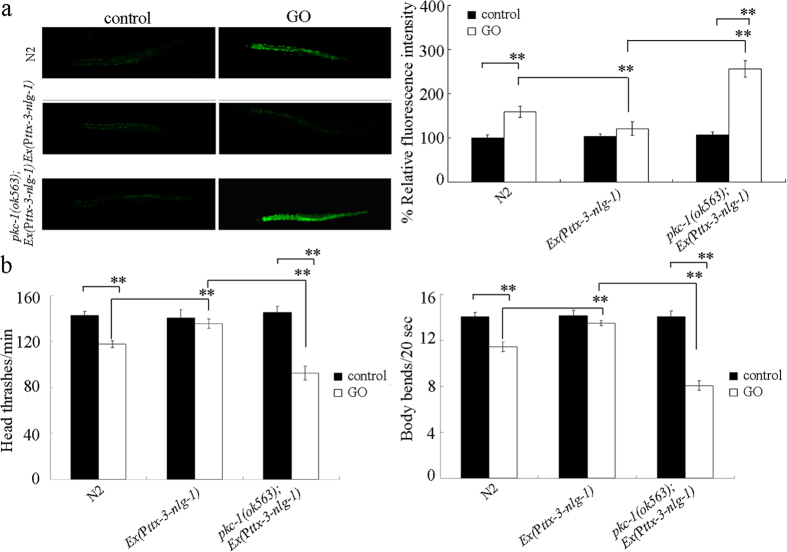
To confirm whether PKC-1 could act downstream of NLG-1 in the AIY interneurons to regulate the response to GO exposure, nlg-1 gene was overexpressed in the AIY interneurons of pkc-1(ok563) mutant nematodes. Using intestinal ROS production and locomotion behavior as the endpoints, we observed that overexpression of nlg-1 gene in the AIY interneurons resulted in a resistant property to GO (1 mg/L) toxicity in inducing intestinal ROS production and in decreasing locomotion behavior (Fig. 6). However, after GO (1 mg/L) exposure, mutation of pkc-1 gene obviously suppressed the resistant property of transgenic nematodes overexpressing NLG-1 in the AIY interneurons to GO toxicity in inducing intestinal ROS production and in decreasing locomotion behavior (Fig. 6). These results suggest that, in the AIY interneurons, PKC-1 can act downstream of NLG-1 to regulate the response to GO exposure.
Figure 6.
Effects of pkc-1 mutation on the induction of intestinal ROS production (a) and the locomotion behavior (b) in GO exposed nematodes overexpressing nlg-1 gene in AIY interneurons. Prolonged exposure was performed from L1-larvae to young adults. GO exposure concentration was 1 mg/L. Bars represent means ± SD. **P < 0.01.
Identification of PKC-1-mediated signaling in the regulation of response to GO exposure
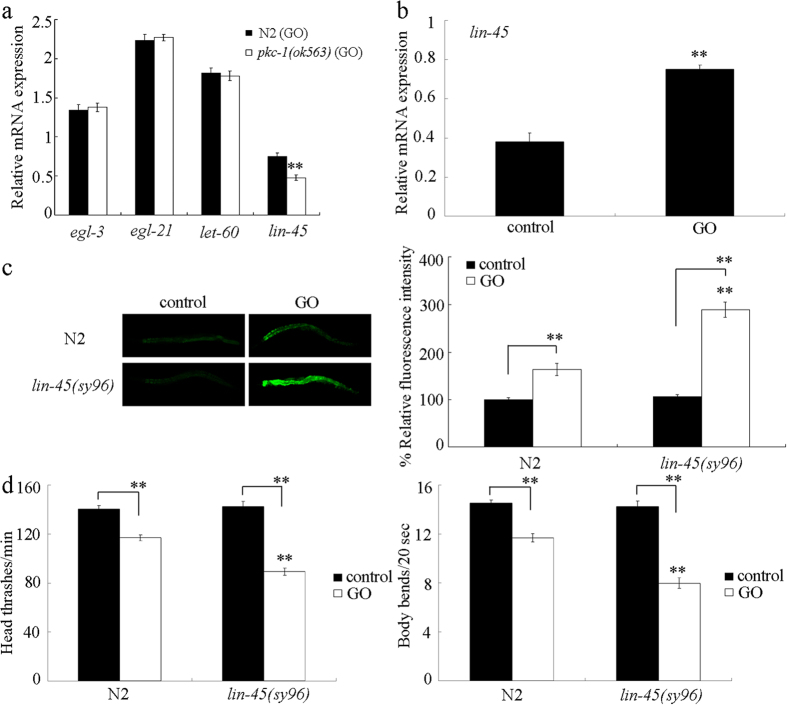
To further identify the functional targets for PKC-1 in the regulation of response to GO exposure, a subset of PKC-1 targets identified in C. elegans were examined42. Among the examined four candidate targeted genes, egl-3 and egl-21 genes encode two proneuropeptide processing enzymes, let-60 gene encodes a Ras protein ERK signaling pathway, and lin-45 gene encodes a Raf protein in ERK signaling pathway. After GO (1 mg/L) exposure, we found that pkc-1 mutation significantly decreased the expression level of lin-45 gene (Fig. 7a). In contrast, after GO (1 mg/L) exposure, we found that pkc-1 mutation did not significantly affect the expression level of egl-3, egl-21, or let-60 gene (Fig. 7a). Meanwhile, in wild-type N2 nematodes, GO (1 mg/L) exposure significantly increased the expression of lin-45 gene (Fig. 7b). These results imply that lin-45 may act as a functional targeted gene for pkc-1 in the regulation of response to GO exposure.
Figure 7. Identification of candidate targets for PKC-1 in regulating response to GO exposure.
(a) Effects of pkc-1 mutation on expression of egl-3, egl-21, let-60, and lin-45 in GO exposed nematodes. Bars represent means ± SD. **P < 0.01 vs N2 (GO). (b) Effect of GO exposure on lin-45 expression in wild-type nematodes. Bars represent means ± SD. **P < 0.01 vs control. (c) Effect of lin-45 mutation on GO toxicity in inducing intestinal ROS production. Bars represent means ± SD. **P < 0.01 vs N2 (if not specially indicated). (d) Effect of lin-45 mutation on GO toxicity in decreasing locomotion behavior. Bars represent means ± SD. **P < 0.01 vs N2 (if not specially indicated). Prolonged exposure was performed from L1-larvae to young adults. GO exposure concentration was 1 mg/L.
Mutation of lin-45 induced a susceptible property to GO toxicity
Using intestinal ROS production and locomotion behavior as the endpoints, after GO (1 mg/L) exposure, we found that mutation of lin-45 gene caused the formation of a susceptible property to GO toxicity in inducing intestinal ROS production and in decreasing locomotion behavior (Fig. 7c,d). Therefore, mutation of lin-45 may induce a susceptible property to GO toxicity.
LIN-45 acted downstream of PKC-1 in the AIY interneurons to regulate the response to GO exposure
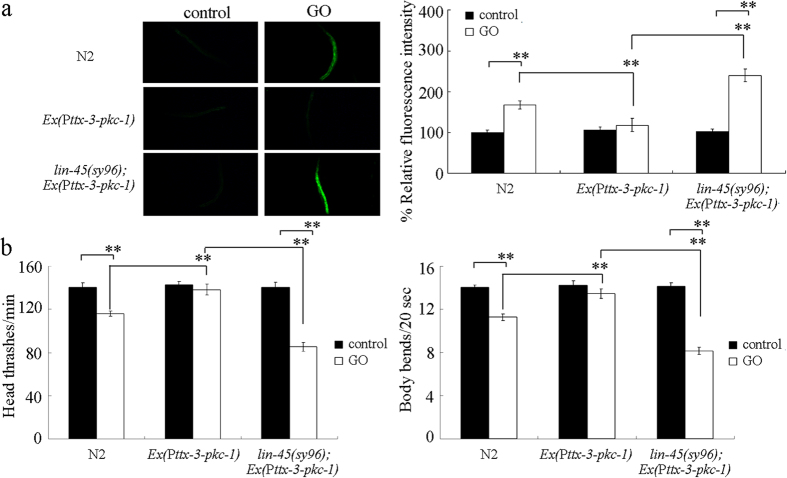
To confirm whether LIN-45 could act downstream of PKC-1 in the AIY interneurons to regulate the response to GO exposure, pkc-1 gene was overexpressed in the AIY interneurons in lin-45(sy96) mutant nematodes. Using intestinal ROS production and locomotion behavior as the endpoints, we observed that overexpression of pkc-1 gene in the AIY interneurons induced in a resistant property to GO (1 mg/L) toxicity in inducing intestinal ROS production and in decreasing locomotion behavior (Fig. 8). However, after GO (1 mg/L) exposure, mutation of lin-45 gene noticeably inhibited the resistant property of transgenic nematodes overexpressing PKC-1 in the AIY interneurons to GO toxicity in inducing intestinal ROS production and in decreasing locomotion behavior (Fig. 8). Therefore, LIN-45 may act downstream of PKC-1 in the AIY interneurons to regulate the response to GO exposure.
Figure 8.
Effects of lin-45 mutation on the induction of intestinal ROS production (a) and the locomotion behavior (b) in GO exposed nematodes overexpressing pkc-1 gene in the AIY interneurons. Prolonged exposure was performed from L1-larvae to young adults. GO exposure concentration was 1 mg/L. Bars represent means ± SD. **P < 0.01.
PKC-1 and LIN-45 could act in the AIY interneurons to regulate response to GO exposure
In C. elegans, both pkc-1 and lin-45 are expressed in the neurons. After GO (1 mg/L) exposure, we found that expression of pkc-1 gene in the AIY interneurons could effectively suppress the susceptible property of pkc-1(ok563) mutant to GO toxicity in inducing intestinal ROS production and in decreasing locomotion behavior (Fig. 9). Similarly, after GO (1 mg/L) exposure, expression of lin-45 gene in the AIY interneurons could also significantly inhibit the susceptible property of lin-45(sy96) mutant to GO toxicity in inducing intestinal ROS production and in decreasing locomotion behavior (Fig. 9). These results imply that PKC-1 and LIN-45 can function in the AIY interneurons to regulate response to GO exposure.
Figure 9. Rescue assay for the function of PKC-1 or LIN-45 expressed in the AIY interneurons in the regulation of response to GO exposure.
(a) Rescue assay for the function of PKC-1 or LIN-45 expressed in the AIY interneurons in regulating GO toxicity in inducing intestinal ROS production. (b) Rescue assay for the function of PKC-1 or LIN-45 expressed in the AIY interneurons in regulating GO toxicity in decreasing locomotion behavior. Prolonged exposure was performed from L1-larvae to young adults. GO exposure concentration was 1 mg/L. Bars represent means ± SD. **P < 0.01 vs N2 (if not specially indicated).
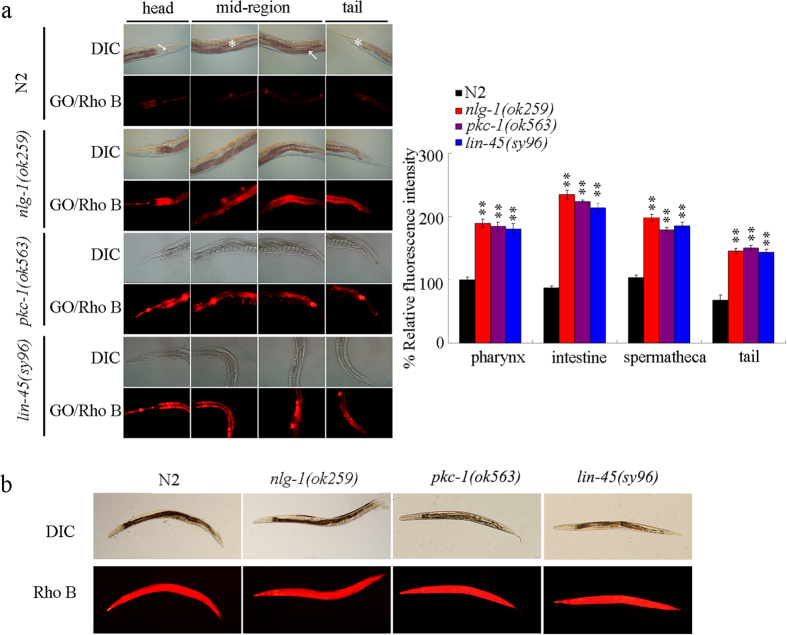
Distribution and translocation of GO in the body of nlg-1, pkc-1, and lin-45 mutant nematodes
Distribution and translocation are crucial for the toxicity formation of certain ENMs including the GO in nematodes17,45. In this study, GO was labeled with molecular probe Rhodamine B (Rho B). After GO/Rho B (1 mg/L) exposure, we observed that mutation of nlg-1, pkc-1, or lin-45 gene significantly increased the distribution of GO/Rho B in the pharynx, the intestine, the spermatheca, and the tail of nematodes compared with wild-type N2 nematodes (Fig. 10a). Compared with distribution of GO/Rho B, exposure to Rho B resulted in a relatively equable distribution of Rho B fluorescence in tissues of wild-type, nlg-1(ok259), pkc-1(ok5630), or lin-45(sy96) mutant nematodes (Fig. 10b). These data suggest that mutation of genes encoding the NLG-1-PKC-1-LIN-45 signaling pathway may affect both the toxicity and the translocation of GO.
Figure 10. Distribution and translocation of GO in wild-type, nlg-1(ok259), pkc-1(ok563), and lin-45(sy96) mutant nematodes.
(a) Distribution of GO/Rho B in wild-type, nlg-1(ok259), pkc-1(ok563), and lin-45(sy96) mutant nematodes. The arrowheads indicate the pharynx and intestine, respectively, in the head region or mid-region in nematodes. The spermatheca (*) in the mid-region and tail (**) are also indicated. Prolonged exposure was performed from L1-larvae to young adults. GO exposure concentration was 1 mg/L. Bars represent means ± SD. **P < 0.01 vs N2. (b) Distribution of Rho B in wild-type, nlg-1(ok259), pkc-1(ok563), and lin-45(sy96) mutant nematodes.
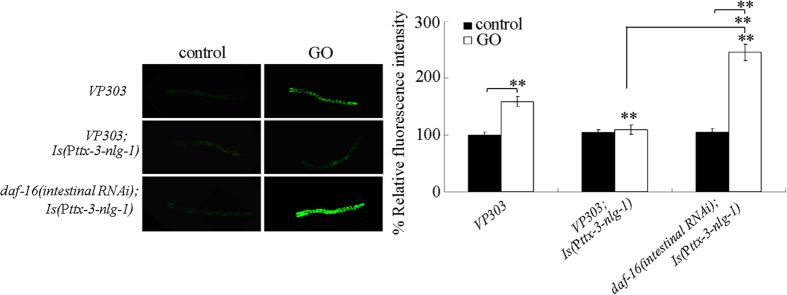
Genetic interaction between NLG-1 and DAF-16 in the regulation of response to GO exposure
Previous study has demonstrated that the insulin signaling could function in the intestinal cells to regulate the GO toxicity25. In the insulin signaling pathway, daf-16 gene encodes a FOXO transcriptional factor, and intestinal RNA interference (RNAi) knockdown of daf-16 gene induced a susceptible property to GO toxicity25. Transgenic strain of VP303 is a powerful tool for the intestine-specific RNAi in nematodes46. Under the normal condition, we observed that overexpression of nlg-1 gene in the AIY interneurons in VP303 did not induce the obvious induction of intestinal ROS production (Fig. 11). After GO (1 mg/L) exposure, VP303 with the overexpression of nlg-1 gene in the AIY interneurons also did not show the noticeable induction of intestinal ROS production (Fig. 11), suggesting the resistant property of the transgenic strain of Is(Pttx-3-nlg-1) to GO toxicity. Moreover, we found that intestinal RNAi knockdown of daf-16 gene caused the significant induction of intestinal ROS production in GO (1 mg/L) exposed transgenic strain of Is(Pttx-3-nlg-1) (Fig. 11), implying that the neuronal NLG-1 may act upstream of DAF-16 to regulate response to GO exposure.
Figure 11. Genetic interaction between NLG-1 and DAF-16 in regulating response to GO exposure.
Prolonged exposure was performed from L1-larvae to young adults. GO exposure concentration was 1 mg/L. Bars represent means ± SD. **P < 0.01 vs VP303 (if not specially indicated).
Discussion
In C. elegans, previous study has suggested that mutation of nlg-1 gene could cause the deficits in a subset of sensory behaviors, such as thermosensation, sensory processing, and longevity35,47, implying that NLG-1 is required for the control of functions of neurons and longevity. In this study, we observed that GO exposure could potentially decrease the expression of NLG-1 (Fig. 1). Meanwhile, our previous studies have demonstrated that GO exposure could decrease the locomotion behavior and reduce the lifespan17,23. Therefore, the reduction of NLG-1 expression may serve as an important contributor to the observed GO toxicity.
Previous studies have suggested that mutation of nlg-1 gene was sensitive to paraquat, a commonly used ROS generator35,36,37. In this study, using the intestinal ROS production and locomotion behavior as the endpoints, we found that mutation of nlg-1 gene induced a susceptible property to GO toxicity (Fig. 2). It was reported that GO exposure could induce the activation of oxidative stress17,23. Compared with the intestinal ROS production in GO exposed wild-type N2 nematodes, the more severe induction of intestinal ROS production in GO exposed nlg-1 mutant nematodes further supports the important role of NLG-1 in inducing the oxidative stress in GO exposed nematodes. Together, these results suggest that NLG-1 is required for the induction of both oxidative stress and stress response.
In C. elegans, NLG-1 is expressed in both the neurons and the muscle35. Because muscle-specific RNAi of nlg-1 did not obviously affect the GO toxicity (data not shown), in this study, we focused on the examination of neuron-specific activity of NLG-1 in the regulation of response to GO exposure. For the neuron-specific activities of NLG-1 in the regulation of response to GO exposure, we found that the activity of NLG-1 in the AIY interneurons was required for the induction of GO toxicity in inducing intestinal ROS production and in decreasing locomotion behavior (Fig. 3). Nevertheless, not the activity of NLG-1 in all the interneurons was required for the induction of GO toxicity, since the expression of NLG-1 in the URB interneurons did not obviously affect the susceptible property of nlg-1(ok259) mutant to GO toxicity (Fig. 3). In this study, with the aid of mec-7 promoter, we observed that the expression of NLG-1 in the PVD sensory neurons did not noticeably influence the susceptible property of nlg-1(ok259) mutant to GO toxicity (Fig. 3). In C. elegans, mec-7 promoter can also drive the genes to be expressed in touch receptor neurons (ALML/R, AVM, PVM, and PLML/R)48, which implies that the activity of NLG-1 in touch sensory neurons may be not required for the induction of GO toxicity. In this study, we also observed that the expression of NLG-1 in the URA motor neurons did not significantly affect the susceptible property of nlg-1(ok259) mutant to GO toxicity (Fig. 3). Considering the fact that both VA and DA are only required for the control of locomotion behavior in nematodes49, we did not examine the activities of NLG-1 in these motor neurons.
In this study, we identified the PKC-1 as a downstream molecular target for NLG-1 in the AIY interneurons to regulate the response to GO exposure. At least three lines of evidence were raised about this. Firstly, after GO exposure, mutation of nlg-1 gene could alter the expression pattern of pkc-1 gene (Fig. 4). Secondly, mutation of pkc-1 gene could induce a susceptible property to GO toxicity (Fig. 5), which was similar to those observed in nlg-1 mutant nematodes. Thirdly, expression of pkc-1 in the AIY interneurons could reverse the susceptible property of pkc-1(ok563) mutant to GO toxicity (Fig. 9). More importantly, mutation of pkc-1 gene could suppress the resistant property to GO toxicity in transgenic strain overexpressing nlg-1 gene in AIY interneurons (Fig. 6). Therefore, NLG-1 may potentially act upstream of PKC-1 in the AIY interneurons to regulate response to GO exposure.
In C. elegans, TTX-3 and CEH-23 control the cell fate specification of AIY interneurons38,50. PTP-3 contains extracellular immunoglobulin-like and fibronectin type III domains and functions in the cell adhesion and the maintenance of presynaptic differentiation39. The observed decrease in the expression of ttx-3, ceh-23, and ptp-3 genes in GO exposed nlg-1(ok259) mutant also implies the possible formation of deficits in the differentiation and development of the AIY interneurons.
In this study, we further identified the potential targeted genes for pkc-1 in the regulation of response to GO exposure. Previous studies have suggested that PKC-1 is required for the secretion of neuropeptides41. However, after GO exposure, mutation of pkc-1 did not alter the expression patterns of egl-3 and egl-21 genes encoding proneuropeptide processing enzymes (Fig. 7a), which implies that the observed susceptible property to GO toxicity in pkc-1 mutant was not due to the altered neuropeptide release. After GO exposure, mutation of pkc-1 gene altered the expression of lin-45 encoding a Raf protein (Fig. 7a), implying that the observed susceptible property to GO toxicity in pkc-1 mutant was due to the altered function of ERK signaling. However, mutation of pkc-1 gene did not affect the expression of let-60 encoding a Ras protein (Fig. 7a), suggesting that not the function of entire ERK signaling pathway was altered in GO exposed pkc-1 mutant. The GO toxicity in lin-45 mutant was similar to that in pkc-1 mutant (Fig. 7c,d). Moreover, lin-45 gene mutation could suppress the resistant property of transgenic nematodes overexpressing PKC-1 in the AIY interneurons to GO toxicity (Fig. 8). Therefore, PKC-1 may function upstream of LIN-15 in the AIY interneurons in the regulation of response to GO exposure. Moreover, we observed that pkc-1 mutation could decrease the lin-45, while lin-45 gene could be significantly induced by GO exposure in wild-type N2 nematodes (Fig. 7a and b). These results imply that mutation of pkc-1 may suppress the protection function of LIN-45-mediated ERK signaling against the GO toxicity in nematodes.
Our results further demonstrated that mutation of genes encoding the NLG-1-PKC-1-LIN-45 signaling pathway may affect the GO translocation. Compared with GO/Rho B exposed wild-type nematodes, we observed the increased accumulation of GO/Rho B in the pharynx, the intestine, the spermatheca, and the tail in nlg-1, pkc-1, or lin-45 mutant nematodes (Fig. 10a). Meanwhile, a relatively equable distribution of Rho B fluorescence was formed in tissues of wild-type N2, nlg-1(ok259), pkc-1(ok5630), or lin-45(sy96) mutant nematodes (Fig. 10b), implying that no noticeable alteration in intestinal permeability was formed in the nlg-1(ok259), pkc-1(ok5630), or lin-45(sy96) mutant compared with wild-type N2 nematodes under the normal condition. It was reported that an elevated levels of oxidized proteins were formed in nlg-1 mutant nematodes35. Therefore, the formed oxidative damage may induce a susceptible property of nlg-1 mutant to environmental toxicants; however, the formed oxidative damage may be not enough to alter the intestinal permeability in nlg-1 mutant nematodes under the normal condition. In contrast to this, after GO exposure, mutation of nlg-1, pkc-1, or lin-45 gene may induce a susceptibility to GO toxicity, which in turn cause the enhanced distribution and translocation of GO in the tissues of nematodes.
Our previous study has identified the insulin signaling as an intestinal signaling to regulate the GO toxicity in nematodes25. In this study, the NLG-1 has been shown to mediate a neuronal signaling in the regulation of response to GO exposure. Moreover, we found that intestinal RNAi knockdown of daf-16 gene encoding the FOXO transcriptional factor in the insulin signaling pathway suppressed the resistant property of transgenic strain overexpressing NLG-1 in the AIY interneurons to GO toxicity (Fig. 11), which suggests that DAF-16 may act downstream of NLG-1 to regulate the response to GO exposure. More importantly, this observation implies the possible existence of an important link between the neuronal NLG-1 signaling and the intestinal insulin signaling. That is, during the response to GO exposure, the neuronal NLG-1 signaling may regulate the functions of certain intestinal signaling pathways such as the intestinal insulin signaling pathway.
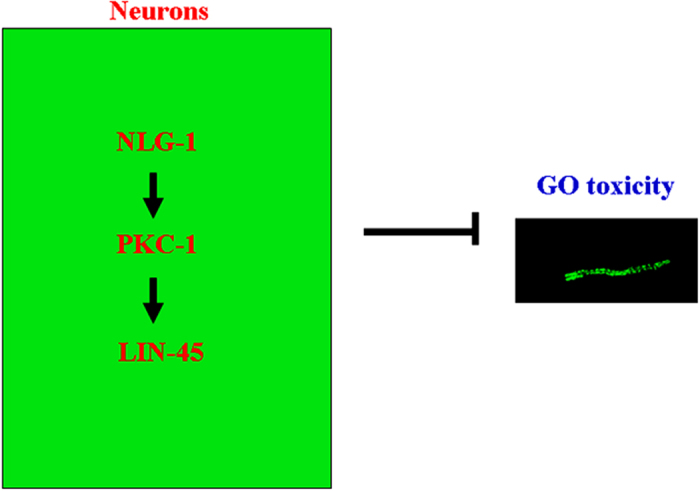
In conclusion, using the in vivo assay system of C. elegans, we here investigated the effect of GO exposure on NLG-1-mediated signaling in neurons. In nematodes, GO exposure significantly decreased the NLG-1 expression, and mutation of nlg-1 gene induced a susceptible property to GO toxicity, suggesting the crucial role of NLG-1 in the induction of GO toxicity. NLG-1 could act in certain interneurons such as the AIY interneurons to regulate the response to GO exposure. In the AIY interneurons, NLG-1 could function upstream of PKC-1, and PKC-1 further acted upstream of LIN-45 to regulate the response to GO exposure. Thus, the NLG-1-PKC-1-LIN-45 signaling cascade was raised in the AIY interneurons to be required for induction of GO toxicity (Fig. 12). Therefore, our results provide the important neuronal basis for the induction of GO toxicity in nematodes. Moreover, our data implies the important link between the neuronal NLG-1 signaling and the intestinal insulin signaling during the control of response to GO exposure.
Figure 12. A diagram showing the role of neuronal NLG-1-PKC-1-LIN-45 signaling cascade in the induction of GO toxicity in nematodes.
In the clinical, mutations of human neuroligin 3 or neuroligin 4 gene are associated with autism spectrum disorders (ASDs)51,52. Moreover, it has been shown that certain mutations in human neuroligin 4 are also associated with other developmental disorders of the nervous system, such as X-linked mental retardation, Tourette syndrome, and schizophrenia34,53,54. The findings in this study implies that the related patients with the mutations of neuroligin 3 or neuroligin 4 gene may need to pay attention to the influences of environmental toxicants, such as the ENMs.
Methods
GO preparation and characterization
According to a modified Hummer’s method, the GO was prepared from a natural graphite powder55. In a 250-mL flask, graphite (2 g) and sodium nitrate (1 g) were first added, and the concentrated H2SO4 (50 mL) was then added on ice, followed by the addition of KMnO4 (7 g). After temperature of the mixture reached to 35 °C, H2O (90 mL) was slowly dripped and stirred at 70 °C for 15 min to dilute the suspension. After treatment with a mixture of 7 mL of 30% H2O2 and 55 mL of H2O, the suspension was then filtered in order to obtain a yellow-brown filter cake. After washing with a solution of 3% HCl, the filter cake was further dried at 40 °C for 24 h. Finally, the GO would be obtained after the ultrasonication of as-made graphite oxide for 1 h.
To determine the physicochemical properties, GO was characterized by Raman spectroscopy, atomic force microscopy (AFM), and zeta potential. To perform the AFM assay, GO suspension was pipetted onto the Si substrates, air-dried, and then examined under the AFM tip (SPM-9600, Shimadzu, Japan). The GO thickness was approximately 1.0 nm based on the AFM assay, which implied the one layer property for the prepared GO (Fig. S1a). Sizes of most of the examined GO after sonication (40 kHz, 100 W, 30 min) were in the range of 40–50 nm (Fig. S1b). Raman spectroscopy was examined using a 632 nm wavelength excitation (Renishaw Invia Plus laser Raman spectrometer, Renishaw, UK). Raman spectroscopy assay indicated that GO had a G band at 1594 cm−1 and a D band at 1335 cm−1 (Fig. S1c). Zeta potential was examined by a Nano Zetasizer (Malvern Instrument Ltd. UK) using a dynamic light scattering (DLS) technique. Zeta potential of GO (100 mg/L) in K-medium was −21.3 ± 2.1 mV.
C. elegans strains and GO exposure
Nematodes used were wild-type N2, mutants of nlg-1(tm1961), pkc-1(ok563), abi-1(tm494), and lin-45(sy96), and transgenic strains of Ex(Pnlg-1-nlg-1::GFP), nlg-1(tm1961)Ex(Pttx-3-nlg-1), nlg-1(tm1961)Ex(Pcfi-1-nlg-1), nlg-1(tm1961)Ex(Pflp-3-nlg-1), nlg-1(tm1961)Ex(Pmec-7-nlg-1), Ex(Pttx-3-nlg-1), pkc-1(ok563);Ex(Pttx-3-nlg-1), Ex(Pttx-3-pkc-1), lin-45(sy96);Ex(Pttx-3-pkc-1), pkc-1(ok563)Ex(Pttx-3-pkc-1), lin-45(sy96)Ex(Pttx-3-lin-45), VP303/kbIs7[nhx-2p::rde-1], Is(Pttx-3-nlg-1), and daf-16(intestinal RNAi);Is(Pttx-3-nlg-1). The wild-type and mutant strains were obtained from the Caenorhabditis Genetics Center (funded by NIH Office of Research Infrastructure Programs (P40 OD010440)). Nematodes were normally maintained at 20 °C as described13. After washing off from the plates into centrifuge tubes, the gravid nematodes were lysed with a bleaching mixture (0.45 M NaOH, 2% HOCl) to obtain the age synchronous L1-larvae populations as described56.
GO at the working concentrations (1, 10, and 100 mg/L) was prepared by diluting the stock solution (1 mg/mL) with K medium after sonication (40 kHz, 100 W, 30 min). Prolonged exposure was performed from L1-larvae to young adults, and GO exposure was performed in the wells of 12-well sterile tissue culture plates at 20 °C with the addition of food (OP50).
Toxicity assessment of GO
The intestinal ROS production was used to reflect the functional state of intestinal cells57. The intestinal ROS production was analyzed as described previously58,59. The nematodes were transferred into 1 μM 5′,6′-chloromethyl-2′,7′-dichlorodihydro-fluorescein diacetate (CM-H2DCFDA; Molecular Probes) to incubate for 3 h at 20 °C in the dark. After that, the nematodes were mounted onto a 2% agar pad for the examination at 510 nm of emission filter and 488 nm of excitation wavelength under a laser scanning confocal microscope (Leica, TCS SP2, Bensheim, Germany). Relative intestinal fluorescence intensity was semi-quantified. After normalization to the intestinal autofluorescence, the semiquantified ROS signals were expressed as relative fluorescence units (RFU). Three replicates were performed, and twenty nematodes were examined per treatment.
The locomotion behaviors of head thrash and body bend were used to reflect the functional state of motor neurons in nematodes60. These locomotion behaviors were analyzed under the dissecting microscope by eyes as described61,62. The head thrash has been defined as a change for the bending direction at the mid body. The body bend has been defined as a change for the direction of the part corresponding to posterior bulb of pharynx along the y axis, assuming that nematodes were traveling along the x axis. Three replicates were performed, and twenty nematodes were examined per treatment.
GO distribution and translocation assay
To examine the GO translocation and distribution, Rho B was loaded on the GO by mixing a Rho B solution (1 mg/mL, 0.3 mL) with an aqueous GO suspension (0.1 mg/mL, 5 mL) as described45. The unbound Rho B would be removed using dialysis against the distilled water over 72 h. The prepared GO/Rho B was stored at 4 °C. Nematodes were incubated with GO/Rho B for 3 h. After washing with three times of M9 buffer, the nematodes were analyzed under a laser scanning confocal microscope (Leica, TCS SP2, Bensheim, Germany). In this study, the Rho B staining alone was used as a control.
Reverse-transcription and quantitative real-time polymerase chain reaction (qRT-PCR) assay
Total nematode RNAs were extracted using an RNeasy Mini kit (Qiagen). After the RNA extraction, the RNAs were reverse transcribed using a PrimeScriptTM RT reagent kit (Takara, Otsu, Shiga, Japan) to obtain the cDNA. The real-time qRT-PCR was performed using a SYBR Premix Ex Taq™ (Takara) for the aim of amplifying PCR products. Real-time qRT-PCR was run at an optimized annealing temperature of 58 °C. The relative quantification of certain targeted gene in comparison to the reference tba-1 gene encoding a tubulin protein was determined. The final results would be expressed as the relative expression ratio between the certain targeted gene and the reference tba-1 gene. Three replicates were performed. The related primer information for qRT-PCR is shown in Table S1.
DNA constructs and germline transformation
To generate entry vector carrying promoter sequence, promoter region for ttx-3 gene specially expressed in the AIY interneurons, cfi-1 gene expressed in the URA neurons, flp-3 gene expressed in the URB neurons, or mec-7 gene expressed in the PVD neurons was amplified by PCR from wild-type C. elegans genomic DNA. These amplified promoter fragments were inserted into the pPD95_77 vector in the sense orientation. nlg-1/C40C9.5e, pkc-1/F57F5.5a, or lin-45/Y73B6A.5b cDNA was amplified by PCR, and further inserted into the corresponding entry vector carrying ttx-3, cfi-1, flp-3, or mec-7 promoter. Germline transformation was performed by coinjecting a testing DNA (10–40 μg/mL) and a marker DNA of Pdop-1::rfp (60 μg/mL) into the gonad of nematodes as described63. The related primer information for DNA constructs is shown in Table S2.
RNAi assay
RNAi was performed by feeding nematodes with certain E. coli strain HT115 (DE3) expressing a double-stranded RNA that is homologous to the targeted gene of daf-16 as described64. E. coli HT115 (DE3) was first grown in the LB broth containing ampicillin (100 μg/mL) at 37 °C overnight, and then plated onto the NGM plates containing ampicillin (100 μg/mL) and isopropyl 1-thio-β-D-galactopyranoside (IPTG, 5 mM). The L2 larvae were transferred onto the RNAi plates, and let to develop into the gravid. The gravid adults were further transferred onto a fresh RNAi plate to lay eggs for 2 h so as to obtain the second generation of RNAi population. The laid eggs were allowed to develop into the young adults for the subsequent assays.
Statistical analysis
Data were expressed as means ± standard deviation (SD). We performed the statistical analysis using a SPSS 12.0 software (SPSS Inc., Chicago, USA), and the differences between the groups were further determined using an analysis of variance (ANOVA). The probability level of 0.05 or 0.01 was considered to be statistically significant.
Additional Information
How to cite this article: Chen, H. et al. Graphene Oxide Dysregulates Neuroligin/NLG-1-Mediated Molecular Signaling in Interneurons in Caenorhabditis elegans. Sci. Rep. 7, 41655; doi: 10.1038/srep41655 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Footnotes
The authors declare no competing financial interests.
Author Contributions Conceived and designed the experiments: D.W. Performed the experiments and analyzed the data: H.C. and H.L. Wrote the paper: D.W.
References
- Geim A. K. Graphene: status and prospects. Science 324, 1530–1534 (2009). [DOI] [PubMed] [Google Scholar]
- Bitounis D., Ali-Boucetta H., Hong B. H., Min D. & Kostarelos K. Prospects and challenges of graphene in biomedical applications. Adv. Mater. 25, 2258–2268 (2013). [DOI] [PubMed] [Google Scholar]
- Chang Y. et al. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 200, 201–210 (2011). [DOI] [PubMed] [Google Scholar]
- Yang K., Li Y., Tan X., Peng R. & Liu Z. Behavior and toxicity of graphene and its functionalized derivatives in biological systems. Small 9, 1492–1503 (2013). [DOI] [PubMed] [Google Scholar]
- Ma Y., Shen H., Tu X. & Zhang Z. Assessment in vivo toxicity of graphene materials: current methods and future outlook. Nanomedicine 9, 1565–1580 (2014). [DOI] [PubMed] [Google Scholar]
- Liang S., Xu S., Zhang D., He J. & Chu M. Reproductive toxicity of nanosclae graphene oxide in male mice. Biomaterials 9, 92–105 (2015). [DOI] [PubMed] [Google Scholar]
- Sydlik S. A., Jhunjhunwala S., Webber M. J., Anderson D. G. & Langer R. In vivo compatibility of graphene oxide with differing oxidation states. ACS Nano 9, 3866–3874 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu G. et al. Graphen oxide induces Toll-like receptor 4 (TLR4)-dependent necrosis in macrophages. ACS Nano 7, 5732–5745 (2013). [DOI] [PubMed] [Google Scholar]
- Chatterjee N., Eom H. & Choi J. A Systems toxicology approach to the surface functionality control of graphene-cell interactions. Biomaterials 35, 1109–1127 (2014). [DOI] [PubMed] [Google Scholar]
- Leung M. C. et al. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol. Sci. 106, 5–28 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.-L., Wu Q.-L., Li Y.-P. & Wang D.-Y. Translocation, transfer, and in vivo safety evaluation of engineered nanomaterials in the non-mammalian alternative toxicity assay model of nematode Caenorhabditis elegans. RSC Adv. 3, 5741–5757 (2013). [Google Scholar]
- Boyd W. A. et al. Developmental effects of the ToxCastTM phase I and II chemicals in Caenorhabditis elegans and corresponding responses in zebrafish, rats, and rabbits. Environ. Health Perspect. 124, 586–593 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.-Y. Biological effects, translocation, and metabolism of quantum dots in nematode Caenorhabditis elegans. Toxicol. Res. 5, 1003–1011 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda-Benitez L. & Olivero-Verbel J. Caenorhabditis elegans, a biological model for research in toxicology. Rev. Environ. Contam. Toxicol. 237, 1–35 (2016). [DOI] [PubMed] [Google Scholar]
- Zhang W. et al. Unraveling stress-induced toxicity properties of graphene oxide and the underlying mechanism. Adv. Mater. 24, 5391–5397 (2012). [DOI] [PubMed] [Google Scholar]
- Wu Q.-L. et al. Contributions of altered permeability of intestinal barrier and defecation behavior to toxicity formation from graphene oxide in nematode Caenorhabditis elegans. Nanoscale 5, 9934–9943 (2013). [DOI] [PubMed] [Google Scholar]
- Yang J.-N., Zhao Y.-L., Wang Y.-W., Wang H.-F. & Wang D.-Y. Toxicity evaluation and translocation of carboxyl functionalized graphene in Caenorhabditis elegans. Toxicol. Res. 4, 1498–1510 (2015). [Google Scholar]
- Zhao Y.-L., Jia R.-H., Qiao Y. & Wang D.-Y. Glycyrrhizic acid, active component from Glycyrrhizae radix, prevents toxicity of graphene oxide by influencing functions of microRNAs in nematode Caenorhabditis elegans. Nanomedicine: Nanotechnol. Biol. Med. 12, 735–744 (2016). [DOI] [PubMed] [Google Scholar]
- Chatterjee N. et al. Screening of toxic potential of graphene family nanomaterials using in vitro and alternative in vivo toxicity testing systems. Environ. Health Toxicol. 30, e2015007 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S. K. et al. Multi-endpoint, high-throughput study of nanomaterial toxicity in Caenorhabditis elegans. Environ. Sci. Technol. 49, 2477–2485 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q.-L., Zhao Y.-L., Li Y.-P. & Wang D.-Y. Molecular signals regulating translocation and toxicity of graphene oxide in nematode Caenorhabditis elegans. Nanoscale 6, 11204–11212 (2014). [DOI] [PubMed] [Google Scholar]
- Wu Q.-L., Zhao Y.-L., Zhao G. & Wang D.-Y. microRNAs control of in vivo toxicity from graphene oxide in Caenorhabditis elegans. Nanomedicine: Nanotechnol. Biol. Med. 10, 1401–1410 (2014). [DOI] [PubMed] [Google Scholar]
- Zhao Y.-L., Wu Q.-L. & Wang D.-Y. A microRNAs-mRNAs network involved in the control of graphene oxide toxicity in Caenorhabditis elegans. RSC Adv. 5, 92394–92405 (2015). [Google Scholar]
- Zhao Y.-L., Yang R.-L., Rui Q. & Wang D.-Y. Intestinal insulin signaling encodes two different molecular mechanisms for the shortened longevity induced by graphene oxide in Caenorhabditis elegans. Sci. Rep. 6, 24024 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.-L., Wu Q.-L. & Wang D.-Y. An epigenetic signal encoded protection mechanism is activated by graphene oxide to inhibit its induced reproductive toxicity in Caenorhabditis elegans. Biomaterials 79, 15–24 (2016). [DOI] [PubMed] [Google Scholar]
- Zhao Y.-L. et al. p38 MAPK-SKN-1/Nrf signaling cascade is required for intestinal barrier against graphene oxide toxicity in Caenorhabditis elegans. Nanotoxicology 10, 1469–1479 (2016). [DOI] [PubMed] [Google Scholar]
- Chatterjee N. et al. A systems toxicology approach reveals the Wnt-MAPK crosstalk pathway mediated reproductive failure in Caenorhabditis elegans exposed to graphene oxide (GO) but not to reduced graphene oxide (rGO). Nanotoxicology, doi: 10.1080/17435390.2016.1267273 (2016). [DOI] [PubMed] [Google Scholar]
- Zhi L.-T., Ren M.-X., Qu M., Zhang H.-Y. & Wang D.-Y. Ligands differentially regulate toxicity and translocation of graphene oxide through different mechanisms in Caenorhabditis elegans. Sci. Rep. 6, 39261 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi L.-T. et al. Graphene oxide induces canonical Wnt/β-catenin signaling-dependent toxicity in Caenorhabditis elegans. Carbon 113, 122–131 (2017). [Google Scholar]
- Yang R.-L., Ren M.-X., Rui Q. & Wang D.-Y. A mir-231-regulated protection mechanism against the toxicity of graphene oxide in nematode Caenorhabditis elegans. Sci. Rep. 6, 32214 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L.-L., Du M., Lin X.-F., Cai T. & Wang D.-Y. Genes required for the functions of olfactory AWA neuron regulate the longevity of Caenorhabditis elegans in an insulin/IGF signaling-dependent fashion. Neurosci. Bull. 26, 91–103 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux G. A. & Ashrafi K. Neural regulatory pathways of feeding and fat in Caenorhabditis elegans. Annu. Rev. Genet. 49, 413–438 (2015). [DOI] [PubMed] [Google Scholar]
- Südhof T. C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J. W. et al. Neuroligin-deficient mutants of C. elegans have sensory processing deficits and are hypersensitive to oxidative stress and mercury toxicity. Dis. Model Mech. 3, 366–376 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z. et al. Neurexin and neuroligin mediate retrograde synaptic inhibition in C. elegans. Science 337, 980–984 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab T. A., Evgrafov O., Knowles J. A. & Sieburth D. Regulation of synaptic nlg-1/neuroligin abundance by the skn-1/Nrf stress response pathway protects against oxidative stress. PLoS Genet. 10, e1004100 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altun-Gultekin Z. F. et al. A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development 128, 1951–1969 (2001). [DOI] [PubMed] [Google Scholar]
- Stryker E. & Johnson K. G. LAR, liprin α and the regulation of active zone morphogenesis. J. Cell Sci. 120, 3723–3728 (2007). [DOI] [PubMed] [Google Scholar]
- Newton A. C. Protein kinase C: structure, function, and regulation. J. Biol. Chem. 270, 28495–28498 (1995). [DOI] [PubMed] [Google Scholar]
- Sieburth D., Madison J. M. & Kaplan J. M. PKC-1 regulates secretion of neuropeptides. Nat. Neurosci. 10, 49–57 (2007). [DOI] [PubMed] [Google Scholar]
- Hyde R., Corkins M. E., Somers G. A. & Hartm A. C. PKC-1 acts with the ERK MAPK signaling pathway to regulate Caenorhabditis elegans mechanosensory response. Gene. Brain Behav. 10, 286–298 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz M. E. et al. Abl kinase inhibits the engulfment of apoptotic cells in Caenorhabditis elegans. PLoS Biol. 7, e99 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K. L. et al. The cell migration molecule UNC-53/NAV2 is linked to the ARP2/3 complex by ABI-1. Development 136, 563–574 (2009). [DOI] [PubMed] [Google Scholar]
- Wu Q.-L. et al. Genome-wide identification and functional analysis of long noncoding RNAs involved in the response to graphene oxide. Biomaterials 102, 277–291 (2016). [DOI] [PubMed] [Google Scholar]
- Wu Q.-L., Zhi L.-T., Qu Y.-Y. & Wang D.-Y. Quantum dots increased fat storage in intestine of Caenorhabditis elegans by influencing molecular basis for fatty acid metabolism. Nanomedicine: Nanotechnol. Biol. Med. 12, 1175–1184 (2016). [DOI] [PubMed] [Google Scholar]
- Shen L.-L., Wang Y. & Wang D.-Y. Involvement of genes required for synaptic function in aging control in C. elegans. Neurosci. Bull. 23, 21–29 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin M., Scott I. M., Way J. C. & Culotti J. G. The mec-7 beta-tubulin gene of Caenorhabditis elegans is expressed primarily in the touch receptor neurons. EMBO J. 11, 2885–2893 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T. et al. An imbalancing act: gap junctions reduce the backward motor circuit activity to bias C. elegans for forward locomotion. Neuron 72, 572–586 (2011). [DOI] [PubMed] [Google Scholar]
- Hu Y.-O., Sun Y., Ye B.-P. & Wang D.-Y. Computational analysis of genetic loci required for amphid structure and functions and their possibly corresponding microRNAs in C. elegans. Neurosci. Bull. 2, 39–20 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S. et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 34, 27–29 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S. et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl. Acad. Sci. USA 105, 1710–1715 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumonnier F. et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am. J. Hum. Genet. 74, 552–557 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M., Südhof T. C. & Biederer T. Synaptic cell adhesion. Cold Spring Harb. Perspect. Biol. 4, a005694 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtyukhova N. I. et al. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 11, 771–778 (1999). [Google Scholar]
- Donkin S. & Williams P. L. Influence of developmental stage, salts and food presence on various end points using Caenorhabditis elegans for aquatic toxicity testing. Environ. Toxicol. Chem. 14, 2139–2147 (1995). [Google Scholar]
- Yang R.-L. et al. Insulin signaling regulates toxicity of traffic-related PM2.5 on intestinal development and function in nematode Caenorhabditis elegans. Toxicol. Res. 4, 333–343 (2015). [Google Scholar]
- Wu Q.-L. et al. Inhibition of ROS elevation and damage on mitochondrial function prevents lead-induced neurotoxic effects on structures and functions of AFD neurons in Caenorhabditis elegans. J. Environ. Sci. 24, 733–742 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang W.-M. et al. Beneficial effects of wheat gluten hydrolysate to extend lifespan and induce stress resistance in nematode Caenorhabditis elegans. PLoS One 8, e74553 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.-P. et al. High concentration of vitamin E decreases thermosensation and thermotaxis learning and the underlying mechanisms in nematode Caenorhabditis elegans. PLoS One 8, e71180 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.-L. et al. Transgenerational effects of traffic-related fine particulate matter (PM2.5) on nematode Caenorhabditis elegans. J. Hazard. Mater. 274, 106–114 (2014). [DOI] [PubMed] [Google Scholar]
- Qiao Y. et al. Full toxicity assessment of Genkwa Flos and the underlying mechanism in nematode Caenorhabditis elegans. PLoS One 9, e91825 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. & Fire A. DNA transformation. Methods Cell. Biol. 4, 8451–482 (1995). [PubMed] [Google Scholar]
- Wu Q.-L., Cao X.-O., Yan D., Wang D.-Y. & Aballay A. Genetic screen reveals link between maternal-effect sterile gene mes-1 and P. aeruginosa-induced neurodegeneration in C. elegans. J. Biol. Chem. 290, 29231–29239 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.