Abstract
Postsurgical relapse remains a common issue for resectable gastric cancer (GC). Here, we investigated the efficacy and safety of an adjuvant treatment combining chemotherapy with cellular immunotherapy (CIT) using autologous natural killer cells, γδT cells, and cytokine‐induced killer cells in the treatment of stage II/III GC. A pilot prospective cohort study was conducted in 169 patients with stage II/III GC who had undergone gastrectomy with D2 lymph node dissection. Patients were assigned into two groups according to the patient choice of treatment, including chemotherapy alone (chemo) or chemotherapy combined with CIT (chemo/CIT). Disease‐free survival (DFS), overall survival (OS), and adverse events were evaluated. Univariate and multivariate Cox models were used to analyze the impact of chemo/CIT on DFS and OS. Kaplan–Meier analysis with the log‐rank test was used to compare the clinical outcome between two groups. Three‐year DFS rate was 60.6% and 74.7% (P = 0.036) and 3‐year OS rate was 64.9% and 83% (P = 0.051) for the chemo and chemo/CIT group, respectively. TNM stage and chemo/CIT were independent prognostic factors for both DFS (for TNM stage, P < 0.001, hazard ratio [HR]: 5.599, 95% confidence interval [CI]: 2.791–11.232; for chemo/CIT, P = 0.013, HR: 0.478, 95% CI: 0.266–0.858) and OS (for TNM stage, P < 0.001, HR: 6.559, 95% CI: 2.903–14.817; for chemo/CIT, P = 0.04, HR: 0.506, 95% CI: 0.264–0.970). In subgroup analysis, 3‐year DFS and OS rates of patients with stage III GC in the chemo/CIT group were significantly higher than those in the chemo group (38.4% vs. 57.1%, P = 0.038; and 45.9% vs. 76%, P = 0.06, respectively), while there was no significant difference between the two groups in patients with stage II GC. Only 15.9% of patients (10/63) in the chemo/CIT group had mild and manageable fever (grades 1 and 2), while no other side effects were observed. The adjuvant treatment combining chemotherapy with cellular immunotherapy is well tolerated and significantly improves the clinical outcome of patients with stage II/III GC, when compared with chemotherapy alone, therefore warrants further attention in treatment for relapsed GC after tumor resection.
Keywords: Cellular immunotherapy, chemotherapy, disease‐free survival, gastric cancer, overall survival
Introduction
Gastric cancer (GC) is the fifth most common malignancy and the fourth leading cause of cancer‐related death worldwide. According to GLOBOCAN, almost one million new cases and over 720,000 deaths were estimated in 2012 1. Almost two thirds of these cases occur in developing countries, while 42% in China 1. Surgical resection is the cornerstone for the treatment of patients with localized gastric cancer. However, postsurgical relapse occurs in approximately 40% of patients within 2 years after surgery 2. Therefore, there has been much interest in using adjuvant therapies to improve the outcomes of GC patients after surgical resection of the primary tumor. Unfortunately, although various strategies have been attempted, few have shown to be clinically beneficial 3, 4, 5, 6, 7, 8. So far, there are only two large randomized phase III studies (i.e., the ACTS GC trial 9 and the CLASSIC trial 10) in Asia that have demonstrated some survival benefit of postoperative chemotherapy following curative D2 lymph node dissection in patients with resectable GC, which therefore support the use of chemotherapy as the standard care for the treatment of these patients after surgery. However, due to high recurrence of tumor, novel therapeutic regimens are urgently needed to improve the efficacy of postoperative adjuvant chemotherapy and thereby clinical outcomes of patients with GC after surgical resection of primary tumor.
Bulk evidence obtained from in vitro and in vivo studies has revealed that surgery as well as GC itself could impair the function of immune system. For example, postoperative changes in the systemic immune response are associated with the degree of surgical trauma, which might contribute to the development of septic complications and tumor metastasis 11. On the other hand, activity of immunocytes is often compromised in GC patients 12. Furthermore, tumor cells can also downregulate major histocompatibility complex (MHC) molecules 13, and thereby protect themselves from recognition and clearance via the immune system (e.g., by cytotoxic T lymphocytes, CTLs) 14. To this end, adoptive cellular immunotherapy (CIT) using non‐MHC‐restricted immune cells (e.g., NK [natural killer], γδT, and CIK [cytokine‐induced killer] cells) has been introduced as an approach to enhance the function of immune system in patients with cancer. Of note, while NK, γδT, and CIK cells function in a similar manner against tumor cells, they exert synergistic effects in killing tumor cells when used in combination 15, suggesting that the combined use of NK, γδT, and CIK cells might improve therapeutic activity of cellular immunotherapy. Interestingly, current adjuvant chemotherapy for treatment of GC patients is based on 5‐fluorouracil in combination with oxaliplatin, which is known to upregulate the expression of natural killer group 2, member D (NKG2D) in tumor cells, and thereby increase their susceptibility to NK, γδT, or CIK cell‐mediated killing 16. Moreover, CIT cells have been shown to improve the efficacy of chemotherapy 17.
It remains to be defined whether CIT would be effective in the adjuvant treatment of patients with local advanced GC. Therefore, this prospective cohort study was designed and conducted to investigate the efficacy and safety of an adjuvant regimen combining chemotherapy with CIT as a novel therapeutic strategy to improve the clinical outcome of patients with stage II/III GC. Here, we report that adjuvant chemotherapy combined with CIT is well tolerable and significantly improves the clinical outcome of patients with stage II/III GC after radical surgery.
Methods
Patients and study design
This is a prospective and observational cohort study approved by the institutional review board of the First Hospital of Jilin University. Patients diagnosed with stage II/III gastric cancer after undergoing a gastrectomy with D2 lymph node dissection were enrolled between 2010 and 2012 at the First Hospital of Jilin University. All patients provided informed consent to participate in this study. Eligible patients met the following criteria: (1) ≥18 years old, (2) diagnosis as stage II/III gastric cancer, with a primary gastric tumor pathologically diagnosed as adenocarcinoma, (3) no more than 4 weeks after a gastrectomy with D2 lymph node dissection, (4) Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and (5) normal liver, renal, and hematologic functions. The World Health Organization Classification of Tumors was used for histological grading and the TNM classification of the American Joint Committee on Cancer (AJCC) was used for tumor staging 18. Exclusion criteria included: (1) other immunotherapies, (2) severe infections, (3) a history of organ transplantation, and (4) pregnancy or breastfeeding.
All the enrolled patients were advised to receive standard 5‐fluorouracil‐ and platinum‐based adjuvant chemotherapy to reduce the risk of recurrence according to the National Comprehensive Cancer Network (NCCN) Guidelines 19. According to the patient choice of treatment, patients were assigned into two groups: chemotherapy alone (chemo) or combined chemotherapy and CIT (chemo/CIT). Patients were followed up every 3 months postoperatively, including complete physical examination, analysis of basic serum chemistry, and contrast‐enhanced computed tomography (CT) of the chest and abdomen.
The primary outcome of this study is disease‐free survival (DFS). The secondary outcomes include overall survival (OS) and systemic side effects of CIT. DFS was defined as the length of time from the date of surgery to the date of progression or to the date of the most recent follow‐up. OS was defined as the length of time from the date of surgery to the date of death or to the date of the most recent follow‐up. Survival status was last updated in August 2015.
Preparation of immunocytes
Peripheral blood mononuclear cells (PBMCs, 1–1.5 × 109 cells) were collected from each patient 1–2 days before adjuvant chemotherapy using Spectra Optia® Apheresis System (Gambro BCT, Inc, Lakewood, CO) as per manufacturer's instructions. After collection, PBMCs was split into two 50‐mL centrifuge tubes that were spun for 10 min at 1734g. The supernatant was discarded and the cell pellets were resuspended in 30 mL of phosphate‐buffered saline (PBS) and placed on top of a 15‐mL Hypaque (Amersham Biosciences, Uppsala, Sweden) in a 50‐mL sterile tube. Lymphocytes were isolated from PBMCs by means of Ficoll‐Hypaque density centrifugation (Ficoll separation) to yield ~1.3 × 109 (1.0–1.8 × 109) PBMCs. Cells were then separated into three pools to induce NK, γδT, and CIK cells through the use of different cytokines as described previously 20. All procedures for preparing the autologous immune cells were carried out under good manufacturing practice conditions approved by the Jilin Provincial Center for Sanitation Inspection and Test (China, certificate ID A20090047).
Characterization of immunocytes
Immunocytes for infusion were characterized using fluorescence‐conjugated primary monoclonal antibodies against specific cell surface markers (NK, CD3−/CD56+; γδT, Vγ9+; CIK, CD3+/CD56+) by four‐color FACSCalibur flow cytometry (BD Biosciences, San Diego, CA) as described previously 20, 21.
Infusion of immunocytes
NK, γδT, and CIK cells were washed three times with normal saline and individually resuspended in 50 mL normal saline. Within half an hour, cells were then administered via an intravenous drip. The number of cells for each infusion ranged from 1.2 to 2.0 × 109 cells. A course of CIT was completed within 3 weeks, with an infusion focus within 14–21 days, after apheresis. For each course, eight sessions of infusion were performed. Collection of PBMCs for the next course started 1–2 days before the last infusion of the previous course. The patients in the chemo/CIT group received continuous treatment with six courses of CIT. The schedule for treatment is shown in Figure 1.
Figure 1.
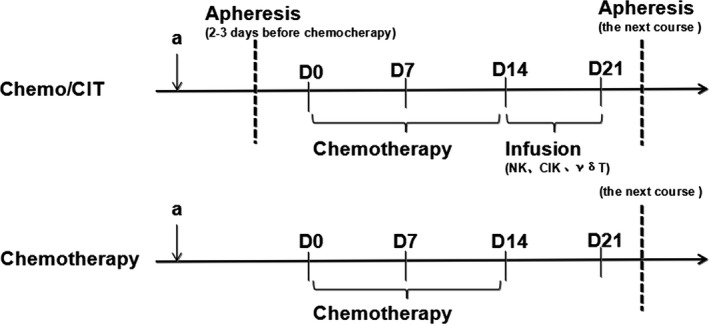
Treatment schedule of chemotherapy and chemoimmunotherapy.
Assessment of treatment‐related side effects
All adverse events experienced by the patients were recorded and graded according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 22.
Statistical analysis
The clinical characteristics were compared between the chemo and the chemo/CIT groups using the chi‐square test. DFS and OS curves were estimated by the Kaplan–Meier analysis, and compared between two groups using the Cox's model for hazard ratio (HR) and 95% confidence interval (CI). Data of immunocyte phenotypes were recorded as the mean ± standard deviation (SD). Percentages of lymphocytes were compared between before and after CIT using the paired sample t‐test. The degree of myelosuppression was compared between two groups using the rank‐sum test. An SPSS software (Version 17.0; SPSS, Inc., Chicago, IL) was used for all statistical analyses. P < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 169 patients were enrolled in this prospective cohort study. Six patients in the chemo group and four patients in the chemo/CIT group were excluded due to loss to follow‐up. As consequence, a total of 159 patients, including 96 patients in the chemo group and 63 in the chemo/CIT group, were subjected to analysis. Patients in these two groups had similar demographics (Table 1). There was no significant difference in sex, age, as well as tumor location, differentiation, and stage between two groups (P > 0.05 for each case). Median follow‐up time was 48.6 months (range: 16.3–69.6 months).
Table 1.
Patient characteristics
| No. of patients | Adjuvant therapy, n (%) | P value | ||
|---|---|---|---|---|
| Chemo (%) | Chemo/CIT (%) | |||
| Sex | ||||
| Male | 123 | 73 (59.3) | 38 (40.7) | 0.624 |
| Female | 36 | 23 (63.9) | 13 (36.1) | |
| Age (years) | ||||
| <60 | 97 | 61 (62.9) | 36 (37.1) | 0.418 |
| ≥60 | 62 | 35 (56.5) | 27 (43.5) | |
| Stage (AJCC) | ||||
| II | 69 | 41 (59.4) | 28 (40.6) | 0.892 |
| III | 90 | 55 (61.1) | 35 (39.6) | |
| Location | ||||
| GEJ | 30 | 14 (46.7) | 16 (53.3) | 0.088 |
| NGEJ | 129 | 82 (63.6) | 47 (36.4) | |
| Differentiation | ||||
| Intermediate | 59 | 33 (55.9) | 26 (44.1) | 0.379 |
| Poor | 100 | 63 (63.0) | 37 (37.0) | |
AJCC, American Joint Committee on Cancer.
Quality of immunocytes
The percentages of NK (CD3−/CD56+), CIK (CD3+/CD56+), and γδT (Vγ9+) cells before and after induction were 11.94% (8.06–15.56%) versus 80.17% (63.2–93.5%), 9.38% (6.39–13.04%) versus 34.4% (27.83–45.71%), and 2.886% (1.07–4.01%) versus 69.66% (50.23–84.01%), respectively (Table 2). Results from a representative patient are shown in Figure 2. Viability of immunocytes exceeded 95%.
Table 2.
Percentage of NK, γδT, and CIK cells before and after induction
| Before (median, range) (%) | After (median, range) (%) | |
|---|---|---|
| NK cells | 11.9 (8.1–15.6) | 80.2 (63.2–93.5) |
| γδT cells | 2.9 (1.1–4.0) | 69.7 (50.2–84.0) |
| CIK cells | 9.4 (6.4–13.0) | 34.4 (27.8–45.7) |
CIK, cytokine‐induced killer.
Figure 2.
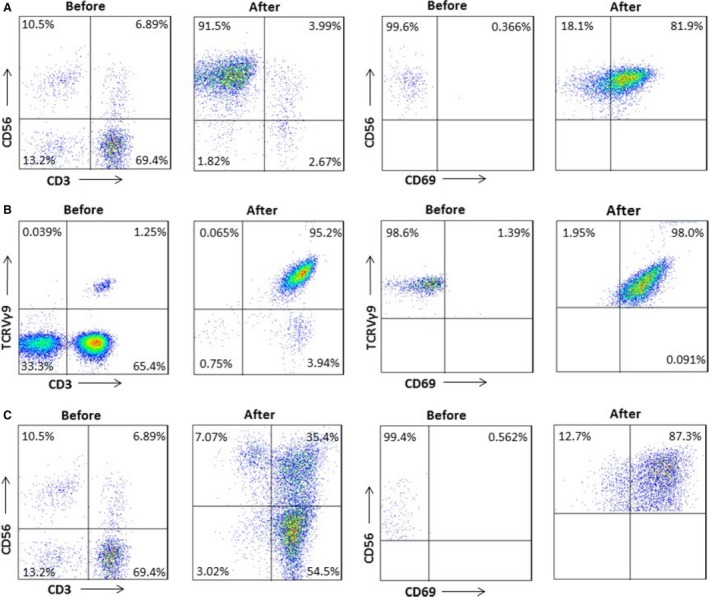
Characterization of NK, γδT, and CIK cells before and after induction. Results from one representative patient are shown. Percentage of activated NK cells (A), γδT cells (B), and CIK cells (C) before and after induction was 0.366% versus 81.9%, 1.39% versus 98%, and 0.562% versus 87.3%, respectively.
Clinical outcomes
To examine efficacy of adjuvant chemotherapy combined with CIT in patients with GC, the Kaplan–Meier analysis was performed to compare DFS and OS rates between the chemo and the chemo/CIT group in a total of 159 assessable patients. As shown in Figure 3, adjuvant treatment combining chemotherapy and CIT significantly increased 3‐year DFS rate (74.7% vs. 60.6%, P = 0.036) and marginally significantly 3‐year OS rate (83% vs. 64.9%, P = 0.051), compared to chemotherapy alone.
Figure 3.
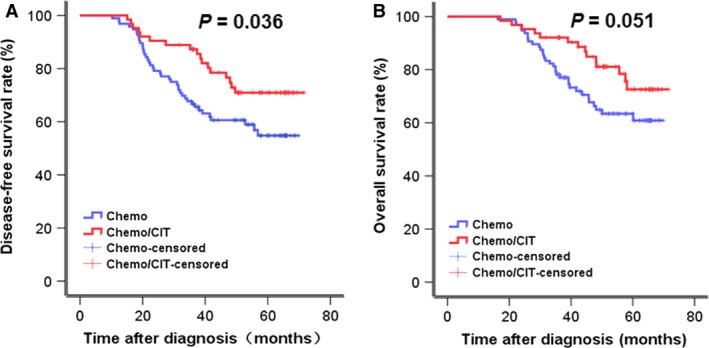
Clinical outcomes of patients who received chemotherapy or chemotherapy plus CIT. Kaplan‐Meier survival analysis was used to compare three‐year DFS and OS rate between the chemo and the chemo/CIT group (DFS, 60.6% vs 74.7%, P = 0.036; OS, 64.9% vs 83%, P = 0.051).
In subgroup analysis (Fig. 4), 3‐year DFS and OS rates of patients with stage II GC in the chemo group were moderately but not significantly lower than those in the chemo/CIT group (DFS, 87.7% vs. 92.4%, P = 0.169; OS, 87.7% vs. 96%, P = 0.138). However, in patients with stage III GC, 3‐year DFS rate for the chemo/CIT group were significantly higher than those in the chemo group (57.1% vs. 38.4%, P = 0.038), while the difference for OS rate was marginally significant (76% vs. 45.9%, P = 0.06).
Figure 4.
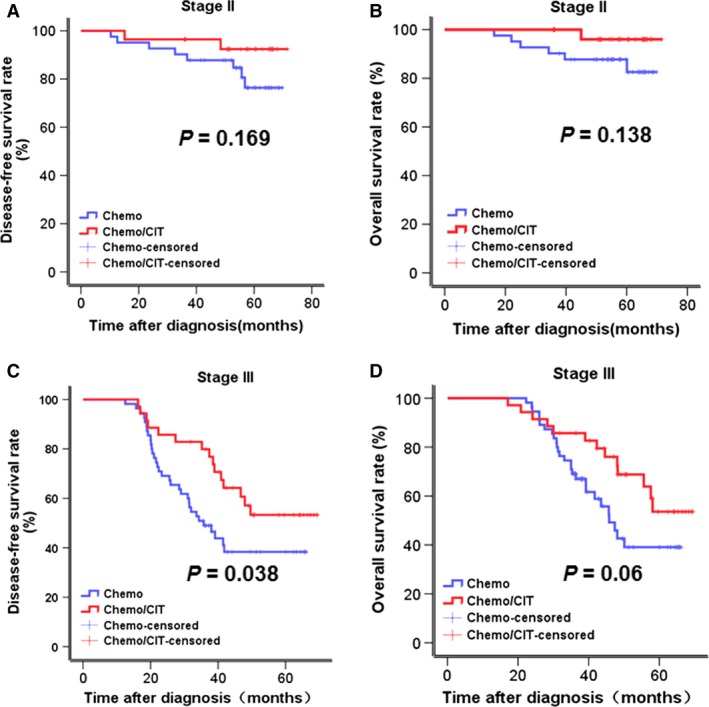
Kaplan–Meier survival analysis was used to compare 3‐year DFS and OS rate between the chemo and the chemo/CIT groups (DFS, 60.6% vs. 74.7%, P = 0.036; OS, 64.9% vs. 83%, P = 0.051). Subgroup analysis of clinical outcomes of patients who received chemotherapy or chemotherapy plus CIT. Three‐year DFS and OS rates were analyzed in patients with stage II (chemo vs. chemo/CIT: DFS, 87.7% vs. 92.4%, P = 0.169; OS, 87.7% vs. 96%, P = 0.138) and III (chemo vs. chemo/CIT: DFS, 38.4% vs. 57.1%, P = 0.038; OS, 45.9% vs. 76%, P = 0.06), respectively.
Analysis of prognostic factors
To estimate the effects of adjuvant chemotherapy combined with CIT on postoperative prognosis of GC patients, univariate Cox regression analyses on DFS (Table 3) and OS (Table 4) were performed in this study cohort. Of note, the results revealed that treatment with combination of chemotherapy and CIT was a significant factor on DFS and OS (HR: 0.549, 95% CI: 0.311–0.970; HR: 0.541, 95% CI: 0.289–1.013, respectively). In addition, TNM stage represented another significant factor (DFS, HR: 5.282, 95% CI: 2.653–10.516; OS, HR: 6.267, 95% CI: 2.794–14.057).
Table 3.
Univariate and multivariate Cox regression analyses of disease‐free survival
| Univariate Cox regression | Multivariate Cox regression | |||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Sex | ||||
| Female | 1.00 | – | 1.00 | – |
| Male | 1.988 | 0.942–4.199 | 2.417 | 1.132–5.161 |
| Age (years) | ||||
| <60 | 1.00 | – | 1.00 | – |
| ≥60 | 0.848 | 0.495–1.453 | 0.867 | 0.502–1.497 |
| Stage (AJCC) | ||||
| II | 1.00 | – | 1.00 | – |
| III | 5.282 | 2.653–10.516 | 5.599 | 2.791–11.232 |
| Location | ||||
| NGEJ | 1.00 | – | 1.00 | – |
| GEJ | 1.309 | 0.705–2.430 | 1.095 | 0.575–2.084 |
| Differentiation | ||||
| Intermediate | 1.00 | ‐ | 1.00 | – |
| Poor | 1.586 | 0.899–2.799 | 1.486 | 0.832–2.655 |
| Adjuvant therapy | ||||
| Chemo | 1.00 | ‐ | 1.00 | – |
| Chemo/CIT | 0.549 | 0.311–0.970 | 0.478 | 0.266–0.858 |
AJCC, American Joint Committee on Cancer; CIT, chemotherapy with cellular immunotherapy.
Table 4.
Univariate and multivariate Cox regression analyses of overall survival
| Univariate Cox regression | Multivariate Cox regression | |||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Sex | ||||
| Female | 1.00 | – | 1.00 | – |
| Male | 1.812 | 0.812–4.045 | 0.889 | 0.484–1.634 |
| Age (years) | ||||
| <60 | 1.00 | – | 1.00 | – |
| ≥60 | 0.842 | 0.464–1.528 | 0.889 | 0.484–1.634 |
| Stage (AJCC) | ||||
| II | 1.00 | – | 1.00 | – |
| III | 6.267 | 2.794–14.057 | 6.559 | 2.903–14.817 |
| Location | ||||
| NGEJ | 1.00 | – | 1.00 | – |
| GEJ | 1.206 | 0.599–2.426 | 1.008 | 0.485–2.094 |
| Differentiation | ||||
| Intermediate | 1.00 | – | 1.00 | – |
| Poor | 1.979 | 1.027–3.813 | 1.938 | 0.990–3.792 |
| Adjuvant therapy | ||||
| Chemo | 1.00 | – | 1.00 | – |
| Chemo/CIT | 0.541 | 0.289–1.013 | 0.506 | 0.264–0.970 |
AJCC, American Joint Committee on Cancer; CIT, chemotherapy with cellular immunotherapy.
Furthermore, multivariate Cox regression analysis was performed to evaluate the robustness of the prognostic values of adjuvant chemotherapy combined with CIT or TNM stage. As shown in Tables 3 and 4, treatment with combination of chemotherapy and CIT (DFS, HR : 0.478, 95% CI: 0.266–0.858; OS, HR: 0.506, 95% CI: 0.264–0.970) and TNM stage (DFS, HR: 5.599, 95% CI: 2.791–11.232; OS, HR: 6.559, 95% CI: 2.903–14.817) were independent prognostic factors of DFS and OS, respectively.
Safety
In the chemo/CIT group, 10 (15.9%) of 63 of patients had grades 1 and 2 fever and shivering after received CIT, which were managed within 2 h by administration of antipyretics. No other CIT‐related adverse effects of were observed. Interestingly, bone marrow suppression occurred modestly less in the chemo/CIT group than the chemo group (52% vs. 59%, P = 0.675, Table 5).
Table 5.
Bone marrow suppression in the chemo and the chemo/CIT group
| Degree | Intervention, n (%) | P value (for I–IV) | |
|---|---|---|---|
| Chemo | Chemo/CIT | ||
| 0 | 39 (40.6%) | 30 (47.6%) | |
| I+II | 39 (40.6%) | 22 (34.9%) | 0.675 |
| III+IV | 18 (18.8%) | 11 (17.5%) | |
Discussion
Postoperative chemotherapy could improve clinical outcome of GC patients who have received curative surgery with D2 lymph node dissection, while its efficacy is comprised by high recurrence and metastasis. The latter is closely associated with immunosuppression in patients with GC 23. In this prospective cohort study, it was observed that adjuvant treatment combining chemotherapy and CIT increased significantly DFS rate, as well as marginally significantly OS rate, of patients with stage II/III GC, when compared to chemotherapy alone, suggesting that this approach may represent a promising treatment to further improve clinical outcome of GC patients after primary tumor resection.
Although a recent clinical study has shown that CIT is effective against GC 24, CIT has however not used as a routine treatment for patients with GC, primarily due to limited evidence of efficacy. The benefits of combining chemo‐ and immunotherapy after surgery may stem from restoration of immune function in GC patients often with immunosuppression because of curative resection of primary tumor, or synergistic effects between chemo‐ and immunotherapy, or both. For example, combining chemotherapy with OK‐432, a Streptococcus‐derived immunotherapeutic agent, is more effective than chemotherapy alone against GC 25. Moreover, a phase III clinical trial has revealed that chemoimmunotherapy significantly prolongs recurrence‐free survival and OS rates of patients with resectable GS, compared to chemotherapy alone 26.
The present study reveals that combined chemotherapy with CIT of NK, γδT, and CIK cells significantly reduced the risk of stage II/III gastric cancer recurrence and metastasis compared with chemotherapy alone, manifested by increased DFS rate. Consistently, OS rate for combined treatment showed a similar tendency, although marginally significant, when compared to chemotherapy alone. Moreover, univariate and multivariate regression analyses of DFS and OS further demonstrate that in this cohort of patients with stage II/III GC, combined chemotherapy with CIT was a more beneficial postoperative adjuvant treatment than chemotherapy only.
Subgroup analysis suggests that patients with stage III GC might benefit more from combination therapy of chemotherapy with CIT than those with stage II GC. Similarly, other studies have also shown that a subset of patients with T3/T4a, N2, or stage III preferably benefit from chemoimmunotherapy 26, 27. One possibility is that immunosuppression might be worse in patients with advanced than early‐stage GC, thereby suggesting that the former may be more susceptible to chemoimmunotherapy.
In this study, after patients received six courses of CIT, only ~16% patients experienced mild and manageable fever, while no other CIT‐related adverse event was observed. These findings support a notion that combining chemotherapy and CIT as adjuvant treatment is well tolerated in patients with GC. Of note, myelosuppression occurred less after treated with chemotherapy in combination with CIT than itself alone, suggesting that addition of CIT might provide more benefits (e.g., reducing chemotherapy‐associated myelosuppression) to patients with GC.
However, there are some limitations in the present study. First, while the maximal six courses of CIT were administrated in this pilot study, it remains to be defined whether further increases in course number and timing of CIT would yield better efficacy of CIT in combination with chemotherapy as postoperative treatment of patients with GC. Thus, future clinical trials designed specifically to determine optimal dosing and schedule of CIT are required to address this issue. Second, although this is a perspective study, assignment of patients into either control (chemotherapy alone) or intervention (chemotherapy plus CIT) group was based on the patient choice of treatment, rather random, suggesting a possibility of selection bias. Therefore, although such a study design was due to a consideration from the standpoint of the current clinical practice, future randomized control trials are required to consolidate the findings of this study. Finally, OS was designed as the secondary, rather primary, outcome because of (1) a relatively short period of follow‐up and (2) varied treatments (e.g., chemotherapy, radiotherapy, chemoradiotherapy, or even no treatment) after tumor recurrence, which influence OS of patients.
In summary, the present findings indicate that combining chemotherapy with CIT after radical surgery improves clinical outcome (e.g., DFS and OS) of patients with advanced GC, especially those with stage III disease, compared to chemotherapy alone. This regimen was well tolerated, while might provide potentially more benefits to patients (e.g., reducing occurrence of chemotherapy‐associated myelosuppression). Therefore, this study lays a foundation for combining chemotherapy and CIT as an adjuvant treatment of GC patients after surgery. Consequently, efforts to develop this regimen further in randomized clinical trials of GC are currently underway.
Conflict of Interest
All authors disclose no commercial, proprietary, or financial interest in the products or companies described in this article.
Acknowledgments
This work was supported by the Jilin Provincial Science and Technology Department (Grants 20111807 and 20140414014GH, and 20150101176 to J. C.), the Key Project of Science and Technology Research of the Ministry of Education (Grant 311015 to J. C.), the Platform Construction Project of Development and Reform Commission of Jilin Province (Grant 2014N147 to J. C.), the Bethune Program B of Jilin University (Grant 2012202 to J. C.), the Key Clinical Project of the Ministry of Health of the People's Republic of China (Grant 2001133 to W. L.), and the National Major Scientific and Technological Special Project (Grant 2013ZX09102032 to J. C.).
Cancer Medicine 2017, 6(1):45–53
Contributor Information
Jiuwei Cui, Email: cuijw@jlu.edu.cn.
Wei Li, Email: weilistudent@sina.com.
References
- 1. Ferlay, J. , Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., et al. 2015. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. D'Angelica, M. , Gonen M., Brennan M. F., Turnbull A. D., Bains M., and Karpeh M. S.. 2004. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann. Surg. 240:808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu, J. K. , Chen Z. X., Zhou Z. G., Zhang B., Tian J., Chen J. P., et al. 2002. Intravenous chemotherapy for resected gastric cancer: meta‐analysis of randomized controlled trials. World J. Gastroenterol. 8:1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group ; X, Paoletti , Oba K., Burzykowski T., Michiels S., Ohashi Y., et al. 2010. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta‐analysis. JAMA 303:1729–1737. [DOI] [PubMed] [Google Scholar]
- 5. De Vita, F. , Giuliani F., Orditura M., Maiello E., Galizia G., Di Martino N., et al. 2007. Adjuvant chemotherapy with epirubicin, leucovorin, 5‐fluorouracil and etoposide regimen in resected gastric cancer patients: a randomized phase III trial by the Gruppo Oncologico Italia Meridionale (GOIM 9602 Study). Ann. Oncol. 18:1354–1358. [DOI] [PubMed] [Google Scholar]
- 6. Di Costanzo, F. , Gasperoni S., Manzione L., Bisagni G., Labianca R., Bravi S., et al. 2008. Adjuvant chemotherapy in completely resected gastric cancer: a randomized phase III trial conducted by GOIRC. J. Natl Cancer Inst. 100:388–398. [DOI] [PubMed] [Google Scholar]
- 7. Kulig, J. , Kolodziejczyk P., Sierzega M., Bobrzynski L., Jedrys J., Popiela T., et al. 2010. Adjuvant chemotherapy with etoposide, adriamycin and cisplatin compared with surgery alone in the treatment of gastric cancer: a phase III randomized, multicenter, clinical trial. Oncology 78:54–61. [DOI] [PubMed] [Google Scholar]
- 8. Sakuramoto, S. , Sasako M., Yamaguchi T., Kinoshita T., Fujii M., Nashimoto A., et al. 2007. Adjuvant chemotherapy for gastric cancer with S‐1, an oral fluoropyrimidine. N. Engl. J. Med. 357:1810–1820. [DOI] [PubMed] [Google Scholar]
- 9. Sasako, M. , Sakuramoto S., Katai H., Kinoshita T., Furukawa H., Yamaguchi T., et al. 2011. Five‐year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S‐1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 29:4387–4393. [DOI] [PubMed] [Google Scholar]
- 10. Bang, Y. J. , Kim Y. W., Yang H. K., Chung H. C., Park Y. K., Lee K. H., et al. 2012. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open‐label, randomised controlled trial. Lancet 379:315–321. [DOI] [PubMed] [Google Scholar]
- 11. Reikreas, O. , Borgen P., Reseland J. E., Lyngstadaas S. P., 2014. Changes in serum cytokines in response to musculoskeletal surgical trauma. BMC Research Notes 7:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kiessling, R. , Wasserman K., Horiguchi S., Kono K., Sjöberg J., Pisa P., et al. 1999. Tumor‐induced immune dysfunction. Cancer Immunol. Immunother. 48:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gilboa, E. 1999. How tumors escape immune destruction and what we can do about it. Cancer Immunol. Immunother. 48:382–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Famault, L. , Sanchoz C., Baier C., Treut T. L., Costello R. T.. 2012. Hematological malignancies escape from NK cell innate immune surveillance: mechanism and therapeutic implications. Clin Dev Immunol. 2012:421702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maniar, A. , Zhang X., Lin W., Gastman B. R., Pauza C. D., Strome S. E., et al. 2010. Human gam‐madelta T lymphocytes induce robust NK cell‐mediated antitumor cytotoxicity through CD137 engagement. Blood 116:1726–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gasser, S. , Orsulic S., Brown E. J., Raulet1 D. H.. 2005. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436:1186–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kershaw, M. H. , Devaud C., John L. B., Westwood J. A., and Darcy P. K.. 2013. Enhancing immunotherapy using chemotherapy and radiation to modify the tumor microenvironment. OncoImmunology 2:e25962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greene, F. L. , Page D. L., Fleming I. D., Fritz A., Balch C. M., Haller D. G.. et al. 2002. AJCC Cancer Staging Manual, 6th ed. Springer‐Verlag, New York. [Google Scholar]
- 19. NCCN Clinical Practice Guidelines in Oncology: Gastric Cancer version 2 . 2009. http://www.nccn.org. (Accessed 3 May 2009).
- 20. Cui, J. W. , Li L. Y., Wang Ch, Jin H., Yao C., Wang Y., et al. 2015. Combined cellular immunotherapy and chemotherapy improves clinical outcome in patients with gastric carcinoma. Cytotherapy 17:979–988. [DOI] [PubMed] [Google Scholar]
- 21. Cui, J. W. , Wang N., Zhao H., Jin H., Wang G., Niu C., et al. 2013. Combination of radiofrequency ablation and sequential cellular immunotherapy improves progression‐free survival for patients with hepatocellular carcinoma. Int. J. Cancer 134:342–351. [DOI] [PubMed] [Google Scholar]
- 22. National Cancer Institute .2010. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v.4.0.
- 23. Xie, Z. J. , Liu Y., Jia L. M., and He Y. C.. 2008. Heparanase expression, degradation of basement membrane and low degree of infiltration by immunocytes correlate with invasion and progression of human gastric cancer. World J. Gastroenterol. 14:3812–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peng, Y. P. , Zhu Y., Zhang J. J., Xu Z. K., Qian Z. Y., Dai C. C., et al. 2013. Comprehensive analysis of the percentage of surface receptors and cytotoxic granules positive natural killer cells in patients with pancreatic cancer, gastric cancer, and colorectal cancer. J. Transl. Med. 11:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sakamoto, J. , Teramukai S., Nakazato H., Sato Y., Uchino J., Taguchi T., et al. 2002. Efficacy of adjuvant immunochemotherapy with OK‐432 for patients with curatively resected gastric cancer: a meta‐analysis of centrally randomized controlled clinical trials. J. Immunother. 25:405–412. [DOI] [PubMed] [Google Scholar]
- 26. Jeung, H. C. , Moon Y. W., Rha S. Y., Yoo N. C., Roh J. K., Noh S. H., et al. 2008. Phase III trial of adjuvant 5‐fluorouracil and adriamycin versus 5‐fluorouracil and adriamycin a polyadenylic polyuridylic acid (poly A:U) for locally advanced gastric cancer after curative surgery: final results of 15 year follow up. Ann. Oncol. 19:520–526. [DOI] [PubMed] [Google Scholar]
- 27. Wick, M. , Dubey P., Koeppen H., Siegel C. T., Fields P. E., Chen L., et al. 1997. Antigenic cancer cells grow progressively in immune hosts without evidence for T cell exhaustion o systemic anergy. J. Exp. Med. 186:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]


