Abstract
Previously, we found that arsenite (AsIII) oxidation could improve the generation of ATP/NADH to support the growth of Agrobacterium tumefaciens GW4. In this study, we found that aioE is induced by AsIII and located in the arsenic island near the AsIII oxidase genes aioBA and co-transcripted with the arsenic resistant genes arsR1-arsC1-arsC2-acr3-1. AioE belongs to TrkA family corresponding the electron transport function with the generation of NADH and H+. An aioE in-frame deletion strain showed a null AsIII oxidation and a reduced AsIII resistance, while a cytC mutant only reduced AsIII oxidation efficiency. With AsIII, aioE was directly related to the increase of NADH, while cytC was essential for ATP generation. In addition, cyclic voltammetry analysis showed that the redox potential (ORP) of AioBA and AioE were +0.297 mV vs. NHE and +0.255 mV vs. NHE, respectively. The ORP gradient is AioBA > AioE > CytC (+0.217 ~ +0.251 mV vs. NHE), which infers that electron may transfer from AioBA to CytC via AioE. The results indicate that AioE may act as a novel AsIII oxidation electron transporter associated with NADH generation. Since AsIII oxidation contributes AsIII detoxification, the essential of AioE for AsIII resistance is also reasonable.
Arsenic (As) is a toxic metalloid widely distributed in environment, being responsible for mass poisoning throughout Asia1,2. In the natural environment, arsenite (AsIII) and arsenate (AsV) are the primary arsenicals3,4, and microbial redox reactions are considered as important contributors to the changes of AsIII and AsV levels5,6,7,8,9,10.
Microbial AsIII oxidation is an elaborate regulation process11,12,13,14. The AsIII oxidase AioBA consists of two heterologous subunits, and is responsible for catalyzing bacterial AsIII oxidation11,15. In some AsIII-oxidizing strains, the three-component system AioXSR sensed the AsIII signal and regulated the expression of AioBA12,14,16. Moreover, the phosphate two-component system PhoBR could be involved in the regulation of aioBA expression13 or bind with the promoter of aioBA directly10; The ArsR repressor, which is involved with the control of the ArsRBC arsenic detoxification system17,18 and the dissimilatory AsV reduction19, is also associated with regulation of phoB1 gene located near the aio locus13, indicating that bacterial AsIII oxidation was co-regulated by the aio, pho and ars regulatory systems. In addition, the AsIII/H+ antiporter Acr3-1 which regulated by ArsR, is essential for AsIII oxidation, suggesting aio, pho and ars gene clusters are all involved in bacterial AsIII oxidation20.
Based on the Mitchellian chemiosmotic energy conversion, the electrochemical disequilibrium between reducing and oxidizing substrates results in the electron transport via the redox reaction, associated with energy generation, which is a common feature of bacteria21,22. Microbial AsIII oxidation is considered as a detoxification mechanism or contributes to energy generation redox reactions depending on the microorganisms9,23,24,25. In some autotrophic AsIII-oxidizing strains, NO3− or O2 is the final electron acceptor of the AsIII oxidation, assisting to generate energy to support bacterial growth23,26. A photosynthetic AsIII-oxidizing bacterium was reported to grow as a photoautotroph using AsIII as the sole photosynthetic electron donor27. In addition, the heterotrophic AsIII-oxidizing bacteria Hydrogenophaga sp. NT-14 and Agobacterium tumefaciens GW4 were also reported to be able to generate energy from AsIII oxidation25,28. A. tumefaciens GW4 is especially effective at improving the generation of both ATP and NADH by AsIII oxidation25. Using O2 as the final electron acceptor, CytC was reported to be the AsIII oxidation electron transporter with the generation of ATP28,29. However, the electron transporter for the production of NADH still unknown.
Recently, using comparative proteomics analysis, we found an oxidoreductase (named AioE) was obviously up-regulated in the presence of AsIII, as well as the AsIII oxidation electron transporter CytC and the large subunit of AsIII oxidase AioA. According to BlastP analysis, AioE belongs to a TrkA superfamily and contains a NAD+ binding domain, which could incorporate one hydroxyl group to carbonyl group by concomitant generation of NADH and H+ 30,31. Such function shares similarities with the reaction converting reduced AioBA back to oxidized AioBA29,31,32. In addition, aioE is located in the arsenic island containing functional aio, pho, pst and ars genes among several available arsenic islands33. Thus, we speculated that AioE may be important for AsIII resistance and oxidation. Herein, the amount of ATP/NADH, the AsIII resistance levels and AsIII oxidation rate were compared between the aioE and cytC mutants. In addition, the redox potential of the AioAB, AioE, and CytC proteins were determined. The summarized results represent a novel contribution and demonstrate that the aioE is involved in AsIII oxidation and resistance. Considering its gene function and encoding protein domains, we propose that AioE may be involved in AsIII oxidation electron transport associating the generation of NADH.
Results
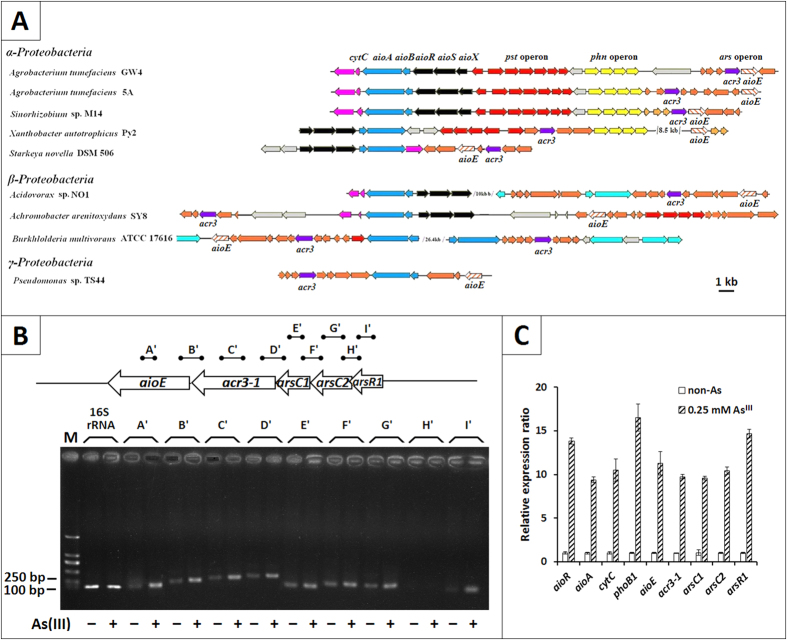
AioE is widely distributed in AsIII oxidizing bacteria
Using comparative proteomics analysis, we found an oxioreductase, AioE, was obviously up-regulated with a 10.5 folds change in the presence of AsIII (unpublished data) in A. tumefaciens GW4 (AWGV01000000). Using BlastP analysis, the AioE showed 91% amino acid identity with an oxidoreductase in Ochrobactrum tritici (AKB90512.1). The aioE gene is located at the downstream of acr3-1 gene within the arsenic island also containing the ars-pho-pst-aio gene cluster in strain GW4 (Fig. 1A). In addition, aioE is widely distributed and consistently located in the arsenic islands in some AsIII-oxidizing strains (Fig. 1A), indicating that the aioE gene is most likely related to AsIII resistance and oxidation. Moreover, aioE of the AsIII oxidizers are phylogenetically clustered into α, β, γ-Proteobacteria (Fig. S1), which is in agreement with 16 S rRNA based phylogenetic analysis (data not shown).
Figure 1.
(A) The coding gene of oxidoreducase AioE was spread in many genomes of AsIII oxidation strains. (B) RT-PCR analysis illustrating the co-transcription of arsR1-arsC1-arsC2-acr3-1-aioE and their enhanced expression with the addition of AsIII. RT-PCRs were performed with the primers in Table S1. A’, 129 bp, B’, 246 bp, C’, 274 bp, D’, 321 bp, E’, 139 bp, F’, 210 bp, G’, 226 bp, H’, 188 bp, and I’, 117 bp. Total RNA was extracted from strain GW4 grown in MMNH4 medium with or without 0.25 mM AsIII. M, the molecular weight marker (DL 2000 plus). All reverse transcriptase reactions contained 15 ng of RNA and each lane was loaded with 5 μL of the PCR product. Amplicon identities were confirmed by DNA sequencing. (C) Quantitative reverse transcriptase-PCR analysis of the genes involved in ars and aio gene island influenced by AsIII. The 16 S rRNA gene was used as a reference. Data are shown as the mean of three replicates, with the error bars representing ± 1 SD. Amplicon identities were confirmed by DNA sequencing.
AioE is induced by AsIII and co-transcribed with arsR1-arsC1-arsC2-acr3-1
To identify the contribution of the aioE gene to AsIII resistance and oxidation, RT-PCR and qRT-PCR were employed to test the transcription level. The PCR which used DNA or RNA as the template respectively confirmed that the reagents and primers both worked well, and the results showed free of DNA contamination in the RNA (Fig. S2). The RT-PCR showed the co-transcription of arsR1-arsC1-arsC2-acr3-1-aioE (Fig. 1B), indicating that the repressor ArsR1 may regulate the transcription of arsC1-arsC2-acr3-1-aioE34,35. With the presence of AsIII, the transcription of aioE was increased by more than 10 folds, which is consistent with the proteomics data. Other genes within the arsC2-arsC1-acr3-1-arsR1 operon, and AsIII oxidation genes aioR and aioA, were also highly induced by AsIII (Fig. 1C). In addition, the phoB1 was also induced by AsIII, which is consistent with results in AsIII-oxidizing strain A. tumefaciens 5A13.
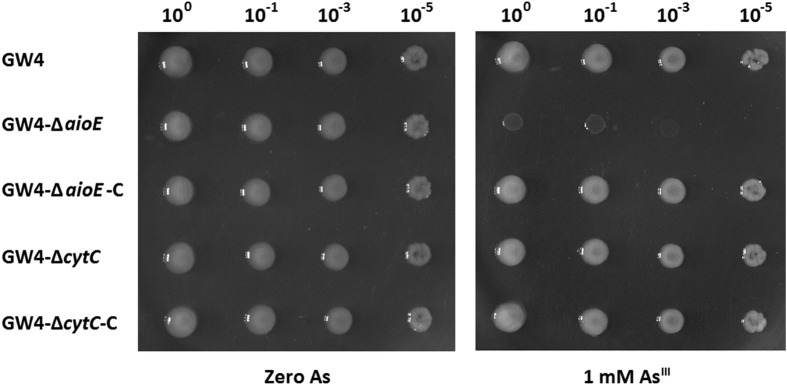
AioE is essential for bacterial AsIII resistance
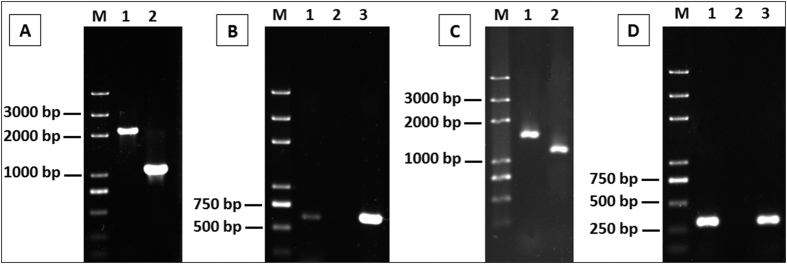
To identify the function of aioE, we constructed aioE deletion mutant GW4-ΔaioE and its complementary strain GW4-ΔaioE-C (Fig. 2). In addition, in order to clarify the AsIII oxidation electron transport function of aioE, we also constructed a cytC deletion mutant GW4-ΔcytC and its complementary strain GW4-ΔcytC-C (Fig. 2). Diagnostic PCRs (Fig. 2) and sequencing (data not shown) confirmed the successful deletion and complementation. Consistent with the decreased AsIII resistance in aioA mutant25, the disruption of aioE also reduced the AsIII resistant level (Fig. 3). However, the AsIII resistance of mutant strain GW4-ΔcytC and the complementary strains were all similar to the wild type strain (Fig. 3). The results indicate that aioE is involved in the bacterial AsIII resistance in strain GW4.
Figure 2.
(A,B) Diagnostic PCR confirming the deletion of aioE to create mutant strain GW4-ΔaioE and complementation to create GW4-ΔaioE-C. (A) PCR amplicons using primers PaioE-1F and PaioE-2R. (B) PCR amplicons using primers IaioE-F and IaioE-R. (C,D) Diagnostic PCR confirming the deletion of cytC to create mutant strain GW4-ΔcytC and complementation to create GW4-ΔcytC-C. (C) PCR amplicons using primers PcytC-1F and PcytC-2R. (D) PCR amplicons using primers IcytC-F and IcytC-R. For panels (A and B): Lane 1, strain GW4, lane 2, aioE gene knock-out strain GW4-ΔaioE and lane 3, the complemented strain GW4-ΔaioE-C. For panels (C and D): Lane 1, strain GW4, lane 2, cytC gene knock-out strain GW4-ΔcytC and lane 3, the complemented strain GW4-ΔcytC-C. M, the molecular weight marker (DL 2000 plus). Amplicon identities were confirmed by DNA sequencing.
Figure 3. AsIII resistance was influenced by the disruption of aioE. Strains GW4, GW4-ΔaioE, GW4-ΔaioE-C, GW4-ΔcytC and GW4-ΔcytC-C were inoculated in MMNH4 medium containing 0.1 mM phosphate.
After 24 h cultivation, 10 μL of each cultures (OD600 = 0.5) were inoculated on the MMNH4 medium plate with or without the addition of 1 mM AsIII, and cultivated at 28 °C for 48 h.
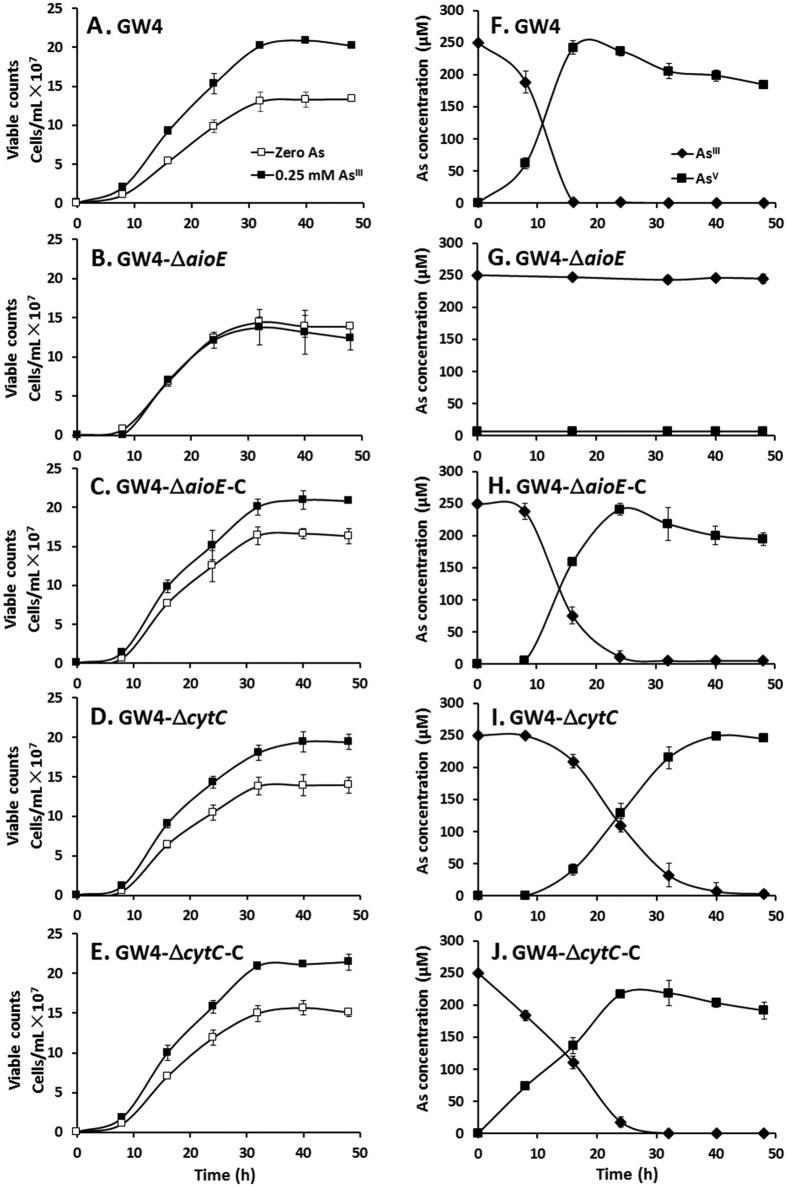
AioE is essential for bacterial AsIII oxidation
The AsIII oxidation efficiencies of the above gene deletion and complemented strains were tested using 0.25 mM AsIII to avoid the effect of the reduced AsIII resistance in GW4-ΔaioE. The addition of 0.25 mM AsIII resulted in enhanced growth for wild type strain GW4 (Fig. 4A), which is consistent with the previous study25. However, the disruption of aioE failed to enhance the bacterial growth with the addition of AsIII (Fig. 4B), while the mutant strain GW4-ΔcytC and the complementary strains GW4-ΔaioE-C and GW4-ΔcytC-C all showed the same growth phenotype with the wild type strain GW4 in the presence of AsIII (Fig. 4C–E). Meanwhile, consistent with the null AsIII oxidation phenotype of deletion mutant GW4-ΔaioA25 (Fig. S3A), the disruption of aioE also caused in deficiency of AsIII oxidation (Figs 4F and S3A), indicating that AsIII oxidation was related to the enhanced bacterial growth25 (Fig. 4A,B,F and G). However, the mutant strain GW4-ΔcytC only showed a reduced AsIII oxidation rate, and did not interrupt the bacterial AsIII oxidation (Fig. 4I). The complementary strains of the two mutants both gained the AsIII oxidation level back to the wild type strain (Figs 4F,H,J and S3A). The results indicated that aioE is essential to AsIII oxidation and enhanced bacterial growth, and that cytC also participates in AsIII oxidation, but its role is less significant compared to aioE in strain GW4. When A. tumefaciens strains grew with AsV, GW4-ΔaioA and GW4-ΔaioE showed AsV reduction phenotypes, while the other A. tumefaciens strains failed to reduce AsV to AsIII (Fig. S3B), indicating that AsV reduction could only occur when AsIII oxidation is disrupted in A. tumefaciens GW4.
Figure 4. AsIII oxidation was influenced by the disruption of aioE.
(A–E) The growth curves of strains GW4, GW4-ΔaioE, GW4-ΔaioE-C, GW4-ΔcytC and GW4-ΔcytC-C in MMNH4 medium containing 0.1 mM phosphate with or without 1 mM AsIII. (F–J) AsIII oxidation profiles of the same strains of B-F. AsIII and AsV concentrations in the culture fluids were measured using HPLC-HG-AFS. The symbols show in panel A are the same as in panels B-E, and the symbols show in panel F are the same as in panels G-J. The data were from triplicates.
AioE is related to the production of NADH
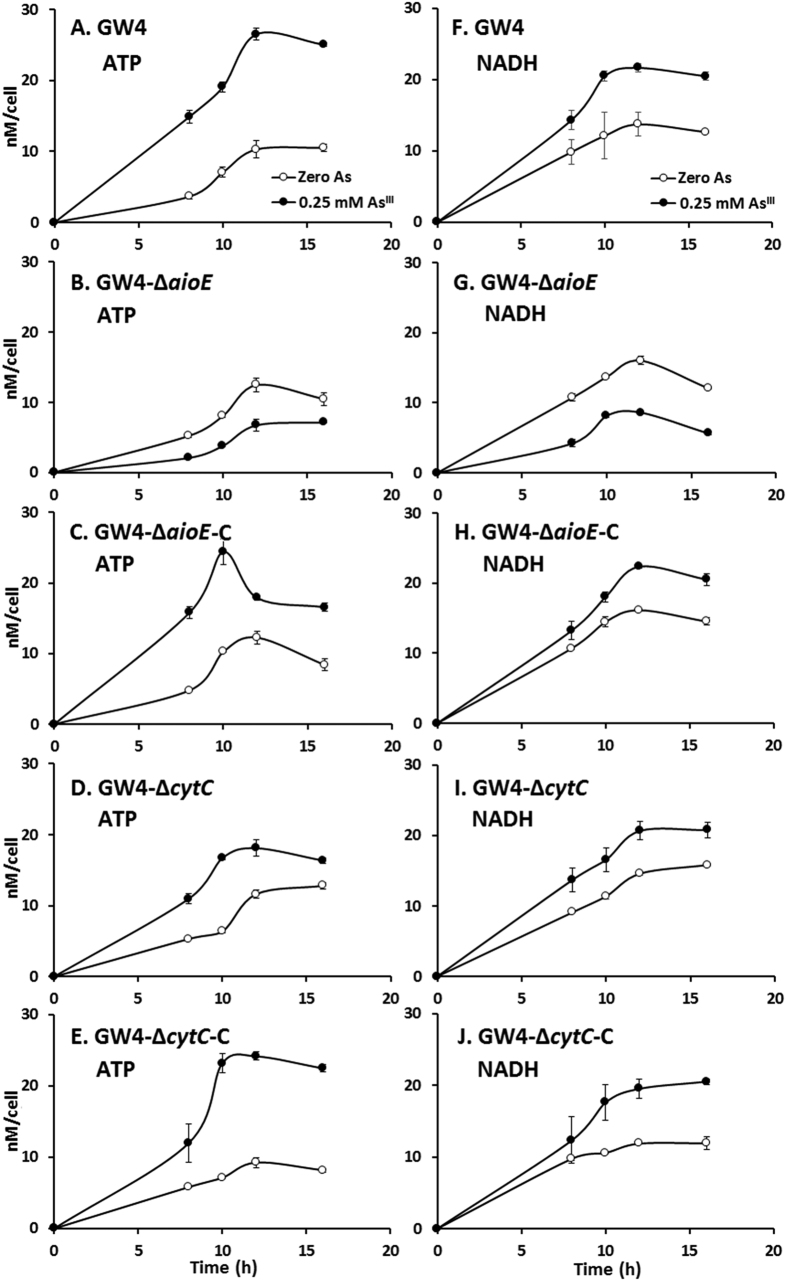
In bacterial cells, NADH and ATP are produced during the electron transport process21,22. We predicted that AioE may be related to AsIII oxidation electron transport and the generation of NADH due to the protein functional domain of AioE29,30,31,32. Thus, the NADH and ATP concentrations in the above mutant and complemented strains and the wild type GW4 were analyzed. The concentrations of cellular ATP and NADH were about 20 nM/cell with the addition of AsIII in cells of strains GW4, GW4-ΔaioE-C and GW4-ΔcytC-C (Fig. 5); in the mutant strain GW4-ΔaioE, the concentrations of ATP and NADH were both decreased with the addition of AsIII (Fig. 5B and G). However, only the concentration of ATP was reduced by the disruption of cytC gene (Fig. 5D), and the NADH concentration in GW4-ΔcytC was similar to that of strain GW4 (Fig. 5I). The tests of bacterial growth, AsIII oxidation, and the contents of NADH/ATP revealed that aioE gene is involved with AsIII oxidation and NADH generation, while cytC gene is related with ATP generation and had a weaker effect on AsIII oxidation than aioE.
Figure 5. The generation of ATP and NADH was influenced by the disruption of aioE.
(A–E) The ATP contents of strains GW4, GW4-ΔaioE, GW4-ΔaioE-C, GW4-ΔcytC and GW4-ΔcytC-C in MMNH4 medium containing 0.1 mM phosphate with or without the addition of 0.25 mM AsIII. (F–J) The NADH contents of strains GW4, GW4-ΔaioE, GW4-ΔaioE-C, GW4-ΔcytC and GW4-ΔcytC-C in MMNH4 medium containing 0.1 mM phosphate with or without 0.25 mM AsIII. The cellular contents of ATP and NADH were tested by HPLC. The symbols shown in panel A are the same as in panels B–E, and the symbols shown in panel F are the same as in panels G-I. The data were from triplicates.
AsIII oxidase AioBA may transport electron to AioE during AsIII oxidation
To further confirm the electron transport possibility among AioBA, AioE, and CytC, the AioBA and AioE proteins were purified (Fig. S4) and their redox potentials (ORP) were obtained by cyclic voltammetry29. The average of the peak potentials showed the formal potential was at pH 6.0. The ORP was +0.297 V vs. NHE for AioBA, and +0.255 V vs. NHE for AioE (Fig. 6). Compared to the reported ORP of +0.217 ~ 0.251 V vs. NHE for CytC29,32, the ORP orders are AioBA > AioE > CytC. Thus, there is a possibility for AioE to participate in the electron transport between AioBA and CytC.
Figure 6.
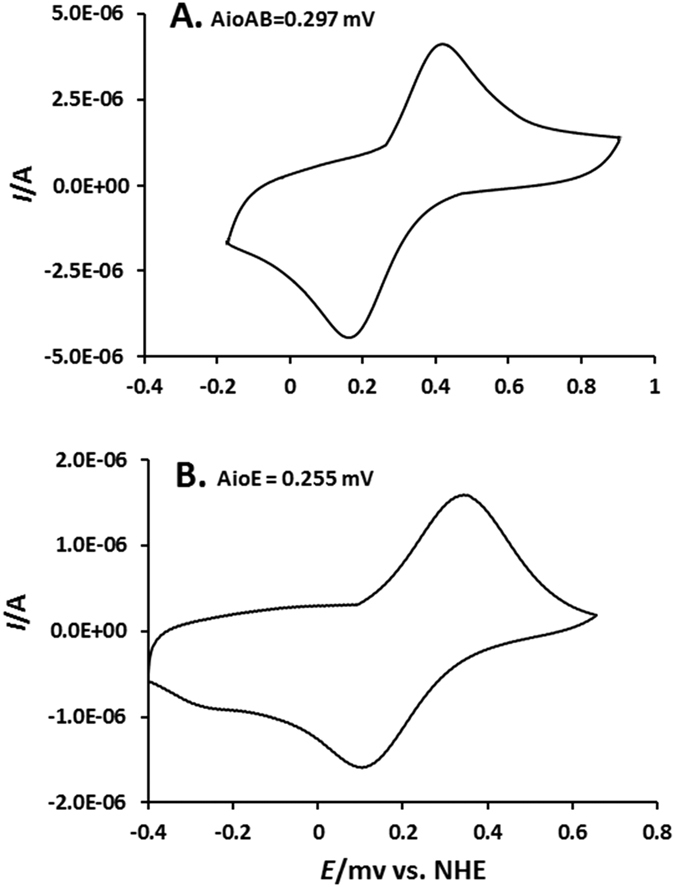
Cyclic voltammetry obtained for 4 μL of AioBA (31.5 μM, A) and AioE (37 μM, B) on Au/MUA electrode in 100 mM phosphate buffer (pH = 6) at a scan rate of 5 mV s−1.
Discussion
The literatures23,26,28 and our previous work25 indicate that bacterial AsIII oxidation is not only a detoxification mechanism, but also related to the energy production. It is likely that electron transport is the cohesive tie between AsIII oxidation and energy production21,22. In Rhizobium sp. NT26, c-type cytochrome CytC was reported as an AsIII oxidation electron transporter29,32. Though electron transport between AsIII oxidase AioAB and CytC was shown, the disruption of cytC did not cause a null phenotype for AsIII oxidation32. In this study, using A. tumefaciens GW4, the disruption of cytC did not interrupt the bacterial AsIII oxidation, but only reduced the AsIII oxidation rate which is consistent with the results from strain NT2632, indicating that the AsIII oxidation electron transport was not be completely blocked without the CytC. Thus, CytC has an additive effect on AsIII oxidation, but is not essential32. This suggests that there should be another protein that can serve as the electron acceptor to the AsIII oxidase AioBA. Herein, we found considerable evidence that aioE is related to the AsIII resistance and oxidation in strain GW4. This conclusion is supported by the AsIII induced expression of aioE (Fig. 1), a decrease of AsIII resistance (Fig. 3), and interruption of AsIII oxidation in aioE mutant (Fig. 4). It also indicates that aioE may be more essential to AsIII oxidation than cytC.
Bacteria often gain energy from the electron transport between the reducing and oxidizing substrates21,22,31. Consistent with our previous study25, AsIII improved the production of both NADH and ATP in strain GW4 (Fig. 5A and F). Being the electronic anchorman of respiratory chain, CytC has multiple copies in bacterial genomes and is reported to be able to produce ATP by transferring the electron to oxygen, as well as in AsIII oxidation process29,32, which is correlated to the reduced cellular concentration of ATP in the cytC mutant (Fig. 5). Compared to the decreased cellular concentration of ATP in cytC mutant, the obvious decreased cellular concentrations of NADH and ATP in aioE mutant, and the reverted NADH and ATP concentrations in strains GW4-ΔcytC-C and GW4-ΔaioE-C (Fig. 5) indicated that AioE may be related with the generation of NADH, and CytC may be involved with the generation of ATP with the addition of AsIII. Because the ORP gradient is AioBA > AioE > CytC (Fig. 6), we infer that the electron may be transferred from AioBA to AioE with the generation of NADH, and then to CytC with the generation of ATP. This hypothesis is agreed with the BlastP predicted protein function of AioE that it produced NADH when catalyzing the reaction of hydroxyl to generate carbonyl, which is similar to the reaction converting reduced AioBA back to oxidized AioBA29,31,32. The AioE may be responsible to the electron transport from the molybdenum ion center of AioBA with the generation of NADH and H+ 29,30,31,32. Though Cytochrome C is an electronic anchorman of Complex III in the respiratory chain, so far, four Complexes of the respiratory chain have been found to be able to create an electrochemical proton gradient that drives the synthesis of ATP. Moreover, Complex I could transfer electrons from the generated NADH to produce proton gradient36, which could link the AsIII oxidation with the respiratory chain, and then produce energy to support the bacterial growth (Fig. 4D).
Acting as an AsIII induced gene (Fig. 1C), aioE is located in the ars operon including genes responsible for AsIII oxidation and resistance in strain GW4, and co-transcribed with the arsR1-arsC1-arsC2-acr3-1. ArsR1 encoded by the arsR1 positioned close nearby acr3-1 may take charge of the regulation of aioE expression. Being described in the literatures published so far, ArsR repressor protein was responsible for the regulation of ars operons invariably37,38,39. Meanwhile, it was also reported to function as a regulator to involve in the expression of pstS1 and phoB1, which are located immediately adjacent to the aio gene cluster and essential for AsIII oxidation13. The regulation of aioE expression provides more evidence for the involvement of ArsR in bacterial AsIII oxidation.
Based on the RT-PCR results, the putative AsV reductase gene arsC and aioE are in the same operon. It is truly interesting that strain GW4 has both AsIII oxidation and AsV reduction function genes in the same operon. When aioE or aioA was deleted, the AsIII oxidation phenotype was disrupted and the AsV reduction phenotype was shown in the mutants which is most probably due to the exist of the arsC (Fig. S3). Generally, AsIII oxidation is also coupled with the enhanced bacterial growth (Fig. 4) via the production of NADH and ATP (Fig. 5) in strain GW4, which is probably more effective than AsV reduction and efflux for bacterial arsenic resistance. This may be a reason for the AsIII oxidation phenotype is dominant in strain GW4. In future studies, it is interesting to confirm if the ArsC is the AsV reductase or if this operon is regulated by ArsR. In addition, it is truly interesting to know that the two opposite function genes in the same operon is associated to the highly arsenite resistance (8 mM) of strain GW4.
In addition, being an AsIII/H+ antiporter, Acr3-1 is generally considered as an AsIII resistance protein40, while its coding gene located in the ars gene clusters isregulated by ArsR37,38,39. Interestingly, the essential of Acr3-1 for bacterial AsIII oxidation was discovered recently20, indicating that the AsIII/H+ antiporter on bacterial membrane was important for AsIII oxidation. AioE may produce H+ when transport electron from hydroxyl31, thus, when AioE transport the electron from AioBA, the generated H+ may involve with the AsIII trafficking across the cytoplasmic membrane, which was proven to be important to AsIII resistance and AsIII oxidation occurred in periplasm20,23,28.
In conclusion, we showed that the oxidoreductase AioE is essential for AsIII oxidation and resistance in heterotrophic AsIII oxidizing bacterium A. tumefaciens GW4. AioE appears to act as a novel electron transporter associating with the generation of NADH during bacterial AsIII oxidation. Since AsIII oxidation contributes the detoxification and the production of energy, the essential of AioE for AsIII resistance is also reasonable.
Methods and Materials
Bacterial strains and culture conditions
Bacterial strains and plasmids used in this study are listed in Table S1. A. tumefaciens strains were grown in a defined minimal mannitol medium (MMNH4)41 at 28 °C containing 0.1 mM phosphate, with or without the presence of 0.25 mM NaAsO2 (AsIII). E. coli strains were grown in Luria-Bertani medium42 at 37 °C. When necessary, kanamycin (Kan, 50 μg/mL), gentamicin (Gen, 50 μg/mL), tetracycline (Tet, 5 μg/mL) or ampicillin (Amp, 100 μg/mL) was added.
Phylogenetic relationship analysis
The aioE sequences was downloaded from National Center for Biotechnology Information Search database (NCBI). Phylogenetic relationships based on neighbor-joining method were then examined by downloading and aligning various sequences using ClustalX v1.8343 with tree constructed using Mega 6.044.
RT-PCR and quantitative RT-PCR analysis
Overnight cultures of strain GW4 were inoculated into 100 mL MMNH4 medium with or without the addition of 0.25 mM AsIII respectively and incubated at 28 °C with 100 rpm shaking. Samples used for RNA isolation were taken after 16 h cultivation. Total RNA was extracted used Trizol Kit (Invitrogen) and incubated with RNase-free DNase I (Takara) at 37 °C to remove the genomic DNA. Then, the reaction was terminated by addition of 50 mM EDTA at 65 °C for 10 min45. After confirming the negative DNA contamination and determining the concentration of RNA by spectrophotometer (NanoDrop 2000, Thermo), RT-PCR for testing the co-transcribe of the ars gene cluster was performed using the primers listed in Table S2, while 300 ng total RNA was reverse transcribed into cDNA with RevertAid First Strand cDNA Synthesis Kit (Thermo). The obtained cDNA was diluted 10-folds for real-time RT-PCR analysis using SYBR® Green Realtime PCR Master Mix (Toyobo)46 with primers listed in Table S2. Quantitative RT-PCR was performed by ABI VIIA7 in 0.1 mL Fast Optical 96-well Reaction Plate (ABI). Each reaction was replicated three times for eliminating the error. Gene expression was normalized by ∆∆CT analysis with an iQ5 Real-Time PCR Detection System (Bio-Rad, USA). All of the PCR products were confirmed by sequencing.
Construction of aioE and cytC mutant and complementation strains
The in-frame deletion in aioE and cytC was respectively constructed using crossover PCR47 with primers listed in Table S2. The PCR products were both cloned into BamHI and XbaI double digested pJQ200SK, respectively. The final constructed pJQ-aioE and pJQ-cytC were separately mobilized into GW4 via conjugation with E. coli strain S17-1. Single cross-over mutants of aioE or cytC were identified on MMNH4 agar plate containing 50 μg/mL Gen, which were then screened on MMNH4 agar with 20% sucrose2548. SucroseR GenSen trans-conjugants were then screened using diagnostic PCR and DNA sequencing to identify a double recombinant GW4-ΔaioE and GW4-ΔcytC.
For complementation, the complete aioE or cytC coding region was PCR-cloned as BamHI-XbaI fragments into palsmid pCPP30, respectively. Using conjugation, the resulting plasmids pCPP30-aioE was transferred into the mutants GW4-ΔaioE, while and pCPP30-cytC was transferred into the mutants GW4-ΔcytC. The mutant and complementary strains were confirmed by PCR using primers listed in Table S2 along with diagnostic sequencing. The successful complementary strain GW4-ΔaioE-C was constructed49.
Analysis of AsIII resistance and oxidation
To investigate the AsIII resistance of mutant strains, overnight cultures of GW4, GW4-ΔaioE, GW4-ΔaioE-C, GW4-ΔcytC, and GW4-ΔcytC-C (OD600 = 0.5–0.6) in MMNH4 medium and three diluted concentrations of these strains were each plated (2 μL) on solid MMNH4 medium containing 0 or 1 mM AsIII. Plates were photographed after 2–3 days at 28 °C until colonies formed. The qualitative AsIII oxidation was performed using AgNO3 staining14. Overnight cultures of GW4, GW4-ΔaioE, GW4-ΔaioE-C, GW4-ΔcytC, GW4-ΔcytC-C the AsIII oxidase large subunit gene aioA mutant and its complementary strain GW4-ΔaioA and GW4-ΔaioA-C constructed in previous work25 were inoculated on MMNH4 agar plates containing 0.1 mM phosphate and 0.25 mM AsIII. After 48 h cultured at 28 °C, the plates were flooded with 0.1 M AgNO314. AsV compounds react with AgNO3 generates brown color colonies indicating AsIII oxidation positive, while AsIII compounds cannot react with AgNO3 to generate brown color product revealing AsIII oxidation negative. The quantitative AsIII oxidation tests were detected using HPLC-HG-AFS (Beijing Titan Instruments Co., Ltd.)25. Overnight cultures of GW4, GW4-ΔaioE, GW4-ΔaioE-C, GW4-ΔcytC and GW4-ΔcytC-C (OD600 = 0.5–0.6) were each inoculated (200 μL) into 100 mL MMNH4 with or without 0.25 mM AsIII and incubated at 28 °C for 48 h with 100 rpm shaking. At designated times, culture samples were taken for viable plate counts and for monitoring AsIII/AsV. The qualitative AsV oxidation was performed using KMnO4 staining36. Overnight cultures of A. tumefaciens strains were inoculated on MMNH4 liquid medium containing 0.1 mM phosphate and 1 mM AsV. After 48 h cultured at 28 °C, 1 mL cultures was mixed with 50 μL 10 mM KMnO4 to detect the presence of AsIII associated with AsV reduction (yellow) or the absence of AsV reduction (pink)36.
Analysis of the amount of ATP and NADH
A. tumefaciens GW4, GW4-ΔaioE, GW4-ΔcytC and the complementary strains were each inoculated into 100 mL MMNH4 medium with or without the addition of 0.25 mM AsIII and incubated at 28 °C with 100 rpm shaking. The bacterial cells were collected by centrifugation (12,600 × g, 5 min, 4 °C) at designated times (during the AsIII oxidation process) and resuspended in 1 mL 0.4 M perchloric acid with 1.0 mM EDTA. After 5 min ultra-sonicated on ice, the unbroken cells were removed by centrifugation (12,600 × g, 5 min, 4 °C). Then the pH of the extracts were adjusted to 7.0 with 1 M K2CO3 and percolated with 0.22 μm filter membrane. The samples were analyzed by HPLC (HPLC 2690 series, Waters, Massachusetts, USA), using the mobile phase containing 90% 50 mM phosphate buffer, 10% acetonitrile, and 3.22 g/L tetrabutylammonium bromide (pH 6.8), and the flow velocity of the mobile phase was 1 mL/min. The amount of ATP and NADH were measured by comparing the retention times to standards50.
Expression and purification of proteins
The AioAB and AioE proteins were expressed using E. coli BL21 StarTM (DE3) pLysS for aioAB on vector pPROEX-HTA and aioE on vector pET-32a(+), respectively. Cells were grown at 37 °C overnight in LB medium containing the required antibiotics. Overnight culture was inoculated into 100 mL of LB and the culture was grown to OD600 of 0.1 and induced with 0.02 mM isopropyl-β-d-thiogalactoside (IPTG) for 16 h. Cells were collected by centrifugation (8,000 r/min for 10 min at 4 °C) after induction, and resuspended in 50 mM Tris-HCl (pH 7.5). After lysed by high pressure cell cracker and centrifuged at 8,000 rpm for 10 min at 4 °C, the cleared lysate of AioBA or AioE was respectively applied on a column of pre-equilibrate ProfinityTM IMAC Resins (Bio-RAD) by gravity flow. Each column was washed with 3 mL of Tris-HCl containing 20 mM imidazole (pH 7.5). Then AioE was eluted with Tris-HCl containing 200 mM imidazole (pH = 7.5), while AioAB was eluted with Tris-HCl containing 40 mM imidazole (pH 7.5). Purified proteins AioAB and AioE were stored at −80 °C, when used, the eluate was dialyzed against PBS to remove imidazole14. The concentrations of the purified AioBA and AioE were determined by Nano Drop 2000 (Thermo Scientific).
Detection of the redox potencial (ORP) of proteins using cyclic voltammetry
The ORP of the proteins were tested using cyclic voltammetry (CV) experiments, performed in PBS buffer, pH = 7.0, at 16 °C using a BAS 100B/W electrochemical workstation coupled with a BAS RDE-3 rotating disk electrode cell stand32. A three-electrode system was employed comprising a gold working electrode, a Pt wire counter, incorporating a saturated calomel electrode (SCE) as the reference. The experiments were carried out with 60 min nitrogen purged solutions and a nitrogen blanket was maintained during the measurement. The Au working electrode was mechanically, chemically, and electrochemically cleaned and polished as described51. The monolayer of 11-mercaptoundecanoic acid (MUA) was prepared on a clean Au electrode by immersion in a 20 mM ethanolic solution of MUA for at least 24 h52. The electrode was subsequently washed with copious amounts of ethanol and water to remove any loosely bound MUA molecules from the electrode surface. The electrode was placed in a solution containing 4 μL AioBA (31.5 μM) or AioE (37 μM) for 16 h at 4 °C, then it was rinsed with PBS buffer (pH = 7.0) to remove all protein molecules that were not immobilized on the surface. The experimental cyclic voltammograms (CVs) were simulated with the Chi660 program53.
Additional Information
How to cite this article: Wang, Q. et al. An Oxidoreductase AioE is Responsible for Bacterial Arsenite Oxidation and Resistance. Sci. Rep. 7, 41536; doi: 10.1038/srep41536 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The present study was supported by the National Natural Science Foundation of China (31500088) and the China Postdoctoral Science Foundation (2014M562038) to Q.W. We would like to express our gratitude to Dr. Timothy R. McDermott (Montana State University) for the scientific comments, and Ms. Tara Saley for the language modification.
Footnotes
The authors declare no competing financial interests.
Author Contributions Q.W. designed and performed the experiments and wrote the manuscript; Y.H., K.S., X.F., L.W. and M.L. participated in the experiments. G.W. designed the study and revised the draft of the manuscript. All authors read and approved the final manuscript.
References
- Chakraborti D. et al. Status of groundwater arsenic contamination in the state of West Bengal, India: a 20-year study report. Mol. Nutr. Food Res. 53, 542–551 (2009). [DOI] [PubMed] [Google Scholar]
- Sun G. et al. Arsenicosis history and research progress in mainland china. Kaohsiung J. Med. Sci. 27(9), 377–381 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft S. E. et al. Alkalilimnicola ehrlichii sp. nov., a novel, arsenite-oxidizing haloalkaliphilic gammaproteobacterium capable of chemoautotrophic or heterotrophic growth with nitrate or oxygen as the electron acceptor. Inter. J. System Evol. Microbiol. 57(3), 504–512 (2007). [DOI] [PubMed] [Google Scholar]
- Zhu Y. G. et al. Earth abides arsenic biotransformations. Annu. Rev. Earth Planet Sci. 42, 443–467 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen W. R. & Reimer K. J. Arsenic speciation in the environment. Chem. Rev. 89, 713–764 (1989). [Google Scholar]
- Inskeep W. P. et al. Arsenic (V)/(III) cycling in soils and natural waters: chemical and microbiological processes. In Frankenberger W. F. Jr. & Macy J. M. (Eds) Environmental Chemistry of Arsenic. pp. 183–215 (2001). [Google Scholar]
- Oremland R. S. & Stolz. J. F. The ecology of arsenic. Science 300, 939–944 (2005). [DOI] [PubMed] [Google Scholar]
- Pontius F. W. et al. Health implications of arsenic in drinking water. J. AWWA. 86, 52–63 (1994). [Google Scholar]
- Stolz J. F. et al. Arsenic and selenium in microbial metabolism. Annu. Rev. Microbiol. 60, 107–130 (2006). [DOI] [PubMed] [Google Scholar]
- Chen F. et al. Regulation of arsenite oxidation by the phosphate two-component system PhoBR in Halomonas sp. HAL1. Front Microbiol. 6, 923 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S. & Phung L. T. Genes and enzymes involved in bacterial oxidation and reduction of inorganic arsenic. Appl. Environ. Microbiol. 71, 599–608 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap D. R. et al. Complex regulation of arsenite oxidation in Agrobacterium tumefaciens. J. Bacteriol. 188, 1081–1088 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y. S. et al. Integrated co-regulation of bacterial arsenic and phosphorus metabolisms. Environ. Microbiol. 14, 3097–3109 (2012a). [DOI] [PubMed] [Google Scholar]
- Liu G. H. et al. A periplasmic arsenite-binding protein involved in regulating arsenite oxidation. Environ. Microbiol. 14, 1624–1634 (2012). [DOI] [PubMed] [Google Scholar]
- Anderson G. L. et al. The purification and characterization of arsenite oxidase from Alcaligenes faecalis, a molybdenum-containing hydroxylase, J. Biol. Chem. 267, 23674–23682 (1992). [PubMed] [Google Scholar]
- Kang Y. S. et al. Involvement of RpoN in regulating bacterial arsenite oxidation. Appl. Environ. Microbiol. 78, 5638–5645 (2012b). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing C. & Rosen B. P. Biogeocycles for redoxactive metal(loids): As, Cu, Mn and Se. In Encyclopedia of Microbiology. Schaechter M. (ed.) Oxford, UK: Elsevier, pp. 205–219 (2006). [Google Scholar]
- Bhattacharjee H. & Rosen B. P. Arsenic metabolism in prokaryotic and eukaryotic microbes. Molecular Microbiology of Heavy Metals. Springer Berlin Heidelberg. pp. 371–406 (2007). [Google Scholar]
- Murphy J. N. & Saltikov C. W. The ArsR repressor mediates arsenite-dependent regulation of arsenate respiration and detoxification operons of Shewanella sp. strain ANA-3. J. Bacteriol. 191, 6722–6731 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y. S. et al. Involvement of the Acr3 and DctA anti-porters in arsenite oxidation in Agrobacterium tumefaciens 5A. Environmental microbiology. 17(6), 1950–1962 (2015). [DOI] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemiosmotic processes. Annu. Rev. Biochem. 46, 996–1005 (1977). [DOI] [PubMed] [Google Scholar]
- Ducluzeau A. L. et al. Was nitric oxide the first deep electron sink? Trends Biochem. Sci. 34, 9–15 (2009). [DOI] [PubMed] [Google Scholar]
- Santini J. M. et al. A new chemolithoautotrophic arsenite-oxidizing bacterium isolated from a gold mine: phylogenetic, physiological, and preliminary biochemical studies. Appl. Environ. Microbiol. 66, 92–97 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Páez-Espino D. et al. Microbial responses to environmental arsenic. Biometals. 22, 117–130 (2009). [DOI] [PubMed] [Google Scholar]
- Wang Q. et al. Fate of arsenate following arsenite oxidation in Agrobacterium tumefaciens GW4. Environ. Microbiol. 17(6), 1926–1940 (2015). [DOI] [PubMed] [Google Scholar]
- Oremland R. S. et al. Anaerobic oxidation of arsenite in Mono lake water and by a facultative, arsenite-oxidizing chemoautotroph, strain MLHE-1. Appl. Environ. Microbiol. 68, 4795–4802 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulp T. R. et al. Arsenic(III) fuels anoxygenic photosynthesis in hot spring biofilms from Mono Lake, California. Science. 321(5891), 967–970 (2008). [DOI] [PubMed] [Google Scholar]
- vanden Hoven R. N. & Santini J. M. Arsenite oxidation by the heterotroph Hydrogenophaga sp. str. NT-14: the arsenite oxidase and its physiological electron acceptor. Biochim. Biophys. Acta. 1656(2–3), 148–155 (2004). [DOI] [PubMed] [Google Scholar]
- Kalimuthu P. et al. Electrochemically driven catalysis of Rhizobium sp. NT-26 arsenite oxidase with its native electron acceptor cytochrome c552. Biochim. Biophys. Acta. 1837(1), 112–120 (2014). [DOI] [PubMed] [Google Scholar]
- Belenky P. et al. NAD+ metabolism in health and disease. Trends Biochem. Sci. 32, 12–19 (2007). [DOI] [PubMed] [Google Scholar]
- Wang Z. et al. Identification of Phytophthora sojae genes involved in asexual sporogenesis. J. Genet. 88(2), 141–148. (2009). [DOI] [PubMed] [Google Scholar]
- Santini J. M. et al. The NT-26 cytochrome c552 and its role in arsenite oxidation. Biochim Biophys Acta. 1767(2), 189–196. (2007). [DOI] [PubMed] [Google Scholar]
- Li H. et al. In silico analysis of bacterial arsenic islands reveals remarkable synteny and functional relatedness between arsenate and phosphate. Front. Microbiol. 4, 347 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher B. G. et al. The chromosomal arsenic resistance genes of Thiobacillus ferrooxidans have an unusual arrangement and confer increased arsenic and antimony resistance to Escherichia coli. Appl. Environ. Microbiol. 66, 1826–1833 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S. et al. Arsenic metabolism: resistance, reduction, and oxidation. In Frankenberger W. F. Jr. & Macy J. M. (Eds) Environmental Chemistry of Arsenic. Marcell Dekker. pp. 247–272. (2002). [Google Scholar]
- Lenoble V. et al. Arsenite oxidation and arsenate determination by the molybdene blue method. Talanta. 61(3), 267–276 (2003). [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Families of arsenic transporters. Trends Microbiol. 7, 207–212 (1999). [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Biochemistry of arsenic detoxification. FEBS Lett. 529, 86–92 (2002). [DOI] [PubMed] [Google Scholar]
- Mukhopadhayay R. et al. Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiol. Rev. 26, 311–325 (2002). [DOI] [PubMed] [Google Scholar]
- Achour A. R. et al. Diversity of arsenite transporter genes from arsenic-resistant soil bacteria. Res. Microbiol. 158, 128–137 (2007). [DOI] [PubMed] [Google Scholar]
- Somerville J. E. & Kahn M. L. Cloning of the glutamine synthetase I gene from Rhizobium meliloti. J. Bacteriol. 156(1), 168–176 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J. et al. Molecular cloning: a laboratory manual (2nd ed.). New York: Cold Spring Harbor Laboratory Press (1989). [Google Scholar]
- Lane D. J. 16S/23S rRNA sequencing, p. 115–147, In Stackebrandt E. & Goodfellow M. (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, New York, NY (1991). [Google Scholar]
- Tamura K. et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. et al. Theoretical prediction and experimental verification of protein-coding genes in plant pathogen genome Agrobacterium tumefaciens strain C58. PloS one. 7(9), e43176. (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duangmano S. et al. Antiproliferative effects of cucurbitacin B in breast cancer cells: down-regulation of the c-Myc/hTERT/telomerase pathway and obstruction of the cell cycle. Int. J. Mol. Sci. 11(12), 5323–5338 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link A. J. et al. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179(20), 6228–6237 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicic V. et al. Generation of unmarked directed mutations in mycobacteria, using sucrose counter‐selectable suicide vectors. Mol. Microbiol. 20(5), 919–925 (1996). [DOI] [PubMed] [Google Scholar]
- Al-Niemi T. S. et al. Regulation of the phosphate stress response in Rhizobium meliloti by PhoB. Appl. Environ. Microbiol. 63(12), 4978–4981 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R. H. et al. Rapid extraction of (di)nucleotides from bacterial cells and determination by ion-pair reversed-phase HPLC. J. Microbiol. Methods. 25, 29–35 (1996). [Google Scholar]
- Lars J. C. et al. Electrochemical origin of hysteresis in the electron-transfer reactions of adsorbed proteins: contrasting behavior of the “Blue” copper protein, azurin, adsorbed on pyrolytic graphite and modified gold electrodes. J. Phys. Chem. B. 105(22), 5271–5282 (2001). [Google Scholar]
- Nakano K. et al. Cytochrome c self-assembly on alkanethiol monolayer electrodes as characterized by AFM, IR, QCM, and direct electrochemistry. Langmuir. 23, 6270–6275 (2007). [DOI] [PubMed] [Google Scholar]
- Brandt U. Energy converting NADH: quinone oxidoreductase (complex I). Annu. Rev. Biochem. 75, 69–92 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.