Abstract
Background:
Patients with schizophrenia and their first-degree relatives' exhibit abnormalities in neuropsychological and electrophysiological measures especially P300. The aim was to study the P300 and neuropsychological measures together in patients with schizophrenia, their unaffected siblings, and normal controls.
Materials and Methods:
Thirty patients diagnosed as schizophrenia, their unaffected biological siblings, and normal controls were included in the study. Inpatient group, the severity of symptoms was assessed by Positive and Negative Syndrome Scale. All subjects were administered P300 event-related potential, which was measured using oddball paradigm and specific neuropsychological tests from NIMHANS neuropsychiatry battery.
Results:
Both patients with schizophrenia and their unaffected biological siblings showed lower P300 amplitude and longer P300 latency when compared with the normal controls. The three groups showed statistically significant differences in digit symbol substitution test, digit vigilance test, Trail making test B and Stroop test (P < 0.001).
Conclusion:
Patients with schizophrenia and their unaffected biological siblings show deficits in both cognitive function tests and P300 event-related potential. Our results suggest a continuum in the electrophysiological and neuropsychological measures among the three groups.
Key words: Neuropsychological tests, P300, schizophrenia, siblings
INTRODUCTION
Schizophrenia is a chronic, heterogeneous, severe mental illness with impairments in biological, psychological, and social functions. Even though the mode of inheritance in schizophrenia is complex and primarily nonmendelian, it is well established that the schizophrenia has a high heritability.[1] Endophenotypes, described as internal phenotypes, are quantifiable characters which are the product of fewer genes when compared to phenotypes and this has the potential to simplify the genetic studies in schizophrenia.[2] Endophenotype abnormalities are seen in patients with schizophrenia well before the start of the illness, and these changes are also seen in first-degree relatives of patients with schizophrenia.[3] Of various markers, cognitive impairments, and event-related potential abnormalities are established endophenotype markers.[4,5]
P300 is a well-researched component of auditory event-related potential that shows abnormality in patients with schizophrenia.[6] It is correlated with information processing in the brain and has high temporal correlation. In most of the studies, P300 is elicited by oddball paradigm. In the oddball paradigm, an odd stimulus is inserted in between a regularly occurring stimulus and the person taking the test responds for the odd stimulus while not the regular stimulus. Since its first report in 1972, studies have shown that various event-related potential abnormalities manifest in persons suffering from schizophrenia, namely, the reduced P300 amplitude and increased latency. Similar abnormalities are also seen in first-degree relatives with schizophrenia probands.[7] Hence, event-related potential abnormalities are considered as the biological vulnerability marker of schizophrenia.
The cognitive defects in schizophrenia have been represented in eight different domains, namely, processing speed, attention/vigilance, working memory, verbal and visual learning/memory, reasoning and problem solving, verbal comprehension and social cognition.[8] Moreover, it is observed that these deficits are also found to be present in unaffected relatives and twins of patients with schizophrenia. Research suggests that the cognitive deficits have a strong genetic linkage, tends to run in families and predispose the unaffected first-degree relatives of patients with schizophrenia to develop the disorder in future.[9]
There are only few studies in Indian literature that has analyzed event-related potentials and neuropsychological measurements together in patients with schizophrenia and their unaffected siblings. The present study was based on the assumption that the P300 and neuropsychological measurements would show a continuum when compared between the patients with schizophrenia, their siblings, and normal controls. It was also hypothesized that the patients with schizophrenia would have the lowest scores in neuropsychological measurements and significant abnormalities in P300 while the siblings and normal controls would follow. For this, the study was designed to measure P300 and neuropsychological functions in patients with schizophrenia, their biological siblings, and normal controls.
MATERIALS AND METHODS
Study population
The current study was conducted at Institute of Mental Health, Madras Medical College, Chennai, Tamil Nadu, India. Thirty consecutive patients with schizophrenia, attending the psychiatry outpatient department, diagnosed using International Classification of Diseases-10 and confirmed using Schedules for Clinical Assessment in Neuropsychiatry (SCAN), were included. The inclusion criteria for the recruitment of patients were age between 18 and 40 years, no other comorbid psychical or mental illness except tobacco use and willing to give informed written consent. The sibling group which included thirty participants were either brothers or sisters of the patients with schizophrenia. The inclusion criteria for the siblings were age between 18 and 40 years, no history of psychical or mental illness except tobacco use and willing to give informed written consent. Normal controls were selected from the relatives and friends of patients who attended the hospital for psychical conditions and the inclusion criteria to recruit normal controls include age group between 18 and 40, having negative family history for psychiatric illness, no psychical or mental illness except tobacco use and willing to give informed written consent. Three groups were matched for age, gender, and education. SCAN was administered to the sibling and normal control group to exclude any psychiatric illness. Ethical Committee of the institute approved the study protocol.
The neuropsychological tests and electrophysiological tests were done in two separate days. The following cognitive function tests were administered to all the participants of this study which include digit symbol substitution test (DSST), Digit vigilance test (DVT), Trail making test (TMT) – B and Stroop test using NIMHANS Neuropsychology Battery-2004-Manual.[10] The total time taken to complete the above battery of tests was nearly one and half hours.
DSST was used to measure the speed, attention, visual scanning, and memory. It consists of an array of nine numbers, each paired with a symbol. Beneath the array is a set of numbers alone and the subject's task is to write the correct symbol under each of these numbers as rapidly as possible. The raw score is computed by counting the number of correct responses made in 120 s.
DVT measures sustained attention. It consists of numbers 1–9 randomly ordered and placed in rows on a page. There are thirty digits per row and fifty rows on the sheet. The subject has to focus on the target digits, i.e., six and nine and cancel them alone amongst other distracter digits. Inability to sustain and focus attention leads to both increased time to complete the test as well as errors.
TMT – B test is considered as a measure of focused attention. Here, subjects have to point to numbers in alternating colors with the successive numbers in ascending order. In each part, a practice form precedes the test. The test is given only after the subject has understood the principle involved and has performed the practice sheet satisfactorily.
The Stroop test is used as a measure of response inhibition. It contains capital letters printed on paper in different colors. The color print does not correspond with the color designated by the words. In the first trial, patients are asked to read the word and in the second trial, name the color in which the word was printed. They should read it column-wise. Timings are recorded for both trials. The reading time is subtracted from the naming time to get the Stroop test effect score.
RMS Neuro Diagnostic System was used to record P300 auditory event-related potentials. For the purpose of this study, the oddball paradigm was developed in the following manner. The auditory stimuli were presented to the subjects in both the ears using headphones. The auditory stimuli were pure tones presented to the subjects at the rate of 1.25 s for 10 min. Each tone was 100 ms in duration at an intensity of 70-db sound level. The pure tones were of 1000 and 2000 Hz in which the rare frequency would be the 2000 Hz. The probability of the occurrence of the rare frequency pure tone was set at 0.2. The subjects who were being tested were instructed to press a button using the right thumb as soon as they hear the rare target stimuli. Using the copper electrodes, the electroencephalogram of the subject was recorded at three sites namely, frontal (Fz), central (Cz), and parietal (Pz) according to the 10–20 international system of electrode placement. The recordings were stored in the computer and were used for further analysis.
Statistical analysis
The results were tabulated and analyzed using the statistical package, SPSS 16.0 (SPSS Inc., Chicako, USA). Age across the three groups was compared using Kruskal–Wallis test while the other socio-demographic variables were compared between the three groups by Chi-square test. The statistical difference among the groups for P300 and neuropsychological measurements were analyzed by analysis of variance. The difference was considered statistically significant at P < 0.01 for all the tests.
RESULTS
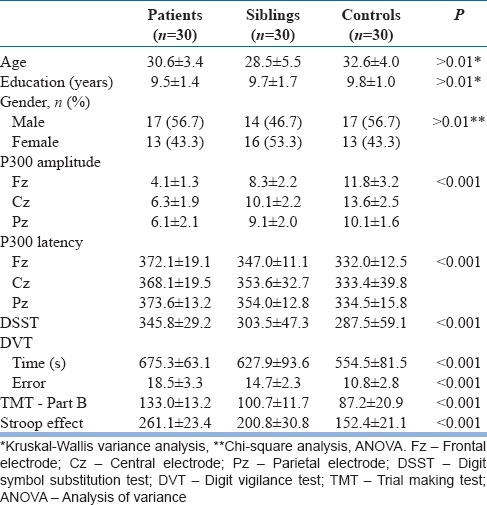
No statistical significance was noted among the three groups with respect to age, sex, and education.
It was observed that there was a statistical difference among three groups in amplitude and latency of P300. The amplitude was lowest in the patient group while the normal controls had the highest and siblings of patients with schizophrenia lying in between them. When the latency of P300 was compared, it was longer in patients when compared with normal controls. Patient group had longer latency when compared with sibling group also. Statistically significant difference was observed among three groups in all the three leads studied.
Table 1 depicts the results of neuropsychological measurements in the participants. Both the patients with schizophrenia and their siblings performed poorly in the neuropsychological tests when compared with the normal controls. Statistically significant difference was seen in all the neuropsychological tests administer among the three groups. The scores in the tests also showed an increasing trend that patients fall in the lowest range while the siblings and normal controls had higher and highest scores, respectively.
Table 1.
Participants characteristics
DISCUSSION
With respect to P300 amplitude measurements in the three groups of subjects, there was a significant difference between the three groups. The difference was more in the case of the amplitude measured in the central and parietal regions when compared with the frontal regions and showed a statistical significance between the groups (F = 77.587). In a study, Kidogami et al. showed that amplitude of P300 in the patients with schizophrenia and the first-degree relatives of patients with schizophrenia was of low amplitude when compared with the control group but no statistically significant difference between the first degree relatives and patients with schizophrenia.[11] In contrast, several studies have shown negative reports regarding P300 abnormalities in unaffected siblings of patients with schizophrenia.[12]
In this study, P300 latency was longer for the patients with schizophrenia followed by siblings and controls and showed a significant difference between the groups. In the study by Araki et al., it was found that latency was prolonged in the case of patients with schizophrenia when compared the controls.[13] In the case of first-degree relatives of patients with schizophrenia, the latency was prolonged when compared to the normal controls and comparable to the patient group.
In DSST, siblings showed a slower rate of mental speed when compared with the control group while patients with schizophrenia had the lowest scores in the test. In a study by Amaresha et al., it was showed that the patients with schizophrenia performed poorly and performance were dependent upon the negative score in Positive and Negative Syndrome Scale.[14] Similarly, there was a decrease in the performance in the tests measuring the speed of processing in the first degree relatives of patients with schizophrenia.
The patients with schizophrenia and their siblings performed poorly with errors when compared to the control group in the continuous performance test. Avila et al. studied the continuous performance test in the schizophrenia spectrum disorders and showed a gradation in the performance with highest deficits in the patients with schizophrenia while lesser impairments in the patients with schizotypal personality disorder.[15] In another study by Bove which studied the continuous performance test in 24 first-degree relatives of patients with schizophrenia, showed a slight decrease in performance in the test when compared with the control group and test performance was not dependent on the basic symptoms.[16]
The present study showed a gradation in the scoring of TMT-B with lowest in the patients with schizophrenia followed by the siblings and the controls. A similar result was reported by Fujiki et al., that the relatives of patients with schizophrenia show difference in the performance in both TMT A and B.[17]
The Stroop effect showed a significant difference between the three groups indicating that the response inhibition was affected in the sibling and patient group when compared to the controls. Similar findings were reported by other studies and reviewed in a meta-analysis. However, in a study by Becker et al., the postconflict related performance in Stroop test had intact results comparable to the general population.[18]
The present study's hypothesis that the patient and sibling groups would exhibit a P300 and neurocognitive performance continuum, with the controls performing the best, followed by the siblings and patients, was supported by the results. We observed a gradation in all the test measurements used in the present study and found a statistically significant difference among the three groups. Similar results were observed in a previous study where a continuum in cognitive performance was exhibited among the groups but did not reach the level of significance.[19]
The major strength of the present study is the evaluation of cognitive functions and P300 in patients with schizophrenia, their healthy siblings and normal controls who were matched for age, gender, and years of education. However, the current study is not without limitations. A major limitations of the study were the small sample size and effect of antipsychotic drugs on P300 and cognition. In the future studies, the patients with schizophrenia could be followed up, and periodic assessment of the event-related potential, P300 and neuropsychological tests could be done to measure the progress of the illness. In the unaffected biological siblings of patients with schizophrenia, these can be used as markers for underlying biological processes and could help in early detection of illness. In conclusion, it is observed that there is a continuum in P300 and neuropsychological measures in patients with schizophrenia, their unaffected siblings and normal controls with an increasing graduation from the patients to controls.
CONCLUSION
The present observation of P300 abnormalities and neuropsychological Performances on various Neuropsychological tests confirms the previous observation that P300 presents as a Biological vulnerability marker for schizophrenia and also a trait marker. However, Future Observation in a homogenous patient sample with an exclusion of confounding variables and application of latest neuro imaging technologies will give a more precise report of P300 as endophenotype vulnerability marker for the Genetic etiology of Schizophrenia, thus helping the Genetic research literature in Schizophrenia
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Coryell W, Zimmerman M. The heritability of schizophrenia and schizoaffective disorder. A family study. Arch Gen Psychiatry. 1988;45:323–7. doi: 10.1001/archpsyc.1988.01800280033005. [DOI] [PubMed] [Google Scholar]
- 2.Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 3.Braff DL, Greenwood TA, Swerdlow NR, Light GA, Schork NJ. Investigators of the Consortium on the Genetics of Schizophrenia. Advances in endophenotyping schizophrenia. World Psychiatry. 2008;7:11–8. doi: 10.1002/j.2051-5545.2008.tb00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: The viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snitz BE, Macdonald AW, 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: A meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–94. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 2004;70:315–29. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: Patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- 8.Fioravanti M, Carlone O, Vitale B, Cinti ME, Clare L. A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychol Rev. 2005;15:73–95. doi: 10.1007/s11065-005-6254-9. [DOI] [PubMed] [Google Scholar]
- 9.Bilder RM, Howe A, Novak N, Sabb FW, Parker DS. The genetics of cognitive impairment in schizophrenia: A phenomic perspective. Trends Cogn Sci. 2011;15:428–35. doi: 10.1016/j.tics.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao SL, Subbakrishna DK, Gopukumar K. NIMHANS Neuropsychology Battery-2004. Bangalore: NIMHANS Publications; 2004. [Google Scholar]
- 11.Kidogami Y, Yoneda H, Asaba H, Sakai T. P300 in first degree relatives of schizophrenics. Schizophr Res. 1991;6:9–13. doi: 10.1016/0920-9964(91)90015-j. [DOI] [PubMed] [Google Scholar]
- 12.Sumich A, Kumari V, Dodd P, Ettinger U, Hughes C, Zachariah E, et al. N100 and P300 amplitude to go and no-go variants of the auditory oddball in siblings discordant for schizophrenia. Schizophr Res. 2008;98:265–77. doi: 10.1016/j.schres.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Araki T, Kasai K, Kirihara K, Yamasue H, Kato N, Kudo N, et al. Auditory P300 latency prolongation with age in schizophrenia: Gender and subcomponent effects. Schizophr Res. 2006;88:217–21. doi: 10.1016/j.schres.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 14.Amaresha AC, Danivas V, Shivakumar V, Agarwal SM, Kalmady SV, Narayanaswamy JC, et al. Clinical correlates of parametric digit-symbol substitution test in schizophrenia. Asian J Psychiatr. 2014;10:45–50. doi: 10.1016/j.ajp.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avila MT, Robles O, Hong LE, Blaxton TA, Myers CS, Wonodi I, et al. Deficits on the continuous performance test within the schizophrenia spectrum and the mediating effects of family history of schizophrenia. J Abnorm Psychol. 2006;115:771–8. doi: 10.1037/0021-843X.115.4.771. [DOI] [PubMed] [Google Scholar]
- 16.Bove EA. Cognitive performance and basic symptoms in first-degree relatives of schizophrenic patients. Compr Psychiatry. 2008;49:321–9. doi: 10.1016/j.comppsych.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Fujiki R, Morita K, Sato M, Kamada Y, Kato Y, Inoue M, et al. Reduced prefrontal cortex activation using the trail making test in schizophrenia. Neuropsychiatr Dis Treat. 2013;9:675–85. doi: 10.2147/NDT.S43137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker TM, Kerns JG, Macdonald AW, 3rd, Carter CS. Prefrontal dysfunction in first-degree relatives of schizophrenia patients during a Stroop task. Neuropsychopharmacology. 2008;33:2619–25. doi: 10.1038/sj.npp.1301673. [DOI] [PubMed] [Google Scholar]
- 19.Sevik AE, Anil Yagcioglu AE, Yagcioglu S, Karahan S, Gürses N, Yildiz M. Neuropsychological performance and auditory event related potentials in schizophrenia patients and their siblings: A family study. Schizophr Res. 2011;130:195–202. doi: 10.1016/j.schres.2011.04.018. [DOI] [PubMed] [Google Scholar]


