Abstract
Background
Syphilis management is complex and demonstration of treatment response requires monitoring of nontreponemal antibody titers for a ≥ 4-fold decline and/or seroreversion to nonreactive titers.
Methods
We evaluated data from a multi-center clinical trial of syphilis treatment conducted from 2000–2009 involving HIV-negative patients age ≥ 18 years with early syphilis. To assess the rate of titer decline and seroreversion after effective therapy, rapid plasma reagin (RPR) titers were analyzed at 1, 3, 6, 9 and 12 months among patients with an appropriate treatment response. We plotted the rate of RPR titer decline after treatment, estimated the frequency of seroreversion, and conducted multivariate analyses to assess characteristics associated with seroreversion.
Results
Among 369/465 (79.4%) HIV-negative patients with early syphilis who had an appropriate treatment response, 333 participants had complete RPR data over 12 months. Although the decline in RPR titers was ≥ 4-fold among 88.0% (293/333) of participants at 3 months and ≥ 8-fold among 77.8% at 6 months, only 9.6% achieved complete RPR seroreversion at 6 months and 17.1% at 12 months after therapy. Male gender (adjusted odds ratio [AOR]: 4.3, 95% confidence interval [CI]: 1.8, 10.5) and baseline RPR titers ≤ 1:32 (AOR: 14.5; 95% CI: 6.8, 31.2) were associated with higher odds of seroreversion compared to females and titers > 1:32, respectively.
Conclusions
Despite a ≥ 4-fold RPR titer decline after treatment, the majority of HIV-negative patients with early syphilis failed to have seroreversion at 12 months. Nontreponemal antibody titers often persist despite an appropriate treatment response.
Keywords: syphilis, serological treatment response, seroreversion
Introduction
The syphilis epidemic is unrelenting and now disproportionately involves men who have sex with men (MSM) in the United States and Western Europe. Traditional syphilis screening and monitoring of therapeutic response rely heavily on serological nontreponemal antibody titers, due to our inability to culture Treponema pallidum subsp. pallidum in vitro. Resolution of signs and symptoms after treatment of primary or secondary syphilis is indicative of response to therapy. However, serological follow-up is recommended for all patients to determine an appropriate serological treatment response defined as ≥ four-fold (or two dilutions; e.g., 1:64 to 1:16) decline from baseline nontreponemal antibody titers or seroreversion to nonreactive.1 The Centers for Disease Control and Prevention (CDC) initially described the appropriate serological treatment response in the 1993 Sexually Transmitted Diseases (STD) Treatment Guidelines based on a study by Brown, et al.,2 which defined a four-fold decrease in Venereal Disease Research Laboratory (VDRL) titers at 3 months as clinical cure after treatment of primary or secondary syphilis. The rate of seroreversion was reported in other studies to vary between 44% – 97% for primary syphilis, 22 – 84% for secondary syphilis, and 13 – 73% for early latent syphilis at 12 months following therapy.3–5
Clinical and serological follow-up after treatment of early syphilis are recommended at 3, 6, 9, 12, and 24 months for HIV-infected persons, and at 6 and 12 months for HIV-negative persons.1 More frequent evaluation is also recommended if the likelihood of follow-up is uncertain or repeat infection is a concern.1 Despite a ≥ 4-fold decline in nontreponemal antibodies after treatment, some patients will have persistent low-level titers over time with lack of complete seroreversion. In the absence of symptoms or possible re-infection, there is considerable uncertainty regarding the clinical significance of patients with persistent nontreponemal antibody titers despite achieving at least a 4-fold decline following therapy, which is especially problematic when a history of prior syphilis treatment or previous nontreponemal titers are not well documented. These patients may undergo unnecessary evaluations, additional therapy, or follow-up visits for serological monitoring.
Characterizing the expected serological outcomes after effective syphilis treatment is thus important to improving our understanding and interpretation of nontreponemal antibody titers following therapy. We previously published data from a randomized controlled trial (RCT) for treatment of early syphilis with benzathine penicillin versus azithromycin involving HIV-negative participants and analyzed their serological outcomes after therapy.6 The majority of 465 participants (79.4%) achieved serological cure with similar cure rates at 6 months between treatment arms.7 In order to evaluate the expected decline in nontreponemal antibody titers and rate of complete seroreversion to nonreactive tests after effective therapy, we conducted further analyses of RCT participants who exhibited an appropriate serological response within 6 months of treatment.
Materials and Methods
We analyzed data from an open-label, RCT of syphilis treatment among HIV-negative patients age ≥ 18 years conducted from June 2000 to March 2009 at five sexually transmitted disease clinics in the U.S. and three clinics in Madagascar.6 Eligible patients were classified with primary, secondary, or early latent syphilis according to standard criteria,8 and underwent treatment randomization to 2.4 million units of benzathine PCN intramuscular injection or azithromycin 2.0 grams orally as directly observed therapy. The master study protocol was approved by the institutional review boards at the University of Alabama at Birmingham and study sites.
Rapid plasma reagin (RPR) titers were obtained at 1, 3, 6, 9 and 12 months after treatment; a subset of patients completed 18 and 24 month follow-up visits after treatment. An appropriate serological response to treatment in the primary study was defined as a ≥ 4-fold (2 dilutions) decrease in RPR titer and/or complete seroreversion at 6 months, defined as a non-reactive RPR after therapy. Patients who exhibited an initial ≥ 4-fold decline after therapy but had persistently positive low-level nontreponemal antibody titers were considered as serological responders who failed to exhibit seroreversion.
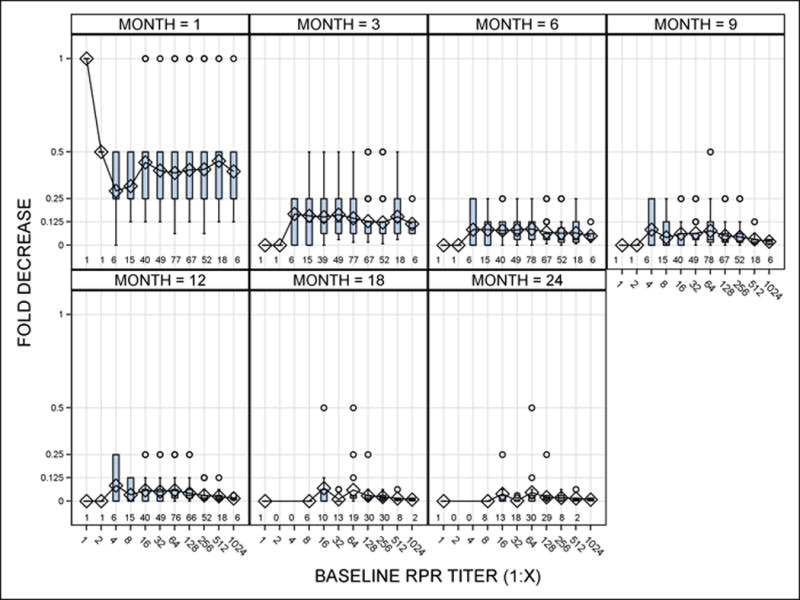
For this analysis, we evaluated participants from the parent study who exhibited a ≥ 4-fold serological titer response at 6 months as previously described by Seña, et al.7 and had at least 12 months of follow-up. We did not include data from study participants who had < 4-fold decline in RPR titers after therapy reported in our prior study,6,7 because our objective was to evaluate the rate of decline and complete seroreversion among those with an appropriate treatment response. In order to plot the rate of titer decline after treatment, we divided the RPR titers obtained at each time point after therapy by the baseline titer to generate decimal values. A 4-fold decline in titers corresponded to a value of 0.25 (e.g. 3 month titer of 1:8 with baseline titer at 1:32 resulting in 8/32 =0.25), whereby ≥ 8-fold decline in titers correspond to values ≤0.125. The decline in titer ratios were then evaluated using box plots for visualization (Figure).
To assess demographic and clinical characteristics associated with seroreversion, bivariate analyses were conducted using odds ratios (OR) and 95% confidence intervals (CI). Model development for characteristics associated with seroreversion was conducted by considering a large set of models, including and excluding variables and using the Akaike information criterion (AIC) to develop the final model.9 The final model was then checked using both forward and backward stepwise selection process to confirm the variable selection process. Adjusted odds ratios (AOR) and estimated 95% CIs are presented in Table 2.
Table 2.
Bivariable analyses of characteristics among participants who exhibited seroreversion versus persistent nontreponemal antibody titers at 12 months (despite an appropriate serological treatment response), and adjusted model for characteristics associated with seroreversion.
| Characteristics | Seroreversion within 12 Months | Reactive RPR at 12 Months | OR (95% CI) | AOR (95% CI) |
|---|---|---|---|---|
| Male | 49 (23.3 %) | 161 (76.7 %) | 4.4 (2.0, 9.6) | 4.3 (1.8, 10.5) |
| Female | 8 (6.5 %) | 115 (93.5 %) | * | * |
| Malagasy | 42 (15.1%) | 237 (85.0 %) | 0.4 (0.1, 1.7) | 0.2 (0.1, 1.3) |
| Black | 12 (26.7 %) | 33 (73.3 %) | 0.7 (0.2, 3.9) | 0.8 (0.1, 4.7) |
| White + other | 3 (33.3 %) | 6 (66.7 %) | * | * |
| Stage: Primary | 32 (37.2 %) | 54 (62.8 %) | 5.1 (2.3, 12.7) | ----- |
| Stage: Secondary | 17 (10.0 %) | 153 (90.0 %) | 1.0 (0.4, 2.4) | ----- |
| Stage: Early Latent | 8 (10.4 %) | 69 (89.6 %) | * | ----- |
| Baseline RPR Titers ≤ 1:32 | 46 (41.1 %) | 66 (58.9 %) | 13.3 (6.5, 27.1) | 14.5 (6.8, 31.2) |
| Baseline RPR Titers > 1:32 | 11 (5.0 %) | 210 (95.0 %) | * | * |
| Penicillin | 32 (19.1 %) | 136 (81.0 %) | 1.3 (0.7, 2.3) | ----- |
| Azithromycin | 25 (15.2 %) | 140 (84.9 %) | * | ----- |
RPR= rapid plasma reagin; OR= odds ratio based on bivariate results; CI, confidence interval; AOR= adjusted odds ratio based on model
Reference category
Results
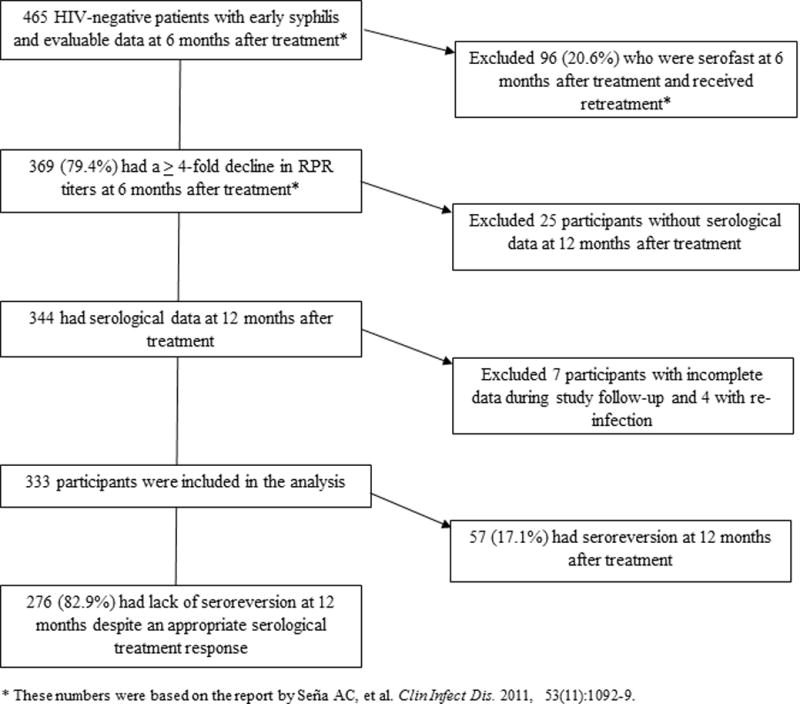
Figure 1 illustrates the flow diagram of HIV-negative participants who were treated for early syphilis in our study, and had serological data over a 12 month period following therapy. There were 369 participants who exhibited at least a 4-fold decline in RPR titers at 6 months after treatment, of which 344 had serological data at the 12 month visit. After excluding 7 participants with incomplete data during follow-up, and 4 participants who had re-infection, we analyzed data from 333 participants who had an appropriate serological treatment response and complete RPR data for each study visit over 12 months. Among these participants, only 9.6% achieved complete seroreversion at 6 months following treatment (Table 1); at 12 months, this proportion increased to only 17.1%, with the majority of participants characterized as serological responders who failed to exhibit seroreversion. The median RPR titer among the 276 patients who did not have seroreversion was 1:2 (range 1:1 – 1:1024) at 12 months after treatment; 13.0% of patients still had persistent titers at ≥ 1:16. The proportion of participants who had seroreversion at 12 months was highest at 37.2% (32/86) among patients with primary syphilis, and lowest among those with secondary syphilis at 10% (17/170); there were no differences in the proportion of seroreversion between secondary and early latent syphilis groups.
Figure 1.
Flow diagram of study participants treated for early syphilis who had an appropriate serological response after therapy and serological data over a 12 month period included in this analyses.
Table 1.
Proportion of patients with seroreversion (negative RPR) after a ≥ 4-fold decline in nontreponemal antibody titers following syphilis treatment, according to stage of infection and time after therapy.
| Stage of syphilis | 3 months | 6 months | 9 months | 12 months |
|---|---|---|---|---|
| Primary (n=86) | 9 (10.5%) | 20 (34.9%) | 30 (34.9%) | 32 (37.2%) |
| Secondary (n=170) | 1 (0.6%) | 7 (4.1%) | 10 (5.9%) | 17 (10.0%) |
| Early latent (n=77) | 5 (6.5%) | 5 (6.5%) | 8 (10.4%) | 8 (10.4%) |
| Total early syphilis (N=333) | 15 (4.5%) | 32 (9.6%) | 48 (14.4%) | 57 (17.1%) |
When we plotted RPR titer decreases from baseline titers by month after therapy (Figure 2), a 2-fold decline in titers was already evident by one month after treatment (corresponding to a value of 0.5). Eighty-eight percent (293/333) of participants had a ≥ 4-fold decline at 3 months after treatment and 77.8% had a ≥ 8-fold among at 6 months, irrespective of baseline RPR titers. The median decline in RPR titers was at least 8-fold (≤ 0.125) by 6 months following therapy, although there were variations in this titer change and outliers up to 24 months after treatment.
Figure 2.
Box plots of decrease in nontreponemal antibody titers based on baseline RPR titers and month (s) after therapy (x-axis). Decline in titers were represented as decimal values in the y-axis, in which 0.5 indicated a 2-fold decline in titers (e.g. 1:16 at one month divided by baseline titer of 1:32, or 16/32=0.5); 0.25 indicated a 4-fold decline; and 0 indicated seroreversion. The box for each baseline titer represented the 25–75th interquartile range, with lines indicating the median value for decline in titers. Each panel also illustrates the range of values outside of the intra-quartile range (whiskers) and outliers (circles) for each baseline titer. The number of observations that corresponded to the baseline titers are included at the bottom of each panel.
Bivariate analyses (Table 2) showed that patient gender, syphilis stage, and baseline RPR titers were each associated with an increased likelihood of complete seroreversion at 12 months. Male participants with early syphilis had a higher odds of achieving seroreversion (OR 4.4; 95% CI: 2.0–9.6) than female participants. Primary syphilis patients had a five-fold higher odds for seroreversion (OR 5.1; 95% CI: 2.3–12.7) compared to those with early latent or secondary syphilis infection. Patients with baseline RPR titers ≤ 1:32 had a significantly higher odds for seroreversion (OR 13.3; 95% CI: 6.5–27.1) compared to those with titers > 1:32.
After adjusting for the effects of gender, race, syphilis stage, baseline titer and syphilis treatment in the multivariate analyses, a higher odds (AOR 4.3, 95% CI: 1.8, 10.5) for seroreversion was found among men compared to women. Additionally, patients with baseline RPR titers ≤ 1:32 had an AOR of 14.5 (95% CI: 6.8, 31.2) for seroreversion at 12 months compared to patients with baseline titers > 1:32.
Discussion
Among HIV-negative patients with early syphilis who demonstrated an appropriate serological treatment response in the six months following therapy, our study demonstrates that a 4-fold decline in RPR titers can occur as early as one month after therapy, and an 8-fold decline as early as 6 months following therapy. The rate of decline appeared to be steepest in the first 3 months following treatment in our study population. This finding generally supports the current treatment guidelines regarding the time points for serological monitoring and expected declines in nontreponemal antibody titers following therapy. However, complete seroreversion occurred in only 17% of early syphilis patients overall by 12 months after treatment. Male gender, primary syphilis stage and lower baseline RPR titers were associated with higher odds of seroreversion.
Nontreponemal tests measure IgM and IgG antibodies to lipoidal antigens, principally cardiolipin, released from damaged host cells and T. pallidum, whose lipid composition is 13% cardiolipin.10–11 Nontreponemal antibody titers tend to correlate with disease activity and are generally higher during early syphilis than during later stages of infection.12 Seroreversion, or the reversion of nontreponemal tests from positive to negative results, was predominantly used by early syphilologists as an indicator of effective treatment.13 Brown, et al. was the first to evaluate the exponential decline in nontreponemal titers in 1985 among 202 patients after treatment of early syphilis.2 In addition to demonstrating up to an 8-fold decline in VDRL titers at 6 months after therapy, they also noted a relationship between duration of syphilis, non-treponemal titers, and serological response, in which seroreversion was more rapid when there was a shorter duration of infection and a lower initial VDRL titer.2,5
Some of the earlier studies suggested that seroreversion occurred in the majority of patients by 1–2 years after treatment of primary and secondary syphilis.14–15 However, the patients in these studies were treated with 4.8 million units of benzathine PCN and were monitored serologically with the VDRL, which may have differences in quantitative titers relative to the RPR. Romanowski, et al.5 noted lower rates of complete seroreversion similar to our findings, reporting that only 43.9% seroreverted with primary syphilis, 21.6% with secondary syphilis, and 13.7% with early latent syphilis at 12 months after treatment. These investigators observed higher proportions of seroreversion at 36 months after therapy for 71.9%, 56.3%, and 26.1% of patients with primary, secondary, and early latent syphilis, respectively. However, the proportion of participants with available RPR titers at ≥ 12 months following therapy was only 41% in this retrospective study.5 We had data on some of our patients up to 24 months after therapy; however, the number with available RPR titers beyond 12 months was relatively small which limited our ability to assess seroreversion rates over a longer period of time.
We found higher odds of complete seroreversion among patients with primary syphilis compared to secondary or early latent syphilis in the bivariate analyses, and among participants with lower baseline RPR titers compared to higher titers in both the bivariate analysis and adjusted model. The relationship between seroreversion and stage of syphilis is not surprising since several studies have found an association between earlier stage of syphilis and serological treatment response.16 Romanowski, et al.5 also demonstrated that seroreversion rates depended on syphilis stage and baseline RPR titers, in which patients with higher initial titers (>1:8) were less likely to serorevert in primary and secondary syphilis. A subsequent analysis of their data further showed that individual serologic response appeared to be a linear function of (log) time, in which the slope of the response line in the first year following therapy was an important predictor of seroreversion along with the pre-treatment titer.17
In contrast, other studies have reported that higher baseline titers (e.g., ≥ 1:32) are significantly associated with a greater likelihood of achieving serological cure among both HIV-infected and HIV-uninfected patients.5, 8, 18–23 Therefore, our findings of an inverse relationship, in which participants with RPR titers ≤ 1:32 had more than a 10-fold higher likelihood of seroreversion after therapy, further complicates the hypotheses regarding the significance of nontreponemal antibody titers during active syphilis infection. Investigators have suggested that persistent nontreponemal antibody titers following therapy may be due to a lingering benign immune response rather than due to residual T. pallidum in the host.16 Translational studies are therefore necessary to investigate these hypotheses and the correlation of quantitative nontreponemal antibody titers with organism burden and the immune response.16 Conducting these studies would be challenging but crucial to our understanding of the antibody responses in patients with syphilis infection before and after treatment.
We also found that male participants, which represented 63.1% of our study population, had a higher likelihood of seroreversion than female patients; however, no associations were noted between participants’ race, treatment arm, and likelihood of seroreversion. A systematic review of other studies evaluating factors that affect serological treatment outcomes reported conflicting results regarding the effect of patient age or gender on these outcomes.16 We found a low rate of seroreversion among HIV-negative patients with early syphilis enrolled from our multicenter study, which may not be generalizable to the syphilis infected population in the US comprised primarily of MSM of whom 51.2% are HIV-infected.24 However, only two out of nine studies investigating the effect of HIV infection on serological treatment outcomes reported that HIV-infection decreased the likelihood and time to serological cure.16,19,25 Based on these findings, HIV-infected patients with early syphilis may experience a similar decline in RPR titers or rate of seroreversion after treatment.
The 2015 CDC STD Treatment Guidelines states that clinical and serologic evaluation should be performed at 6 and 12 months after treatment of primary and secondary syphilis, and at 6, 12, and 24 months for latent syphilis.1 We used a more stringent criteria of a ≥ 4-fold decline at 6 months due to the initial design of the clinical trial.6 Therefore, our findings represent the response that would be characteristic of patients with a more rapid treatment response, and not all patients who may subsequently exhibit a 4-fold decline in nontreponemal antibody titers at ≥ 12 months after therapy. We did not assess the rate of seroreversion among patients with late latent syphilis in our study, so additional prospective studies involving diverse populations with longer follow-up periods would be ideal to determine clinical outcomes among persons with persistent nontreponemal antibody titers following therapy. Lastly, patients with a history of prior syphilis infection may have a different rate of seroreversion after treatment, but only 17 of our participants had a syphilis history limiting our ability to assess this variable in our analyses.
Based on our findings, clinicians should be aware that most patients who exhibit ≥ 4-fold decline in nontreponemal antibody titers in the first year after treatment for early syphilis may continue to have reactive serological tests over time. Longer periods of serological monitoring would be required if complete seroreversion after syphilis treatment should be the desired outcome; however, the benefits of such an approach are unproven and unlikely to be significant when an appropriate treatment response has already been documented. Given that only a small proportion of patients have seroreversion after an appropriate serological treatment response, follow-up serological monitoring may not be necessary for all patients who already demonstrate a 4-fold decline in titers. The need for follow-up periods longer than one year should be determined on a case-by-case basis depending on resources, patient compliance and risk for subsequent reinfections.
Acknowledgments
We would like to acknowledge Dr. Myron Cohen of the University of North Carolina at Chapel Hill for his support for the STI Clinical Trails Group; Suzette Lugo from FHI360 for her administrative support; Dr. Kathleen van Damme for assisting with participant recruitment and enrollment; and the research staff at each institution who contributed their time and efforts for the RCT.
Funding: This work was supported by the NIAID through contracts N01 A1 75329 (STD Clinical Trials Unit, Myron Cohen, MD, PI) and HHSN 26620040073C (STI Clinical Trials Group, Edward W. Hook, III, MD,PI.)
Footnotes
Conflict of Interest: Dr. Hook reports personal fees from Rib-X (Melinta), grants from Cempra, grants from Becton Dickinson, grants from Hologic, personal fees from Roche Molecular, personal fees from McGraw Hill, personal fees from Cepheid, outside the submitted work. All other authors declare no conflict of interests.
References
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR Recomm Rep. 2015;64(3):34–48. [Google Scholar]
- 2.Brown ST, Zaidi A, Larsen SA, Reynolds GH. Serological response to syphilis treatment. A new analysis of old data. JAMA. 1985;253(9):1296–9. [PubMed] [Google Scholar]
- 3.Schroeter AL, Lucas JB, Price EV, Falcone VH. Treatment of early syphilis and reactivity of serologic tests. JAMA. 1972 Jul 31;221(5):471–6. [PubMed] [Google Scholar]
- 4.Petersen CS, Jørgensen BB, Pedersen NS. Treatment of early syphilis infection in Denmark. A retrospective serological study. Dan Med Bull. 1984 Feb;31(1):70–2. [PubMed] [Google Scholar]
- 5.Romanowski B, Sutherland R, Fick GH, et al. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114(12):1005–9. doi: 10.7326/0003-4819-114-12-1005. [DOI] [PubMed] [Google Scholar]
- 6.Hook EW, Behets F, Van Damm K, Leone P, Seña AC, Martin D, Langley C, McNeil L, Wolff M. A phase III equivalence trial of azithromycin vs benzathine penicillin for treatment of early syphilis. Journal of Infectious Diseases. 2010;201(11):1729–35. doi: 10.1086/652239. [DOI] [PubMed] [Google Scholar]
- 7.Seña AC, Wolff M, Martin DH, et al. Predictors of serological cure and serofast state after treatment in HIV-negative persons with early syphilis. Clin Infect Dis. 2011;53(11):1092–9. doi: 10.1093/cid/cir671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen SA, Pope V, Johnson RE, Kennedy EJ., Jr . A Manual of Tests for Syphilis. Washington DC: American Public Health Association; 1998. [Google Scholar]
- 9.Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F, editors. 2nd International Symposium on Information Theory Akademia Kiado; Budapest. 1973. pp. 267–281. [Google Scholar]
- 10.Belisle JT, Brandt ME, Radolf JD, Norgard MV. Fatty acids of Treponema pallidum and Borrelia burgdorferi lipoproteins. J Bacteriol. 1994;176(8):2151–7. doi: 10.1128/jb.176.8.2151-2157.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews HM, Yang TK, Jenkin HM. Unique lipid composition of Treponema pallidum (Nichols virulent strain) Infect Immun. 1979;24(3):713–9. doi: 10.1128/iai.24.3.713-719.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samoff E, Koumans EH, Gibson JJ, Ross M, Markowitz LE. Pre-treatment syphilis titers: distribution and evaluation of their use to distinguish early from late latent syphilis and to prioritize contact investigations. Sex Transm Dis. 2009;36(12):789–93. doi: 10.1097/OLQ.0b013e3181b3566b. [DOI] [PubMed] [Google Scholar]
- 13.Kampmeier RH. Essentials of Syphilology. JB Lippincott Company; Philadelphia: 1943. pp. 192–193. [Google Scholar]
- 14.Fiumara NJ. Treatment of seropositive primary syphilis: an evaluation of 196 patients. Sex Transm Dis. 1977;4(3):92–5. doi: 10.1097/00007435-197707000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Fiumara NJ. Treatment of secondary syphilis: an evaluation of 204 patients. Sex Transm Dis. 1977;4(3):96–9. doi: 10.1097/00007435-197707000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Seña AC, Zhang XH, Li T, Zheng HP, et al. A systematic review of syphilis serological treatment outcomes in HIV-infected and HIV-uninfected persons: rethinking the significance of serological non-responsiveness and the serofast state after therapy. BMC Infect Dis. 2015;15:479. doi: 10.1186/s12879-015-1209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose MS, Fick GH, Romanowski B, Love EJ. First year serologic response to treatment for syphilis: a model for prediction of seroreversion. Stat Med. 1997;16(18):2103–15. doi: 10.1002/(sici)1097-0258(19970930)16:18<2103::aid-sim640>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 18.Riedner G, Rusizoka M, Todd J, et al. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N Engl J Med. 2005;353(12):1236–44. doi: 10.1056/NEJMoa044284. [DOI] [PubMed] [Google Scholar]
- 19.Ghanem KG, Erbelding EJ, Wiener ZS, et al. Serological response to syphilis treatmentin HIV-positive and HIV-negative patients attending sexually transmitted diseases clinics. Sex Transm Infect. 2007;83(2):97–101. doi: 10.1136/sti.2006.021402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dionne-Odom J, Karita E, Kilembe W, et al. Syphilis treatment response among HIV-discordant couples in Zambia and Rwanda. Clin Infect Dis. 2013;56(12):1829–37. doi: 10.1093/cid/cit146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jinno S, Anker B, Kaur P, Bristow CC, Klausner JD. Predictors of serological failure after treatment in HIV-infected patients with early syphilis in the emerging era of universal antiretroviral therapy use. BMC Infect Dis. 2013;13:605. doi: 10.1186/1471-2334-13-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tittes J, Aichelburg MC, Antoniewicz L, Geusau A. Enhanced therapy for primary and secondary syphilis: a longitudinal retrospective analysis of cure rates and associated factors. Int J STD AIDS. 2013;24(9):703–11. doi: 10.1177/0956462413480721. [DOI] [PubMed] [Google Scholar]
- 23.Tong ML, Lin LR, Liu GL, et al. Factors associated with serological cure and the serofast state of HIV-negative patients with primary, secondary, latent, and tertiary syphilis. PLoS One. 2013;8(7):e70102. doi: 10.1371/journal.pone.0070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. 2014 Sexually Transmitted Diseases Surveillance. http://www.cdc.gov/std/stats14/syphilis.htm Last accessed on June 23, 2016.
- 25.Rolfs RT, Joesoef MR, Hendershot EF, et al. A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. New Eng J Med. 1997;337(5):307–14. doi: 10.1056/NEJM199707313370504. [DOI] [PubMed] [Google Scholar]


