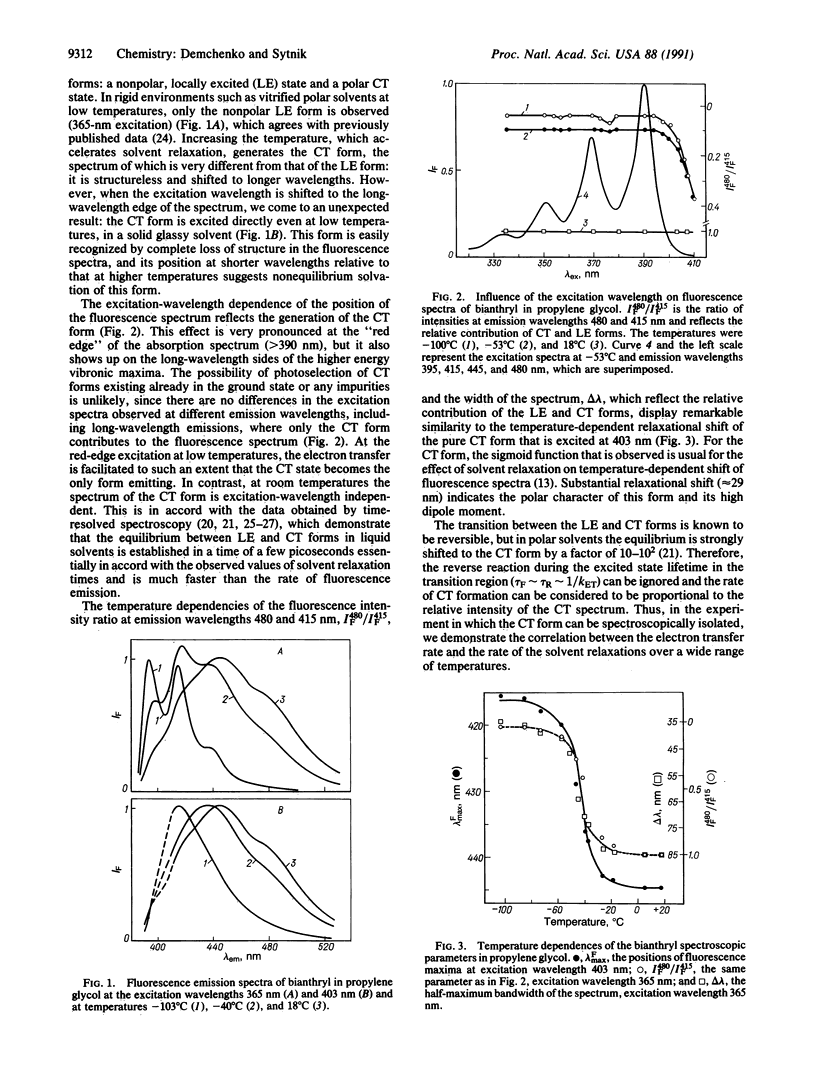
Abstract
Polar solvents are characterized by statistical distributions of solute-solvent interaction energies that result in inhomogeneous broadening of the solute electronic spectra. This allows photoselection of the high interaction energy part of the distribution by excitation at the red (long-wavelength) edge of the absorption bands. We observe that intramolecular electron transfer in the bianthryl molecule from the locally excited (LE) to the charge-transfer (CT) state, which requires solvent relaxation and does not occur in vitrified polar solutions, is dramatically facilitated in low-temperature propylene glycol glass by the red-edge excitation. This allows one to obtain spectroscopically the pure CT form and observe its dependence upon the relaxational properties of the solvent. A qualitative potential model of this effect is presented.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beece D., Eisenstein L., Frauenfelder H., Good D., Marden M. C., Reinisch L., Reynolds A. H., Sorensen L. B., Yue K. T. Solvent viscosity and protein dynamics. Biochemistry. 1980 Nov 11;19(23):5147–5157. doi: 10.1021/bi00564a001. [DOI] [PubMed] [Google Scholar]
- Demchenko A. P., Shcherbatska N. V. Nanosecond dynamics of charged fluorescent probes at the polar interface of a membrane phospholipid bilayer. Biophys Chem. 1985 Aug;22(3):131–143. doi: 10.1016/0301-4622(85)80035-1. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Wolynes P. G. Rate theories and puzzles of hemeprotein kinetics. Science. 1985 Jul 26;229(4711):337–345. doi: 10.1126/science.4012322. [DOI] [PubMed] [Google Scholar]
- Galley W. C., Purkey R. M. Role of heterogeneity of the solvation site in electronic spectra in solution. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1116–1121. doi: 10.1073/pnas.67.3.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroncelli M., Macinnis J., Fleming G. R. Polar solvent dynamics and electron-transfer reactions. Science. 1989 Mar 31;243(4899):1674–1681. doi: 10.1126/science.243.4899.1674. [DOI] [PubMed] [Google Scholar]



