Traumatic spinal cord injury (SCI) remains a devastating neurological disorder leading to severe consequences for the affected individual and their families. Further, socioeconomic implications should not be neglected as well. Although life expectancy after SCI increased tremendously, therapeutic treatment options remain limited. Within the last decades we have experienced serious progress in operative and intensive care management after SCI, which most likely affects the outcome for patients and their relatives positively.
In most cases, the initial trauma leads to cell disruption and intramedullary petechial bleedings. After that a plethora of secondary events in the pathophysiological cascade is initiated leading to further damage of the spinal cord and potentially to clinical deterioration. Theoretically, some of these secondary events might be ameliorated with appropriate treatment. After traumatic SCI, the spinal canal is often narrowed due to fracture dislocation, hematoma or traumatic disc injuries. The degree and duration of on-going spinal cord compression due to spinal canal narrowing have been linked to injury severity and outcome in experimental studies. Numerous animal studies have shown that early osseous decompression is capable of alleviating secondary sequels in the pathophysiology of SCI and improving neurobehavioural deficits (Batchelor et al., 2013). The ultimate goal would be that saving spinal cord tissue (neuroprotection) would lead to improved preconditions for neuroregenerative treatment strategies as well.
Nowadays, early surgical intervention is recommended (Walters et al., 2013). However, the timing and role of surgery per se remains controversial for some clinicians. The multicenter, international, prospective cohort “STASCIS trial” (Surgical Timing in Acute Spinal Cord Injury) with more than 300 patients provided strong evidence that the time point of decompression (here within 24 hours) positively influences neurological outcome compared to later intervention without increasing complication rates (Fehlings et al., 2012). Studies from Central Europe indicate that “ultra-early” decompression within the first 8 hours might be even more beneficial. Neurologic recovery was superior if surgical intervention occurred within the first 8 hours versus 8–24 hours (Jug et al., 2015). The primary outcome was the change in American Spinal Injury Association Impairment Scale (AIS Scale) grade after 6 months of follow-up period (= AIS conversion). Additionally, we have shown that early surgical management within 8 hours improves the functional outcome of SCI patients. This was assessed via the Spinal Cord Independence Measure (SCIM) score – an outcome tool solely developed for individuals with SCI – after a 1-year follow-up period. The rate of perioperative complications did not differ between both groups. In our study we were able to demonstrate that rapid intervention might lead to a significant gain of segmental medullary function as indicated by more caudal neurological and motor levels in the early decompressed group. The results were also reflected by significantly improved AIS grades, a higher AIS conversion rate and higher upper extremity and total motor scores in the early-operated patient cohort (Grassner et al., 2016b). Especially in patients with cervical SCI, gaining one or two levels of segmental spinal cord function affects the patients autonomy significantly. Both studies show that early surgery within the first few hours is possibly achievable, if extra- and intramural factors are optimized. Sometimes extramural hesitation may not be influenced easily. Early transport to a hospital capable of providing adequate surgical and intensive care is crucial. However, in some studies significant delay occurred once patients were already admitted to the hospital due to healthcare related issues. We think that there is some potential in order to facilitate prompt operative care.
The mentioned studies show among many others that there has been a shift towards more rapid surgical intervention. In our opinion, traumatic SCI is a neurologic emergency, which needs to be treated in an adequate center where multi-disciplinary management is provided as fast as possible. Early rehabilitation in a SCI center has been shown to improve the outcome. Adequate acute care is possibly capable of providing additional improvements for affected patients. Further, we are experiencing a paradigm shift in the acute setting. In the past, surgery for patients with clinically complete syndromes (AIS A according to ISNCSCI examination – International Standards for Neurological Classification of Spinal Cord Injury) was thought to be not as urgently required as for incomplete patients. However, one should keep in mind that a clinical complete syndrome does not equal anatomical completeness of the lesion. Further, the neurological examination in the acute setting has a relatively low predictive value and might be influenced by numerous confounders shortly after the injury. Hence, recent studies show that this cohort has probably a high potential for recovery and we do see relatively high conversion rates to higher AIS grades in initially AIS A patients (Bourassa-Moreau et al., 2016). Therefore, regardless of their initial clinical presentation rapid surgical management seems to be justified in most patients (with some exceptions mentioned below).
As we do see a relatively high number of patients who do not show clinical improvement after SCI, it is to date not clear if all patients profit from early surgery. This is especially true for patients with central cord syndromes – nowadays the most prevalent traumatic SCI syndrome. Usually, these patients are elderly with preexisting degenerative spine diseases and multiple-comorbidities. Although rapid surgery might have a positive impact in this cohort as well, the individual medical preconditions might lead to increased morbidity in early-operated patients. In general, surgery may need to be delayed due to polytrauma or concomitant traumatic brain injury. Currently it is not clear if there is a “time-window” for some patients (e.g., if there is delayed transfer) where surgery should be postponed due to pathophysiological sequels after SCI. If a patient shows rapid improvement of neurological symptoms shortly after the trauma, we tend to wait sometimes to perform surgery. Hence, treatment decisions need to be individualized according to the patients need.
The circumstance of not having level-1 evidence recommending early surgical intervention has been criticized. However we strongly believe that this argument is not supportable. With all the evidence mentioned above, we think that it is unethical to perform a “true” randomized controlled clinical trial. Therefore, studies protocols like the STASCIS trial or the SCI-POEM study (Prospective, Observational European Multicenter study on the efficacy of acute surgical decompression after traumatic Spinal Cord Injury) warrant enough evidence to guide clinical decision-making.
Over the first 5–7 days after injury, maintaining mean arterial pressure (MAP) above 85–90 mmHg is recommended – especially for patients with cervical injuries (Ryken et al., 2013). Blood pressure augmentation most likely has a positive impact on the final outcome. The rationale behind this strategy is to increase the spinal cord perfusion pressure (SCPP). Theoretically, this should enhance perfusion of the perilesional penumbra and therefore save tissue at risk. However, SCPP might be influenced in a multi-faceted way and several contributors need to be addressed. In a relevant proportion of SCI patients, intramedullary posttraumatic edema leads to increased intraspinal pressure (ISP) (Grassner et al., 2016a; Saadoun and Papadopoulos, 2016). At some point, the spinal cord is further constricted by the relatively firm spinal dura mater. Hence, a “spinal compartment syndrome” might arise in some individuals. In these patients we are solely restoring spinal realignment and stability, since neural elements are not really decompressed (an illustrative case example is shown in Figure 1). A new technology has been introduced to measure ISP at the injury site via a subdural probe. This has been shown to be safe and SCPP can be computed reliably (SCPP = MAP-ISP) (Saadoun and Papadopoulos, 2016).
Figure 1.
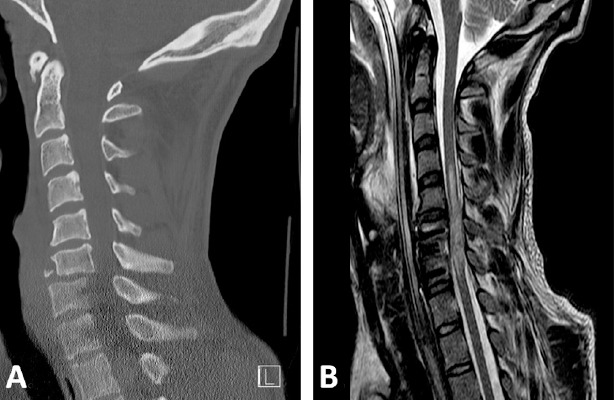
Spinal cord compartment syndrome.
Imaging studies of a 24 y/o male are provided, who was transferred to our institution after a swimming accident. The patient was tetraplegic immediately after the insult. Follow-MR imaging 3 days after the injury shows restoration of spinal realignment, while the spinal cord is compressed against the spinal canal and constricted from spinal meninges. Hence, the spinal cord is not adequately “decompressed”. (A) Initial sagittal CT showing a C6/7 luxation fracture. (B) Sagittal T2w MR image showing intramedullary hyperintensities and profound edema of the spinal cord. The perimedullar subarachnoid space is absent around the lesion site. y/o: Years old; MR: magnetic resonance; CT: computer tomography; C: cervical.
Some treatment paradigms from patients with traumatic brain injury (TBI) might be adopted into the SCI field. However, relevant differences regarding vascularization and autoregulation between the brain and spinal cord are present. Elevated ISP might be reduced by performing duraplasty after spinal stabilization. Recent data indicates that augmentative duraplasty reduces ISP, increases SCPP and improves autoregulation. In our opinion, this strategy is really promising and will potentially affect our acute care treatment regimens. However, to date it is not performed routinely and might also bear increased complication rates. Additionally, it is clear if all patients require expansive duroplasty. More minimal invasive implantation techniques might be helpful. However, first observations from these studies might already be implemented in our postoperative routine. A ring-shaped pillow to reduce pressure at the laminectomized levels reduces ISP. Additionally, it has been shown that a 10 mmHg increase of SCPP increases methylprednisolone levels at the injury site significantly (Saadoun and Papadopoulos, 2016). If optimized SCPP could really allow increased drug delivery at the injury site, results and designs of several past, on-going and future clinical trials using systemic administration of therapeutic drugs in order to achieve neuroprotection or neuroregeneration would have to be revisited. In summary, reducing ISP remains a major goal of surgery and has gained more attention recently (Leonard and Vink, 2015; Saadoun and Papadopoulos, 2016).
In conclusion, early surgical management seems to be justified in most SCI patients. In recent years, the current practice changed towards “ultra-early decompression” and we have seen a paradigm shift, where clinical complete patients are considered as a neurologic emergency. The goal is fast and adequate management in specialized SCI centers. Efforts are necessary to decrease intra- and extramural delays. In the future, advanced neuromonitoring will probably deliver important new insights and will allow an optimized and individualized acute care of SCI patients.
The authors thank Orpheus Mach for his scientific coordination.
References
- Batchelor PE, Wills TE, Skeers P, Battistuzzo CR, Macleod MR, Howells DW, Sena ES. Meta-analysis of pre-clinical studies of early decompression in acute spinal cord injury: a battle of time and pressure. PLoS One. 2013;8:e72659. doi: 10.1371/journal.pone.0072659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa-Moreau E, Mac-Thiong JM, Li A, Ehrmann Feldman D, Gagnon DH, Thompson C, Parent S. Do patients with complete spinal cord injury benefit from early surgical decompression? Analysis of neurological improvement in a prospective cohort study. J Neurotrauma. 2016;33:301–306. doi: 10.1089/neu.2015.3957. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Vaccaro A, Wilson JR, Singh A, D WC, Harrop JS, Aarabi B, Shaffrey C, Dvorak M, Fisher C, Arnold P, Massicotte EM, Lewis S, Rampersaud R. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) PLoS One. 2012;7:e32037. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassner L, Winkler PA, Strowitzki M, Buhren V, Maier D, Bierschneider M. Increased intrathecal pressure after traumatic spinal cord injury: an illustrative case presentation and a review of the literature. Eur Spine J. 2016a doi: 10.1007/s00586-016-4769-9. doi: 10.1007/s00586-016-4769-9. [DOI] [PubMed] [Google Scholar]
- Grassner L, Wutte C, Klein B, Mach O, Riesner S, Panzer S, Vogel M, Buhren V, Strowitzki M, Vastmans J, Maier D. Early decompression (< 8 h) after traumatic cervical spinal cord injury improves functional outcome as assessed by Spinal Cord Independence Measure (SCIM) after 1 year. J Neurotrauma. 2016b;33:1658–1666. doi: 10.1089/neu.2015.4325. [DOI] [PubMed] [Google Scholar]
- Jug M, Kejzar N, Vesel M, Al Mawed S, Dobravec M, Herman S, Bajrovic FF. Neurological recovery after traumatic cervical spinal cord injury is superior if surgical decompression and instrumented fusion are performed within 8 hours versus 8 to 24 hours after injury: a single center experience. J Neurotrauma. 2015;32:1385–1392. doi: 10.1089/neu.2014.3767. [DOI] [PubMed] [Google Scholar]
- Leonard AV, Vink R. Reducing intrathecal pressure after traumatic spinal cord injury: a potential clinical target to promote tissue survival. Neural Regen Res. 2015;10:380–382. doi: 10.4103/1673-5374.153683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryken TC, Hurlbert RJ, Hadley MN, Aarabi B, Dhall SS, Gelb DE, Rozzelle CJ, Theodore N, Walters BC. The acute cardiopulmonary management of patients with cervical spinal cord injuries. Neurosurgery 72 Suppl. 2013;2:84–92. doi: 10.1227/NEU.0b013e318276ee16. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC. Spinal cord injury: is monitoring from the injury site the future? Critical care. 2016;20:308. doi: 10.1186/s13054-016-1490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters BC, Hadley MN, Hurlbert RJ, Aarabi B, Dhall SS, Gelb DE, Harrigan MR, Rozelle CJ, Ryken TC, Theodore N American Association of Neurological S, Congress of Neurological S. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 60 Suppl. 2013;1:82–91. doi: 10.1227/01.neu.0000430319.32247.7f. [DOI] [PubMed] [Google Scholar]


