Keywords: nerve regeneration, neurons, isoflurane, patch clamp, cell membrane, synaptic response, inhalational anesthesia, electrophysiology
Abstract
Isoflurane is a widely used inhaled anesthetic in the clinical setting. However, the mechanism underlying its effect on consciousness is under discussion. Therefore, we investigated the effect of isoflurane on the hippocampus and cortex using an in vivo field recording approach. Our results showed that 1.3%, 0.8%, and 0.4% isoflurane exerted an inhibitory influence on the mouse hippocampus and cortex. Further, high frequency bands in the cortex and hippocampus showed greater suppression with increasing isoflurane concentration. Our findings suggest that in vivo field recordings can monitor the effect of isoflurane anesthesia on the mouse cortex and hippocampus.
Introduction
Isoflurane is a widely used inhaled anesthetic, although its mechanisms for inducing unconsciousness in the central nervous system (CNS) are poorly understood. It has been shown that presynaptic selectivity in conjunction with postsynaptic and extrasynaptic gamma-aminobutyric acid (GABA)A receptor potentiation are potential CNS synaptic pathways (Kotani and Akaike, 2013; Westphalen et al., 2013). However, in vivo field recordings reflecting neural processing of different concentrations of anesthetics have not been reported.
Therefore, we used an in vivo field recording approach to determine whether the same mechanism occurs in living anesthetized animals. Field recording methods enable CNS recordings in intact animals (Sakmann, 2006; Furue et al., 2007) in which accumulating extracellular signals due to synaptic excitation can easily be recorded with a field electrode. Furthermore, this method reflects in vivo neuronal activity, and provides sufficient signal quality to define synaptic events for understanding neural processing during normal and drug-mediated behavior (Kolb et al., 2013). In this study, we introduce our in vivo field recording method, and use it to examine cortical and hippocampal synaptic response after inhalation of different isoflurane concentrations.
Materials and Methods
Animal preparation
All animal experiments were approved by the Animal Care Committee of the China-Japan Friendship Hospital and performed in accordance with the United States National Institutes of Health Guideline for the Care and Use of Laboratory Animals. All attempts were made to minimize the number of animals used.
A recording chamber was prepared prior to surgery. A conical stone drill bit (CA1063, Minimo Precision Instruments and Tools, Daido Mecha Tronics Co., Ltd., Shanghai, China) and high speed dental drill (OS-40, Osada Electric. Co., Ltd., Tokyo, Japan) were used to drill a hole of approximately 2 cm in diameter through a 35 mm plastic dish. Before craniotomy, the skin above the recording area was cut using scissors, and the recording chamber placed directly onto the skull and glued using cyanoacrylic glue (UHU).
Craniotomies were performed on 10 female and male C57/BL6 mice, aged 3 to 4 months old (Chinese Academy of Medical Science Institute of Experimental Animal, Beijing, China). Mice were placed in a stereotaxic frame (New Standard 51700, Friends Honestly Life Science Company, Ltd., Beijing, China), and deeply anesthetized throughout surgery using 1.7% isoflurane (YBH40052005; Abbott Laboratories, Shanghai, China) (Silva et al., 2010) delivered in 2 L/min oxygen through a securely fitted mask. Erythromycin eye ointment (150502; Beijing Shuangji Pharmaceuticals, Beijing, China) was applied to protect the eyes from injury. Anesthesia was obtained when there was no response to pinching. Mice were allowed to spontaneously breathe, and their body temperature was monitored with a rectal probe and maintained at 37°C with a thermostatically-controlled electric heating pad (CWE, Inc., Ardmore, PA, USA). Hair was removed using a trimmer and human hair removal cream (L’Oreal, Paris, France), and an incision made in the skin using a scalpel. After subcutaneous injection of 1% xylocaine (6H98J2, China Otsuka Pharmaceutical Co. Ltd., Tianjin, China), muscle was exposed using cotton-swab applicators. The skull was washed using the same xylocaine solution and dried with compressed air. Fascia was removed using a scalpel blade. Next, the area around the intended craniotomy (center 2.5 mm anterior of bregma, 2.5 mm lateral, and 3–4 mm diameter) was thinned using a high-speed drill (ZH-GSZ; Anhui Zhenghua Biological Equipment Co. Ltd., Anhui, China) with a small tip steel burr (0.5 mm in diameter). The area was then gently perforated with forceps to disclose the dura mater, which was removed with scissors. The cortex was cleaned with artificial cerebrospinal fluid (119 mM NaCl, 26.2 mM NaHCO3, 2.5 mM KCl, 1 mM NaH2PO4, 1.3 mM MgCl2, and 10 mM glucose). Subsequently, 5% CO2/95% O2 was administered for 10–15 minutes, and then 2.5 mM CaCl2 added. The filtrate was obtained using a 0.22-μm filter apparatus (597316; Friends Honestly Life Science Company, Ltd., Beijing, China) sterilized and stored at 4°C (HW Press, 2011). The cortex was kept humid. The left and right side of the holders were gently adjusted until they were parallel to the recording chamber. Artificial cerebrospinal fluid filled the recording chamber and ensured there was no leakage along the edge between the recording chamber and cortex.
Electrophysiological recordings
The surface of the exposed brain was irrigated with artificial cerebrospinal fluid. In vivo field recordings from the cortex and hippocampus were performed using a patch electrode (B100-50-7.5-HP; Friends Honestly Life Science Company, Ltd.) (thin-walled borosilicate glass capillary, tip 4–5 μm diameter, and resistance of 2–3 MΩ), filled with the following internal solution: 110 mM potassium gluconate, 20 mM KCl, 10 mM HEPES, 10 mM EGTA, 2 mM MgATP, 5 mM Na2ATP, and 0.1 mM Na-GTP; pH 7.3. The correct electrode location was determined using physiological and stereotaxic indicators. Under positive pressure (100–200 mbar), the electrode pipette was slowly advanced into the cortex and hippocampus at a speed of 0.2 mm/min to minimize trauma to the brain tissue. The hippocampal electrode was inserted into the CA1 area (2.0 mm posterior, 2.0 lateral to bregma, and 1.5 mm ventral to the dura mater) (Paxinos and Watson, 2005), with a prominent amplitude oscillation recorded at a frequency of 6–7 oscillations/second. The cortical electrode was inserted into the superficial cortical layer (1.2 mm anterior, 2.0 lateral to bregma, and 0.5 mm ventral to the dura mater).
Cortical and hippocampal extracellular field recordings were monitored under different isoflurane concentrations, with 1.7%, 1.3%, 0.8%, and 0.4% isoflurane administered via an anesthesia machine (AS-01-007; Friends Honestly Life Science Company, Ltd.). The recording time was 15 minutes except for 0.4% isoflurane, which was only 5 minutes.
Current-clamp recordings were performed using an Axopatch 200B amplifier (Molecular Devices, Foster City, CA, USA). Extracellular field potentials were recorded at a frequency band of 0.05–3,000 Hz and amplified 200 times. No significant changes in access resistance were observed throughout the experiments. At the end of the experiment, mice were deeply anesthetized with supplemental isoflurane.
Data analysis
Data were analyzed using pCLAMP software (version 10, Axon Instruments/Molecular Devices, Sunnyvale, USA). Data are expressed as the mean ± SD. Group differences were tested by one-way analysis of variance (ANOVA) and Dunnett's test (post hoc test). P < 0.05 was considered statistically significant.
Results
Electroencephalogram (EEG) of the cortex and hippocampus
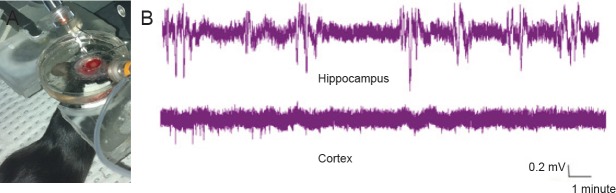
Eight stable in vivo field recordings of the cortex and hippocampus were obtained. Figure 1 shows in vivo cortical and hippocampal field recordings in mice under 0.4% isoflurane anesthesia.
Figure 1.
In vivo field recording of the cortex and hippocampus (A) and their spontaneous discharges (B).
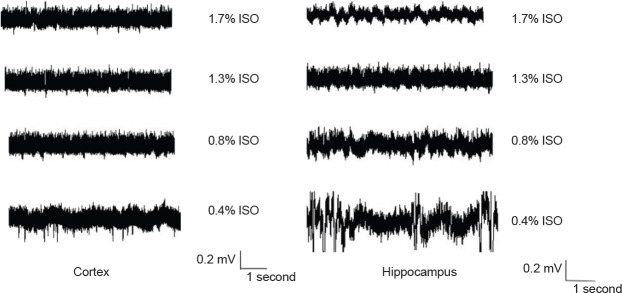
Next, the effect of isoflurane on cortical and hippocampal field recordings was investigated. As shown in Figure 2, under different isoflurane concentrations, hippocampal and cortical field recordings had different discharge levels. Further, with increasing isoflurane, the discharge reduced.
Figure 2.
Extracellular cortical and hippocampal field recordings under different isoflurane concentrations.
ISO: Isoflurane.
Hippocampal and cortical frequency bands
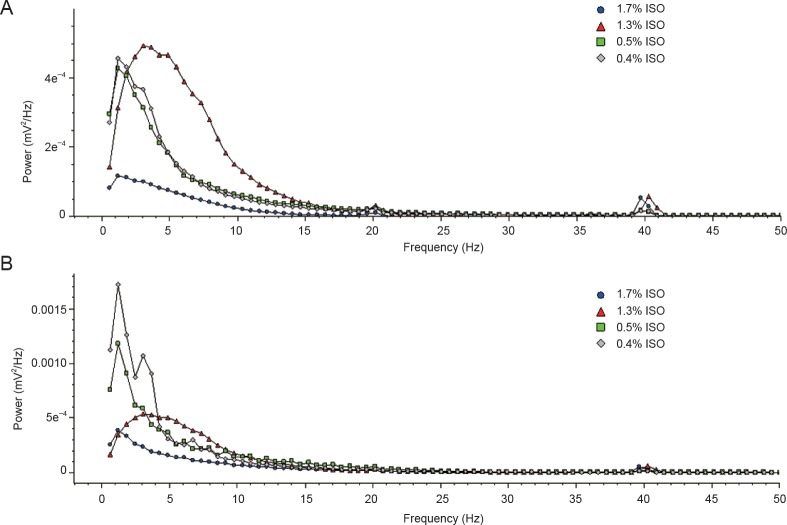
The isoflurane dose-response on hippocampal and cortical frequency bands was examined. In the cortex, there were significant differences in β, θ, and α signals between 1.7% and 1.3% isoflurane. Further, 1.3% and 0.8% isoflurane showed significant differences in θ signal frequency, with γ signal differences also observed between 1.7%, 1.3%, and 0.8% isoflurane. In the hippocampus, δ signal power increased as isoflurane changed from 1.7% to 1.3% and 0.4%. Significant differences were found between 1.7% and 1.3% isoflurane, and 1.3% and 0.8% isoflurane in θ and α signals. Hippocampal β and γ signal power increased as isoflurane changed from 1.7% to 0.4%. Additionally, there was a significant difference in β signal between 1.3% and 0.8% isoflurane (Table 1). Figure 3 shows the relationship between power and different frequency bands under different isoflurane concentrations delivered within the cortical and hippocampal area. There was an increase in power at about 40 Hz under all isoflurane concentrations, however this did not differ significantly between each group.
Table 1.
Power spectrum in the hippocampus and cortex using different isoflurane concentrations
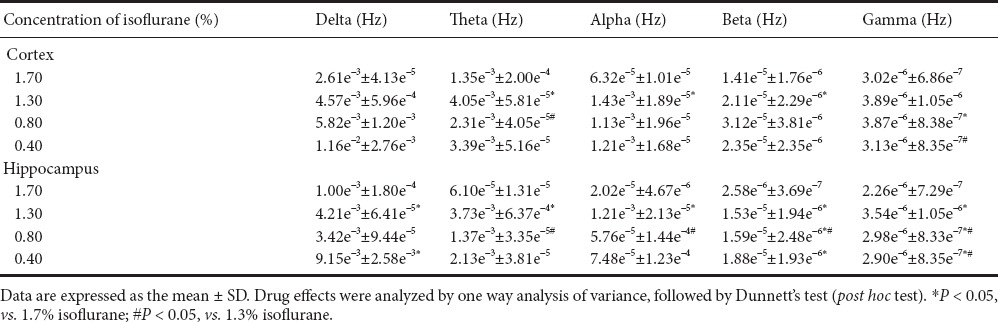
Figure 3.
Power spectrum in the hippocampus and cortex under different isoflurane concentrations.
ISO: Isoflurane.
In summary, low frequency bands were relatively resistant to isoflurane in both hippocampal and cortical recordings, except for 1.7% isoflurane. The suppression effect of isoflurane on high frequency bands was dose-dependent.
Discussion
Here, we have used in vivo field recording to monitor changes in the mouse cortex and hippocampus. Cortical and hippocampal neurons are involved in physiological CNS processes such as learning and memory. Consequently, studying the effect of isoflurane on spontaneous electrical brain activity may help understanding of neural events during anesthetic-induced unconsciousness. We found that with increasing isoflurane concentration, spontaneous cortical and hippocampal activity was suppressed. Specifically, we observed a decrease in low frequency bands in the hippocampus (including δ, θ, and α signals) between 1.7% and 1.3% isoflurane, and 1.3% and 0.8% isoflurane. Similarly, higher frequency bands showed a declining trend in a dose-dependent manner. With regards cortical frequency bands, only higher frequency bands showed a decrease between 1.7% and 1.3% isoflurane. Further, we also found an increasing trend at 40 Hz under all isoflurane concentrations in both the hippocampus and cortex, although none were significantly different. These findings are consistent with our unpublished data on EEG recordings in the mouse hippocampus and cortex following midazolam treatment.
It is known that the δ, θ, and α powers are commonly used signal variables for assessing cognitive function. Accumulative evidence supports hippocampal θ as a synchronized rhythmic oscillation of field potentials at 14–12 Hz in memory formation (Luft et al., 2013). In this study, we found that high isoflurane concentrations depressed δ, θ, and α power, which contributes to anesthetic-induced amnesia (Perouansky et al., 2010). In the mammalian cortex, neural communication is organized by 30–100 Hz γ oscillation, with γ frequency related to processing speed in neural networks (Insel et al., 2012). Indeed, 40-Hz oscillation is associated with various aspects of the conscious process (Desmedt and Tomberg, 1994; Joliot et al.,1994; Tallon-Baudry et al., 1996). Numerous studies have addressed the effect of anesthetic agents on oscillations in both humans and animals (Uchida et al., 2000; John et al., 2001; Sleigh et al., 2001). Hudetz et al. (2011) found that isoflurane at moderate concentrations did not suppress cortical oscillations at the 40 Hz frequency. In contrast, high-frequency γ power activity at 70–140 Hz was notably suppressed by isoflurane in a concentration-dependent manner. Similar observations were previously shown in the rat hippocampus (Ma et al., 2002). Sensory stimulation may produce cortical arousal even during isoflurane anesthesia, which may be responsible for enhanced γ oscillations under anesthesia (Hudetz, 2008). Consistent with these studies, we show that cortical and hippocampal γ signals are sensitive to isoflurane and decreased with increasing concentration, thereby inducing unconsciousness.
We also applied electrophysiological methods to examine CNS mechanisms underlying anesthetic agents. Intracranial techniques such as local field potentials (LFPs) are not influenced by electromyographic activity, and can analyze higher frequencies such as γ-bands. Thus, LFPs may more accurately reflect the effect of anesthetics on the brain than electroencephalograms, which enables them to precisely examine anesthetic performance (Silva et al., 2010). There are three points to attain a successful stable recording: first, during recordings, experimental animals should be maintained in a physiological situation; second, setting of recording chambers should be accurate and careful; third, placing of recording electrodes onto designated areas must be based on good anatomical knowledge and recognition of characteristic neuronal rhythms. Although we examined features and changes in electrophysiological activity of cortical and hippocampal neurons following isoflurane anesthesia, behavioral manifestations or single cell electrophysiological changes still needs to be investigated in detail.
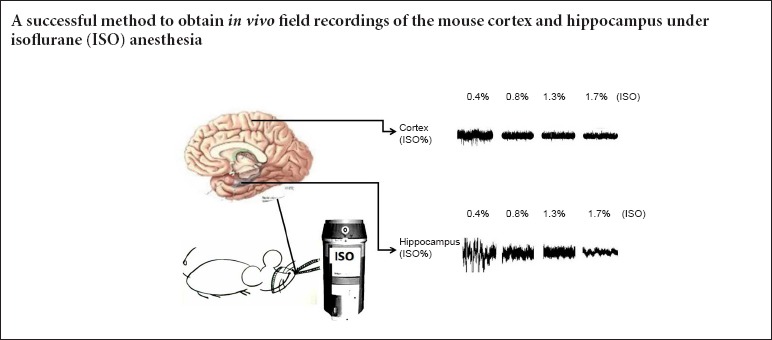
In conclusion, our study provides a successful method to obtain stable in vivo field recordings in the mouse cortex and hippocampus under isoflurane anesthesia. Furthermore, we show that high frequency cortical and hippocampal bands are more sensitive to the suppression of different isoflurane concentrations.
Footnotes
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by James R, Stow A, Yin YQ, Yu J, Li CH, Li JY, Song LP, Zhao M
References
- Desmedt JE, Tomberg C. Transient phase-locking of 40 Hz electrical oscillations in prefrontal and parietal human cortex reflects the process of conscious somatic perception. Neurosci Lett. 1994;168:126–129. doi: 10.1016/0304-3940(94)90432-4. [DOI] [PubMed] [Google Scholar]
- Furue H, Katafuchi T, Yoshimura M. In: In Vivo Patch-Clamp Technique. In: Patch-Clamp Analysis Advanced Techniques. Walz W, editor. Totowa: Humana Press; 2007. [Google Scholar]
- Hudetz AG. Are we unconscious during general anesthesia? Int Anesthesiol Clin. 2008;46:25–42. doi: 10.1097/AIA.0b013e3181755db5. [DOI] [PubMed] [Google Scholar]
- Hudetz AG, Vizuete JA, Pillay S. Differential effects of isoflurane on high-frequency and low-frequency γ oscillations in the cerebral cortex and hippocampus in freely moving rats. Anesthesiology. 2011;114:588–595. doi: 10.1097/ALN.0b013e31820ad3f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HW Press. Artificial cerebrospinal fluid (ACSF) Cold Spring Harbor Protocols. 2011 doi: 10.1101/pdb.rec094359. doi:10.1101/pdb.rec065730. [DOI] [PubMed] [Google Scholar]
- Insel N, Patron LA, Hoang LT, Nematollahi S, Schimanski LA, Lipa P, Barnes CA. Reduced gamma frequency in the medial frontal cortex of aged rats during behavior and rest: implications for age-related behavioral slowing. J Neurosci. 2012;32:16331–16344. doi: 10.1523/JNEUROSCI.1577-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John ER, Prichep LS, Kox W, Valdés-Sosa P, Bosch-Bayard J, Aubert E, Tom M, diMichele F, Gugino LD. Invariant reversible QEEG effects of anesthetics. Conscious Cogn. 2001;10:165–183. doi: 10.1006/ccog.2001.0507. [DOI] [PubMed] [Google Scholar]
- Joliot M, Ribary U, Llinás R. Human oscillatory brain activity near 40 Hz coexists with cognitive temporal binding. Proc Natl Acad Sci U S A. 1994;91:11748–11751. doi: 10.1073/pnas.91.24.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb I, Holst G, Goldstein B, Kodandaramaiah SB, Boyden ES, Culurciello E, Forest CR. Automated, in-vivo, whole-cell electrophysiology using an integrated patch-clamp amplifier. BMC Neurosci. 14:P131. [Google Scholar]
- Kotani N, Akaike N. The effects of volatile anesthetics on synaptic and extrasynaptic GABA-induced neurotransmission. Brain Res Bull. 2013;93:69–79. doi: 10.1016/j.brainresbull.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Luft CD, Nolte G, Bhattacharya J. High-learners present larger mid-frontal theta power and connectivity in response to incorrect performance feedback. J Neurosci. 2013;33:2029–2038. doi: 10.1523/JNEUROSCI.2565-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Shen B, Stewart LS, Herrick IA, Leung LS. The septohippocampal system participates in general anesthesia. J Neurosci. 2002;22:RC200. doi: 10.1523/JNEUROSCI.22-02-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. London: Academic Press; 2005. [Google Scholar]
- Perouansky M, Rau V, Ford T, Oh SI, Perkins M, Eger EI, Pearce RA. Slowing of the hippocampal θ-rhythm correlates with anesthetic-induced amnesia. Anesthesiology. 2010;113:1299–1309. doi: 10.1097/ALN.0b013e3181f90ccc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B. Patch pipettes are more useful than initially thought: simultaneous pre- and postsynaptic recording from mammalian CNS synapses in vitro and in vivo. Pflugers Arch. 2006;453:249–259. doi: 10.1007/s00424-006-0172-4. [DOI] [PubMed] [Google Scholar]
- Silva A, Cardoso-Cruz H, Silva F, Galhardo V, Antunes L. Comparison of anesthetic depth indexes based on thalamocortical local field potentials in rats. Anesthesiology. 2010;112:355–363. doi: 10.1097/ALN.0b013e3181ca3196. [DOI] [PubMed] [Google Scholar]
- Sleigh JW, Steyn-Ross DA, Steyn-Ross ML, Williams ML, Smith P. Comparison of changes in electroencephalographic measures during induction of general anaesthesia: influence of the gamma frequency band and electromyogram signal. Br J Anaesth. 2001;86:50–58. doi: 10.1093/bja/86.1.50. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci. 1996;16:4240–4249. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Nakayama H, Maehara T, Hirai N, Arakaki H, Nakamura M, Nakabayashi T, Shimizu H. Suppression of gamma activity in the human medial temporal lobe by sevoflurane anesthesia. Neuroreport. 2000;11:39–42. doi: 10.1097/00001756-200001170-00008. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Desai KM, Hemmings HC. Presynaptic inhibition of the release of multiple major central nervous system neurotransmitter types by the inhaled anaesthetic isoflurane. Br J Anaesth. 2013;110:592–599. doi: 10.1093/bja/aes448. [DOI] [PMC free article] [PubMed] [Google Scholar]