Keywords: nerve regeneration, Yougui Pill, glucocorticoid, apoptosis, Bcl-2, cytochrome c, caspase-3, caspase-9, mitochondrial membrane potential, neural regeneration
Abstract
Long-term glucocorticoid use may result in sustained suppression of one or more secreted components from the hypothalamo-pituitary-adrenal axis, and often results in apoptosis. Yougui Pill (YGP), a 10-component traditional Chinese herbal medicine, has been shown to be clinically effective for glucocorticoid-induced suppression of the hypothalamo-pituitary-adrenal axis. However, the pharmacological and molecular mechanisms remain unclear. We hypothesized that YGP would exert an anti-apoptosis effect on dexamethasone-treated anterior pituitary cells. In vivo experiments showed that YGP significantly reduced the number of apoptotic cells, down-regulated mRNA expression of cytochrome c, caspase-3, and caspase-9, and up-regulated mRNA expression of Bcl-2. These findings suggest that YGP reduced glucocorticoid-induced apoptosis in rat anterior pituitary cells by regulating the mitochondria-mediated apoptosis pathway.
Introduction
Glucocorticoids have been widely applied to treat autoimmune-related diseases, such as systemic lupus erythematosus, scleroderma, and rheumatoid arthritis (Buttgereit et al., 2002; Bijlsma et al., 2005). Long-term glucocorticoid use prohibits secretion of compounds from the hypothalamo-pituitary-adrenal (HPA) axis, which results in a series of adverse reactions. Previous studies have shown that glucocorticoid use results in sustained suppression of one or more secreted components from the HPA axis, both in humans (Lamberts et al., 1997; Richter et al., 2002) and in rats (Nicholson et al., 1984; Calogero et al., 1990; Huang, 1994). Apoptosis is thought to play an important role in the negative effects of glucocorticoids (Nolan et al., 1998; Nolan and Levy, 2001).
Yougui Pill (YGP), a 10-component Chinese herbal mixture (Radix Rehmanniae Preparata, Radix Aconiti Lateralis Preparata, cinnamon, yam, Macrocarpium officinale, Cuscuta reflexa, Lycium barbarum, antler glue, Cortex Eucommiae, and Radix Angelicae Sinensis), has been used to improve symptoms caused by glucocorticoids, although the underlying mechanisms remain unknown. The present study aimed to explore the anti-apoptotic effects of YGP on rat anterior pituitary cells treated by dexamethasone.
Material and Methods
Experimental animals
Male, specific pathogen-free, 3-month-old, Sprague-Dawley rats weighing 180–200 g, were purchased from the Department of Laboratory Animal of China Medical University (Shenyang, Liaoning Province, China). Ten animals per cage were housed in temperature- and humidity-controlled rooms, and were maintained on a 12-hlight/dark cycle and allowed free access to water and food. The experimental protocol was approved by the committee for experimental animals of China Medical University Laboratory Animal Center (Approval No. CMUEC2008035) and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Precautions were taken to minimize suffering and the number of animals used in each experiment.
YGP preparation
The ten YGP components were purchased from Tongrentang Pharmaceutical Group (Beijing, China). Briefly, all YGP components (24 g Radix Rehmanniae Preparata, 6 g Radix Aconiti Lateralis Preparata, 6 g cinnamon, 12 g yam, 6 g Macrocarpium officinale, 12 g Cuscuta reflexa, 9 g Lycium barbarum, 12 g antler glue, 12 g Cortex Eucommiae, and 9 g Radix Angelicae Sinensis) were respectively broken down into a coarse powder, and then mixed together. The mixture was mixed for 1 hour with distilled water to a final concentration of 10%, heated to the boiling point, and then decocted at a simmer for 1 hour. After filtering out the crude herbs, the residuals were decocted twice using the same method. The filtrates were then collected and mixed, then centrifuged at 400× g for 5 minutes to remove residual sediment. The extracting solution was concentrated to 0.45 g/mL in a 90°C water bath. Finally, the drug-containing mixture was cooled down, and stored at 4°C until further use.
Treatment
A total of 80 male rats were randomly assigned to four groups. For the dexamethasone group, the rats were administered an intramuscular injection of 1 mg/kg dexamethasone (Tiantai Mountain Chengdu Pharmaceutical Co., Ltd., Chengdu, Sichuan Province, China) once daily for 15 days, followed by 0.5 mg/kg dexamethasonefor 15 days. For the dexamethasone plus YGP group, the rats were intramuscularly injected with 0.5 mg/kg dexamethasone once daily for 15 days, followed by intragastric administration of 10 mL/kg YGP once daily for another 15 days. For the YGP group, the rats were intramuscularly injected with 1 mL/kg normal saline once daily for 15 days, followed by a half dosage of normal saline and intragastric administration of 10 mL/kg YGP once daily for another 15 days. For the control group, rats were intramuscularly injected with normal saline once daily for 15 days, followed by intragastric administration of normal saline (10 mL/kg) once daily for another 15 days. All treatments were performed from 8:00 a.m. to 9:00 a.m. The rats were sacrificed by decapitation at day 31, and the anterior pituitaries were dissected for observation.
Preparation of tissue sections
Immediately after decapitation, the anterior pituitary glands were carefully removed and fixed in 4% formaldehyde in phosphate-buffered saline (PBS) for 48 hours, washed twice with fresh PBS, and embedded in paraffin wax. A series of 5-μm-thick axial sections was cut from each pituitary for immunohistochemistry and terminal deoxynucleotide transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay.
Immunohistochemical staining
Immunohistochemistry was performed to analyze expressions of various apoptotic regulatory factors on paraffin sections of pituitary glands from all groups, as previously described (Gu et al., 2012). Antigen retrieval was performed by incubating for 10 minutes in a 700-W microwave oven in 10 mM citric acid, pH = 6.0, to increase sensitivity of antigen detection. The rabbit anti-rat primary antibodies (Boster, Wuhan, China) used in this study included Bcl-2 (1:80), cytochrome c (1:80), caspase-3 (1:80), and caspase-9 (1:80) at 37°C for 20 minutes. All sections were stained using the streptavidin-biotin peroxidase complex (SABC) method with goat anti-rabbit antibodies (Boster, Wuhan, China) as the secondary antibody at 37°C for 30 minutes, and then 4°C overnight. Brown-yellow granules in the cytoplasm represented a positive staining. Six views were randomly quantified from each section under a microscope (Olympus CX31-LV320 Olympus (China) Co., Ltd., Shanghai, China). The results are expressed as a percentage of positive cells.
TUNEL assay
Apoptotic activity was analyzed by TUNEL staining using a commercial kit (Boster) on paraffin sections of pituitary tissue. Briefly, anterior pituitary sections were treated with proteinase K (1:200 in Tris-HCl buffered saline (TBS)) for 15 minutes at 37°C prior to the TUNEL reaction. After incubation with DIG-dUTP and TdT enzyme for 2 hours at 37°C, the sections were incubated with anti-DIG-biotin (1:100) for 30 minutes at 37°C, and then developed with SABC and diaminobenzidine (DAB) for 10 minutes. Apoptosis was evaluated by randomly quantifying six views from each section under a microscope (Olympus (China) Co., LTD., Shanghai, China). The results are expressed as a percentage of apoptosis.
Real-time quantitative PCR
Real-time quantitative PCR was performed to identify differentially expressed apoptosis-related genes. Total RNA was extracted from anterior pituitaries as described in the TRIZOL Reagent protocol (Invitrogen, Shanghai, China) with some modifications (Zhang et al., 2014). Total RNA was treated with deoxyribonuclease I (DNase I; Sigma) and reverse-transcribed using Prime Script™ reverse-transcriptase (Takara, Dalian, China). Real-time quantitative PCR reactions were performed using a Light Cycler (Roche, Shanghai, China) and the fluorescent reporter dye SYBR Green I (Molecular Probes, Takara, Dalian, Liaoning Province, China). Primers (designed and compounded by Takara Japan) were as follows: Bcl-2, upstream primer: 5′-TGA ACC GGC ATC TGC ACA C-3′, downstream primer: 5′-CGT CTT CAG AGA CAG CCA GGA G-3′; cytochrome c, upstream primer: 5′-GGA GGC AAG CAT AAG CAT GG-3′, downstream primer: 5′-CTG CCC TTT CTC CCT TCT TC-3′; caspase-3, upstream primer: 5′-GCA GCA GCC TCA AAT TGT TGA CTA-3′, downstream primer: 5′-TGC TCC GGC TCA AAC CAT C-3′; caspase-9, upstream primer: 5′-AGG AAC TCA CAG CTC CAT TAC-3′, downstream primer: 5′-CAG CAT TAG CGA CCC TAA GCA-3′; GAPDH, upstream primer 5′-AGA AGG CTG GGG CTC ATT TG-3′, downstream primer 5′-AGG GGC CAT CCA CAG TCT TC-3′. The relative amount of mRNA for a particular sample was represented by the threshold cycle (Ct) of amplification. The relative quantitation of transcripts was calculated using the comparative Ct method (Livak and Schmittgen, 2001). Expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control. The GAPDH Ct value was subtracted from the target gene Ct value to obtain a ΔCt. Likewise, the difference between the ΔCt values of samples for the target gene and the ΔCt values of the calibrator (untreated control) were determined. The relative and normalized quantitative apoptosis-related gene expression levels (2–ΔΔCt) were calculated.
Preparation of YGP-containing serum
Twenty rats were equivalently randomized into two groups, followed by daily administration of either distilled water or YGP (10 mL/kg) by gavage for 11 days. Before the last administration, the rats were fasted all night, and blood samples were collected 3 hours after the final administration. Serum from YGP-treated and distilled water-treated rats were respectively obtained by centrifugation at 800 × g for 10 minutes and then filtered through a 0.22-μm filter membrane. All samples were stored at −20°C.
Anterior pituitary cell culture and treatment
Six normal rats were sacrificed by decapitation. The anterior pituitary glands were removed, washed several times with low-glucose-DMEM (HyClone, Beijing, China), and then cut into small fragments. The cells obtained from the gland fragments by enzymatic dispersion (trypsin/deoxyribonuclease I; Solarbio, Beijing, China) were washed twice and suspended in low-glucose DMEM. Cell viability assessed by trypan blue exclusion was > 90%. All cells were seeded onto 96-well tissue culture plates (1 × 106 cells/well) for 3 days (37°C, 5% CO2 in air) in low-glucose DMEM supplemented with 100 U/mL streptomycin (Solarbio), 100 U/mL penicillin (Solarbio), and 10% FBS (HyClone). The cultured cells were then divided into three groups: dexamethasone, dexamethasone plus YGP, and control. There were four samples in each group. The medium was replaced with fresh low-glucose DMEM plus test substances and the cells were incubated for an additional 3 days. The test substances were dexamethasone (1 × 10–4 M) plus 10% serum from distilled water-treated rats, dexamethasone (1 × 10–4 M) plus 10% serum from YGP-treated rats, and 10% serum from those controls, respectively. Afterwards, the cells were assayed using flow cytometry (BD Biosciences, San Diego, CA, USA).
Detection of changes in mitochondrial membrane potential according to flow cytometry
The mitochondrial membrane potential (Δψm) was assessed using rhodamine 123, a mitochondrial potential sensor (Molecular Probes), as previously described (Mondal et al., 2014). Trypsin/EDTA was added to the cells and cell suspensions (1 × 106 cells/mL, 500 μL/well) were obtained. The medium in each well was discarded and treated with 500 μL of medium containing rhodamine 123 at 10 μg/mL for 30 minutes at 37°C and 5% CO2 in the dark. The cells were washed three times in PBS, and then analyzed using flow cytometry (BD Biosciences).
Detection of cell apoptosis by flow cytometry
The cells were harvested, then rinsed with ice-cold PBS and re-suspended in 100 μL of reaction buffer. Annexin V-FITC stock solution (5 μL, Boster, Wuhan, China) and 5 μL propidium iodide (PI) were added to the cells and incubated for 15 minutes at 25°C in the dark, as previously described (Lin et al., 2013). Subsequently, 400 μL of reaction buffer was added to the cells and immediately analyzed on a FACSort equipped with CellQuest software (BD Biosciences).
Statistical analysis
All data are expressed as the mean ± SD. Statistical analyses were evaluated by one-way analysis of variance and post-hoc Student-Nevman-Keuls with SPSS 13.0 (SPSS, Chicago, IL, USA). A P value < 0.05 was considered statistically significant.
Results
YGP reduced cell apoptosis in the dexamethasone-stimulated anterior pituitary
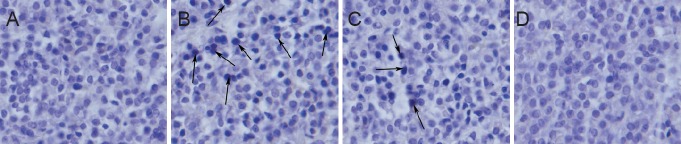
TUNEL staining revealed a significantly increased number of apoptotic cells in the dexamethasone group compared with the control group (11.58 ± 0.95% vs. 0.03 ± 0.01%; P < 0.001). There were less TUNEL-positive cells in the dexamethasone plus YGP group (7.60 ± 0.48%) than in the dexamethasone group (P < 0.001). There were no visible TUNEL-positive cells in the YGP and control groups (Figure 1).
Figure 1.
Effect of YGP on cell apoptosis in the dexamethasone-stimulated anterior pituitary (TUNEL staining, × 200).
(A) Control group: rats were intramuscularly injected with normal saline, and intragastrically administered normal saline; (B) dexamethasone group: rats were injected intramuscularly with dexamethasone; (C) dexamethasone plus YGP group: rats were intramuscularly injected with dexamethasone, and intragastrically administered YGP; (D) YGP groups: rats were intramuscularly injected with normal saline, and intragastrically administered YGP. Arrows indicate apoptotic cells. There are less TUNEL-positive cells in the dexamethasone plus YGP group than in the dexamethasone group. There are no visible TUNEL-positive cells in the YGP and control groups. YGP: Yougui Pill; TUNEL: terminal dexoynucleotide transferase (TdT)-mediated dUTP nick-and labeling.
Effect of YGP on apoptosis-related factors in the dexamethasone-stimulated anterior pituitary
Compared with the dexamethasone group, expression of the anti-apoptosis protein Bcl-2 was significantly increased, while expressions of the pro-apoptosis proteins cytochrome c, caspase-3, and caspase-9 were significantly decreased in the dexamethasone plus YGP group (P < 0.01). There were no significant differences in pro-apoptotic protein expression between the YGP and control groups (P > 0.05) (Table 1, Figure 2).
Table 1.
Effect of YGP on the percentage of cells expressing apoptosis-related factors in the dexamethasone-stimulated anterior pituitary
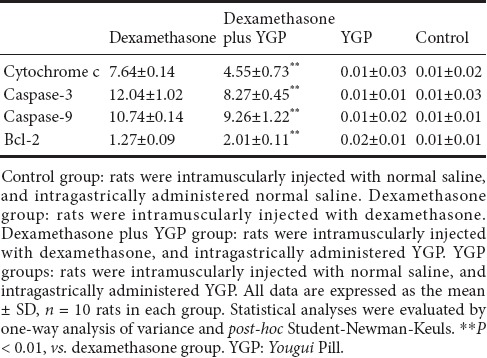
Figure 2.
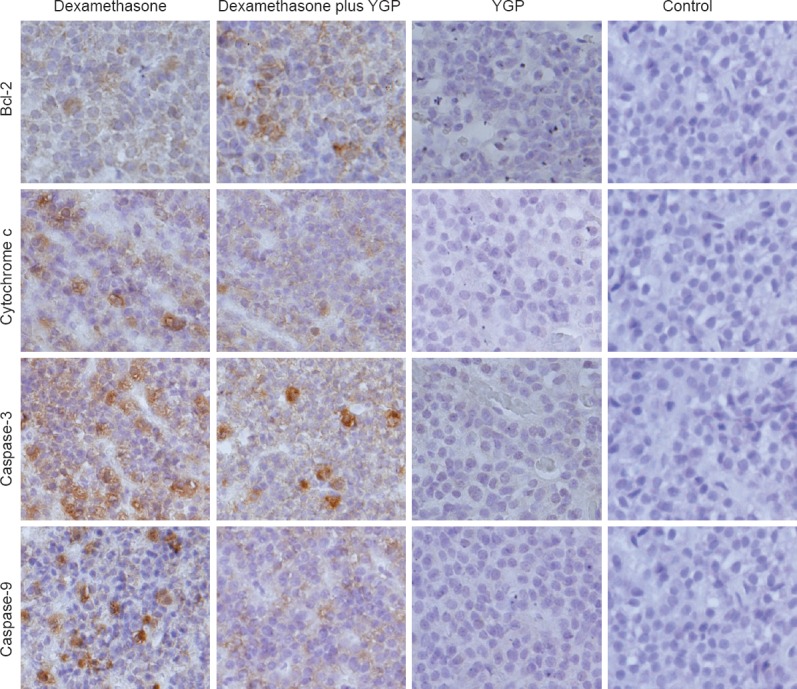
Effect of YGP on apoptosis-related factor expression in the dexamethasone-stimulated anterior pituitary (immunohistochemical staining, × 200).
Control group: rats were intramuscularly injected with normal saline, and intragastrically administered normal saline; dexamethasone group: rats were intramuscularly injected with dexamethasone; dexamethasone plus YGP group: rats were intramuscularly injected with dexamethasone, and intragastrically administered YGP; YGP group: rats were intramuscularly injected with normal saline, and intragastrically administered YGP. Brown staining represents a positive immunoreaction. Bcl-2 expression increased in the dexamethasone plus YGP group compared with the dexamethasone group. However, expressions of cytochrome c, caspase-3, and caspase-9 were opposite. Expression of the pro-apoptotic factors did not significantly differ between the YGP and control groups. YGP: Yougui Pill.
Effect of YGP on apoptosis-related mRNA expressions in the dexamethasone-stimulated anterior pituitary
Apoptosis-related mRNA expression trends were almost consistent with their immunoreactivities in the corresponding groups. There was a significant increase in Bcl-2 mRNA expression, and a significant decrease in mRNA expressions of the pro-apoptosis factors cytochrome c, caspase-3, and caspase-9 in the dexamethasone plus YGP group compared with the dexamethasone group (P < 0.01). The above indices show no significant differences between the YGP and control groups (P < 0.01) (Table 2).
Table 2.
Effect of YGP on apoptosis-related mRNA expressions in the dexamethasone-stimulated anterior pituitary
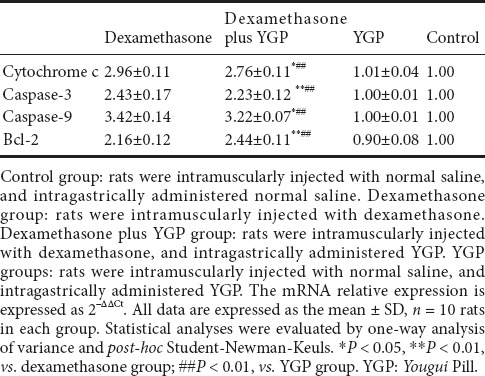
YGP reduced apoptosis in cultured anterior pituitary cells treated with dexamethasone
Flow cytometry showed that a greater number of Annexin V+/PI– cells (early-stage apoptotic cells) in the dexamethasone group than in the control and dexamethasone plus YGP groups (Figure 3).
Figure 3.
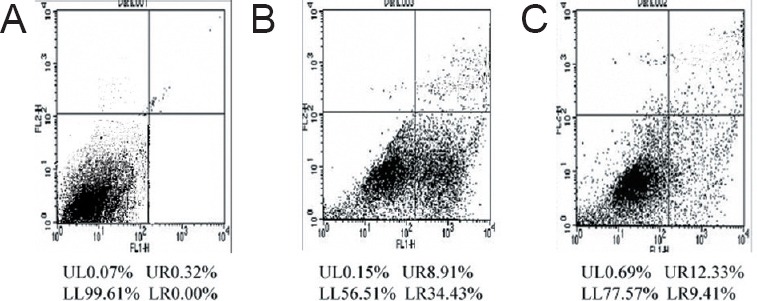
Effect of YGP on apoptosis in cultured anterior pituitary cells treated with dexamethasone.
(A) Control group: anterior pituitary cells were cultured with 10% serum from distilled water-treated rats; (B) dexamethasone group: anterior pituitary cells were cultured with dexamethasone and 10% serum from the distilled water-treated rats; (C) dexamethasone plus YGP group: anterior pituitary cells were cultured with dexamethasone and 10% serum from YGP-treated rats. There were more Annexin V+/PI– cells (in right lower quadrant) in the dexamethasone group than in the dexamethasone plus YGP and control groups (detected by flow cytometry, stained with Annexin V-FITC and PI). The experiments were repeated at least three times. YGP: Yougui Pill; PI: propidium iodine; V-FITC: Annexin V-fluorescein isothiocyanate.
Effect of YGP on changes in mitochondrial membrane potential of cultured anterior pituitary cells treated with dexamethasone
Flow cytometry revealed a significantly increased percentage of cells with reduced mitochondrial membrane potential in the dexamethasone group than in the control and dexamethasone plus YGP groups (P < 0.01) (Figure 4).
Figure 4.
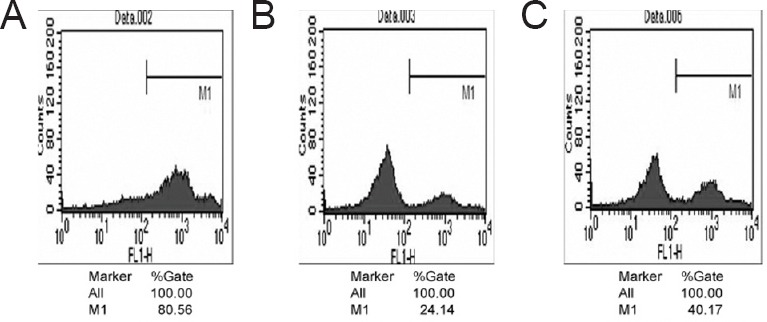
Effect of YGP on changes in mitochondrial membrane potential of cultured anterior pituitary cells treated with dexamethasone.
(A) Control group: anterior pituitary cells were cultured with 10% serum from distilled water-treated rats; (B) dexamethasone group: anterior pituitary cells were cultured with dexamethasone and 10% serum from the distilled water-treated rats; (C) dexamethasone plus YGP group: anterior pituitary cells were cultured with dexamethasone and 10% serum from YGP-treated rats. The percentage of cells with reduced mitochondrial membrane potential was less in the dexamethasone plus YGP group than in the dexamethasone group (determined by flow cytometry, stained with Rhodamine 123). The experiments were repeated at least three times. YGP: Yougui Pill.
Discussion
Because of the potential health benefits, Chinese herbal medicines have long been used as an alternative therapy in China, Japan, and Korea, and are even popular in Western Society (No authors listed, 1996; Eisenberg et al., 1998). One advantage of Chinese herbal medicines over Western medicine has been attributed to the interactions among the herbal components (Ikegami et al., 2006), which are thought to be synergistic, enhancing, and immuno-suppressive. Unlike modern Western pharmaceutical agents, traditional herbal medicines have an overall combined effect that is greater than the effect of each individual component (Zhang et al., 2014). Herbal medicines have been extensively used for preventing and treating many diseases, including diarrhea (Li et al., 2013), diabetes (Ghorbani, 2014), and various types of cancers (Ling et al., 2014a; Safarzadeh et al., 2014). Interestingly, traditional Chinese medicine has been reported to play an important regulatory role in gene therapy (Ling et al., 2014b). In particular, YGP has been commonly used, although the mechanisms of action remain unclear. Our study specifically focused on the anti-apoptotic effect of these herbs on dexamethasone-stimulated anterior pituitary cells.
Apoptosis constitutes a highly regulated mechanism for removal of old, damaged, or mutated cells through two distinct molecular mechanisms (Galluzzi et al., 2007). On one hand, the extrinsic apoptotic pathway mediates cell death in response to extracellular stimuli, which occurs either through ligand-induced activation of death receptors at the plasma membrane (Wajant, 2002; Peter and Krammer, 2003), or via signaling cascades emanating from dependency receptors in the absence of their ligands (Bredesen et al., 2005). On the other hand, intrinsic apoptosis is regulated by mitochondria, which integrate lethal and pro-survival signals, eventually deciding the fate of a cell (Green and Kroemer, 1998).
In vertebrate animals, the mitochondrial pathway is the major pathway for apoptosis, and the critical event responsible for caspase activation is mitochondrial outer membrane permeabilization (MOMP), which has been referred to as the “point of no return” of cell death (Green and Kroemer, 2004). In the mitochondria, death signals lead to changes in mitochondrial membrane permeability and subsequent release of pro-apoptotic factors, including cytochrome c, apoptosis-inducing factor, second mitochondria-derived activator of caspase (Smac/DIABLO), and endonuclease G. This release leads to the cytoplasmic assembly of procaspase-9, cytochrome c, and apoptosis protease-activating factor 1 into an initiation complex known as the apoptosome (Ghosh et al., 2002; Düssmann et al., 2003; Delivani and Martin, 2006), which results in activation of caspase-9 and subsequent activation of “executioner” caspases, such as caspase-3.
The key regulatory proteins of mitochondria-mediated apoptosis belong to the Bcl-2 family and contain both pro-apoptotic (Bax, Bad, Bak, Bim) and anti-apoptotic (Bcl-2, Bcl-xL) members. Pro-apoptotic proteins normally reside in the cytosol (Wolter et al., 1997), but target the mitochondria during apoptotic signaling, causing the release of apoptotic signaling molecules, such as cytochrome c, SMAC/DIABLO, and apoptosis-inducing factor (Arden and Betenbaugh, 2004). Conversely, anti-apoptotic members of the Bcl-2 family maintain mitochondrial membrane potential and prevent the release of cytochrome c during apoptotic insults (Kluck et al., 1997).
In the current study, we demonstrated that intrinsic apoptosis pathways were involved in the anti-apoptotic effects of YGP on dexamethasone-treated anterior pituitary cells. The anti-apoptotic protein Bcl-2 was up-regulated, whereas the pro-apoptotic proteins cytochrome c, caspase-3, and caspase-9 were down-regulated, further potentiating the process. This herbal medicine concoction could act by regulating gene expression of mitochondrial pathways.
In conclusion, our study demonstrated that long-term dexamethasone use leads to apoptosis of anterior pituitary cells, and YGP administration reduces the number of apoptotic cells, suggesting that YGP exhibits anti-apoptosis effects by regulating mitochondrial function in apoptosis pathways. Further studies are needed to identify the types of anterior pituitary cells that are sensitive to YGP therapy, as well as to provide a better understanding of the molecular mechanisms involved.
Acknowledgments
We thank the Laboratory of Cell Biology in China Medical University for providing technological assistance.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 30471576, and 30872277; and a grant from the Innovative Research Program in Universities of the Ministry of Education of China, No. IRT0760.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Cooper C, Stow A, Ji YZ, Yu J, Li CH, Li JY, Song LP, Zhao M.
References
- Arden N, Betenbaugh MJ. Life and death in mammalian cell culture: strategies for apoptosis inhibition. Trends Biotechnol. 2004;22:174–180. doi: 10.1016/j.tibtech.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bijlsma JW, Cutolo M, Straub RH, Masi AT. Clinical aspects of immune neuroendocrine mechanisms in rheumatic diseases. Rheumatic Disease Clinics. 2005;31:xiii–xvi. doi: 10.1016/j.rdc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Bredesen DE, Mehlen P, Rabizadeh S. Receptors that mediate cellular dependence. Cell Death Differ. 2005;12:1031–1043. doi: 10.1038/sj.cdd.4401680. [DOI] [PubMed] [Google Scholar]
- Buttgereit F, Da Silva JA, Boers M, Burmester GR, Cutolo M, Jacobs J, Kirwan J, Kohler L, van Riel P, Vischer T, Bijlsma JW. Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: current questions and tentative answers in rheumatology. Ann Rheum Dis. 2002;61:718–722. doi: 10.1136/ard.61.8.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calogero AE, Kamilaris TC, Johnson EO, Tartaglia ME, Chrousos G. Recovery of the rat hypothalamic-pituitary-adrenal axis after discontinuation of prolonged treatment with the synthetic glucocorticoid agonist dexamethasone. Endocrinology. 1990;127:1574–1579. doi: 10.1210/endo-127-4-1574. [DOI] [PubMed] [Google Scholar]
- Düssmann H, Kögel D, Rehm M, Prehn JH. Mitochondrial membrane permeabilization and superoxide production during apoptosis. A single-cell analysis. J Biol Chem. 2003;278:12645–12649. doi: 10.1074/jbc.M210826200. [DOI] [PubMed] [Google Scholar]
- Delivani P, Martin SJ. Mitochondrial membrane remodeling in apoptosis: an inside story. Cell Death Differ. 2006;13:2007–2010. doi: 10.1038/sj.cdd.4402049. [DOI] [PubMed] [Google Scholar]
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L, Kroemer G. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–1243. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- Ghorbani A. Clinical and experimental studies on polyherbal formulations for diabetes: current status and future prospective. J Integr Med. 2014;12:336–345. doi: 10.1016/S2095-4964(14)60031-5. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Majumder M, Steele R, Liu TJ, Ray RB. MBP-1 mediated apoptosis involves cytochrome c release from mitochondria. Oncogene. 2002;21:2775–2784. doi: 10.1038/sj.onc.1205384. [DOI] [PubMed] [Google Scholar]
- Green D, Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 1998;8:267–271. doi: 10.1016/s0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Gu Y, Lin S, Li JL, Nakagawa H, Chen Z, Jin B, Tian L, Ucar DA, Shen H, Lu J, Hochwald SN, Kaye FJ, Wu L. Altered LKB1/CREB-regulated transcription co-activator (CRTC) signaling axis promotes esophageal cancer cell migration and invasion. Oncogene. 2012;31:469–479. doi: 10.1038/onc.2011.247. [DOI] [PubMed] [Google Scholar]
- Huang TS. Corticotropin secretagogues facilitate recovery of the hypothalamus-pituitary-adrenal axis suppressed by prolonged treatment with dexamethasone. Metabolism. 1994;43:544–548. doi: 10.1016/0026-0495(94)90193-7. [DOI] [PubMed] [Google Scholar]
- Ikegami F, Sumino M, Fujii Y, Akiba T, Satoh T. Pharmacology and toxicology of Bupleurum root-containing Kampo medicines in clinical use. Hum Exp Toxicol. 2006;25:481–494. doi: 10.1191/0960327106het654oa. [DOI] [PubMed] [Google Scholar]
- Iwama H, Amagaya S, Ogihara Y. Effect of shosaikoto, a Japanese and Chinese traditional herbal medicinal mixture, on the mitogenic activity of lipopolysaccharide: a new pharmacological testing method. J Ethnopharmacol. 1987;21:45–53. doi: 10.1016/0378-8741(87)90093-6. [DOI] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Lamberts SW, Bruining HA, de Jong FH. Corticosteroid therapy in severe illness. New Engl J Med. 1997;337:1285–1292. doi: 10.1056/NEJM199710303371807. [DOI] [PubMed] [Google Scholar]
- Li J, Wu XL, Chen Y, Tang Z, Xu YH, Jiang JM, Gu YY, Chen JP, Yang DP, Wang DM. Antidiarrheal properties of different extracts of Chinese herbal medicine formula Bao-Xie-Ning. J Integr Med. 2013;11:125–134. doi: 10.3736/jintegrmed2013019. [DOI] [PubMed] [Google Scholar]
- Lin S, Tian L, Shen H, Gu Y, Li JL, Chen Z, Sun X, You MJ, Wu L. DDX5 is a positive regulator of oncogenic NOTCH1 signaling in T cell acute lymphoblastic leukemia. Oncogene. 2013;32:4845–4853. doi: 10.1038/onc.2012.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling CQ, Yue XQ, Ling C. Three advantages of using traditional Chinese medicine to prevent and treat tumor. J Integr Med. 2014a;12:331–335. doi: 10.1016/S2095-4964(14)60038-8. [DOI] [PubMed] [Google Scholar]
- Ling CQ, Wang LN, Wang Y, Zhang YH, Yin ZF, Wang M, Ling C. The roles of traditional Chinese medicine in gene therapy. J Integr Med. 2014b;12:67–75. doi: 10.1016/S2095-4964(14)60019-4. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mondal J, Bishayee K, Panigrahi AK, Khuda-Bukhsh AR. Low doses of ethanolic extract of Boldo (Peumus boldus) can ameliorate toxicity generated by cisplatin in normal liver cells of mice in vivo and in WRL-68 cells in vitro, but not in cancer cells in vivo or in vitro. J Integr Med. 2014;12:425–438. doi: 10.1016/S2095-4964(14)60045-5. [DOI] [PubMed] [Google Scholar]
- Nicholson S, Campbell E, Torrellas A, Beckford U, Altaher R, Sandford R, Scraggs R, Gillham B, Jones M. Recovery of the hypothalamo-pituitary-adrenocortical axis in the rat after long-term dexamethasone treatment. Neuroendocrinology. 1984;39:343–349. doi: 10.1159/000124002. [DOI] [PubMed] [Google Scholar]
- No authors listed. Complementary medicine is booming worl1dwide. BMJ. 1996;313:131–133. [PMC free article] [PubMed] [Google Scholar]
- Nolan L, Levy A. Anterior pituitary trophic responses to dexamethasone withdrawal and repeated dexamethasone exposures. J Endocrinol. 2001;169:263–270. doi: 10.1677/joe.0.1690263. [DOI] [PubMed] [Google Scholar]
- Nolan LA, Kavanagh E, Lightman SL, Levy A. Anterior pituitary cell population control: basal cell turnover and the effects of adrenalectomy and dexamethasone treatment. J Neuroendocrinol. 1998;10:207–215. doi: 10.1046/j.1365-2826.1998.00191.x. [DOI] [PubMed] [Google Scholar]
- Peter ME, Krammer PH. The CD95(APO-1//Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- Richter B, Neises G, Clar C. Glucocorticoid withdrawal schemes in chronic medical disorders. A systematic review. Endocrinol Metab Clin North Am. 2002;31:751–778. doi: 10.1016/s0889-8529(02)00008-7. [DOI] [PubMed] [Google Scholar]
- Safarzadeh E, Sandoghchian Shotorbani S, Baradaran B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull. 2014;4:421–427. doi: 10.5681/apb.2014.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–1636. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Wang Y, Yusufali AH, Ashby F, Zhang D, Yin ZF, Aslanidi GV, Srivastava A, Ling CQ, Ling C. Cytotoxic genes from traditional Chinese medicine inhibit tumor growth both in vitro and in vivo. J Integr Med. 2014;12:483–494. doi: 10.1016/s2095-4964(14)60057-1. [DOI] [PubMed] [Google Scholar]