Keywords: nerve regeneration, spinal cord injury, electroacupuncture, Governor Vessel, Dazhui (GV14) acupoint, Mingmen (GV4) acupoint, Wnt/β-cateninsignaling pathway, neuroprotection, neural regeneration
Abstract
Electroacupuncture at Dazhui (GV14) and Mingmen (GV4) on the Governor Vessel has been shown to exhibit curative effects on spinal cord injury; however, the underlying mechanism remains poorly understood. In this study, we established rat models of spinal cord injury using a modified Allen's weight-drop method. Ninety-nine male Sprague-Dawley rats were randomly divided into three equal groups: sham (only laminectomy), SCI (induction of spinal cord injury at T10), and EA (induction of spinal cord injury at T10 and electroacupuncture intervention at GV14 and GV4 for 20 minutes once a day). Rats in the SCI and EA groups were further randomly divided into the following subgroups: 1-day (n = 11), 7-day (n = 11), and 14-day (n = 11). At 1, 7, and 14 days after electroacupuncture treatment, the Basso, Beattie and Bresnahan locomotor rating scale showed obvious improvement in rat hind limb locomotor function, hematoxylin-eosin staining showed that the histological change of injured spinal cord tissue was obviously alleviated, and immunohistochemistry and western blot analysis showed that Wnt1, Wnt3a, β-catenin immunoreactivity and protein expression in the injured spinal cord tissue were greatly increased compared with the sham and SCI groups. These findings suggest that electroacupuncture at GV14 and GV4 upregulates Wnt1, Wnt3a, and β-catenin expression in the Wnt/β-catenin signaling pathway, exhibiting neuroprotective effects against spinal cord injury.
Introduction
Spinal cord injury (SCI) is a consequence of mechanical trauma that leaves a damaged core area in the spinal cord. SCI results in motor, sensory, and autonomic impairments that greatly diminish the quality of life of an individual. SCI places social, psychological, and financial burdens on patients and their families (Varma et al., 2013). However, the therapies currently available for SCI are minimally effective and produce limited results (Silva et al., 2014; Yilmaz and Kaptanoğlu, 2015). Therefore, an effective therapy for SCI is urgently needed.
Electroacupuncture (EA) is a therapy in which an electrical current is applied to acupuncture needles after they have been inserted into the body, and is one of the most popular types of acupuncture (Ulett et al., 1998; Mitchell, 2009). EA has been shown to affect neuron proliferation and differentiation in animal models of intervertebral disc protrusion (Jiang et al., 2015), demyelinating diseases (Huang et al., 2011; Ding et al., 2015; Liu et al., 2015), hypoxic-ischemic encephalopathy (Xu et al., 2014a), and stroke (Tao et al., 2010; Yu et al., 2012; Hong et al., 2013). Clinically, EA is an effective analgesic (Colak et al., 2010; Xue et al., 2012). EA has also been proven effective in many neurological disorders (Yu et al., 2012; Sun et al., 2013; Zhang et al., 2013a, b). Preclinical and clinical studies have demonstrated that EA can induce functional improvement in motor function after SCI (Wong et al., 2003).
EA displays neuroprotective effects and promotes dorsal neuronal function recovery after SCI in rats; in particular, EA stimulation at GV14 and GV4 can greatly promote neuronal function recovery through upregulating the expression of neurotrophic factors (Liu et al., 2014) such as NT-3 (Mo et al., 2016) and brain-derived neurotrophic factor (Zhang et al., 2012). Therefore, EA at the acupoints GV14 and GV4 can be used as an alternative treatment for SCI; however, the underlying mechanisms remain unclarified.
Wnt signaling has been studied as a potential therapeutic approach for SCI (Lambert et al., 2015). Canonical Wnt signaling, represented by Wnts such as Wnt1 and Wnt3A, is involved in many processes in the nervous system, including neurulation, neural induction, proliferation, axonal growth, fate determination and cell specification, migration, and differentiation (O’Connell and Weeraratna, 2009; Xu et al., 2014b).
There have been a limited number of studies on the effects of EA on the Wnt/β-catenin signaling pathways in SCI, and the mechanisms are presently unknown. In this study, we investigated the effects of EA on SCI rats and the role of the Wnt/β-catenin signaling pathway, with an aim to provide laboratory evidence for a new and effective therapy for SCI.
Materials and Methods
Animals
All experiments were approved by the Institutional Animal Care and Use Committee of Beijing University of Chinese Medicine, China. Ninety-nine male Sprague-Dawley rats (180–220 g body weight) were randomly divided into three equal groups: sham (laminectomy only), SCI (SCI induction at the T10 spinal segment), and EA (SCI induction at the T10 spinal segment and EA intervention at GV14 and GV4). Rats in the SCI and EA groups were randomly divided into the following subgroups: 1-day (n = 11), 7-day (n = 11), and 14-day (n = 11). All animals were housed in individual cages with free access to food and water. Room temperature was maintained at 25 ± 3°C.
Spinal cord injury models
The surgical procedure for inducing SCI was conducted according to established methods (Xu et al., 2010) (Figure 1). Rats were anesthetized with 10% chloral hydrate (0.35 mL/100 g body weight) via intraperitoneal injection. After anesthesia, a 2.5 cm median longitudinal incision was made dorsally on the rats, with the T10 spinous process as the center to expose the T9–11 spinous processes and laminae. The whole T10 lamina was resected and an approximately 10 mm segment of spinal cord was exposed. The SCI model was then produced using a modified Allen device (NYU/MASCIS impactor device; Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China) at 40 g•cm at the T10 segment in the SCI and EA groups. All SCI animals included in the study met the following injury criteria: spinal cord ischemia and edema around the wound, formation of tail sway reflex, flicking of both body and legs, and the appearance of sluggish paralysis. Rats in the sham group underwent T10 total laminectomy without injury to the spinal cord. The animals were returned to individual cages with sufficient water and food, and were then treated with an intraperitoneal injection of gentamicin at a dose of 2,000 U/day. The bladder was manually squeezed every 8 hours to assist urination until spontaneous voiding recurred.
Figure 1.
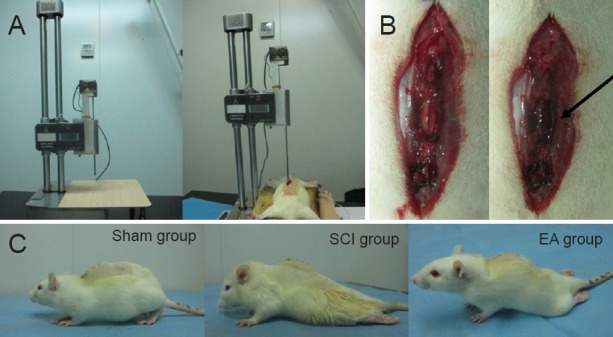
Establishment of a rat model of spinal cord injury (SCI).
(A) Modified Allen's weight-drop apparatus made of stainless steel, with a pouching rod of 3.0 mm diameter and adjustable height. The device precisely measures the movement of a 5 g rod dropped 80 mm onto the T9–10 spinal cord exposed by laminectomy. (B) Before SCI, the spinal cord was smooth; after SCI, the spinal cord was congested (black arrow). (C) After SCI, rats in the SCI and EA groups showed hind limb dyskinesia, while hind limb movement was normal in the sham group (laminectomy only). EA: Electroacupuncture.
Electroacupuncture administration
In the EA group, EA was performed once daily at GV14 and GV4 (Figure 2). According to the Laboratory Animal Acupuncture Atlas developed by the National Acupuncture Society for Experimental Research, GV14 is located on the posterior midline below the spinous process of the seventh cervical vertebra, and GV4 is located on the posterior midline below the spinous process of the second lumbar vertebra (Geng et al., 2013). A sterilized stainless steel needle (0.30 mm × 25 mm; Beijing Zhongyan Taihe Medical Instrument Co., Ltd., China) was inserted obliquely at an angle of 15–45° and a depth of 0.5–0.7 cm at each acupoint. The needles were connected to the output terminals of an EA apparatus (HANS-LH202 nerve stimulator; Beijing Huawei Industrial Development Co., Ltd., Beijing, China). To minimize any discrepancies due to stress, rats in the SCI and sham groups were also immobilized in a manner to the same as that used in the EA group. Electrical stimulation parameters were set at a frequency of 2 Hz and intensity of 1 mA for 20 minutes. The EA group underwent EA treatment 2 hours after establishment of SCI and anesthesia recovery. One hour before euthanasia, at the 24th hour postoperatively, rats in the 1-day EA group were given a second treatment. Rats in the 7-day and 14-day EA groups were given the same treatment once per day until they were euthanized.
Figure 2.
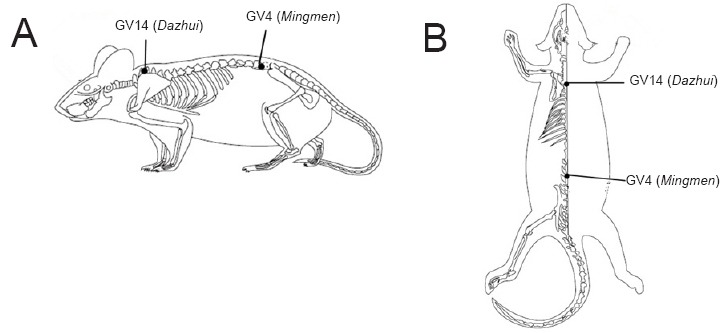
Location of the acupoints GV14 and GV4.
(A) Lateral view; (B) dorsal view.
Behavioral tests
The hind limb motor function of rats in the sham, SCI, and EA groups was assessed by two unbiased observers independently by two observers who were blinded to grouping using the Basso, Beattie and Bresnahan (BBB) hind limb locomotor rating scale test on days 1, 7, and 14 post-injury (Basso et al., 1995). The BBB rating scale is a 21-point system based on operationally defined behavioral features, which follows the recovery progression from complete paralysis to normal locomotion. The BBB rating scale ranges from 0 to 21, with a score of 0 indicating complete hind limb paralysis and a score of 21 denoting completely normal locomotor function. The average rat hindlimb motor function scores were recorded after modeling and before euthanasia.
Sample preparation and hematoxylin-eosin staining
At every time point (days 1, 7, and 14), six of 11 rats in each group were euthanized at a specified postoperative time and transcardially perfused with 0.9% sodium chloride, and then with 4% paraformaldehyde in phosphate-buffered saline (4% PFA) for 30 minutes. The spinal cord was obtained from the upper 1cm to the lower 1cm of the striking point, and then fixed. The spinal cord was sectioned into two paraffin blocks with the striking point as the center, and the striking point was used as the studying range. Spinal cords were dissected and kept in 4% paraformaldehyde overnight. After dehydration, the spinal cords were embedded with paraffin, and sliced into serial coronal sections of 4 μm thickness. Five sections were then stained with hematoxylin and eosin to assess the histopathologic changes.
Immunohistochemical staining
The streptavidin-biotin complex method was used to perform immunohistochemical staining (Zheng et al., 2013). The sections were stained using a streptavidin-biotin complex kit (Jiehao, Shanghai, China). Endogenous peroxidase activity was quenched with a 10-minute incubation in 3% H2O2 at 37°C. The sections were rinsed three times in phosphate-buffered saline for 2 minutes each time, incubated in normal non-specific goat serum for 10 minutes at 37°C, and incubated overnight with primary antibodies at 4°C. Sections were then incubated for 1 hour with biotinylated secondary antibody (1:200) (KIT-5004; horseradish peroxidase-polymer anti-rabbit immunohistochemistry kit, Fuzhou Maixin, China) and for 1 hour with horseradish peroxidase-conjugated-avidin at 37°C. Finally, the reaction product was visualized with diaminobenzidine in 0.1M phosphate-buffered saline for 30 seconds. Sections were mounted on glass slides, dehydrated through an ascending series of alcohol, cleared with xylene, and cover-slipped. The following antibodies were used: anti-Wnt1 antibody (ab85060) (1:2,200; Abcam, Cambridge, MA, USA), anti-Wnt3a antibody (09-162) (1:300; Millipore, Billerica, MA, USA), and anti-β-catenin[E247] antibody (ab32572) (1:100; Abcam). Immunohistochemical staining results were observed under a light microscope (Olympus, Tokyo, Japan), and quantitative assessment of relative changes in immunohistochemical staining was performed by Image Pro Plus 6.0 software (Media Cybernetics, Atlanta, GA, USA).
Western blot analysis
The remaining five of 11 rats in each group were deeply anesthetized with 10% chloral hydrate and euthanized at an appointed time. A 1 cm segment of spinal cord centered at the injury epicenter was quickly dissected. Protein homogenates of the spinal cord were prepared by rapid homogenization in 50 μL of a lysis buffer. Samples were centrifuged at 10,000 r/min for 10 minutes at 4°C. Protein concentrations were determined using the Bradford method. For electrophoresis, protein samples (40 μg each) were dissolved in a sample buffer, and heated at 100°C for 5 minutes. Samples were then resolved on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The filters were blocked with 5% low-fat milk and incubated overnight at 4°C with primary antibody: rabbit anti-Wnt1 antibody (ab85060) (1:3,000; Abcam), rabbit anti-Wnt3a antibody (09-162-KL) (1:2,000; Millipore), and rabbit anti-β-catenin [E247] antibody (ab32572) (1:2,000; Abcam). After being washed, the membranes were incubated with horseradish peroxidase-goat-anti-rabbitIgG (ZB-2301) (1:2,000; ZSGB-BIO, Beijing, China). Proteins were visualized by ECL chemiluminescence (sc-2048; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Bands were quantified using Image-Pro Plus image analysis software (Media Cybernetics, Rockville, MD, USA). The final results were expressed as relative optical density: an optical density ratio of target protein to GAPDH (an internal control).
Statistical analyses
Data are presented as the mean ± SEM. SPSS 20.0 (IBM Corporation, Armonk, NY, USA) was used for data analysis with one-way analysis of variance after testing for normal distribution and homogeneity of variance, followed by the post hoc least significant difference test to compare differences between two groups. Statistical significance was set at P < 0.05.
Results
Effects of electroacupuncture on neurological function after spinal cord injury
BBB scale scores were significantly lesser in the SCI and EA groups compared with the sham group at all time points (P < 0.01). BBB scale scores were significantly greater in the EA group compared with the SCI group on days 7 and 14 post-injury (P < 0.01; Figure 3).
Figure 3.
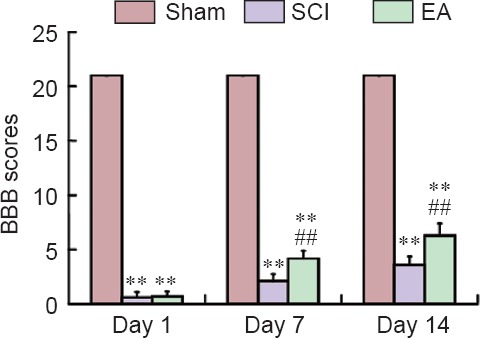
Effects of electroacupuncture (EA) on neurological function of rat models of spinal cord injury (SCI).
BBB scale scores were used to assess the neurological function of rats, with higher score representing better neurological function. Data are expressed as the mean ± SEM (n = 11 per group at each time point). **P < 0.01, vs. the sham group; ##P < 0.01, vs. the SCI group (one-way analysis of variance followed by post hoc least significant difference test). BBB: Basso, Beattie and Bresnahan hind limb locomotor rating scale. The sham group underwent laminectomy only, the SCI group underwent laminectomy + SCI induction at T10, and the EA group underwent laminectomy + SCI induction at T10 + EA intervention.
Effect of electroacupuncture on histological changes in injured spinal cord
The spinal cord tissues were normal in the sham group. In contrast, the spinal cords in the SCI and EA groups demonstrated obvious signs of compression following SCI, with destruction of organizational structures. In the SCI group on day 1 post-injury, there were a few scattered small foci of hemorrhage from small veins and capillaries, nerve cells were swollen, and necrosis was observed (Figure 4). In the SCI group on day 7 post-injury, nerve cell necrosis and interstitial inflammatory cell infiltration were markedly worsened, and there was extensive hemorrhage and nerve cell necrosis. In the SCI group on day 14 post-injury, there was less bleeding, few gray matter neurons, and a large number of proliferated microglia. In the EA group on day 1 post-injury, there were no histomorphological changes observed, although there was bleeding and swelling. In the EA group on day 7 post-injury, there was less bleeding and the pathological changes started to improve. On day 14 post-injury, the EA group had no bleeding, and the edge of the neurons began to be clear; microglial proliferation appeared, but it was relatively less than that in the SCI group (Figure 4).
Figure 4.
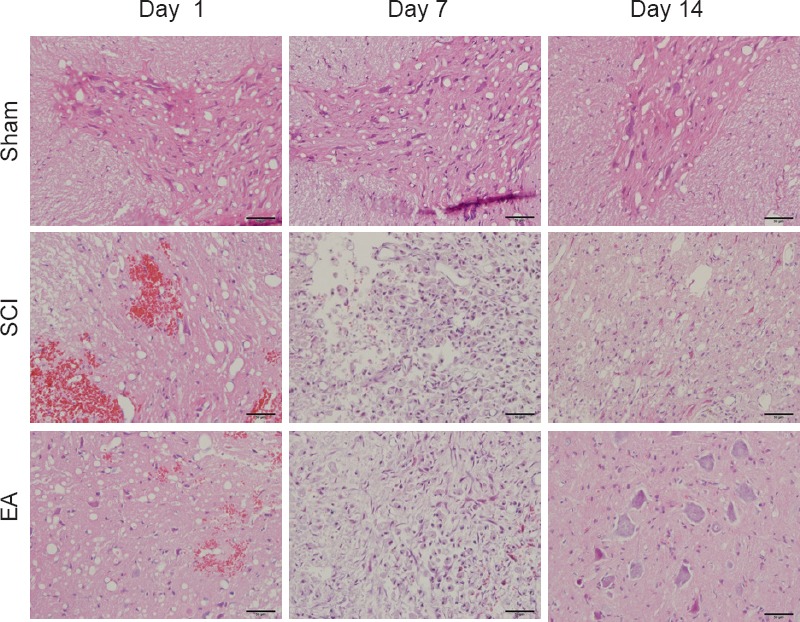
Representative hematoxylin-eosin stained images of spinal cord sections (original magnification ×400).
On day 1 post-injury, there was bleeding and swelling in the sham and SCI groups, but there were no histomorphological changes observed in the EA group; on day 7 post-injury, there was less bleeding, and the pathological changes started to improve in the EA group; on day 14 post-injury, the EA group had no bleeding, the edge of the neurons began to be clear, and there was less microglial proliferationthan in the SCI group. Scale bars: 50 μm. The sham group underwent laminectomy only, the SCI group underwent laminectomy + SCI induction at T10, and the EA group underwent laminectomy + SCI induction at T10 + EA intervention. SCI: Spinal cord injury; EA: electroacupuncture.
Electroacupuncture regulated expression of Wnt1, Wnt3a and β-catenin after spinal cord injury in rats
Immunohistochemical results
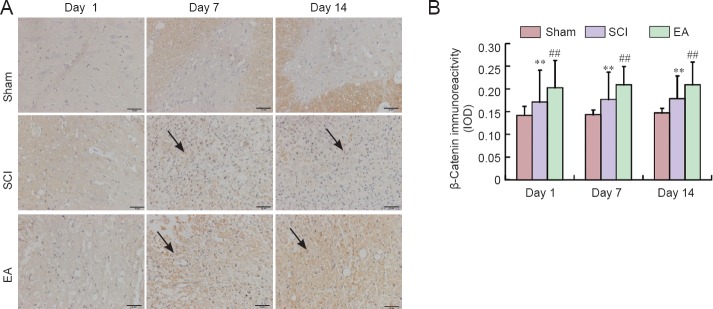
In the sham group, Wnt1, Wnt3a, and β-catenin were obviously expressed. On days 7 and 14 post-injury, Wnt1, Wnt3a, and β-catenin immunoreactivities were increased and were continuously stronger in the SCI group than in the sham group (Figures 5–7). Wnt1, Wnt3a, and β-catenin immunoreactivities were more greatly increased in the EA group than in the SCI group on days 1, 3 and 7 post-injury.
Figure 5.
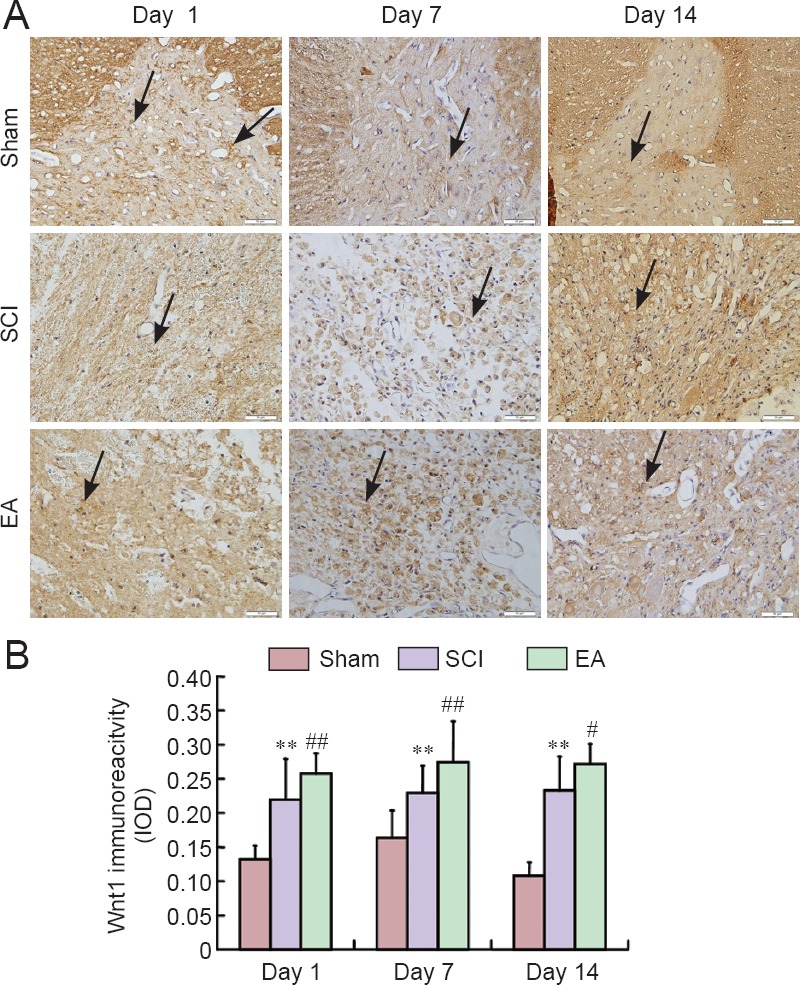
Effects of electroacupuncture (EA) on Wnt1 immunoreactivity in the injured spinal cord of rats.
(A) Representative immunohistochemical staining for Wnt1 expression (original magnification ×400). Red arrows indicate Wnt1-immunoreactive cells. Scale bars: 50 μm. (B) Semi-quantification of Wnt1 immunoreactivity in the sham, SCI, and EA groups on days 1, 7, and 14 post-injury. Data are expressed as the mean ± SEM (n = 5 rats per group). **P < 0.01, vs. the sham group; #P < 0.05, ##P < 0.01, vs. the SCI group (one-way analysis of variance followed by post hoc least significant difference test). The sham group underwent laminectomy only, the SCI group underwent laminectomy + SCI induction at T10, and the EA group underwent laminectomy + SCI induction at T10 + EA intervention. SCI: Spinal cord injury; IOD: integrated optical density.
Figure 7.
Effects of electroacupuncture (EA) on β-catenin immunoreactivity in the injured spinal cord of rats.
(A) Representative immunohistochemical staining for β-catenin expression (original magnification ×400). Red arrows indicate β-catenin-immunoreactive cells. Scale bars: 50 μm. (B) Semi-quantification of β-catenin immunoreactivity in the sham, SCI, and EA groups on days 1, 7, and 14 post-injury. Data are expressed as the mean ± SEM (n = 5 rats per group). **P < 0.01, vs. the sham group; #P < 0.05, ##P < 0.01, vs. the SCI group (one-way analysis of variance followed by post hoc least significant difference test). The sham group underwent laminectomy only, the SCI group underwent laminectomy + SCI induction at T10, and the EA group underwent laminectomy + SCI induction at T10 + EA intervention. SCI: Spinal cord injury; IOD: integrated optical density.
Figure 6.
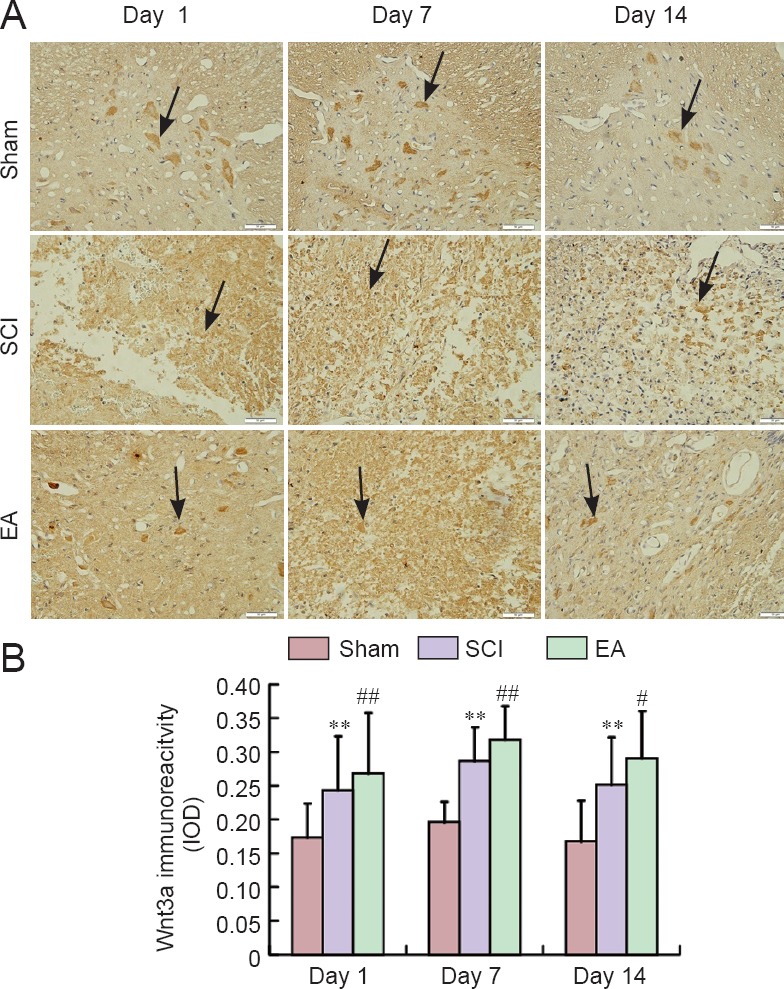
Effects of electroacupuncture (EA) on Wnt3a immunoreactivity in the injured spinal cord of rats.
(A) Representative immunohistochemical staining for Wnt3a expression (original magnification ×400). Red arrows indicate Wnt3a-immunoreactive cells. Scale bars: 50 μm. (B) Semi-quantification of Wnt3a immunoreactivity in the sham, SCI, and EA groups on days 1, 7, and 14 post-injury. Data are expressed as the mean ± SEM (n = 5 rats per group). **P < 0.01, vs. the sham group; #P < 0.05, ##P < 0.01, vs. the SCI group (one-way analysis of variance followed by post hoc least significant difference test). The sham group underwent laminectomy only, the SCI group underwent laminectomy + SCI induction at T10, and the EA group underwent laminectomy + SCI induction at T10 + EA intervention. SCI: Spinal cord injury; IOD: integrated optical density.
Western blot analysis results
Western blot analysis confirmed the immunohistochemical results for Wnt1, Wnt3a and β-catenin (Figure 8).
Figure 8.
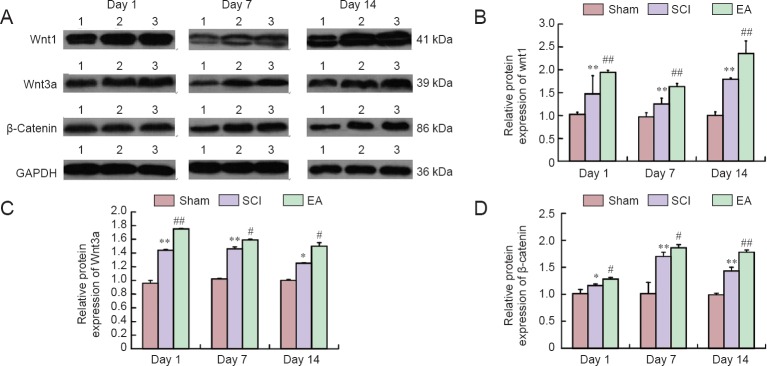
Effect of electroacupuncture (EA) treatment on protein expression of Wnt1, Wnt3a and β-catenin in injured spinal cord of rats (western blot analysis).
(A) Western blots of Wnt1, Wnt3a, and β-catenin. 1: Sham group; 2: spinal cord injury (SCI) group; 3: EA group. (B–D) Quantification of protein expression of Wnt1 (B), Wnt3a (C), and β-catenin (D) in the sham, SCI, and EA groups (n = 5 per group). *P < 0.05, **P < 0.01, vs. the sham group; #P < 0.05, ##P < 0.01, vs. the SCI group (one-way analysis of variance followed by post hoc least significant difference test). The sham group underwent laminectomy only, the SCI group underwent laminectomy + SCI induction at T10, and the EA group underwent laminectomy + SCI induction at T10 + EA intervention.
In the SCI group, protein expression levels of Wnt1, Wnt3a, and β-catenin were significantly increased on days 1, 7 and 14 post-injury (P < 0.05 or P < 0.01; Figure 8), whereas the expression level of Wnt1 was decreased on day 7 post-injury, and protein expression levels of Wnt3a and β-catenin were decreased on day 14 post-injury. Wnt1, Wnt3a, and β-catenin protein expression levels in spinal cord tissues were significantly higher in the EA group than in the SCI group on days 1, 7, and 14 post-injury (P < 0.05 or P < 0.01; Figure 8).
Discussion
The Wnt/β-catenin signaling pathway is closely related to nervous system development, stem cell proliferation, and differentiation (Yao et al., 2011). There are currently 19 known Wnt genes, which can be grouped into two categories: Wnt1 and Wnt5a families. The Wnt1 family includes Wnt1, Wnt3a, and Wnt7a, whereas the Wnt5a family includes Wnt4 and Wnt5a (He, 2003). The Wnt1 family is especially important for the development of the nervous system. There is evidence that Wnt1 and Wnt3a in particular are closely related to neural stem cell proliferation and differentiation (Vangipuram and Lyman, 2012).
The Wnt/β-catenin signaling pathway begins with the binding of the Frizzled receptors and requires low-density lipid receptor-related protein 6, which acts as a co-receptor for the Wnt ligand (Caspi et al., 2014). At the intracellular level, the canonical Wnt pathway requires the intracellular β-catenin protein, which is maintained at low levels by the action of a so-called destruction complex formed by several proteins including axin, adenomatous polyposis coli, casein kinase I, and the enzyme glycogen synthase kinase 3β. This complex drives β-catenin phosphorylation and stimulates its destruction through the proteosomal pathway (Vangipuram and Lyman, 2012). The canonical Wnt signaling activation leads to the dissociation of the β-catenin destruction complex by a series of phosphorylations that inhibit the activity of glycogen synthase kinase 3β; as a consequence, β-catenin accumulates in the cytoplasm and translocates into the nucleus, promoting its interaction with the T-cell specific transcription factor and the lymphoid enhancer-binding factor (Caspi et al., 2014). The Wnt/β-catenin signaling pathway plays an important role in SCI, especially Wnt1 and Wnt3a, and it is involved in many processes in the nervous system, including neurulation, neural induction, proliferation, axonal growth, fate determination and cell specification, and migration and differentiation (Xu et al., 2014b).
In traditional Chinese medicine, Governor Vessel injury is the main reason for SCI. When the Governor Vessel is injured, Qi and blood cannot reach the four limbs, causing tendons and muscles in the four limbs to lose nourishment, which produces disuse and atrophy because of the obstruction of meridians and collaterals. Therefore, acupoints of the Governor Vessel are the first choices during acupuncture treatment of paraplegia brought on by SCI.
GV14 is the confluence point of the Governor Vessel and the Hand and Foot Three Yang Meridians. Acupuncture stimulation of GV14 can activate channel-Qi, and regulate and inspire systemic Yang-Qi to dredge the meridian. GV4is also an acupoint of the Governor Vessel, and is the intersection of the Governor Vessel and the Belt Vessel. GV4 gathers genuine-Yin and genuine-Yang of the human body, which is the root of Yuan-Qi and the gateway of life. Stimulating GV4 can regulate these channels and activate collaterals, tonify Yang, and strengthen the kidneys. Previous experimental research showed that acupuncture at GV14 and GV4 in SCI rats regulates the physiological functions of the Governor Vessel and clears out the meridian (Xie et al., 2006).
The application of EA for the treatment of SCI has shown positive results in the alleviation of patient suffering (Wong et al., 2003). The use of EA on the Governor Vessel has successfully treated secondary damage after SCI in both human and animal models (Han et al., 2005; Xie et al., 2006).
In the present study, the BBB scale score results of the rats in the EA group showed an increase in recovery compared with the SCI group, and EA reversed the histological changes that occurred after SCI. Furthermore, EA on the Governor Vessel promoted the expression of Wnt1, Wnt3a and β-catenin expressions in the damaged spinal area. The levels of Wnt1, Wnt3a and β-catenin in the SCI group were significantly higher than the levels in the sham group, which points to an association with spinal cord tissue self-repair after SCI; however, this self-repair function was limited, while EA appreciably activated the Wnt/β-catenin pathway and the motor function of SCI rats.
The Wnt/β-catenin signaling pathway plays an important role in SCI. Briefly, a signal is received by β-catenin from membrane receptors and then transmitted to the nucleus, where some transcription factors are bound (Gao et al, 2015). As the central player, the β-catenin protein promotes the regeneration of axons, inhibits apoptosis, and improves functional recovery after SCI. Studies on the Wnt/β-catenin pathway have provided some original mechanisms in treating SCI (González-Fernández et al., 2014; Galliera et al., 2016; Han et al., 2016). In the present study, functional recovery in a rat SCI model occurred through activation of the Wnt/β-catenin signaling pathway. Western blot and immunohistochemical staining results showed that the expression of β-catenin was significantly increased with EA treatment after SCI. The results show that EA activated the Wnt/β-catenin signaling pathway, which suggests that the effect of EA has some underlying mechanism to be applied clinically.
In summary, EA stimulation of GV14 and GV4 is effective for the treatment of SCI by upregulating the Wnt/β-catenin signaling pathway.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81373728.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Zammit K, Raye W, Li CH, Song LP, Zhao M
References
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Caspi M, Perry G, Skalka N, Meisel S, Firsow A, Amit M, Rosin-Arbesfeld R. Aldolase positively regulates of the canonical Wnt signaling pathway. Mol Cancer. 2014;13:164. doi: 10.1186/1476-4598-13-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colak MC, Kavakli A, Kilinç A, Rahman A. Postoperative pain and respiratory function in patients treated with electroacupuncture following coronary surgery. Neurosciences (Riyadh) 2010;15:7–10. [PubMed] [Google Scholar]
- Ding Y, Zhang RY, He B, Liu Z, Zhang K, Ruan JW, Ling EA, Wu JL, Zeng YS. Combination of electroacupuncture and grafted mesenchymal stem cells overexpressing TrkC improves remyelination and function in demyelinated spinal cord of rats. Sci Rep. 2015;5:9133. doi: 10.1038/srep09133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliera E, Marazzi MG, Drago L, Banfi G, Luzzati A, CorsiRomanelli MM. Wnt signaling pathway inhibitors as promising diagnostic serum markers of osteolytic bone metastasis. J Biol Regul Homeost Agents. 2016;30:399–408. [PubMed] [Google Scholar]
- Gao K, Wang YS, Yuan YJ, Wan ZH, Yao TC, Li HH, Tang PF, Mei XF. Neuroprotective effect of rapamycin on spinal cord injury via activation of the Wnt/β-catenin signaling pathway. Neural Regen Res. 2015;10:951–957. doi: 10.4103/1673-5374.158360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Sun T, Li JH, Zhao N, Wang Y, Yu HL. Electroacupuncture in the repair of spinal cord injury: inhibiting the Notch signaling pathway and promoting neural stem cell proliferation. Neural Regen Res. 2015;10:394–403. doi: 10.4103/1673-5374.153687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Fernández C, Fernández-Martos CM, Shields SD, Arenas E, Javier Rodríguez F. Wnts are expressed in the spinal cord of adult mice and are differentially induced after injury. J Neurotrauma. 2014;31:565–581. doi: 10.1089/neu.2013.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han QM, Xie J, Chai ST, Fang J, Liu Q. Effect of Governer Meridian electro-acupuncture on water channel aquaporin-4 in experimental spinal cord injured rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25:637–639. [PubMed] [Google Scholar]
- Han XG, Wang DW, Bi ZG, Gao F. Regulatory effect of estrogen receptor-α-mediated Wnt/β-catenin signaling pathway on osteoblast proliferation. J Biol Regul Homeost Agents. 2016;30:381–387. [PubMed] [Google Scholar]
- He X. A Wnt-Wnt situation. Dev Cell. 2003;4:791–797. doi: 10.1016/s1534-5807(03)00165-5. [DOI] [PubMed] [Google Scholar]
- Hong J, Wu G, Zou Y, Tao J, Chen L. Electroacupuncture promotes neurological functional recovery via the retinoic acid signaling pathway in rats following cerebral ischemia-reperfusion injury. Int J Mol Med. 2013;31:225–231. doi: 10.3892/ijmm.2012.1166. [DOI] [PubMed] [Google Scholar]
- Huang SF, Ding Y, Ruan JW, Zhang W, Wu JL, He B, Zhang YJ, Li Y, Zeng YS. An experimental electro-acupuncture study in treatment of the rat demyelinated spinal cord injury induced by ethidium bromide. Neurosci Res. 2011;70:294–304. doi: 10.1016/j.neures.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Jiang DX, Lu ZS, Li GB, Sun SY, Mu X, Lee P, Chen W. Electroacupuncture improves microcirculation and neuronal morphology in the spinal cord of a rat model of intervertebral disc extrusion. Neural Regen Res. 2015;10:237–243. doi: 10.4103/1673-5374.152377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert C, Cisternas P, Inestrosa NC. Role of Wnt signaling in central nervous system injury. Mol Neurobiol. 2016;53:2297–2311. doi: 10.1007/s12035-015-9138-x. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zheng SS, Dan QQ, Liu J, Wang TH. Effects of Governor Vessel electroacupuncture on the systematic expressions of NTFs in spinal cord transected rats. Neuropeptides. 2014;48:239–247. doi: 10.1016/j.npep.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Liu Z, He B, Zhang RY, Zhang K, Ding Y, Ruan JW, Ling EA, Wu JL, Zeng YS. Electroacupuncture promotes the differentiation of transplanted bone marrow mesenchymal stem cells preinduced with neurotrophin-3 and retinoic acid into oligodendrocyte-like cells in demyelinated spinal cord of rats. Cell Transplant. 2015;24:1265–1281. doi: 10.3727/096368914X682099. [DOI] [PubMed] [Google Scholar]
- Mitchell D. Acupuncture immunomodulation. J Complement Med. 2009:8. [Google Scholar]
- Mo YP, Yao HJ, Lv W, Song LY, Song HT, Yuan XC, Mao YQ, Jing QK, Shi SH, Li ZG. Effects of electroacupuncture at Governor Vessel acupoints on neurotrophin-3 in rats with experimental spinal cord injury. Neural Plast 2016. 2016:2371875. doi: 10.1155/2016/2371875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell MP, Weeraratna AT. Hear the WntRor: how melanoma cells adjust to changes in Wnt. Pigment Cell Melanoma Res. 2009;22:724–739. doi: 10.1111/j.1755-148X.2009.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Sun J, Sang H, Yang C, Dong H, Lei C, Lu Y, Ma Y, Zhou X, Sun X, Xiong L. Electroacupuncture improves orthostatic tolerance in healthy individuals via improving cardiac function and activating the sympathetic system. Europace. 2013;15:127–134. doi: 10.1093/europace/eus220. [DOI] [PubMed] [Google Scholar]
- Tao J, Xue XH, Chen LD, Yang SL, Jiang M, Gao YL, Wang XB. Electroacupuncture improves neurological deficits and enhances proliferation and differentiation of endogenous nerve stem cells in rats with focal cerebral ischemia. Neurol Res. 2010;32:198–204. doi: 10.1179/174313209X414506. [DOI] [PubMed] [Google Scholar]
- Ulett GA, Han S, Han JS. Electroacupuncture: mechanisms and clinical application. Biol Psychiatry. 1998;44:129–138. doi: 10.1016/s0006-3223(97)00394-6. [DOI] [PubMed] [Google Scholar]
- Vangipuram SD, Lyman WD. Ethanol affects differentiation-related pathways and suppresses Wnt signaling protein expression in human neural stem cells. Alcohol ClinExp Res. 2012;36:788–797. doi: 10.1111/j.1530-0277.2011.01682.x. [DOI] [PubMed] [Google Scholar]
- Varma AK, Das A, Wallace G, 4th, Barry J, Vertegel AA, Ray SK, Banik NL. Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem Res. 2013;38:895–905. doi: 10.1007/s11064-013-0991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AM, Leong CP, Su TY, Yu SW, Tsai WC, Chen CP. Clinical trial of acupuncture for patients with spinal cord injuries. Am J Phys Med Rehabil. 2003;82:21–27. doi: 10.1097/00002060-200301000-00004. [DOI] [PubMed] [Google Scholar]
- Xie J, Fang J, Feng X, Liu Q. Effect of electroacupuncture at acupoints of the governor vessel on aquaporin-4 in rat with experimental spinal cord injury. J Tradit Chin Med. 2006;26:148–152. [PubMed] [Google Scholar]
- Xu D, Zhao W, Pan G, Qian M, Zhu X, Liu W, Cai G, Cui Z. Expression of Nemo-like kinase after spinal cord injury in rats. J Mol Neurosci. 2014b;52:410–418. doi: 10.1007/s12031-013-0191-5. [DOI] [PubMed] [Google Scholar]
- Xu T, Li W, Liang Y, Yang Z, Liu J, Wang Y, Su N. Neuroprotective effects of electro acupuncture on hypoxic-ischemic encephalopathy in newborn rats Ass. Pak J Pharm Sci. 2014a;27(6 Suppl):1991–2000. [PubMed] [Google Scholar]
- Xu YQ, Feng SQ, Wang P. Axonal regeneration promotion by chondroitinase ABC after spinal cord injury in rats. Orthop J Chin. 2010;7:016. [Google Scholar]
- Xue CC, Helme RD, Gibson S, Hogg M, Arnold C, Somogyi AA, Da Costa C, Wang Y, Lu SC, Zheng Z. Effect of electroacupuncture on opioid consumption in patients with chronic musculoskeletal pain: protocol of a randomised controlled trial. Trials. 2012;13:169. doi: 10.1186/1745-6215-13-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Ashihara E, Maekawa T. Targeting the Wnt/β-catenin signaling pathway in human cancers. Expert Opin Ther Targets. 2011;15:873–887. doi: 10.1517/14728222.2011.577418. [DOI] [PubMed] [Google Scholar]
- Yılmaz T, Kaptanoğlu E. Current and future medical therapeutic strategies for the functional repair of spinal cord injury. World J Orthop. 2015;6:42–55. doi: 10.5312/wjo.v6.i1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu KW, Lin CL, Hung CC, Chou EC, Hsieh YL, Li TM, Chou LW. Effects of electroacupuncture on recent stroke inpatients with incomplete bladder emptying: a preliminary study. Clin Interv Aging. 2012;7:469–474. doi: 10.2147/CIA.S37531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kang T, Li L, Zhang J. Electroacupuncture reduces hemiplegia following acute middle cerebral artery infarction with alteration of serum NSE, S-100B and endothelin. Curr Neurovasc Res. 2013a;10:216–221. doi: 10.2174/15672026113109990005. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Zou Y, Zhao Y, Wang TH, Zhang W. Effects of electroacupuncture of “governor vessel” acupoints on changes of BDNF in the cortical motor area of mice with spinal cord transection. Sichuan Daxue Xuebao Yixue Ban. 2012;43:250–253. [PubMed] [Google Scholar]
- Zhang ZJ, Ng R, Man SC, Li JT, Wong W, Wong HK, Wang D, Wong MT, Tsang AW, Yip KC, Sze SC. Use of electroacupuncture to accelerate the antidepressant action of selective serotonin reuptake inhibitors: a single-blind, randomised, controlled study. Hong Kong Med J. 2013b;19(Suppl 9):12–16. [PubMed] [Google Scholar]
- Zheng LR, Zhu XQ, Huang XM, Gu Q, Xie DH. Morphological study on early development of brain derived neurophic factor-positive neurons in the frontal lobe of human fetus. Zhongguo Yixue Kexue Yuan Xuebao. 2013;35:260–264. doi: 10.3881/j.issn.1000-503X.2013.03.004. [DOI] [PubMed] [Google Scholar]