Abstract
Obesity paradox (OP) describes a widely observed clinical finding of improved cardiovascular fitness and survival in some overweight or obese patients. The molecular mechanisms underlying OP remain enigmatic partly due to a lack of animal models mirroring OP in patients. Using apolipoprotein E knock-out (apoE−/−) mice on a high fat (HF) diet as an atherosclerotic obesity model, we demonstrated 1) microRNA-155 (miRNA-155, miR-155) is significantly up-regulated in the aortas of apoE−/− mice, and miR-155 deficiency in apoE−/− mice inhibits atherosclerosis; 2) apoE−/−/miR-155−/− (double knock-out (DKO)) mice show HF diet-induced obesity, adipocyte hypertrophy, and present with non-alcoholic fatty liver disease; 3) DKO mice demonstrate HF diet-induced elevations of plasma leptin, resistin, fed-state and fasting insulin and increased expression of adipogenic transcription factors but lack glucose intolerance and insulin resistance. Our results are the first to present an OP model using DKO mice with features of decreased atherosclerosis, increased obesity, and non-alcoholic fatty liver disease. Our findings suggest the mechanistic role of reduced miR-155 expression in OP and present a new OP working model based on a single miRNA deficiency in diet-induced obese atherogenic mice. Furthermore, our results serve as a breakthrough in understanding the potential mechanism underlying OP and provide a new biomarker and novel therapeutic target for OP-related metabolic diseases.
Keywords: atherosclerosis, inflammation, liver, microRNA (miRNA), obesity
Introduction
Metabolic syndrome (MetS)4 is a recently coined clinical term that refers to the presence of at least three of the following five conditions: central obesity, elevated triglycerides, diminished high density lipoprotein cholesterol, hypertension, and insulin resistance. Individuals diagnosed with MetS have a 2-fold increased risk of suffering from stroke, myocardial infarction, atherosclerosis, or succumbing to cardiovascular disease (CVD)-associated mortality and are 5 times more likely to develop type II diabetes (1). In addition, non-alcoholic fatty liver disease (NAFLD) is a clinical condition hallmarked by the abnormal accumulation of triglycerides within the liver and is considered a hepatic manifestation of MetS (2). In contrast to the low mortality risk found in the normal body mass index (BMI) range of 20–24.9 kg/m, BMI values in the overweight and obese categories usually have adverse effects on cardiac structure and function, with a prevalence of CVD markedly increasing with the degree of obesity (3). As an exception to this widely accepted concept, recent clinical data has shown that in some patients with chronic diseases, including chronic heart failure or coronary artery disease, the best survival outcome is observed in overweight or obese patients. This phenomenon is termed “obesity paradox” (4–7). However, the molecular and regulatory mechanisms underlying the obesity paradox remain unknown, which results partially from the fact that animal models mirroring obesity paradox, have not been established.
Chronic inflammation is a common mechanism of both obesity (8) and atherosclerosis (9), the latter of which can give rise to complications, such as myocardial infarction, stroke, and peripheral artery disease, which are the leading cause of morbidity and mortality in the United States (10). We and others previously reported that hyperlipidemia and other risk factors promote vascular inflammation in various aspects, such as endothelial cell (EC) activation and injury (11, 12), increasing monocyte recruitment and differentiation (13–15), and decreasing regulatory T cell inflammation suppression (16, 17). However, the interplay between obesity and atherosclerosis in the pathology of obesity paradox remains unknown.
As we recently reviewed, the discovery of non-coding RNAs, including microRNAs (miRs), has revolutionized the way that we examine the genome, RNA products, and the regulation of transcription and translation (18, 19). By facilitating mRNA degradation and translation repression, miRs regulate inflammatory responses (20), EC activation (21), atherosclerosis (22), obesity (23), and NAFLD (24), etc. Due to the well characterized proinflammatory nature of miR-155, its role in chronic vascular inflammatory diseases has been critically investigated. However, the reported roles of miR-155 in vascular pathology have been controversial. miR-155 promotes EC dysfunction by targeting the 3′-untranslated region of endothelial nitric-oxide synthase. In fact, inhibition of miR-155 increases endothelial nitric-oxide synthase expression and improves EC function (25). However, other reports found that miR-155 reduces the expression of vascular cell adhesion molecule-1 (VCAM-1), leukocyte-EC interaction, and EC migration (26) via inhibition of proinflammatory extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) in ECs (27) and up-regulation of anti-inflammatory heme oxygenase-1 (28). Along the same line, the role of miR-155 in atherosclerosis also remains controversial. miR-155 expression can be induced by tumor necrosis factor-α (TNF-α) (29) in atherogenic apolipoprotein E-deficient (apoE−/−) mouse aorta and in plasma samples from patients with coronary artery disease. In addition, it has been shown that hematopoietic deficiency of miR-155 leads to more advanced atherosclerotic lesions in low density lipoprotein receptor knock-out (LDLR−/−) mice (25), whereas intravenous delivery of miR-155 “agomiRs” to overexpress miR-155 reduces atherosclerotic lesion in apoE−/− mice (30). In contrast to these findings, however, it has been reported that miR-155 promotes type 1 CD4+ T helper cell (Th1) differentiation (31, 32) and that leukocyte-specific miR-155 deficiency reduces plaque size in apoE−/− mice via directly targeting an NF-κB negative regulator, Bcl-6 (33). Thus, the controversial roles of miR-155 in EC activation and atherogenesis need to be further elucidated.
In addition, miR-155 appears to play an important role in adipose tissue. It is enriched in mouse brown adipose tissue, and miR-155-deficient mice exhibit increased brown adipose tissue function and browning of white fat tissue (34). miR-155 is also expressed in human omental and subcutaneous adipose tissue where miR-155 expression is negatively correlated with adipocyte volume (35). However, an important question remains of whether miR-155 regulates the fat deposition differentially in aorta, liver, and white adipose tissue (WAT).
In this study, we examined the role of miR-155 in atherosclerosis and obesity using our newly generated apoE−/−/miR-155−/− double knock-out (DKO) mice. By using polymerase chain reaction (PCR) array, histological, flow cytometry, nuclear magnetic resonance (NMR), and metabolic analyses, we found that miR-155 promotes aortic EC activation and atherosclerosis and consequently that apoE−/−/miR-155−/− DKO mice have less atherosclerotic plaques. Paradoxically, we showed that miR-155 inhibits WAT adipogenesis and NAFLD and, consequently, that DKO mice have significantly increased body weight and augmented WAT and NAFLD. These results suggest that miR-155 promotes aortic EC activation and atherogenesis but inhibits WAT development and the pathogenesis of NAFLD, which make miR-155 deficiency in apoE−/− background a novel model for studying the pathogenesis of obesity paradox. The establishment of this novel mouse model for the investigation of obesity paradox would have significant impact on the determination of the molecular mechanisms underlying obesity paradox and the identification of novel therapeutics for related diseases.
Results
MiR-155 Is Significantly Up-regulated in the Aortas of ApoE−/− Mice during Early Atherosclerosis
To determine the roles of miRNAs in atherosclerosis development as well as measure their relevancies against other miRNAs, we utilized a PCR array to screen 84 of the most abundant and well characterized miRNAs in miRBase. We compared the aortic miRNA expression profiles of apoE−/− mice fed normal chow diet versus apoE−/− mice fed a high fat diet for 12 weeks. Analysis of the array data revealed several miRNAs that were differentially regulated (Fig. 1A). We found that 16 miRNAs were up-regulated by at least 4-fold (miR-880, miR-295, miR-155, miR-21a, miR-302d, miR-291a, and miR-744) or down-regulated by at least 4-fold (miR-99a, miR-199a, miR-335, miR-142–3p, miR-142–5p, miR-19b, miR-101a, miR-19a, and miR-22) (Fig. 1B). The atherosclerosis-induced down-regulations of miR-19b and miR-22 were predicted in our bioinformatics analysis (18). Of note, atherosclerosis-induced up-regulations of five miRs, including miR-880, miR-295, miR-302d, miR-291a, and miR-744, in mouse aorta have not been reported (36, 37). In addition, atherosclerosis-induced down-regulations of eight miRs have not been reported in the atherosclerotic aorta, the exception being miR-19a (38, 39). miR-155 was one of 3 miRNAs with a 10-fold or greater change in expression, and its well characterized proinflammatory role suggested that it might be involved in the development of atherosclerosis. Of note, the unavailability of miR-880- and miR-295-deficient mice prevented us from performing further atherosclerotic studies on those miRs right away. Next, we confirmed our miR-155 screening results with qRT-PCR (Fig. 1C). The statistical significance for miR-155 up-regulation was observed after only 3 weeks of a high fat diet (HF) diet. Moreover, the -fold change and significance continued to increase with duration of feeding (Fig. 1C). Our data on the increased plasma levels of cholesterol and triglycerides in apoE−/− mice fed with a high fat diet for 0 weeks, 3 weeks, 6 weeks, and 12 weeks (Fig. 1, D and E) suggest that up-regulation of miR-155 in aortas is correlated well with elevated levels of plasma cholesterol and triglycerides. This dramatic shift in miR-155 expression during atherosclerosis indicated that it might play a critical role in atherosclerosis and that it could be one of the most pertinent contributing miRNAs. In addition, because lipopolysaccharide (LPS) has been found to contribute to atherosclerosis (38), we also examined the expression of miR-155 and other miRs in LPS-stimulated wild-type mouse aortas using PCR array. The results showed that LPS significantly up-regulated miR-155 and miR-22 in wild-type (WT) mouse aortas (Fig. 1, F and G). Quantitative PCR confirmed that LPS induced miR-155 up-regulation by >10-fold in WT mouse aortas (Fig. 1H). Collectively, these findings demonstrate that aortic miR-155 expression is up-regulated during atherogenesis, indicating a pertinent role for miR-155 in atherosclerosis.
FIGURE 1.
Aortic miR-155 expression was augmented with atherosclerosis development and during LPS-induced acute inflammation. The aortas of apoE−/− mice on NC or HF diets for 12 weeks (12W) were excised, and RNA was isolated. A, Scatter plot analysis of the log10 of normalized gene expression levels of the 84 most abundant miRNAs in miRBase in high fat-fed mouse aortas (y axis) versus normal chow fed mouse aortas (x axis). The colored symbols outside the 4-fold threshold line area indicate -fold differences larger than 4-fold. Red triangles denote miRNAs having a >4-fold increase. Blue squares denote miRNAs having a >4-fold decrease. B, list of miRNAs that were either up-regulated or down-regulated by at least 4-fold. C, aortic miR-155 expression in apoE−/− mice fed a HF diet for 0 weeks (n = 6), 3 weeks (n = 7), 6 weeks (n = 7), and 12 weeks (n = 4) measured by qRT-PCR. D and E, plasma levels of cholesterol and triglyceride in apoE−/− mice fed with HF diet for 0 weeks (n = 6), 3 weeks (n = 3), 6 weeks (n = 6), and 12 weeks (n = 3). F, Scatter plot analysis of the 84 most abundant miRNAs in miRBase in WT LPS-challenged aortas (y axis) versus PBS-treated aortas (x axis). G, list of miRNAs that were up-regulated in mouse aorta stimulated with LPS by at least 4-fold. H, WT mice on NC injected intraperitoneally with LPS (20 μg/g) or PBS. After 4 h, aortas were excised and RNA was isolated. miR-155 expression was confirmed by qRT-PCR. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
MiR-155 Promotes Atherosclerosis and Endothelial Cell Activation in ApoE−/− Mice
Thus far, there have been contradicting reports on the contributions of miR-155 in atherosclerosis development. A previous paper published by Nazari-Jahantigh et al. (33) detailed a pro-atherogenic role for miR-155 through its promotion of a proinflammatory macrophage response. In contrast, Donners et al. (25) report that hematopoietic miR-155 deficiency enhances atherosclerosis and decreases plaque stability in hyperlipidemic mice. Due to the conflicting reports within the scientific community, we felt that atherosclerosis assessment within our model would provide useful and significant insight into the topic. As part of this assessment, we organized a table identifying direct targets of miR-155 in atherosclerosis from previous studies (supplemental Table 1). Furthermore, our new results demonstrating that 4-h LPS stimulation significantly up-regulates miR-155 expression in WT aortas uniquely highlight the potential contributions of vascular cell miR-155 expression to atherogenesis in the absence of significant macrophage recruitment into the aorta under these conditions (39). We performed several experiments to determine the role of miR-155 in vascular ECs and atherosclerosis. Investigation of atherosclerosis within our models yielded novel results. First, we found aortic intercellular adhesion molecule-1 (ICAM-1), a prototypic EC adhesion molecule, to be significantly diminished in miR-155−/− mice in comparison with that of WT mice in non-stimulated and LPS-stimulated mice (Fig. 2, A and C). This suggests that miR-155 promotes ICAM-1 expression under both physiological conditions and during times of inflammation where it facilitates aortic EC activation. The constitutive expression of ICAM-1 and insignificant up-regulation of ICAM-1 in WT aortas in response to 4-h LPS stimulation may be due to a high constitutive expression of ICAM-1 or possibly a delayed ICAM-1 up-regulation in response to LPS stimulation. Second, we found no difference in the expression of aortic VCAM-1, another prototypical EC adhesion molecule, between miR-155−/− and WT mice (Fig. 2, A and B). This suggests that miR-155 selectively impacts ICAM-1 expression. To further extend this finding, we examined miR-155 expression in human aortic ECs (HAECs) stimulated by LPS and pro-atherogenic inflammatory cytokines TNF-α and IL-1β as well as oxidized low density lipoprotein-derived pro-atherogenic lipids, lysophosphatidic acid and lysophosphatidylcholine. Although the results of TNF-α-induced up-regulation of miR-155 in human umbilical vein ECs have been reported (29), we are the first to report that stimulation by LPS, TNF-α, IL-1β, lysophosphatidic acid, and lysophosphatidylcholine induces miR-155 up-regulation in atherosclerosis-related human aortic ECs (Fig. 2D). Similar to our data, other studies show that TNF-α, IL-1β, and IL-6 serve as proinflammatory mediators of miR-155's pro-atherosclerotic function (33, 40). Our findings of miR-155 up-regulation in aortic ECs in response to pro-atherogenic stimuli and of miR-155 promotion of ICAM-1 expression in mouse aortas strongly suggest an important role for miR-155 in promoting aortic EC activation. To directly examine this issue, we used a static EC adhesion assay as a functional read-out for aortic EC activation. HAECs were transfected with various doses of miR-155 mimic (6.25, 12.5, 25, and 50 ng) and then permitted to interact with non-treated human peripheral blood mononuclear cells as we have previously reported (12). Our results showed that enhanced miR-155 expression in aortic ECs significantly increased monocyte adhesion to miR-155 mimic-transfected HAECs in comparison to HAECs transfected with mimic controls, indicating that miR-155 plays a functional role in EC activation and leukocyte adhesion (Fig. 2E).
FIGURE 2.
MiR-155 increased untreated monocyte adhesion to aortic endothelial cells by promoting ICAM-1 expression during homeostasis and LPS-induced inflammation. A, wild-type and miR-155−/− VCAM-1 and ICAM-1 protein expression under homeostatic and inflammatory conditions (20 μg/g LPS stimulation for 4 h) in the mouse aorta. Quantification of Western blots. B, VCAM-1. C, ICAM-1. D, miR-155 expression is elevated in a dose-dependent manner by atherogenic endotoxin, cytokines, and lipids within HAECs in vitro. E, static human aortic endothelial cell adhesion assay after treatment with miR-155 mimics and incubation with untreated human primary peripheral blood mononuclear cells. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Proinflammatory immune responses play a critical role in the pathogenesis of MetS, obesity, and atherosclerosis (41), and miR-155 has been indicated as a mediator of several cellular processes related to these pathologies (33). In addition, our results on miR-155 promotion of aortic EC activation implicate miR-155 as a player in atherogenesis. We used apoE−/−/miR-155−/− DKO mice to determine whether miR-155 promotes early atherosclerosis, which was not examined previously (33, 42). At 8 weeks of age, several groups of male mice were fed with a high fat diet for 0, 3, 6, or 12 weeks, after which aortas were excised for enface analysis (Fig. 3, A and B). As expected, we observed virtually no plaque area in the 0- and 3-week high fat-fed mice as well as no discernible differences between apoE−/− and DKO mice. However, after 6 weeks of a high fat diet, a noticeable phenotype began to develop despite being statistically insignificant, as DKO mice had 32% less aortic lesions. This indicates that miR-155 in vascular cells, including aortic ECs, contributes to early atherosclerosis (i.e. the stage before intermediate lesion formation in apoE−/− mouse aortas; Ref. 43) as we reported in a similar study (11). Observation at 12 weeks of a high fat diet resulted in statistically significant findings, translating to a 45% decrease in lesion area in DKO mice in comparison to their apoE−/− equivalents (p < 0.05) (Fig. 3, A and B). We then confirmed this 12-week high fat data by examining atherosclerotic plaque development within the aortic root using histological approach. Again, we observed a significant statistical difference in plaque area in the aortic root between apoE−/− and DKO mice (p < 0.05) (Fig. 3C). These new findings along with our new EC activation data strongly indicate that miR-155 is pro-EC-activating and pro-atherogenic in nature, which significantly strengthens the macrophage data published by Nazari-Jahantigh et al. (33) and Du et al. (42).
FIGURE 3.
apoE−/−/miR-155−/− DKO mice have reduced atherosclerosis development. A, the aortas of apoE−/− and DKO mice were excised after noted durations of a HF diet feeding and stained with Sudan IV (en face staining). B, the ratios of the plaque area stained red with Sudan IV over total aorta area were quantitated in the aortas of DKO and apoE−/− control mice fed HF diet for 0 weeks (apoE−/−, n = 5; DKO, n = 5): 3 weeks (3W) (apoE−/−, n = 10; DKO, n = 10); 6W (apoE−/−, n = 10; DKO, n = 10); 12W (apoE−/−, n = 10; DKO, n = 10). C, representative cross-sections of aortic roots stained with Oil Red O (left panel). The atherosclerotic plaque lesions were quantitated in the DKO aortic roots (n = 10) and apoE−/− control aortic roots (n = 10) (right panel). *, p < 0.05. D, data-mining analysis showed that miR-155 expression is down-regulated in diet-induced obese white adipose tissue in comparison to that of wild-type mice. These data were obtained from our database mining analysis of microarray experimental datasets (GEO DataSets ID: GDS3985) deposited by others in the NIH-Geo Datasets database (for the details of the microarray experiments, see Yadav et al. (69). DIO, diet-induced obese; n = 4.
MiR-155 Inhibits HF-induced Obesity and Augmented Gonadal White Adipose Tissue Mass in ApoE−/− Mice
The incidence of overweight and obese individuals is a growing epidemic worldwide. As a result, individuals afflicted with MetS and CVD have simultaneously skyrocketed, posing a major health problem (41). In contrast to traditional health outcomes, the best survival of patients with chronic heart failure and coronary artery disease is observed in overweight or obese patients, a finding termed as the obesity paradox (4–7). Although a previous report suggests that miR-155 inhibits the differentiation of brown adipose tissue (34), it remains unclear whether miR-155 regulates the adipogenesis of WAT. The following findings indicate that miR-155 may promote atherosclerosis but inhibit white adipocyte hypertrophy. 1) miR-155 promotes atherosclerosis reported here and also by others (34, 44). 2) miR-155 is expressed in human intra-abdominal omental adipose tissue (WAT) (35). 3) miR-155 expression is down-regulated during adipogenesis (34, 40). 4) Our in silico analysis (National Institutes of Health Geo Datasets database) found that miR-155 expression is decreased in WAT samples from high fat diet-induced obese mice when compared with non-obese controls (Fig. 3D). 5) An inverse correlation exists between the expression of miR-155 in human intra-abdominal omental adipocytes and mean adipocyte volume (35). 6) Overexpression of miR-155 during adipogenesis of primary human stromal cells inhibits adipogenesis (40). Thus, we examined a novel hypothesis that miR-155 promotes atherosclerosis but inhibits white adipocyte hypertrophy. Our corollary hypothesis was that depletion of miR-155 in an apoE−/− background would result in mice with decreased atherosclerosis and increased obesity, similar to the findings found in patients with obesity paradox (4–7). To test these hypotheses, we used the well established method of high fat diet-induced obesity on WT mice, miR-155−/− mice, apoE−/− mice (44), and apoE−/−/miR-155−/− DKO mice (Fig. 4) to explore the role of miR-155 in obesity and MetS. We first examined body mass, where we found that both male and female mice from the WT group and the miR-155−/− group gained significant body weight, starting gradually after 2–3 weeks of a HF diet (supplemental Fig. 1, A–D), suggesting that the high fat diet-induced obese model worked as expected. However, there were no differences in body weight between the WT group and the miR-155−/− group on either normal chow (NC) or HF diet except in female WT versus miR-155−/− mice fed NC, which exhibited a significant difference only until 13 weeks old (Fig. 4, A–D). In contrast, when challenged with a high fat diet (44), male and female DKO mice exhibited a clear phenotype with body mass augmentation in comparison to apoE−/− mice (p < 0.05) (Fig. 4, G and H). This difference rapidly manifested in male DKO mice showing statistical difference after only 1 week of high fat feed and persisting throughout the duration of the study (p < 0.05) (Fig. 4G). Female DKO mice also demonstrated significant distinction from apoE−/− counterparts on a high fat feed, although this phenotype was delayed in onset and not as dramatic as that witnessed in the males (p < 0.05) (Fig. 4H). Our data suggest that miR-155 plays an integral part in maintaining metabolic homeostasis during times of caloric surplus in a vascular disease setting.
FIGURE 4.
ApoE−/−/miR-155−/− (DKO) mice have augmented body weight. By comparison, miR-155−/− mice have no consistent phenotypic difference in body weight from wild-type controls. Body weight measurements of mice: high fat diet feeding starting at 8 weeks of age (A′); male (A) and female (B) miR-155−/− and WT mice on normal chow (NC); male (C) and female (D) miR-155−/− and WT mice on HF diet; male (E) and female (F) apoE−/− and DKO mice on an NC diet; male (G) and female (H) DKO mice on a HF diet. Red data points denote mice fed with a high fat diet. Black data points denote mice fed with normal chow. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Because the observed augmentation in body weight within our DKO mice could be due to a change in body composition, we further investigated this matter. Using an NMR method as reported (45), we were able to evaluate mouse body fat percentage, lean mass percentage, and free fluid percentage. We found that the absolute body fat in grams and body fat percentage of both male and female miR-155−/− mice on NC diet were significantly higher than those of WT controls (p < 0.05) (Fig. 5, A and B), whereas the absolute body fat in grams and body fat percentages of both male and female miR-155−/− mice on a high fat diet were also higher than that of WT controls but without significance (p > 0.05) (Fig. 5, A and B). In addition, male DKO mice on normal or HF diet had a significantly elevated body fat percentage when compared with apoE−/− counterparts (Fig. 5G). These differences equated to a 19% and 46% increase in body fat percentage within the NC and HF diet-fed groups, respectively. This trend was also observed in the female DKO mice (Fig. 5H). The evaluation of the female high fat diet groups revealed a statistically significant 30% augmentation in body fat percentage, whereas the same trend was observed in the NC groups despite not reaching statistical significance. These data definitively showed that the elevated body mass in the DKO mice is a result of augmented body fat. In addition, the smaller distinction in body fat percentage among female mice was in accordance with our body weight observations, showing the continuity among our data.
FIGURE 5.
MiR-155−/− mice have augmented body fat and corresponding reductions in lean mass compared with wild-type controls, and DKO mice have augmented body fat and corresponding reductions in lean mass compared with apoE−/− controls. NMR body composition analysis was performed. Body fat in grams (left panel) and by percentage (right panel) was calculated for male (A) and female (B) miR-155−/− and WT mice. Lean mass in grams (left panel) and by percentage (right panel) was calculated for male (C) and Female (D) miR-155−/− and WT mice. Free fluid in grams (left panel) and by percentage (right panel) was calculated for male (E) and Female (F) miR-155−/− and WT mice. WT male on normal chow (NC), n = 11; miR-155−/− male on NC, n = 10; WT male on high fat diet (HF for 12 weeks throughout this experiment), n = 12; miR-155−/− male on HF diet, n = 4; WT female on NC, n = 10; miR-155−/− female on NC, n = 10; WT female on HF diet, n = 12; miR-155−/− female on HF diet, n = 10. NMR body composition analysis was performed. Body fat in grams (left panel) and by percentage (right panel) was calculated for male (G) and female (H) DKO and apoE−/− mice. Lean mass in grams (left panel) and by percentage (right panel) was calculated for male (I) and female (J) DKO and apoE−/− mice. Free fluid in grams (left panel) and by percentage (right panel) was calculated for male (K) and female (L) DKO and apoE−/− mice. apoE−/− male on NC, n = 9; DKO male on NC, n = 10; apoE−/− male on HF diet, n = 11; DKO male on HF diet, n = 10; apoE−/− female on NC, n = 10; DKO female on NC, n = 9; apoE−/− female on HF diet, n = 10; DKO female on HF diet, n = 10. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Analysis of the lean mass data generated by NMR, as expected, yielded an inverse relationship of the lean mass with body mass percentage. This is anticipated as an increase in body fat percentage must result in a reduction in lean mass percentage. Specifically, we found that lean mass percentage was significantly reduced in male DKO mice in comparison to male apoE−/− mice on HF diet (Fig. 5I). Furthermore, the same trend was observed in NC groups, although statistical significance was not achieved. Investigation of the lean mass data in female DKO mice on a HF diet also revealed a significant reduction when compared with apoE−/− equivalents (Fig. 5J). Also like the males, a statistically significant distinction between female mice on NC could not be established even though the same trend was observed. These lean mass data provide additional support to our findings on mouse body fat percentages.
The final measurement recorded with NMR was free body fluid mass and percentage. In this case we observed a difference between male apoE−/− mice and male DKO mice on a high fat diet (Fig. 5K) but found no differences within diet groups for females (Fig. 5L). This uniformity among apoE−/− and DKO mice is important as free body fluid percentage serves as a comparable baseline between groups for the validity of the body fat percentage and lean mass percentage measurements.
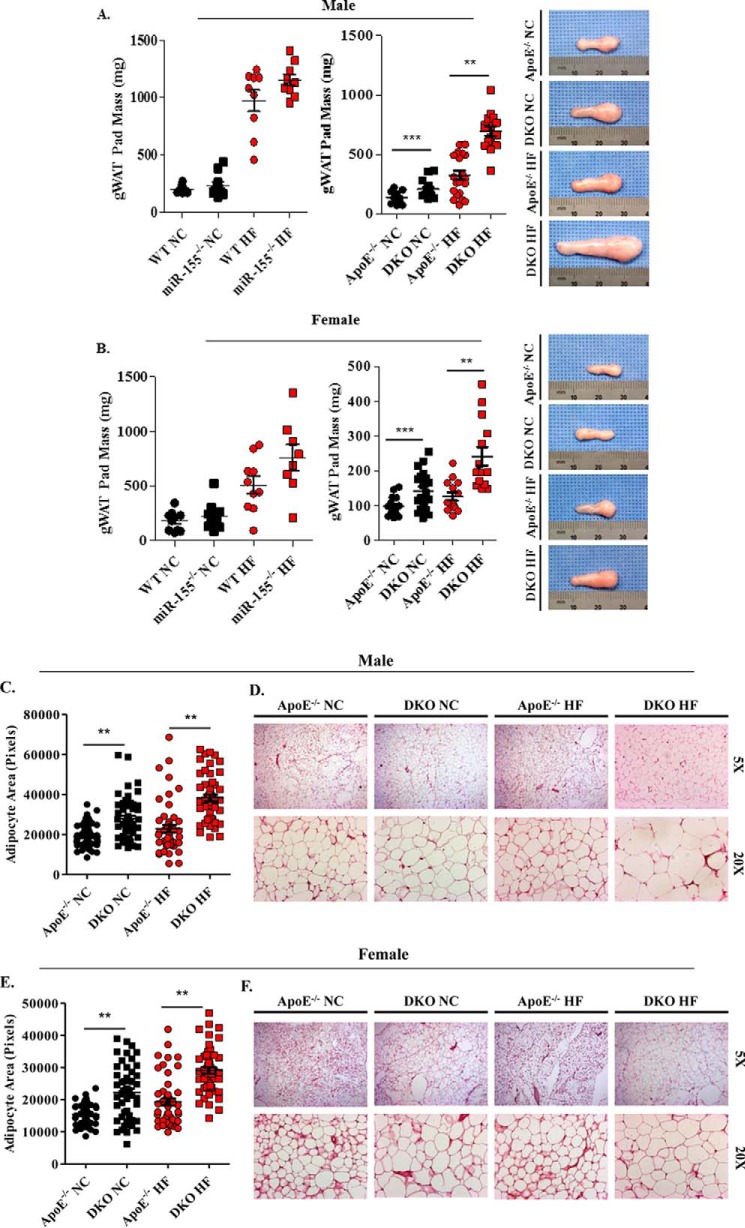
The lack of previous publications by the scientific community in regard to our findings on body mass and body fat percentage prompted us to further confirm these novel findings through fat pad examination. Although there are several sites where adipose tissue resides, the gonadal WAT (gWAT) deposit was chosen for further analysis. This site was selected for its size, comprising up to 50% of murine adipose tissue, and easy excision. The left gWAT pad mass of miR-155−/− mice was heavier than that of WT mice despite not reaching statistical significance (Fig. 6). Analysis of the left gWAT pad revealed a significant increase in mass among male and female DKO mice on either a NC or HF diet when compared with apoE−/− equivalents (Fig. 6). This observation equated to a 43 and 112% increase in male gWAT pad mass in NC and HF diet-fed mice, respectively (Fig. 6A). In accordance with this finding, female DKO mice also had larger gWAT deposits amounting to a 45 and 57% increase in gWAT mass in the NC and HF groups, respectively (Fig. 6B). Our finding of augmented gWAT mass supports our previous body composition data.
FIGURE 6.
DKO mice have augmented gonadal white adipose tissue mass and adipocyte hypertrophy. After gonadal white adipose tissue pads (gWAT) were excised, gWAT mass, gross morphology, and histological analysis were performed. A and B, weighed. A, male (WT on NC, n = 10; miR-155−/− on NC, n = 10; WT on HF diet, n = 9; miR-155−/− on HF diet, n = 9) and (apoE−/− on NC, n = 21; DKO on NC, n = 14; apoE−/− on HF diet, n = 21; DKO on HF diet, n = 15). Gonadal WAT weighed and pictured for DKO and apoE−/− on NC and HF diet. B, female (WT on NC, n = 10; miR-155−/− on NC, n = 10; WT on HF diet, n = 10; and miR-155−/− on HF diet, n = 8) and (apoE−/− on NC, n = 15; DKO on NC, n = 20; apoE−/− on HF diet, n = 14; DKO on HF diet, n = 14). Gonadal WAT was weighed and pictured for DKO and apoE−/− on NC and HF diet. C–F, adipocyte area measured. C, male (apoE−/− on NC, n = 9; DKO on NC, n = 10; apoE−/− on HF diet, n = 11; DKO on HF diet, n = 10). E, female (apoE−/− on NC, n = 10; DKO on NC, n = 9; apoE−/− on HF diet, n = 10; DKO on HF diet, n = 10). apoE−/− and DKO gWAT pads were fixed, sectioned, and subjected to H&E staining. The representative gWAT pad histological analyses (D and F) were presented. **, p < 0.01; ***, p < 0.001.
MiR-155 Inhibits HF Diet-induced Adipocyte Hypertrophy in ApoE−/− Gonadal White Adipose Tissue
Recent progress shows that the generation of new adipocytes is constantly occurring (46, 47). Thus, expansion of adipose tissue mass can occur by two processes: hyperplasia, the increase of mature adipocyte cell number, or by adipocyte hypertrophy, the enlargement of adipocyte cellular volume. During periods of positive caloric imbalance, adipocyte hypertrophy is believed to be the main contributor of augmented adipose tissue mass (48). To determine if adipocyte hypertrophy was the source for the increased adipocyte mass in our DKO mice, histological evaluation of adipocyte area within gWAT was performed. Our results revealed a clear and significant phenotype. Male and female DKO adipocyte cell areas were drastically larger than adipocyte cell areas of apoE−/− equivalents (Fig. 6, C and E). This was not the case, however, when we looked at brown adipose tissue in our apoE−/− and DKO mice, as we found no significant difference in histological analysis (supplemental Fig. 2). These data support our earlier observations of increased gWAT mass and provide evidence that the observed increase in body fat is due to adipocyte hypertrophy, which is well correlated with CVD and MetS (49).
MiR-155 Inhibits HF Diet-induced NAFLD in ApoE−/− Mice
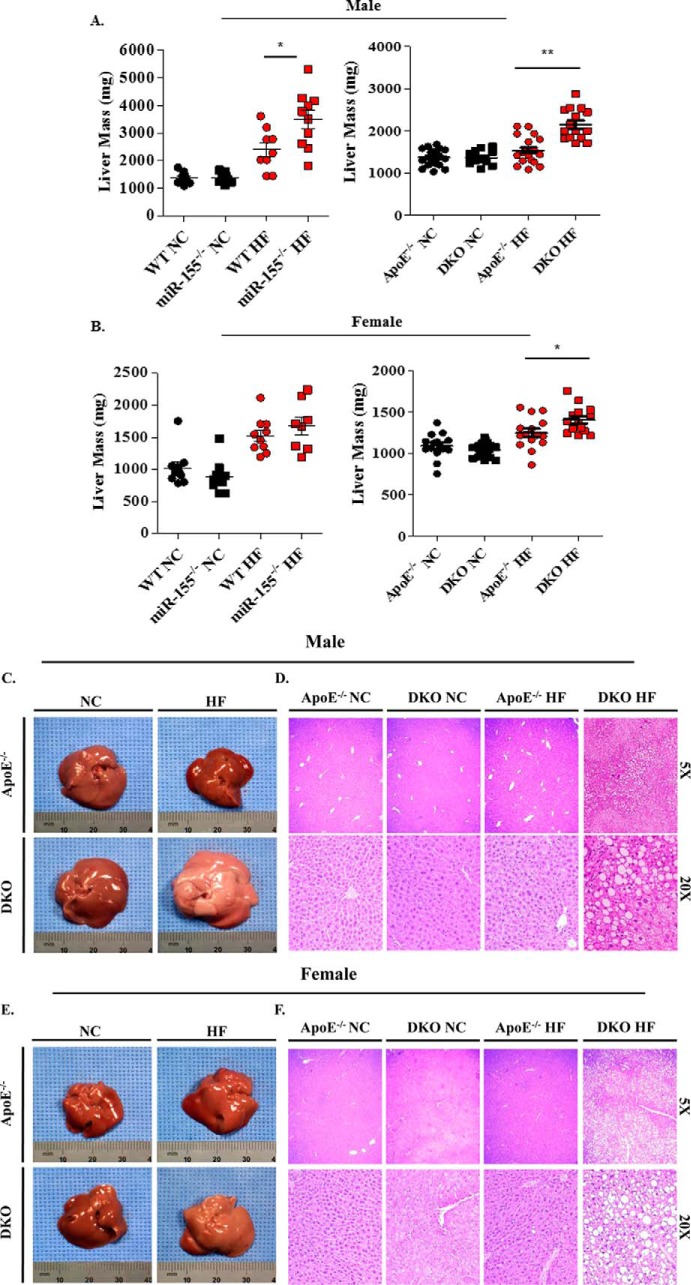
NAFLD is a clinical condition hallmarked by the abnormal accumulation of triglycerides within the liver and is often considered a hepatic manifestation of MetS due to their tight affiliation (2). Although miR-155 is not on the list of the 10 most up-regulated miRs or 10 down-regulated miRs in NAFLD (50), miR-155 was found to inhibit NAFLD (hepatosteatosis) in 6-month HF diet-induced NAFLD in wild-type mice (51). However, the effects of miR-155 deficiency in an apoE−/− background in regard to NAFLD remain unknown. We postulated that miR-155 would impact hepatic lipid accumulation to a great enough extent to cause a discrepancy in hepatic weight. We first examined WT mice and miR-155−/− mice fed with a HF diet in comparison to WT and miR-155−/− mice fed with NC. We found a statistically significant increase in hepatic mass in male miR-155−/− mice on a high fat diet when compared with WT equivalents, whereas no difference in NC groups was noted (Fig. 7A, left panel). This correlated well with previous reports (51). On the other hand, no significant difference was seen between female WT and miR-155−/− mice on HF or NC (Fig. 7B, left panel). In contrast, we found a statistically significant 40% increase in hepatic mass in male DKO mice on HF diet when compared with apoE−/− equivalents, whereas no difference in NC groups was noted. The same trend was witnessed in female mice where a 16% increase in hepatic weight in DKO mice on HF diet was detected, but no difference between NC groups was seen (Fig. 7, A and B, right panels). These liver mass data are in accordance with our reports of triglyceride accumulation within DKO mice adipocytes (Fig. 6). To directly demonstrate that increased liver mass results from accumulation of fat in livers, we examined the morphology, coloration, and histology of apoE−/− and DKO livers. Livers excised from both male and female DKO mice were more pale and whitish in color, a result of hepatic lipid accumulation, than their deeply pigmented counterparts from apoE−/− mice (Fig. 7, C and E). The hepatic steatosis was so pervasive that side by side unaided observation of whole livers from apoE−/− and DKO mice clearly reveals a distinction in hepatic coloration between the two. Widespread hepatic accumulation of triglycerides was seen in the male and female DKO mouse livers (Fig. 7, D and F). Our new data clearly demonstrate that miR-155 significantly inhibits the development of NAFLD in both male and female apoE−/− mice.
FIGURE 7.
DKO mice on high fat diet had increased hepatic mass, altered gross morphology, and tissue remodeling. Mouse livers were excised and weighed. A, male (WT on NC, n = 10; miR-155−/− on NC, n = 10; WT on HF diet, n = 9; miR-155−/− on HF diet, n = 10) and (apoE−/− on NC, n = 9; DKO on NC, n = 10; apoE−/− on HF diet, n = 11; DKO on HF diet, n = 10). B, female (WT on NC, n = 10; miR-155−/− on NC, n = 10; WT on HF diet, n = 10; miR-155−/− on HF diet, n = 8) and (apoE−/− on NC, n = 10; DKO on NC, n = 9; apoE−/− on HF diet, n = 10; DKO on HF diet, n = 10). Pictures were then taken so gross morphology and pigmentation changes could be visualized. C, male. E, female. Livers from male (D) and Female (F) apoE−/− and DKO mice were then subjected to H&E staining. *, p < 0.05; **, p < 0.01.
MiR-155 Inhibits HF Diet-induced Elevation of Plasma Leptin, Resistin, Fed-state Insulin, and Fasting Insulin but Does Not Affect Glucose Tolerance, Insulin Tolerance, and Body Weight Tolerance to Fasting in ApoE−/− Mice
Adipocytes from visceral fat are reported to be more lipolytically active than adipocytes from other fat pads and are closely linked with the deregulation of adipokines, including adiponectin, resistin, and leptin in obese individuals (52). In light of this, we decided to examine the plasma levels of these three critical adipokines. Analysis of plasma leptin levels within male DKO and apoE−/− control mice revealed a statistically significant distinction in the male DKO HF groups (p < 0.05) and a similar albeit non-significant trend in the NC groups in comparison to apoE−/− control mice (p = 0.0665). Quantification of this phenotype revealed an 86 and 101% increase in DKO leptin levels among the normal and high fat chow groups, respectively, when compared with apoE−/− counterparts (Fig. 8A). The high leptin levels in DKO mice were similar to the clinical observation of elevated circulating leptin in obese individuals in comparison to healthy controls (53). These results suggest that miR-155 inhibits high leptin levels in apoE−/− mice. Next, we examined plasma resistin levels and found a statistically significant 70% increase in circulating resistin levels among male DKO mice on HF feed compared with apoE−/− equivalents (Fig. 8B), which was in accordance with previous reports on obese mice and humans (54). However, we did not find any significant difference in adiponectin levels among our mice (Fig. 8C). Furthermore, our analysis also revealed a significant increase in fed-state insulin and fasting insulin levels in DKO mice among the high fat diet groups when compared with apoE−/− counterparts (p < 0.05) (Fig. 8, D and E). Finally, we found that plasma fed-state insulin levels in DKO mice fed with NC were also significantly increased when compared with apoE−/− mice (p < 0.05), whereas the plasma fasting insulin levels were not different between apoE−/− mice and DKO mice fed NC. These results suggest that miR-155 inhibits high fat diet-induced elevations of plasma leptin, resistin, fed-state insulin, and fasting insulin in apoE−/− mice. We also looked at plasma triglyceride levels and found no significant difference (supplemental Fig. 3A).
FIGURE 8.

DKO mice have augmented levels of adipokines known to influence glucose regulation as well as elevated levels of insulin when compared with apoE−/− controls. Adipokine levels of apoE−/− and DKO mice on NC and HF diet are shown. A, leptin levels (apoE−/− on NC, n = 10; DKO on NC, n = 8; apoE−/− on HF diet, n = 20; DKO on HF diet, n = 20). B, resistin levels (apoE−/− on NC, n = 10; DKO on NC, n = 9; apoE−/− on HF diet, n = 20; DKO on HF diet, n = 20). C, adiponectin levels (apoE−/− on NC, n = 9; DKO on NC, n = 9; apoE−/− on HF diet, n = 20; DKO on HF diet, n = 20). D, fed-state insulin levels (apoE−/− on NC, n = 10; DKO on NC, n = 8; apoE−/− on HF diet, n = 12; DKO on HF diet, n = 20). E, fasting insulin levels (apoE−/− on NC, n = 9; DKO on NC, n = 6; apoE−/− on HF diet, n = 8; DKO on HF diet, n = 8). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Because obesity is a risk factor for type II diabetes (55), elevated levels of fed-state insulin and fasting insulin in DKO mice suggest that glucose metabolism within DKO mice may be affected by miRNA-155 deficiency. To examine this possibility, we performed glucose tolerance and insulin tolerance tests. The results showed that in both NC and HF diet-fed mice, DKO mice showed no differences in the glucose tolerance tests (Fig. 9A) and insulin tolerance tests when compared with apoE−/− mice (Fig. 9C) (p > 0.05). Further quantitation analysis of the curves as the area under the curve (AUC) showed no statistical differences in male and female mice fed with NC or HF diet (Fig. 9, B and D). Moreover, to consolidate the results from the glucose tolerance test and insulin tolerance test, we measured body weights of apoE−/− and DKO mice in their tolerance to fasting. The results showed no difference in body weight in both male and female DKO mice compared with apoE−/− control mice fed with NC or HF diet (Fig. 10). These results further confirm our findings of no differences between DKO mice and apoE−/− mice in glucose tolerance and insulin tolerance. The data suggest that miR-155 deficiency leads to obesity but does not cause insulin resistance and glucose intolerance.
FIGURE 9.
DKO mice showed no difference in glucose tolerance or insulin tolerance compared with apoE−/− mice. Mice were administered the following treatment: glucose (1g/kg) GTT (A) or insulin (0.75 units/kg) for ITT (C), and blood was drawn from apoE−/− and DKO mice fed with either NC or HF diet for detection of glucose levels. B and D, area under the curve (AUC) was calculated. E, male fed-state glucose levels (apoE−/− on NC, n = 16; DKO on NC, n = 15; apoE−/− on HF diet, n = 9; DKO on HF diet, n = 21) and fasting glucose levels (apoE−/− on NC, n = 20; DKO on NC, n = 20; apoE−/− on HF diet, n = 14; DKO on HF diet, n = 17). F, female fed-state glucose levels (apoE−/− on NC, n = 19; DKO on NC, n = 17; apoE−/− on HF diet, n = 16; DKO on HF diet, n = 17) and fasting glucose levels (apoE−/− on NC, n = 19; DKO on NC, n = 17; apoE−/− on HF diet, n = 21; DKO on HF diet, n = 19). There was no difference in blood glucose levels among corresponding fed or fasted apoE−/− and DKO mice of either sex.
FIGURE 10.

apoE−/− and DKO mice have equal body weight tolerance to fasting. Male (A) and female (B) mice were fasted overnight, and body mass loss was recorded. The sample size (n) for each group is listed as follows: apoE−/− males on NC, 21; DKO males on NC, 20; apoE−/− males on HF, 26; DKO males on HF, 18; apoE−/− females on NC, 19; DKO females on NC, 13; apoE−/− females on HF, 16; DKO females on HF, 19.
MiR-155 Inhibits White Adipose Tissue Adipogenesis by Decreasing the Expression of Three Adipogenic Transcription Factors, C/EBP-α, C/EBP-β, and PPAR-γ, in WAT and Liver
Because the three transcription factors, CCAAT/enhancer-binding protein-α (C/EBP-α), C/EBP-β, and peroxisome proliferator-activated receptor-γ (PPAR-γ), play critical roles in promoting adipogenesis, we hypothesized that these three transcription factors are modulated by miR-155 expression in adipose tissue and in liver. To test this hypothesis, we performed quantitative RT-PCRs. The results showed that the expression levels of the three adipogenesis transcription factors in WAT and liver were up-regulated in DKO mice in comparison to those of apoE−/− mice on HF and NC feeding (Fig. 11). Of note, a previous report showed that PPAR-γ and C/EBP-β are miR-155 targets in brown adipose tissue (34), but our results demonstrated that these adipogenic transcription factors are also miR-155 targets in both WAT and liver. Furthermore, previous studies demonstrate that miR-155 deficiency or overexpression respectively increased or decreased the levels of these transcription factors (supplemental Table 2). These results suggest that the adipogenic regulations in WAT and liver are shared in miR-155 regulatory pathways.
FIGURE 11.

DKO mice have increased gWAT and liver expression of key adipogenic transcription factors: C/EBP-α, C/EBP-β, and PPAR-γ. ApoE−/− and DKO murine gWAT expression of indicated transcription factors on NC (A) or HF (B) diet. ApoE−/− and DKO murine hepatic expression of indicated transcription factors on NC (C) or HF (D) diet. The sample size (n) for each group are listed as follows: gWAT (A and B): C/EBP-α: all groups, n = 8; C/EBP-β: apoE−/− NC, n = 7; DKO NC, n = 8; apoE−/− HF, n = 6; DKO HF, n = 8; PPAR-γ: apoE−/− NC, n = 6; DKO NC, n = 7; apoE−/− HF, n = 7; DKO HF, n = 6. Liver (C and D): C/EBP-α: apoE−/− NC, n = 6; DKO NC, n = 6; apoE−/− HF, n = 8; DKO HF, n = 8; C/EBP-β: all groups, n = 8; PPAR-γ: all groups, n = 8. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Discussion
It is widely accepted that the prevalence of CVD markedly increases with increasing severity of obesity (3). However, an exception to this widely accepted concept was observed clinically in some patients with chronic diseases, such as chronic heart failure or coronary artery disease, where the best survival, or prognosis, is observed in overweight or obese patients compared with ideal weight patients. This phenomenon is termed the obesity paradox (4–7). Currently, the regulatory mechanisms underlying the obesity paradox remain unknown, which may be partially due to the fact that animal models mirroring obesity paradox in patients have not been established. We set out to address this issue with the use of a high fat diet-induced atherogenesis and adipogenesis model in apoE−/− mice. By performing array, histological, flow cytometry, NMR, and metabolic analyses, we were able to demonstrate the following exciting findings: first, miR-155 is significantly up-regulated in the aortas of apoE−/− mice fed with a HF diet for only 3 weeks and in the aortas of 4-h LPS-stimulated WT mice; second, miR-155 promotes human aortic EC activation and atherosclerosis; third, miR-155 inhibits HF diet-induced obesity and augmented gonadal WAT mass in apoE−/− mice; fourth, miR-155 inhibits HF diet-induced adipocyte hypertrophy in apoE−/− gonadal WAT; fifth, miR-155 inhibits HF diet-induced NAFLD in apoE−/− mice; sixth, miR-155 inhibits HF diet-induced elevations of plasma leptin, resistin, fed-state insulin, and fasting insulin but does not affect glucose tolerance, insulin tolerance, and body weight tolerance to fasting in apoE−/− mice; seventh, miR-155 inhibits WAT adipogenesis by decreasing the expression of three adipogenic transcription factors, C/EBP-α, C/EBP-β, and PPAR-γ, in WAT and liver. These results suggest that miR-155 promotes aortic EC activation and atherogenesis but inhibits WAT development and the pathogenesis of NAFLD, which makes our DKO mice a novel model for studying the pathogenesis of obesity paradox.
In 2002, Gruberg et al. observed better prognosis in overweight and obese patients with coronary heart disease undergoing percutaneous coronary intervention compared with their normal weight counterparts (56). Although there are extensive debates over whether obesity paradox exists (57), many teams have reported the obesity paradox in various chronic diseases, including peripheral artery disease, stroke, type 2 diabetes, chronic heart failure, etc. (4–7, 58). The obesity paradox may be partly explained by cardiovascular fitness as less coronary atherosclerosis was found in the autopsies of severely obese subjects (59). However, as aforementioned, the molecular mechanisms underlying the generation of obesity paradox remain largely unknown (58), which may partially result from the fact that animal models mirroring obesity paradox have not been established. Our findings in apoE−/−/miR-155−/− (DKO) mice, which have features of decreased atherosclerosis but increased obesity and NAFLD in comparison to apoE−/− mice, suggest that our DKO mice constitute a novel mouse model for studying obesity paradox. Since it was first reported that spontaneous hypercholesterolemia and atherosclerotic lesions develop in mice lacking apoE in 1992 (60), many genes and factors have been identified in regulating atherogenesis, including inflammatory factors (61). Similarly, we would expect that our DKO mice would significantly facilitate the research in further dissecting the genes and factors contributing to the obesity paradox.
Our and Nazari-Jahantigh et al. (33) congruent murine findings appear to translate to humans as well. Raitoharju et al. (62) completed human miRNA expression profiling with various arterial beds and found the expression of several miRNAs to be modulated. Specifically, miR-155 expression was up-regulated in the femoral artery and aorta of patients with atherosclerosis when compared with healthy patients. Additionally, in a second study, the levels of miR-155 were found to be elevated in human carotid artery plaques when compared with non-plaque areas of the vessel wall (33).
It has been previously reported that miR-155 plays a critical role in adipose tissue function, specifically brown adipose tissue (34). However, no reports have investigated the role of miR-155 in WAT, which is physiologically distinct from brown adipose tissue. Therefore, our findings of miR-155 regulation in gWAT are novel. The classification of gWAT as visceral fat implicates miR-155 as a mediator of CVD and MetS pathologies as both are tightly correlated with visceral fat abundance. Our new data obtained with our DKO mice have directly demonstrated the cause-effect relationship that miR-155 inhibits high fat diet-induced WAT hypertrophy in mouse WAT.
Metabolically healthy obesity (MHO) is a condition where some obese patients retain a healthy metabolic profile that is defined by the guidelines of having none of the following: elevated blood pressure, increased fasting glucose levels, elevated triglycerides, and decreased high density lipoproteins (63). In other words, these patients do not have MetS. In our model, we have demonstrated that DKO mice lack elevated fasting glucose levels and triglycerides. Furthermore, our mice do not show a decrease in high density lipoproteins (supplemental Fig. 3B). In contrast to other proposed models for MHO study, which display only one or two of the criteria for MHO (64), our model incorporates multiple key traits of MHO. We, therefore, propose apoE−/−/miR-155−/− DKO mice as a novel integrated mouse model exhibiting the key characteristics of metabolically healthy obesity giving rise to the observed obesity paradox.
Taken together, based on our findings and the reports of others, we propose a novel working model where miR-155 expression contributes to the obesity paradox (Fig. 12) as follows: 1) Pro-atherogenic stimuli, including oxidized LDL, lysolipids, LPS, TNF-α, and IL-1β up-regulate miR-155 in mouse aortas. miR-155 then promotes atherogenesis by inducing EC activation (shown in this study) and by promoting proinflammatory macrophage response in atherosclerosis through direct repression of B-cell lymphoma 6 (BCL6), a transcription factor known to repress NF-κB signaling (33) and direct repression of suppressor of cytokine signaling 1 (SOCS1) (65). 2) Adipogenesis signals in white and brown fat tissues, along with transforming growth factor (TGF)-β (34), inhibit miR-155 expression, which leads to the up-regulation of miR-155 target genes, including PPAR-γ, C/EBP-α, and C/EBP-β, in adipose tissues and NAFLD. Consequently, miR-155 deficiency/down-regulation leads to augmentation of WAT mass, obesity and NAFLD. 3) Because administration of recombinant resistin in healthy animals leads to diminished glucose tolerance and insulin sensitivity (66), increased plasma resistin levels and increased PPAR-γ expression in DKO mice would be expected to contribute to insulin insensitivity. As a consequence, insulin production would increase in a compensatory manner, resulting in the observed hyperinsulinemia in the DKO mice without the other accompanying MetS features, including hyperglycemia, insulin resistance, and type 2 diabetes. 4) Although our results on obesity paradox were obtained mostly in DKO mice, miR-155 up-regulation is also induced by various pro-atherogenic stimuli in HAECs as well as atherogenic apoE−/− aortas. In addition, it has been reported that miR-155 is expressed in human intra-abdominal omental adipose tissue (WAT) (35), and miR-155 expression is down-regulated during adipogenesis (34, 40). Moreover, an inverse correlation has been found between the expression of miR-155 in human intra-abdominal omental white fat adipocytes and the mean white fat adipocyte volume (35). Furthermore, overexpression of miR-155 during adipogenesis of primary human stromal cells inhibits adipogenesis (40). Taken together, these miR-155 findings in human cells suggest that our obesity paradox model could truly mimic human molecular pathways of miR-155, which supports the concept that apoE−/−/miR-155−/− DKO mice are a novel mouse model for studying obesity paradox in humans. Future work will be needed to verify the deficiency or down-regulation of miR-155 expression in aortas, liver, and adipose tissues in patients with obesity paradox. The establishment of this obesity paradox mouse model would significantly improve our understanding of the obesity paradox and facilitate the future characterization of its molecular mechanisms. Hopefully these advances will lead to the identification of novel therapeutics for related metabolic CVDs.
FIGURE 12.
The downregulation or deficiency of miR-155 in mice serves as a novel mouse model of obesity paradox with decreased atherosclerosis but increased obesity. Our new working model suggests that: A, during the development of atherosclerosis, pro-atherogenic signals (e.g. oxLDL, LPS, lysolipids, and/or pro-inflammatory cytokines) activate their respective receptors to promote miR-155 expression or activity within aorta, leading to endothelial cell activation, monocyte/macrophage recruitment, and therefore atherosclerosis initiation. Thus, deficiency or down-regulation of miR-155 leads to decreased atherosclerosis. On the other hand, in white adipose tissue, pro-adipogenic signals and TGF-β inhibit miR-155 expression or activity. Consequently, deficiency or down-regulation of miR-155 leads to increased expression of miR-155-suppressed molecular targets including pro-adipogenic transcription factors (e.g. C/EBP-α, C/EBP-β, and PPAR-γ), leading to NAFLD, adipocyte hypertrophy and eventual obesity. The resulting obesity promotes resistin levels that would normally create an insulin insensitive milieu. However, the increased levels of PPAR-γ inhibit this process, thus insulin sensitivity is maintained. B, our findings show that miR-155 deficiency or down-regulation results in decreased atherosclerosis, but increased white adipocyte hypertrophy, obesity, NAFLD and hyperinsulinemia amidst euglycemia.
Experimental Procedures
Animal Care, Mouse Genotyping, and Age Development
All animal use was authorized by, and performed in accordance with, Institutional Animal Care and Use Committee (IACUC) guidelines. In addition, all laboratory animal work was approved by the Experimental Animal Committee of Temple University School of Medicine.
ApoE mutant mice, miR-155 mutant mice, and WT mice were of a C57BL/6 background and purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in the Temple University Central Animal Facility under controlled conditions, given ad libitum access to standard chow diet and water and put on a 12-h light-dark cycle. Novel miR-155−/−/apoE−/− (DKO) mice were generated by crossing miR-155−/− mice with apoE−/− mice. In all experiments mice were age-matched and gender-specified. At 8 weeks of age, mice were either maintained on their current normal chow diet (5% fat, Labdiet 5001; St. Louis, MO) or switched to a diet supplemented with 0.2% (w/w) cholesterol and 21.2% (w/w) fat (high fat diet) (TD 88137, Harlan Teklad) for specified periods before being sacrificed for experimental use.
Mice were genotyped for a specific gene of interest with PCR followed by agarose gel analysis. First, mouse tail or ear tissue was collected and digested with 600 μl of tissue lysis buffer (10 mm Tris (pH 8.0), 100 mm NaCl, 10 mm EDTA (pH 8.0), and 0.5% SDS) containing 0.4 mg/ml proteinase K (EMD Millipore) at 55 °C overnight. Tissue lysate was then centrifuged at 13,000 rpm for 20 min. Supernatant containing genomic DNA was then collected, and DNA was precipitated in 700 μl of 100% ethyl alcohol before being dissolved in 200 μl of distilled deionized water at 37 °C overnight.
Mouse genomic DNA was next amplified with the use of gene of interest-specific primer sets and PCR. Murine miR-155 allele was examined using the following primers: miR-155 common primer 5′-GTGCTGCAAACCAGGAAGG-3′, miR-155 wild-type primer 5′-CTGGTTGAATCATTGAAGATGG-3′, and miR-155 knock-out primer 5′-CGGCAAACGACTGTCCTGGCCG-3′. Optimized miR-155 allele PCR was performed under conditions of 94 °C for 30 s, 61.8 °C for 60 s, and 72 °C for 60 s for a total of 35 cycles. The PCR products were then separated by gel electrophoresis on a 1.5% agarose gel with the wild-type band resolving at 465 bp and the knock-out band at 600 bp. The murine apoE allele was examined using the following primers: apoE common primer 5′-GCCTAGCCGAGGGAGAGCCG-3′, apoE wild-type primer 5′-TGTGACTTGGGAGCTCTGCAGC-3′, and apoE mutant primer 5′-GCCGCCCCGACTGCATCT-3′. Optimized apoE allele PCR was performed under conditions of 94 °C for 30 s, 68 °C for 40 s, and 72 °C for 60 s for 35 cycles. The PCR products were then separated on a 2% agarose gel by gel electrophoresis with the apoE wild-type band resolving at 155 bp and the mutant band at 245 bp. All gels were supplemented with 0.1 mg/ml ethidium bromide and visualized using ultraviolet detection from a Foto® analyst image system (Fotodyne, WI).
Two weeks after birth, the development of male and female apoE−/− and DKO mice was determined by weighing their mass on a weekly basis with an ISO9001 scale from Satorius® (Elk Grove, IL). At 4 weeks of age, mice were weaned into gender-specific cages with 2–5 mice per cage. After maturation at 8 weeks of age, mice were then either kept on normal chow or put on high fat feed until 20 weeks of age when they were sacrificed for experiment.
Body Composition Analysis
At 20 weeks of age, body composition analysis was performed on male and female apoE−/− and DKO mice fed either normal chow diet or high fat diet (Harlan Teklad). In brief, mice were euthanized and frozen at −80 °C until collection of the total experiment population. Mice were then thawed, and NMR body composition analysis was performed with a LF50 minispec from Bruker®. This analysis provided total body mass, fat mass, lean body mass, and free fluid mass measurements.
gWAT Pad Analysis
Male and female apoE−/− and DKO mice on normal or high fat chow were sacrificed at 20 weeks of age, and the left gWAT pad was then excised, washed in phosphate buffered saline (PBS), and measured with an AB54-S scale from Mettler Toledo (Switzerland). The gWAT was then imaged with NIS Elements software on a radiograph copy stand from Ilex optical company with a Nikon® DS-Fi1 camera. A surgical ruler was used as a point of reference in all pictures.
The right gWAT was also excised and fixed in 5 ml of Bouin Solution (75 ml of picric acid, 25 ml of 37–40% formaldehyde, and 5 ml glacial acetic acid) for 24 h before paraffin embedding (described below). Paraffin blocks were then sent to the AML Laboratories (Baltimore, MD) for sectioning and hematoxylin and eosin (H&E) staining. Slides were then imaged using an Akioskop2 Plus microscope and Axiocam camera (Carl Zeiss Inc.). Adipocyte sizes within a 20× magnification field were then determined using Adobe® Photoshop CS5 software.
Hepatic Tissue Analysis
Livers from euthanized male and female apoE−/− and DKO mice on normal and high fat chow were excised, washed in PBS, and weighed with an AB54-S scale from Mettler Toledo®. Segments of liver were then fixed in 4% paraformaldehyde (Sigma) for 24 h before being embedded in paraffin (described below). Paraffin blocks were then sent to AML Laboratories for sectioning and H&E staining. Histological evaluation of the sections was then performed, and images were taken using an Akioskop2 Plus microscope and AxioCam camera. In addition to the livers used for histological purposes, other whole livers were imaged on a radiograph copy stand from Ilex Optical Co. with NIS Elements software and a Nikon® DS-Fi1 camera. A surgical ruler was used as a point of reference in all pictures.
Paraffin Embedding
Tissue samples were fixed as previously described before paraffin embedding. After fixation, samples were dehydrated with various concentrations of isopropyl alcohol (Fisher). In brief, samples were put in 50% isopropyl alcohol for 45 min and then in 70% isopropyl alcohol for at least 2 h. After this, the tissues were submerged in 95% isopropyl alcohol for 45 min. Next, samples were soaked in 100% isopropyl alcohol for 30 min (twice), and then the tissue was dehydrated in xylene (Sigma) for 45 min (twice). At this point, the samples were put in melted paraffin (Fisher), which was then allowed to harden. The paraffin was then melted in a 57 °C incubator for 2–3 h, and the samples were moved to fresh liquid paraffin and kept at 57 °C for 1 h. Finally, the tissue was moved to steel molds where fresh paraffin was poured on top and covered with a plastic mold (Fisher). The liquid paraffin was then allowed to harden, and the molds were placed in a refrigerator at 4 °C. After 1 h, the steel molds were removed leaving the tissue-containing paraffin block attached to the plastic mold.
Murine Metabolic Evaluation
Utilizing metabolic cages from Tecniplast® (Italy), male and female apoE−/− and DKO mice were evaluated for water consumption, food consumption, urine excretion, and fecal excretion. At 8 weeks of age, mice were individually housed within a metabolic cage and given ad libitum access to diet gel (Fisher) and water. After 24 h of acclimation, water bottles, food cups, feces, and urine were weighed on a daily basis for the next 7 days. In addition, mice were weighed on a daily basis to ensure overall health.
Lipid, Lipoprotein, and Metabolic Parameter Analysis
Whole blood was collected from anesthetized animals via the inferior vena cava and deposited into mini-centrifuge tubes containing 25 μl of 5% EDTA. Plasma was then isolated by centrifugation at 4 °C for 15 min at 3000 rpm and frozen at −80 °C. The samples were then packed on dry-ice and shipped overnight to either the National Mouse Metabolic Phenotyping Center in Yale University School of Medicine (New Haven, CT) for measurement of low density lipoprotein, high density lipoprotein, total cholesterol, non-esterified fatty acids, and triglycerides or to the National Mouse Metabolic Phenotyping Center in the University of Massachusetts (Amherst, MA) for measurement of glucose, insulin, glucagon, leptin, adiponectin, and resistin.
Glucose Tolerance Test (GTT)
GTTs were performed on normal- or high fat-fed male and female apoE−/− and DKO mice at 20 weeks of age as previously described (67, 68). In brief, after an overnight fast of 16 h, mice were restrained, and their tails nicked for blood collection. Blood glucose levels were then determined by gently massaging a small drop of blood from the tail and onto a glucometer strip. The strip was then analyzed with a Glucose 201 glucometer from HemoCue® (Brea, CA). After determination of fasted blood glucose levels, each mouse received a 1 g/kg intraperitoneal (i.p.) injection of 10% d-glucose (Sigma). Blood glucose levels were then measured at 30, 60, 90, and 120 min post injection and recorded. In addition, each animal was weighed before and after fasting to ensure health before testing.
Insulin Tolerance Test (ITT)
ITTs were carried out on male and female apoE−/− and DKO in a similar fashion as described above. After baseline blood glucose levels were determined, each mouse received a 0.75 unit/kg i.p. injection of human insulin (Sigma). Blood glucose levels were then determined 30, 60, 90, and 120 min post injection.
RNA Extraction, Isolation, and Quantification
As described by the manufacturer, total RNAs, including miRNAs, were isolated from murine aorta, liver, and gWAT with the miRNeasy isolation kit (Qiagen). In brief, cultured cells were grown in 35-mm cell culture dishes and twice rinsed with ice-cold PBS before being lysed with 1 ml of Qiazol® reagent (Qiagen). After being collected into a micro-centrifuge tube, lysed cells were shaken vigorously for 15 s and then incubated for 5 min at room temperature to allow for complete dissociation of nucleoprotein complexes. Next, 140 μl of chloroform was added to each sample, and the mixture was shaken vigorously for 15 s before being allowed to stand for 2–3 min at room temperature. After this, the samples were centrifuged at 13,000 rpm for 15 min at 4 °C. The upper aqueous phase was then collected, and the RNA was precipitated by the addition of 1.5 volumes of 100% ethyl alcohol (Pharmco-Aaper). The solution was then added to Qiagen® mini spin columns and centrifuged at 12,000 rpm for 60 s. Flow-through was then discarded, and the column was washed with 700 μl of Qiagen® RWT buffer. Again the flow-through was discarded, and the column was washed twice with Qiagen® RPE buffer. After centrifugation, the column was gently removed and placed into a clean micro-centrifuge tube. The RNA was then solubilized in 30 μl of nuclease-free water and passed off the column. RNA generated from murine tissue was isolated with homogenization with a Pro200 homogenizer from Scientific Pro® in 900 μl of Qiazol® Reagent and purified in a similar fashion. The quality and concentration of all RNA samples were then determined with a Nanodrop 2000 from Thermo Scientific®.
RNA Reverse Transcription
Total RNA was reverse-transcribed to generate complementary DNA using the High Capacity cDNA Reverse Transcription kit (Invitrogen, catalog #4368814) as per the manufacturer's instructions. Meanwhile, reverse transcription to generate complementary DNA for miRNAs was accomplished with the miScript II RT kit (Qiagen, catalog #218160) according to the manufacturer's instructions. All cDNA products were then stored at −80 °C until further use.
Quantitative Real-time PCR
The mRNA expression levels of protein coding genes as well as miRNA expression were determined by quantitative real-time PCR performed with the StepOnePlus PCR system (Applied Biosystems). SYBR Green dye (Invitrogen) was used to quantify mRNA expression levels with transcripts being normalized to the constitutive mRNA expression of β-actin or GAPDH for all human genes and murine genes, respectively. The expression of miRNA was also detected by SYBR-green dye (Qiagen), and transcripts were normalized to the constitutively expressed ribosomal RNA, U6. The specific primers used are listed: murine GAPDH (forward primer 5′-GAGGCCGGTGCTGAGTATGTCGTGGA-3′, reverse primer 5′-CACACCCATCACAAACTGGGGGCAT-3′); murine C/EBP-β (forward primer 5′-CACCACGACTTCCTCTCCGACCTCT-3′, reverse primer 5′-GTACTCGTCGCTCAGCTTGTCCACCC-3′); murine C/EBP-α (forward primer5′-CGAGGAGGACGAGGCGAAGCA-3′, reverse primer 5′-TGCGCAGGCGGTCATTGTCAC-3′); murine PPAR-γ (forward primer 5′-TCCGTAGAAGCCGTGCAAGAGATCA-3′, reverse primer 5′-CAGCAGGTTGTCTTGGATGTCCTCG-3′); murine miR-155 (Purchased from Qiagen); murine/human U6 (purchased from Qiagen); murine ICAM-1 (forward primer 5′-GTTCTCTAATGTCTCCGAGGC-3′; reverse primer 5′-CTTCAGAGGCAGGAAACAGG-3′) and murine VCAM-1 (forward primer 5′-GCAAAGGACACTGGAAAAGAG-3′, reverse primer 5′-TCAAAGGGATACACATTAGGGAC-3′).
miFinder PCR Array
Total RNAs, including miRNAs, were isolated from the aortas of apoE−/− mice on 12 weeks of normal or high fat chow and reverse-transcribed as previously described. The cDNA from five individual mice of the same experimental group was then equally mixed together and added to a miFinder PCR array (Qiagen), and quantitative real-time PCR was performed. The data from each experimental array plate was then analyzed with software provided on the Qiagen website.
Atherosclerotic Lesion Analysis
Murine hearts and aortas were perfused with ice-cold PBS, harvested, and fixed overnight with 4% paraformaldehyde. Fixed tissues were then kept in 20% (v/v) sucrose (Sigma) for 24 h. After this, the lower ventricular portion of the mouse heart was removed with a scalpel, and the upper cardiac portion was embedded with Optimal Cutting Temperature compound (OCT) (Fisher) and frozen. Mouse aortas were transferred to mini-centrifuge tubes containing PBS and stored at 4 °C. The hearts were then cross-sectioned for observation of the aortic root with a Microm HM 560 cryostat (Thermo Scientific). Mounted onto Superfrost® slides (Fisher), 10-μm sections were taken from the point where the aortic valves first appeared until a total of 80 sections were collected serially across 10 slides. Slides were next stained with Oil red O (Sigma) and alum hematoxylin (Fisher). Briefly, fixed sections were rinsed with 60% isopropyl alcohol and stained with freshly prepared Oil red O (0.3% Oil Red O in 60% isopropyl alcohol) for 18 min. After this, slides were rinsed with 60% isopropyl alcohol, dipped in alum hematoxylin, and rinsed with distilled water. The stained sections were then mounted with aqueous mounting medium (Sigma) and stored at room temperature until imaging. Meanwhile, the aortas were used for enface staining. After fully removing all adipose and connective tissue, the aortas were stained with Sudan IV (5 mg/ml in 70% isopropyl alcohol) (Sigma) for 40 min at 37 °C. After this, the aortas were incubated in 70% isopropyl alcohol for 5 min. Next, a longitudinal cut was made to expose the intimal surface of the vessel and pinned open on a black silicone tray for imaging.
Images of the aortic root were captured with an AxioCam camera mounted to an Axioskop 2 microscope, whereas images of the aorta were captured with the same camera mounted to a Stemi 2000-C microscope (Carl Zeiss). Atherosclerotic lesion area within the aortic sinus was defined as the area stained with Oil red O and measured with ImageJ (National Institutes of Health). Evaluating all eight aortic sections per slide, the percentage of lesion area was calculated by dividing the lesion area by the total sinus area and averaging the values for the eight sections. Meanwhile, the lesion area in the whole aorta was defined as the red area stained with Sudan IV. The summated lesion area was then divided by the total aorta area to tabulate the atherosclerotic lesion area.
Protein Extraction, Bicinchoninic Acid (BCA) Assay and Western Blot Analysis
Protein extracts were prepared from murine aortas. In brief, aortas were snap-frozen in liquid nitrogen until put in PBS supplemented with 0.2% 500× EDTA (Sigma), 0.4% 250× PMSF) (Sigma), and 2.0% proteinase tablet (Roche Applied Science). Aortas were then cut with fine dissection scissors, sonicated, and boiled for 5 min. The samples were then centrifuged at 13,000 rpm for 2 min, and the pellets were then resuspended in 150 μl of sample buffer (0.75% SDS, 0.03 m Tris-HCl stock (pH 6.8), 5.6% glycerol, 1 mm EDTA, 0.04 mg/ml PMSF, 0.2% proteinase tablet) and frozen until quantification.
Unknown protein samples were quantified using a pierce BCA protein assay kit (Fisher) and a standard curve of bovine serum albumin. Samples were loaded in duplicates onto a 96-well plate (Sigma), and 5 μl of unknown protein samples and standards were incubated with 200 μl of BCA reagent (200 parts of solution A and 1 part of solution B) for 30 min at 37 °C. After this, the absorbance of each sample at a wavelength of 490 nm was measured with an ELx800 Microplate reader (Bio-TEK Instrument Inc., VT). Using the standard curve, the concentrations of the unknown samples were then calculated.
Equivalent amounts of total protein from samples were loaded and separated on an 8% SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane (GE Healthcare). Membranes were next blocked by 5% nonfat milk (Santa Cruz) in TBS containing 0.01% Tween 20 (TBST) (Fisher). After this, membranes were incubated with primary antibodies overnight at 4 °C before washing extensively with TBST (3–5-min washes) and incubated with the appropriate horseradish peroxidase-labeled secondary antibodies for 1 h at room temperature. Afterward, membranes were incubated with enhanced chemiluminescence substrate for horseradish peroxidase (Thermo Scientific). The chemiluminescence was then detected by X-ray film exposure in a dark room and developed with a SRX-101A medical film processor (Konica Minolta). The expression levels of proteins were then quantified with ImageJ software (National Institutes of Health).
Statistical Analysis
All statistical analyses were performed with the GraphPad Prism 5 software. For statistical comparison of single parameters between 2 groups, an independent t test was used. Statistical significance was defined as p < 0.05.
Author Contributions
A. V. carried out data gathering and analysis and drafted the manuscript. C. J, J. L.-P., Y. S., H. F., Y.-F. L, Y. Y., J. M., X. L., V. R., M. T., Z. B., H. S., and X. J. provided material input. H. W. and X.-F. Y. supervised experimental design and data analysis. A. V. and X.-F. Y. wrote manuscript.
Acknowledgments
Part of this work was performed at the National Mouse Metabolic Phenotyping Center at University of Massachusetts Medical School funded by National Institutes of Health Grant U24-DK093000.
This work was supported by National Institutes of Health Grants R01 HL108910-01, R01 HL116917-01, and R01 HL131460-1 (to X.-F. Y. and H. W.). This work was also supported by American Heart Association predoctoral fellowship 12PRE11640013 (A. V.). The authors declare that they have no conflicts of interest with the contents of this article. This content is solely the responsibility of the authors and does not necessarily represent the official views of National Institutes of Health.

This article contains supplemental Figs. 1–3 and Tables 1 and 2.
- MetS
- metabolic syndrome
- CVD
- cardiovascular disease
- NAFLD
- non-alcoholic fatty liver disease
- EC
- endothelial cell
- HF
- high fat diet
- miR
- microRNA
- VCAM-1
- vascular cell adhesion molecule-1
- WAT
- white adipose tissue
- gWAT
- gonadal WAT
- DKO
- double knock-out
- qRT
- quantitative real-time
- ICAM-1
- intercellular adhesion molecule-1
- HAEC
- human aortic EC
- NC
- normal chow
- C/EBP-α
- CCAAT/enhancer-binding protein-α
- PPAR-γ
- peroxisome proliferator-activated receptor-γ
- MHO
- metabolically healthy obesity
- GTT
- glucose tolerance test
- ITT
- insulin tolerance test.
References
- 1. Hansson G. K., and Hermansson A. (2011) The immune system in atherosclerosis. Nat. Immunol. 12, 204–212 [DOI] [PubMed] [Google Scholar]
- 2. Anstee Q. M., Targher G., and Day C. P. (2013) Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 10, 330–344 [DOI] [PubMed] [Google Scholar]
- 3. Lavie C. J., Alpert M. A., Arena R., Mehra M. R., Milani R. V., and Ventura H. O. (2013) Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 1, 93–102 [DOI] [PubMed] [Google Scholar]
- 4. Niedziela J., Hudzik B., Niedziela N., Gsior M., Gierlotka M., Wasilewski J., Myrda K., Lekston A., Poloński L., and Rozentryt P. (2014) The obesity paradox in acute coronary syndrome: a meta-analysis. Eur. J. Epidemiol. 29, 801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gurm H. S., Brennan D. M., Booth J., Tcheng J. E., Lincoff A. M., and Topol E. J. (2002) Impact of body mass index on outcome after percutaneous coronary intervention (the obesity paradox). Am. J. Cardiol. 90, 42–45 [DOI] [PubMed] [Google Scholar]
- 6. Khattab A. A., Daemen J., Richardt G., Rioux P., Amann F. W., Levy R., Horvath I. G., Teles R. C., Ordoubadi F., Pieters M., Wittebols K., Stoll H. P., and Serruys P. W. (2008) Impact of body mass index on the one-year clinical outcome of patients undergoing multivessel revascularization with sirolimus-eluting stents (from the Arterial Revascularization Therapies Study Part II). Am. J. Cardiol. 101, 1550–1559 [DOI] [PubMed] [Google Scholar]
- 7. Lavie C. J., De Schutter A., and Milani R. V. (2015) Healthy obese versus unhealthy lean: the obesity paradox. Nat. Rev. Endocrinol. 11, 55–62 [DOI] [PubMed] [Google Scholar]
- 8. Gregor M. F., and Hotamisligil G. S. (2011) Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 [DOI] [PubMed] [Google Scholar]
- 9. Rocha V. Z., and Libby P. (2009) Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 6, 399–409 [DOI] [PubMed] [Google Scholar]
- 10. Mestas J., and Ley K. (2008) Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc. Med. 18, 228–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yin Y., Li X., Sha X., Xi H., Li Y. F., Shao Y., Mai J., Virtue A., Lopez-Pastrana J., Meng S., Tilley D. G., Monroy M. A., Choi E. T., Thomas C. J., Jiang X., Wang H., and Yang X. F. (2015) Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arterioscler. Thromb. Vasc. Biol. 35, 804–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sha X., Meng S., Li X., Xi H., Maddaloni M., Pascual D. W., Shan H., Jiang X., Wang H., and Yang X. F. (2015) Interleukin-35 inhibits endothelial cell activation by suppressing MAPK-AP-1 pathway. J. Biol. Chem. 290, 19307–19318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fang P., Zhang D., Cheng Z., Yan C., Jiang X., Kruger W. D., Meng S., Arning E., Bottiglieri T., Choi E. T., Han Y., Yang X. F., and Wang H. (2014) Hyperhomocysteinemia potentiates hyperglycemia-induced inflammatory monocyte differentiation and atherosclerosis. Diabetes 63, 4275–4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang D., Fang P., Jiang X., Nelson J., Moore J. K., Kruger W. D., Berretta R. M., Houser S. R., Yang X., and Wang H. (2012) Severe hyperhomocysteinemia promotes bone marrow-derived and resident inflammatory monocyte differentiation and atherosclerosis in LDLr/CBS-deficient mice. Circ. Res. 111, 37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang D., Jiang X., Fang P., Yan Y., Song J., Gupta S., Schafer A. I., Durante W., Kruger W. D., Yang X., and Wang H. (2009) Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine β-synthase-deficient mice. Circulation 120, 1893–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xiong Z., Song J., Yan Y., Huang Y., Cowan A., Wang H., and Yang X. F. (2008) Higher expression of Bax in regulatory T cells increases vascular inflammation. Front. Biosci. 13, 7143–7155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xiong Z., Yan Y., Song J., Fang P., Yin Y., Yang Y., Cowan A., Wang H., and Yang X. F. (2009) Expression of TCTP antisense in CD25(high) regulatory T cells aggravates cuff-injured vascular inflammation. Atherosclerosis 203, 401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Virtue A., Mai J., Yin Y., Meng S., Tran T., Jiang X., Wang H., and Yang X. F. (2011) Structural evidence of anti-atherogenic microRNAs. Front. Biosci. (Landmark Ed.) 16, 3133–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Virtue A., Wang H., and Yang X. F. (2012) MicroRNAs and toll-like receptor/interleukin-1 receptor signaling. J. Hematol. Oncol. 5, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Connell R. M., Rao D. S., and Baltimore D. (2012) microRNA regulation of inflammatory responses. Annu. Rev. Immunol. 30, 295–312 [DOI] [PubMed] [Google Scholar]
- 21. Sun X., Belkin N., and Feinberg M. W. (2013) Endothelial microRNAs and atherosclerosis. Curr. Atheroscler. Rep. 15, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raitoharju E., Oksala N., and Lehtimäki T. (2013) MicroRNAs in the atherosclerotic plaque. Clin. Chem. 59, 1708–1721 [DOI] [PubMed] [Google Scholar]
- 23. Deiuliis J. A. (2015) MicroRNAs as regulators of metabolic disease: pathophysiologic significance and emerging role as biomarkers and therapeutics. Int. J. Obes. (Lond) 40, 88–101 ( 10.1038/ijo.2015.170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferreira D. M., Simão A. L., Rodrigues C. M., and Castro R. E. (2014) Revisiting the metabolic syndrome and paving the way for microRNAs in non-alcoholic fatty liver disease. FEBS J. 281, 2503–2524 [DOI] [PubMed] [Google Scholar]
- 25. Donners M. M., Wolfs I. M., Stöger L. J., van der Vorst E. P., Pöttgens C. C., Heymans S., Schroen B., Gijbels M. J., and de Winther M. P. (2012) Hematopoietic miR155 deficiency enhances atherosclerosis and decreases plaque stability in hyperlipidemic mice. PloS ONE 7, e35877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu N., Zhang D., Chen S., Liu X., Lin L., Huang X., Guo Z., Liu J., Wang Y., Yuan W., and Qin Y. (2011) Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis 215, 286–293 [DOI] [PubMed] [Google Scholar]
- 27. Cheng W., Liu T., Jiang F., Liu C., Zhao X., Gao Y., Wang H., and Liu Z. (2011) microRNA-155 regulates angiotensin II type 1 receptor expression in umbilical vein endothelial cells from severely pre-eclamptic pregnant women. Int. J. Mol. Med. 27, 393–399 [DOI] [PubMed] [Google Scholar]
- 28. Pulkkinen K. H., Ylä-Herttuala S., and Levonen A. L. (2011) Heme oxygenase 1 is induced by miR-155 via reduced BACH1 translation in endothelial cells. Free Radic. Biol. Med. 51, 2124–2131 [DOI] [PubMed] [Google Scholar]
- 29. Sun H. X., Zeng D. Y., Li R. T., Pang R. P., Yang H., Hu Y. L., Zhang Q., Jiang Y., Huang L. Y., Tang Y. B., Yan G. J., and Zhou J. G. (2012) Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension 60, 1407–1414 [DOI] [PubMed] [Google Scholar]
- 30. Zhu J., Chen T., Yang L., Li Z., Wong M. M., Zheng X., Pan X., Zhang L., and Yan H. (2012) Regulation of microRNA-155 in atherosclerotic inflammatory responses by targeting MAP3K10. PloS ONE 7, e46551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodriguez A., Vigorito E., Clare S., Warren M. V., Couttet P., Soond D. R., van Dongen S., Grocock R. J., Das P. P., Miska E. A., Vetrie D., Okkenhaug K., Enright A. J., Dougan G., Turner M., and Bradley A. (2007) Requirement of bic/microRNA-155 for normal immune function. Science 316, 608–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thai T. H., Calado D. P., Casola S., Ansel K. M., Xiao C., Xue Y., Murphy A., Frendewey D., Valenzuela D., Kutok J. L., Schmidt-Supprian M., Rajewsky N., Yancopoulos G., Rao A., and Rajewsky K. (2007) Regulation of the germinal center response by microRNA-155. Science 316, 604–608 [DOI] [PubMed] [Google Scholar]
- 33. Nazari-Jahantigh M., Wei Y., Noels H., Akhtar S., Zhou Z., Koenen R. R., Heyll K., Gremse F., Kiessling F., Grommes J., Weber C., and Schober A. (2012) MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. The Journal of clinical investigation 122, 4190–4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen Y., Siegel F., Kipschull S., Haas B., Fröhlich H., Meister G., and Pfeifer A. (2013) miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat. Commun. 4, 1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klöting N., Berthold S., Kovacs P., Schön M. R., Fasshauer M., Ruschke K., Stumvoll M., and Blüher M. (2009) MicroRNA expression in human omental and subcutaneous adipose tissue. PloS ONE 4, e4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Economou E. K., Oikonomou E., Siasos G., Papageorgiou N., Tsalamandris S., Mourouzis K., Papaioanou S., and Tousoulis D. (2015) The role of microRNAs in coronary artery disease: from pathophysiology to diagnosis and treatment. Atherosclerosis 241, 624–633 [DOI] [PubMed] [Google Scholar]
- 37. Hosin A. A., Prasad A., Viiri L. E., Davies A. H., and Shalhoub J. (2014) MicroRNAs in atherosclerosis. J. Vasc. Res. 51, 338–349 [DOI] [PubMed] [Google Scholar]
- 38. Doherty T. M., Shah P. K., and Arditi M. (2005) Lipopolysaccharide, toll-like receptors, and the immune contribution to atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 25, e38. [DOI] [PubMed] [Google Scholar]
- 39. Nogueras S., Merino A., Ojeda R., Carracedo J., Rodriguez M., Martin-Malo A., Ramírez R., and Aljama P. (2008) Coupling of endothelial injury and repair: an analysis using an in vivo experimental model. Am. J. Physiol. Heart Circ. Physiol. 294, H708–H713 [DOI] [PubMed] [Google Scholar]
- 40. Skårn M., Namløs H. M., Noordhuis P., Wang M. Y., Meza-Zepeda L. A., and Myklebost O. (2012) Adipocyte differentiation of human bone marrow-derived stromal cells is modulated by microRNA-155, microRNA-221, and microRNA-222. Stem Cells Dev. 21, 873–883 [DOI] [PubMed] [Google Scholar]
- 41. Coppieters K. T., and von Herrath M. G. (2014) Metabolic syndrome; removing roadblocks to therapy: antigenic immunotherapies. Mol. Metab. 3, 275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Du F., Yu F., Wang Y., Hui Y., Carnevale K., Fu M., Lu H., and Fan D. (2014) MicroRNA-155 deficiency results in decreased macrophage inflammation and attenuated atherogenesis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 34, 759–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakashima Y., Plump A. S., Raines E. W., Breslow J. L., and Ross R. (1994) apoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 14, 133–140 [DOI] [PubMed] [Google Scholar]
- 44. Huang Y., Liu X. Q., Rall S. C. Jr, Taylor J. M., von Eckardstein A., Assmann G., and Mahley R. W. (1998) Overexpression and accumulation of apolipoprotein E as a cause of hypertriglyceridemia. J. Biol. Chem. 273, 26388–26393 [DOI] [PubMed] [Google Scholar]
- 45. Tang T., Zhang J., Yin J., Staszkiewicz J., Gawronska-Kozak B., Jung D. Y., Ko H. J., Ong H., Kim J. K., Mynatt R., Martin R. J., Keenan M., Gao Z., and Ye J. (2010) Uncoupling of inflammation and insulin resistance by NF-κB in transgenic mice through elevated energy expenditure. J. Biol. Chem. 285, 4637–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Spalding K. L., Arner E., Westermark P. O., Bernard S., Buchholz B. A., Bergmann O., Blomqvist L., Hoffstedt J., Näslund E., Britton T., Concha H., Hassan M., Rydén M., Frisén J., and Arner P. (2008) Dynamics of fat cell turnover in humans. Nature 453, 783–787 [DOI] [PubMed] [Google Scholar]
- 47. Sun K., Kusminski C. M., and Scherer P. E. (2011) Adipose tissue remodeling and obesity. J. Clin. Investig. 121, 2094–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Q. A., Tao C., Gupta R. K., and Scherer P. E. (2013) Tracking adipogenesis during white adipose tissue development, expansion, and regeneration. Nat. Med. 19, 1338–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Van Gaal L. F., Mertens I. L., and De Block C. E. (2006) Mechanisms linking obesity with cardiovascular disease. Nature 444, 875–880 [DOI] [PubMed] [Google Scholar]
- 50. Lakner A. M., Bonkovsky H. L., and Schrum L. W. (2011) microRNAs: fad or future of liver disease. World J. Gastroenterol. 17, 2536–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miller A. M., Gilchrist D. S., Nijjar J., Araldi E., Ramirez C. M., Lavery C. A., Fernández-Hernando C., McInnes I. B., and Kurowska-Stolarska M. (2013) MiR-155 has a protective role in the development of non-alcoholic hepatosteatosis in mice. PloS ONE 8, e72324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bjørndal B., Burri L., Staalesen V., Skorve J., and Berge R. K. (2011) Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J. Obes. 2011, 490650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Considine R. V., Sinha M. K., Heiman M. L., Kriauciunas A., Stephens T. W., Nyce M. R., Ohannesian J. P., Marco C. C., McKee L. J., and Bauer T. L. (1996) Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 334, 292–295 [DOI] [PubMed] [Google Scholar]
- 54. Degawa-Yamauchi M., Bovenkerk J. E., Juliar B. E., Watson W., Kerr K., Jones R., Zhu Q., and Considine R. V. (2003) Serum resistin (FIZZ3) protein is increased in obese humans. J. Clin. Endocrinol. Metab. 88, 5452–5455 [DOI] [PubMed] [Google Scholar]
- 55. Wang B., Chandrasekera P. C., and Pippin J. J. (2014) Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr. Diabetes Rev. 10, 131–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gruberg L., Weissman N. J., Waksman R., Fuchs S., Deible R., Pinnow E. E., Ahmed L. M., Kent K. M., Pichard A. D., Suddath W. O., Satler L. F., and Lindsay J. Jr. (2002) The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J. Am. Coll. Cardiol. 39, 578–584 [DOI] [PubMed] [Google Scholar]
- 57. Dixon J. B., Egger G. J., Finkelstein E. A., Kral J. G., and Lambert G. W. (2015) “Obesity paradox” misunderstands the biology of optimal weight throughout the life cycle. Int. J. Obes. (Lond) 39, 82–84 ( 10.1038/ijo.2014.59) [DOI] [PubMed] [Google Scholar]
- 58. Hainer V., and Aldhoon-Hainerová I. (2013) Obesity paradox does exist. Diabetes care 36, S276–S281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kortelainen M. L., and Porvari K. (2011) Extreme obesity and associated cardiovascular disease verified at autopsy: time trends over 3 decades. Am. J. Forensic Med. Pathol. 32, 372–377 [DOI] [PubMed] [Google Scholar]
- 60. Zhang S. H., Reddick R. L., Piedrahita J. A., and Maeda N. (1992) Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 258, 468–471 [DOI] [PubMed] [Google Scholar]
- 61. Bäck M., and Hansson G. K. (2015) Anti-inflammatory therapies for atherosclerosis. Nat. Rev. Cardiol. 12, 199–211 [DOI] [PubMed] [Google Scholar]
- 62. Raitoharju E., Lyytikäinen L. P., Levula M., Oksala N., Mennander A., Tarkka M., Klopp N., Illig T., Kähönen M., Karhunen P. J., Laaksonen R., and Lehtimäki T. (2011) miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis 219, 211–217 [DOI] [PubMed] [Google Scholar]
- 63. Ortega F. B., Lavie C. J., and Blair S. N. (2016) Obesity and cardiovascular disease. Circ. Res. 118, 1752–1770 [DOI] [PubMed] [Google Scholar]
- 64. Wang F., Deeney J. T., and Denis G. V. (2013) Brd2 gene disruption causes “metabolically healthy” obesity: epigenetic and chromatin-based mechanisms that uncouple obesity from type 2 diabetes. Vitam. Horm. 91, 49–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Androulidaki A., Iliopoulos D., Arranz A., Doxaki C., Schworer S., Zacharioudaki V., Margioris A. N., Tsichlis P. N., and Tsatsanis C. (2009) The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity 31, 220–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Steppan C. M., Bailey S. T., Bhat S., Brown E. J., Banerjee R. R., Wright C. M., Patel H. R., Ahima R. S., and Lazar M. A. (2001) The hormone resistin links obesity to diabetes. Nature 409, 307–312 [DOI] [PubMed] [Google Scholar]
- 67. Banerjee R. R., Rangwala S. M., Shapiro J. S., Rich A. S., Rhoades B., Qi Y., Wang J., Rajala M. W., Pocai A., Scherer P. E., Steppan C. M., Ahima R. S., Obici S., Rossetti L., and Lazar M. A. (2004) Regulation of fasted blood glucose by resistin. Science 303, 1195–1198 [DOI] [PubMed] [Google Scholar]
- 68. Wu D., Molofsky A. B., Liang H. E., Ricardo-Gonzalez R. R., Jouihan H. A., Bando J. K., Chawla A., and Locksley R. M. (2011) Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yadav H., Quijano C., Kamaraju A. K., Gavrilova O., Malek R., Chen W., Zerfas P., Zhigang D., Wright E. C., Stuelten C., Sun P., Lonning S., Skarulis M., Sumner A. E., Finkel T., and Rane S. G. (2011) Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell. Metab. 14, 67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]