Abstract
Group I metabotropic glutamate receptors (mGluRs) play important roles in various neuronal processes and elicit changes in synaptic efficacy through AMPA receptor (AMPAR) endocytosis. Trafficking of mGluRs plays an important role in controlling the precise localization of these receptors at specific region of the cell; it also regulates the activity of these receptors. Despite this obvious significance, we know very little about the cellular mechanisms that control the trafficking of group I mGluRs. We show here that ligand-mediated internalization of group I mGluRs is ubiquitination-dependent. A lysine residue (Lys1112) at the C-terminal tail of mGluR1 (a member of the group I mGluR family) plays crucial role in this process. Our data suggest that Lys63-linked polyubiquitination is involved in the ligand-mediated endocytosis of mGluR1. We also show here that the mGluR1 internalization is dependent on a specific E3 ubiquitin ligase, Siah-1A. Furthermore, acute knockdown of Siah-1A enhances the mGluR-mediated AMPAR endocytosis. These studies reveal a novel function of ubiquitination in the regulation of group I mGluRs, as well as its role in mGluR-dependent AMPAR endocytosis.
Keywords: endocytosis, G protein-coupled receptor (GPCR), metabotropic glutamate receptor (mGluR), neurotransmitter receptor, receptor internalization, ubiquitin, trafficking
Introduction
Glutamate is a major excitatory neurotransmitter in the mammalian brain, and it functions by activating two distinct types of receptors, viz. ionotropic and metabotropic glutamate receptors (1–3). Ionotropic receptors are ion channels that allow cations to go through them. There are three types of ionotropic glutamate receptors present in the brain, viz. NMDA, AMPA, and kainate receptors. On the other hand, metabotropic glutamate receptors (mGluRs)2 are members of the G protein-coupled receptor (GPCR) superfamily (2, 3). Group I mGluRs (mGluR1 and mGluR5) are predominantly localized at the membrane of the post-synaptic cells and regulate variety of physiological functions through positive coupling with Gq/11 (4–7). These receptors have been implicated in various forms of synaptic plasticity, including learning and memory, as well as in various neuropsychiatric disorders like fragile X syndrome, autism, etc. (8–13). As with many other GPCRs, uncoupling of group I mGluRs from the heterotrimeric G proteins represents an essential feedback mechanism to protect the cells from receptor overstimulation (2, 14). Subsequent to desensitization, these receptors undergo internalization, and internalized receptors recycle back to the surface through a protein phosphatase-dependent manner that could serve as a mechanism to resensitize the receptor (15, 16). Although trafficking of mGluRs plays a critical role in the regulation of the receptor activity and thus in turn regulates physiological functions carried out by those receptors, the molecular mechanisms underlying these processes are not fully understood.
Ubiquitination, originally identified as a process for protein degradation by the 26yS proteasome, also regulates the internalization of several plasma membrane proteins. Ubiquitin (Ub) is a 76-amino acid protein, which is recognized by Ub-binding domains, found in proteins of the endocytic sorting machinery (17). Ubiquitination of proteins involves sequential action of three enzymes: ubiquitin activating enzyme (E1), ubiquitin carrying enzyme (E2), and ubiquitin protein ligase (E3) (18). As stated before, the trafficking of some GPCRs is ubiquitin-dependent. For example, ubiquitination regulates internalization of the yeast Ste2 and Ste3 receptors. Studies indicate that monoubiquitination is both necessary and sufficient for constitutive and agonist-induced internalization of Ste2 and Ste3 receptors (19, 20). In contrast, multiple studies have suggested that for many mammalian GPCRs, ubiquitination does not play a direct role in the internalization of the receptor, but it functions indirectly (21, 22). One report has suggested that ubiquitination somehow regulates the mGluR-dependent synaptic plasticity in the hippocampus (23). However, the role of ubiquitination in the regulation of group I mGluRs and its physiological significance have not been investigated in detail.
We show here that the internalization of both mGluR1 and mGluR5 critically depends on the process of ubiquitination. Inhibition of the E1 activating enzyme by a pharmacological inhibitor, PYR-41, resulted in the inhibition of the ligand-mediated endocytosis of group I mGluRs. Our data also suggest that Lys63-linked polyubiquitination is involved in the internalization of these receptors. Importantly, the lysine residue at the 1112 position of the C-terminal tail of mGluR1 seems to be critical for the endocytosis of the receptor. Mutation of Lys1112 to arginine resulted in the inhibition of the internalization of mGluR1. We further show here that the E3 ubiquitin ligase, Siah-1A, is involved in this process. Acute knockdown of this ligase resulted in the complete inhibition of ligand-mediated internalization of mGluR1. Finally, cells in which endogenous Siah-1A was knocked down showed enhanced mGluR-mediated AMPA receptor (AMPAR) endocytosis compared with control cells. These results suggest that ubiquitination plays a critical role in the trafficking of group I mGluRs, and there exists an ubiquitin-independent mGluR-mediated AMPAR endocytosis pathway that limits a step in the multistep process leading from mGluR activation to AMPAR endocytosis.
Results
Ubiquitination Is Essential for the Endocytosis of Group I mGluRs
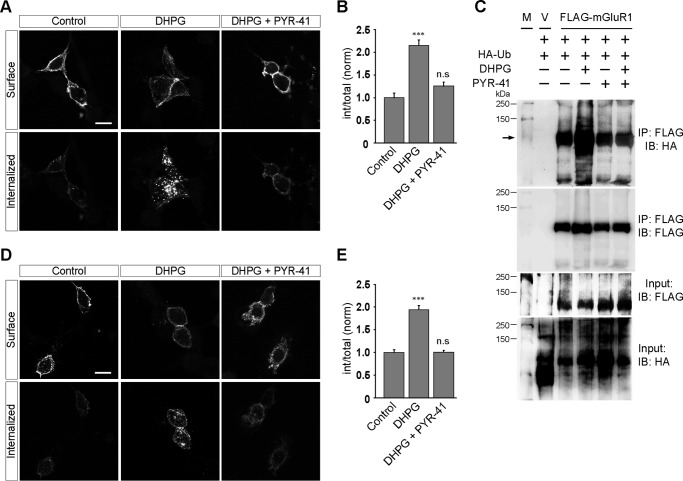
To investigate whether protein ubiquitination is required for the internalization of group I mGluRs, we used PYR-41/4[4-(5-nitro-furan-2-ylmethylene)-3,5-dioxo-pyrazolidin-1-yl]-benzoic acid ethyl ester. PYR-41 is a cell-permeable compound that has been demonstrated to irreversibly inhibit the E1-activating enzyme of the ubiquitin cascade inhibiting the E1-ubiquitin thioester bond formation (24). We have used 50 μm PYR-41 in all our assays because at this concentration PYR-41 has been reported to inhibit the formation of E1-ubiquitin thioester bonds by 95%; it also robustly inhibits several ubiquitin-dependent intracellular processes (24–27). Initially we studied the role of ubiquitination, if any, in the endocytosis of mGluR1 and mGluR5 in HEK293 cells. Live HEK293 cells expressing myc-mGluR1 were pretreated with 50 μm PYR-41 for 30 min at 37 °C and were stained with anti-myc primary antibody following that. Subsequent application of 100 μm R,S-DHPG led to the internalization of myc-mGluR1 at 30 min in PYR-41 untreated cells, whereas PYR-41-treated cells did not show significant internalized receptors on R,S-DHPG application, and most of the receptors were observed to be localized at the cell surface (control, 1 ± 0.1; DHPG, 2.15 ± 0.12; DHPG + PYR-41, 1.2 ± 0.09) (Fig. 1, A and B). Furthermore, although application of R,S-DHPG led to an increase in the ubiquitination of mGluR1 in wild type cells, the presence of PYR-41 inhibited the R,S-DHPG-mediated increase in the ubiquitination of the receptor (Fig. 1C). PYR-41 inhibited the R,S-DHPG-mediated internalization of another member of the group I mGluR family, and myc-mGluR5 as well in HEK293 cells (control, 1 ± 0.06; DHPG, 1.94 ± 0.09; DHPG + PYR-41, 1 ± 0.04) (Fig. 1, D and E).
FIGURE 1.
Ubiquitination regulates the group I mGluR internalization in HEK293 cells. A, representative images showing inhibition of DHPG-mediated endocytosis of myc-mGluR1 on application of PYR-41 in HEK293 cells. B, quantitation of the effect of PYR-41 on the internalization of myc-mGluR1. C, co-immunoprecipitation assays demonstrate that application of PYR-41 inhibits ligand-induced ubiquitination of mGluR1. M, molecular weight markers; V, empty vector. D and E, representative images (D) and quantitation (E) of DHPG-induced myc-mGluR5 internalization suggest that application of PYR-41 also inhibits the endocytosis of myc-mGluR5. All the results are represented as means ± S.E., collected from three independent experiments. Scale bar, 10 μm. ***, p < 0.001; n.s, p > 0.05.
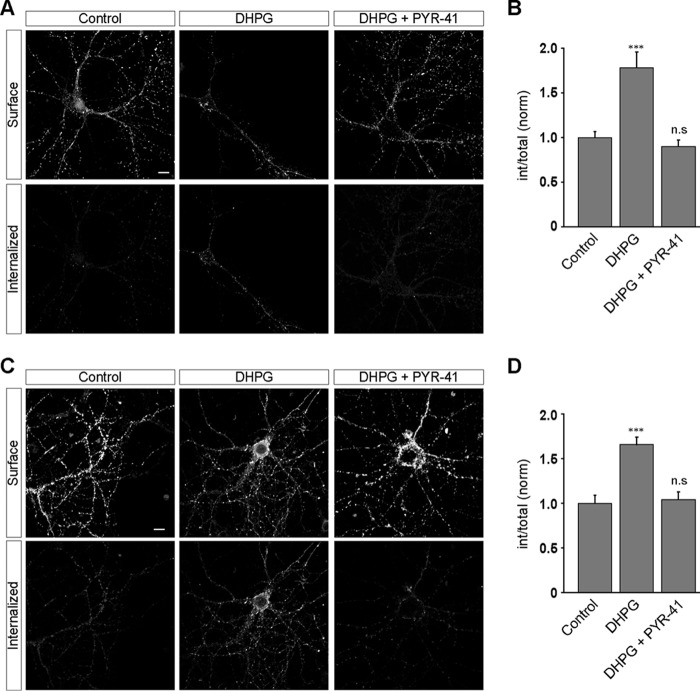
We next investigated the effect of ubiquitination on group I mGluR internalization in primary hippocampal neurons. myc-mGluR1 expressing live primary hippocampal neurons were first labeled with anti-myc primary antibody followed by the application of 100 μm R,S-DHPG, which led to the internalization of the receptor in PYR-41-untreated cells (control, 1 ± 0.07; DHPG, 1.78 ± 0.18) (Fig. 2, A and B). Application of 50 μm PYR-41 resulted in the complete inhibition of R,S-DHPG-mediated myc-mGluR1 endocytosis (DHPG + PYR-41, 0.9 ± 0.07). We obtained similar results when we investigated the effect of PYR-41 in the internalization of myc-mGluR5. The receptor internalized in PYR-41-untreated cells, whereas PYR-41-treated hippocampal neurons showed complete inhibition of R,S-DHPG-mediated myc-mGluR5 endocytosis (control, 1 ± 0.09; DHPG, 1.66 ± 0.08; DHPG + PYR-41, 1 ± 0.08) (Fig. 2, C and D). These results suggest that ubiquitination is critical for the internalization of both members of group I mGluRs, viz. mGluR1 and mGluR5. Because trafficking of both mGluR1 and mGluR5 were equally affected by PYR-41, we concentrated on mGluR1 for rest of the study.
FIGURE 2.
Ligand-mediated internalization of group I mGluRs depends on the ubiquitin system in primary hippocampal neurons. A, myc-mGluR1 internalized on 100 μm R,S-DHPG exposure as shown in the representative images. Application of PYR-41 completely inhibited the ligand-mediated internalization of the receptor. B, quantitation showing the effect of PYR-41 on the endocytosis of myc-mGluR1 in primary hippocampal neurons. C and D, similarly, exposing cells to PYR-41 also inhibited the DHPG-mediated internalization of myc-mGluR5 in primary hippocampal neurons as shown by the representative images (C) and quantitation (D). All the results are represented as means ± S.E., collected from three independent experiments. Scale bar, 10 μm. ***, p < 0.001; n.s, p > 0.05.
Endocytosis of mGluR1 Is Lys63-linked Polyubiquitination-dependent
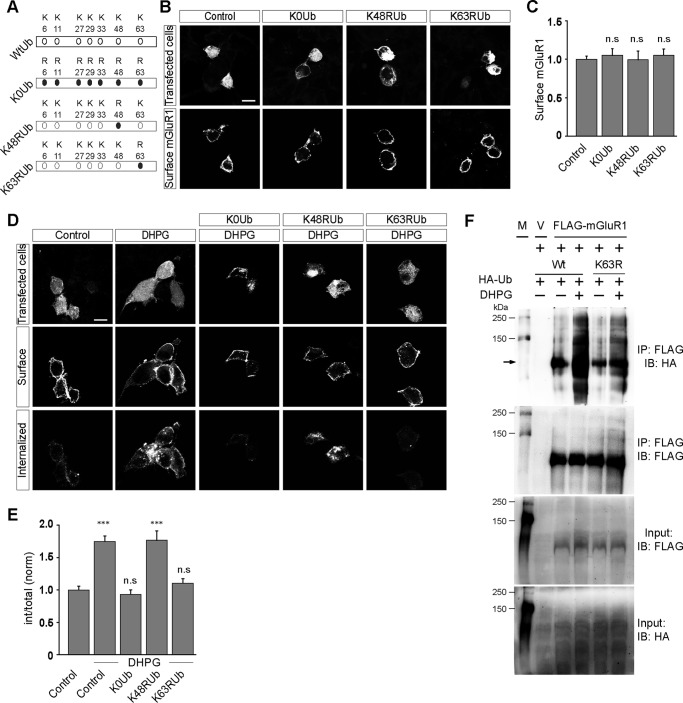
Ubiquitin can be attached to proteins as a single unit on one or multiple lysine residues, yielding monoubiquitinated and multimonoubiquitinated proteins, respectively (28, 29). Ubiquitin contains seven Lys residues and an N-terminal Met (Met1) that can serve as acceptor sites for additional ubiquitin molecules generating polyubiquitinated proteins (28–30). Thus, ubiquitin chains can also be connected through seven lysine residues present in ubiquitin. However, the most common polyubiquitin chains are formed through linkages at Lys48 and Lys63. Extensive studies on Lys48-linked and Lys63-linked chains established essential roles in proteasomal degradation for Lys48 and in cell signaling for Lys63-linked chains (31, 32). It has been reported that in the case of some GPCRs, ubiquitination plays a critical role in the internalization of those receptors (19, 20, 33). To investigate what type of ubiquitination leads to the internalization of mGluR1, we used various ubiquitin mutants, viz. K0Ub, K48RUb and K63RUb. All the lysines in K0Ub construct have been mutated to arginine, overexpression of which will result in only monoubiquitination and multimonoubiquitination of proteins. In K48RUb construct changing the lysine residue to arginine in position 48 of ubiquitin conserves the positive charge of the lysine but eliminates the ϵ-amino group that is required for Lys48-linked ubiquitin conjugation. Similarly in K63RUb, changing the lysine residue to arginine at 63 position of ubiquitin eliminates the Lys63-linked conjugations (Fig. 3A). Overexpression of these three mutants of ubiquitin in HEK293 cells did not have any effect on the surface expression of myc-mGluR1 (control, 1 ± 0.04; K0Ub, 1.05 ± 0.09; K48RUb, 1 ± 0.11; K63RUb, 1.05 ± 0.08) (Fig. 3, B and C). We next investigated the effect of overexpression of these constructs on the DHPG-mediated internalization of mGluR1. Although the wild type cells showed normal endocytosis of myc-mGluR1 on 100 μm R,S-DHPG application, cells overexpressing K0Ub did not show any significant endocytosis of the receptor (control, 1 ± 0.06; DHPG, 1.75 ± 0.09; K0Ub, DHPG: 0.93 ± 0.07) (Fig. 3, D and E). These results suggest that monoubiquitination or multimonoubiquitination is not sufficient for the ligand-mediated endocytosis of mGluR1. We therefore investigated the role of polyubiquitination, if any, in the internalization of the receptor. As stated before, a polyubiquitin chain linked through Lys48 of ubiquitin is the type of modification most commonly used for recognition and degradation by the proteasome. To determine whether polyubiquitin chains formation through Lys48 is required for ubiquitin to serve as an internalization signal of the receptor, we studied the internalization of the receptor in cells overexpressing K48RUb. Overexpression of K48RUb had no effect on the internalization of myc-mGluR1 in HEK293 cells (K48Rub, DHPG, 1.77 ± 0.15). The receptor internalized with almost similar extent on R,S-DHPG application in control cells as well in cells overexpressing K48RUb. In addition to Lys48, ubiquitin carries six other lysines in its sequence, and alternate types of polyubiquitin chains have been shown to form through Lys63. We therefore investigated the role of Lys63-linked ubiquitination, if any, on the internalization of mGluR1 by overexpressing K63RUb in HEK293 cells. Importantly, overexpression of K63RUb completely inhibited the R,S-DHPG-mediated endocytosis of myc-mGluR1 (K63RUb, DHPG, 1.1 ± 0.07). Furthermore, overexpressed K63RUb got incorporated in mGluR1 on 100 μm R,S-DHPG application (Fig. 3F). These results suggest that mGluR1 undergoes endocytosis subsequent to R,S-DHPG application through the Lys63 residue of ubiquitin-dependent polyubiquitination chain formation.
FIGURE 3.
Endocytosis of mGluR1 is Lys63-linked polyubiquitination-dependent. A, schematic showing the ubiquitin constructs (K0Ub, K48Rub, and K63RUb) used for this study. B, surface expression of myc-mGluR1 upon overexpression of various ubiquitin constructs as shown in representative images. C, quantitation shows that overexpression of K0Ub, K48Rub, and K63RUb did not have any effect on the surface expression of the receptor. D and E, however, overexpression of both K0Ub and K63RUb inhibited the DHPG-mediated internalization of myc-mGluR1 as shown by the representative images (D) and quantitation (E). On the other hand, K48RUb overexpression had no effect on the endocytosis of the receptor. F, co-immunoprecipitation assays demonstrate that application of R,S-DHPG resulted in the incorporation of HA-K63RUb in mGluR1. M, molecular weight markers; V, empty vector; IB, immunoblot; IP, immunoprecipitation. All the results are represented as means ± S.E., collected from three independent experiments. Scale bar, 10 μm. ***, p < 0.001; n.s, p > 0.05.
The C-terminal Lysine Residue of mGluR1 Plays a Critical Role in the Endocytosis of the Receptor
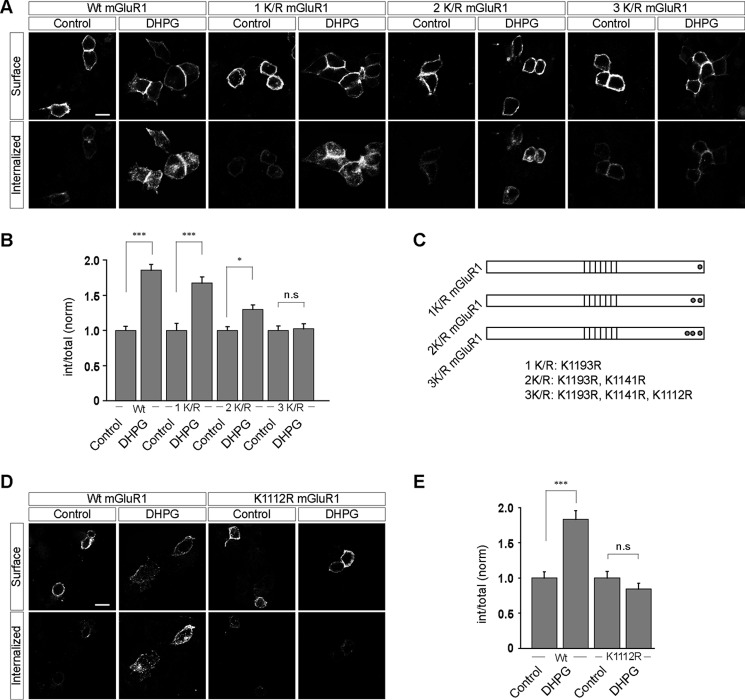
To define the importance of the post-translational modification of lysine residues with ubiquitin in the regulation of mGluR1 trafficking, we made various mGluR1 constructs having the lysine residues mutated to arginines. The mGluR1 C-terminal tail contains 16 lysine residues. We targeted the last three lysine residues at the extreme C-terminal tail of the receptor. As expected, the wild type myc-mGluR1 internalized on 100 μm R,S-DHPG application in HEK293 cells (WT, control, 1 ± 0.06; DHPG, 1.86 ± 0.08) (Fig. 4, A and B). However, myc-mGluR1 1K/R showed little decrease in the R,S-DHPG-mediated endocytosis, which decreased even further when the last two lysine residues of mGluR1 were mutated to arginines (myc-mGluR1 2K/R) (1K/R, control, 1 ± 0.1; DHPG, 1.67 ± 0.09; 2K/R, control: 1 ± 0.05; DHPG, 1.3 ± 0.07). Importantly, the third mutant, myc-mGluR1 3K/R, did not internalize at all on application of 100 μm R,S-DHPG (3K/R, control, 1 ± 0.07; DHPG, 1.02 ± 0.07). In this construct, the last three lysine residues of mGluR1 were mutated to arginines. Fig. 4C depicts the mutant receptors used in these experiments and the positions of the mutations in the cytoplasmic tail of the receptor. Because mutation of the 1112 lysine residue of the receptor to arginine in myc-mGluR1 3K/R mutant completely abolished the R,S-DHPG-mediated endocytosis of mGluR1, we asked whether mutation of this residue alone could affect the endocytosis of mGluR1. Indeed, mutation of Lys1112 to Arg alone in myc-mGluR1 completely blocked the 100 μm R,S-DHPG-induced endocytosis of the receptor in HEK293 cells (WT, control, 1 ± 0.09; DHPG, 1.83 ± 0.12; K1112R, control: 1 ± 0.09; DHPG, 0.84 ± 0.08) (Fig. 4, D and E).
FIGURE 4.
Lys1112 residue of mGluR1 plays critical role in the ubiquitin-dependent internalization of the receptor in HEK293 cells. A, representative images showing the internalization of various mutants of mGluR1 (1K/R, 2K/R, and 3K/R) on application of 100 μm R,S-DHPG in HEK293 cells. B, quantitation suggested that myc-mGluR1 1K/R internalized normally like the wild type receptor, whereas myc-mGluR1 2K/R showed lesser internalization on application of the ligand. Finally, myc-mGluR1 3K/R failed to internalize on application of 100 μm R,S-DHPG. C, schematic showing the position of various mutations in the 1K/R, 2K/R, and 3K/R constructs. D, mutation of Lys1112 residue of mGluR1 to arginine alone completely inhibited the ligand-mediated endocytosis of the receptor as shown in the representative images (D) and quantitation (E). All the results are represented as means ± S.E., collected from three independent experiments. Scale bar, 10 μm. ***, p < 0.001; *, p < 0.05; n.s, p > 0.05.
The role of Lys1112 residue of mGluR1 on the endocytosis of the receptor in primary hippocampal neurons was subsequently investigated. Mutating the Lys1112 residue to arginine did not affect the surface localization of the receptor at the dendrites of the hippocampal neurons (WT, 1 ± 0.06; K1112R, 0.95 ± 0.06) (Fig. 5, A and B). However, similar to in HEK293 cells, the K1112R myc-mGluR1 did not internalize on application of 100 μm R,S-DHPG, whereas the wild type myc-mGluR1 showed normal endocytosis on application of the ligand in hippocampal neurons (WT, control, 1 ± 0.07; DHPG, 1.78 ± 0.18; K1112R, control, 1 ± 0.03; DHPG, 0.87 ± 0.06) (Fig. 5, C and D). These results suggest that the 1112 lysine residue at the C-terminal tail of mGluR1 is critical for the endocytosis of the receptor.
FIGURE 5.
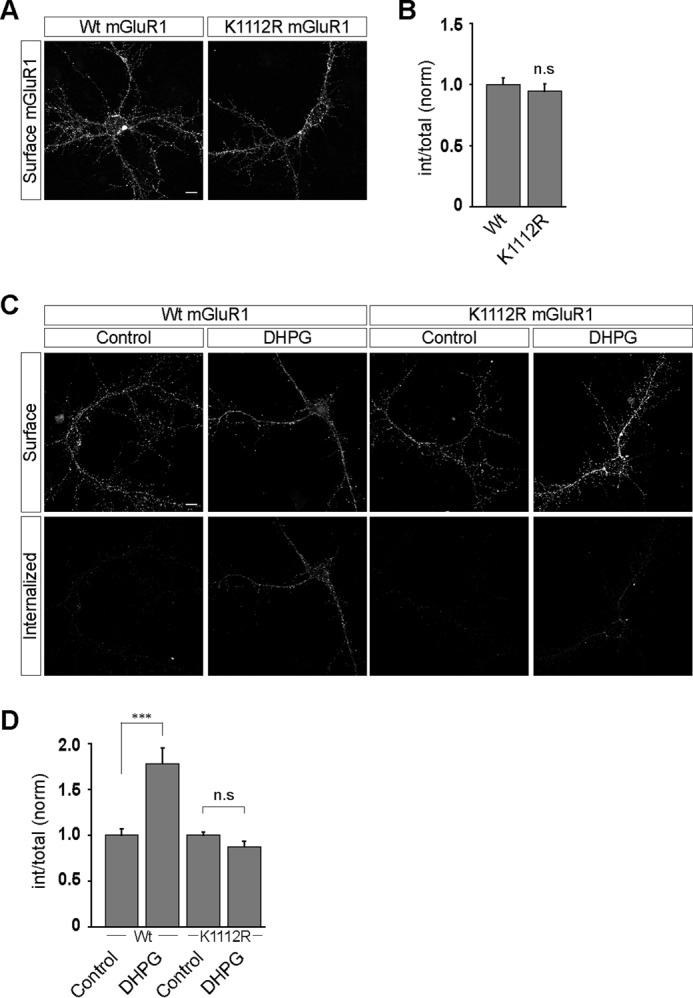
Mutation of lysine 1112 residue to arginine in mGluR1 blocks the ligand-mediated endocytosis of the receptor in primary hippocampal neurons. A, mutating the lysine 1112 residue at the C-terminal tail of mGluR1 to arginine had no effect on the surface expression of the receptor in the primary hippocampal neurons as shown in representative images. B, quantitation also suggested that both wild type and K1112R myc-mGluR1 manifested similar level of expression at the cell surface. C, representative images showing that although wild type myc-mGluR1 internalized normally on R,S-DHPG application, K1112R myc-mGluR1 did not internalize on ligand application. D, quantitation also suggested that mutation of Lys1112 to Arg in mGluR1 resulted in the complete blocking of the DHPG-mediated internalization of the receptor. All the results are represented as means ± S.E., collected from three independent experiments. Scale bar, 10 μm. ***, p < 0.001; n.s, p > 0.05.
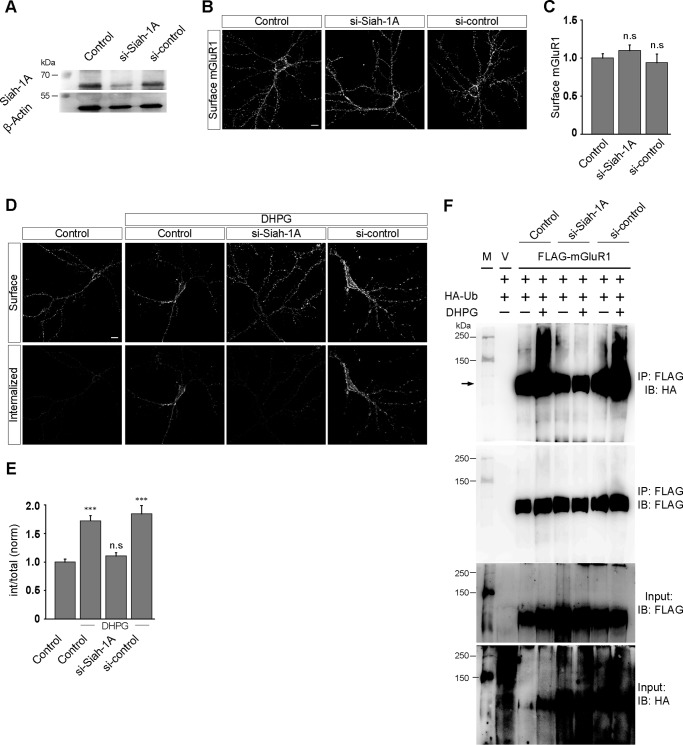
Agonist-mediated Endocytosis of mGluR1 Is Siah-1A-dependent
Drosophila Sina (seven in absentia) and its mammalian homolog Siah (seven in absentia homolog) are members of the E3 ubiquitin ligase family (34). In mice, there are three Siah proteins: Siah-1A, Siah-1B, and Siah-2 (34). Siah-1A has been demonstrated to bind to the C-terminal domain of group I mGluRs and modulates group I mGluR-mediated signaling (35–37). It has also been reported that Siah-1A mediates ubiquitination of group I mGluRs. We therefore investigated the role of Siah-1A, if any, in the internalization of mGluR1. Primary hippocampal neurons were co-transfected with myc-mGluR1 cDNA and siRNA against endogenous Siah-1A (ON-TARGET plus) or scrambled siRNA (si-control) at 8–9 days in vitro, and experiments were performed when the cells were at 12–13 days in vitro according to the method described under “Experimental Procedures.” Knockdown of endogenous Siah-1A was checked by Western blotting analyses. Our data suggested that the siRNA efficiently knocked down the endogenous Siah-1A, whereas the si-control did not show any significant knockdown (Fig. 6A). Acute knockdown of endogenous Siah-1A did not affect the surface expression of myc-mGluR1 in primary hippocampal neurons (control, 1 ± 0.05; si-Siah-1A, 1.1 ± 0.07; si-control, 0.94 ± 0.11) (Fig. 6, B and C). Importantly, knockdown of endogenous Siah-1A resulted in the complete inhibition of the R,S-DHPG-mediated internalization of myc-mGluR1. The receptor internalized normally upon application of 100 μm R,S-DHPG in control cells as well as in si-control transfected cells (control, 1 ± 0.05; control + DHPG, 1.72 ± 0.09; si-Siah-1A + DHPG, 1.1 ± 0.06; si-control + DHPG, 1.84 ± 0.15) (Fig. 6, D and E). Furthermore, knockdown of Siah-1A led to the inhibition of R,S-DHPG-mediated increase in the ubiquitination of the receptor. On the other hand, 100 μm R,S-DHPG application resulted in an increase in the ubiquitination of the receptor in both wild type cells and si-control treated cells (Fig. 6F). These results suggest that Siah-1A plays an important role in the ligand-mediated endocytosis of mGluR1.
FIGURE 6.
The ligand-mediated internalization of mGluR1 is Siah-1A-dependent. A, Western blotting analyses confirming that the endogenous Siah-1A was efficiently knocked down by si-Siah-1A in primary neurons. B, representative images show that acute knockdown of endogenous Siah-1A had no effect on the surface expression of myc-mGluR1 in primary hippocampal neurons. C, quantitation of the effect of Siah-1A knockdown on the surface expression of myc-mGluR1. D, the effect of endogenous knockdown of Siah-1A on the internalization of myc-mGluR1. Representative images showing that knockdown of Siah-1A led to the inhibition of DHPG-mediated internalization of the receptor. E, quantitation also suggested that knockdown of Siah-1A resulted in the inhibition of myc-mGluR1 endocytosis. F, co-immunoprecipitation experiments suggest that knockdown of Siah-1A resulted in the inhibition of the ligand-induced increase in the ubiquitination of mGluR1. M, molecular weight markers; V, empty vector; IB, immunoblot; IP, immunoprecipitation. All the results are represented as means ± S.E., collected from three independent experiments. Scale bar, 10 μm. ***, p < 0.001; n.s, p > 0.05.
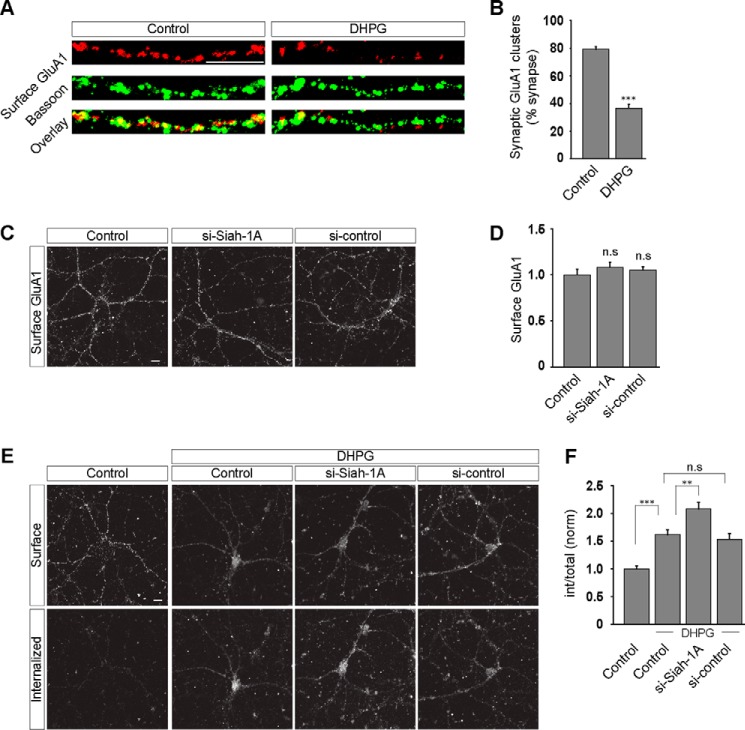
Inhibition of Ubiquitination Results in the Enhanced mGluR-mediated AMPAR Endocytosis
Rapid endocytosis of surface AMPARs can be triggered in cultured hippocampal neurons by application of various glutamate receptor agonists, including glutamate itself, NMDA, AMPA, and group I mGluR agonists (23, 38–40). mGluR-mediated AMPAR endocytosis is believed to be the cellular correlate for the mGluR-dependent synaptic plasticity (8, 41–43). Because the goal of this study was to elucidate the role of ubiquitination in group I mGluR trafficking and whether it in turn affects the mGluR-dependent synaptic AMPAR endocytosis, it was important to first establish that group I mGluR activation did in fact lead to the loss of synaptic AMPARs under our experimental conditions. To address whether mGluR activation using our protocol results in the endocytosis of synaptic AMPARs, we quantified the proportion of synapses containing detectable levels of surface AMPARs by staining for surface GluA1 containing clusters and counterstaining for Bassoon, a core component of the active zone that is commonly used to identify presynaptic terminals (44). Application of R,S-DHPG (100 μm for 5 min) in the presence of both AMPAR and NMDAR antagonists (20 μm 6,7-dinitroquinoxaline-2,3-dione and 50 μm 2-amino-5-phosphonopentanoic acid, respectively) substantially reduced the proportion of surface GluA1 puncta that co-localized with Bassoon puncta (control, 79.15 ± 2.1%; DHPG, 36.61 ± 2.6%; number of puncta per condition, 1000–1100) (Fig. 7, A and B). These results confirm that in our assays brief application of R,S-DHPG caused the internalization of synaptic AMPARs. To examine whether ubiquitination affects the mGluR-mediated AMPAR endocytosis, we acutely knocked down endogenous Siah-1A in primary hippocampal neurons. Siah-1A acute knockdown did not have any effect on the surface expression of GluA1 containing receptors (control, 1 ± 0.06; si-Siah-1A, 1.08 ± 0.05; si-control, 1.05 ± 0.04) (Fig. 7, C and D). On the other hand knockdown of endogenous Siah-1A caused an enhancement of the dendritic AMPAR endocytosis triggered by the application of R,S-DHPG (100 μm) compared with control cells. The si-control had no effect on the mGluR-mediated AMPAR endocytosis (control, 1 ± 0.05; control + DHPG, 1.62 ± 0.09; si-Siah-1A + DHPG, 2.08 ± 0.12; si-control + DHPG, 1.53 ± 0.1) (Fig. 7, E and F). It has been reported that activation of group I mGluRs does not increase the ubiquitination of AMPA receptors (45). Altogether these results not only suggest the existence of an ubiquitin-independent mGluR-mediated AMPAR endocytosis pathway, they also imply that the ubiquitin system limits a step in the multistep process leading from mGluR activation to AMPAR endocytosis.
FIGURE 7.
Ubiquitination regulates the mGluR-mediated AMPAR trafficking. A, examples of dendritic staining for surface GluA1 (red) and Bassoon (green) after R,S-DHPG treatment. B, quantitation of synaptic GluA1 clusters (synapses defined by Bassoon staining) before and after the R,S-DHPG treatment. C, representative images showing that acute knockdown of Siah-1A did not have any effect on the surface expression of GluA1 containing receptors. D, quantitation of the effect of acute knockdown of endogenous Siah-1A on the surface expression of GluA1 containing receptors. E and F, on the other hand, cells in which endogenous Siah-1A was knocked down showed enhanced mGluR-mediated AMPAR endocytosis as compared with control cells on application of 100 μm R,S-DHPG as shown in representative images (E) and quantitation (F). All the results are represented as means ± S.E., collected from three independent experiments. Scale bar, 10 μm. ***, p < 0.001; **, p < 0.01; n.s, p > 0.05.
Discussion
In this study we have defined a novel role for ubiquitination in the ligand-mediated internalization of group I mGluRs. We demonstrate that application of the ligand leads to the increase in ubiquitination of mGluR1. Importantly, the finding that pharmacologically blocking the ubiquitination of mGluR1 using the drug PYR-41 resulted in the inhibition of ligand-mediated internalization of the receptor suggests that ubiquitination plays crucial role in the trafficking of group I mGluRs. The above results were further strengthened by the observation that mutation of a lysine residue at the C terminus of mGluR1, viz. Lys1112 to arginine completely inhibited the DHPG-mediated internalization of the receptor. Our data also suggest that Lys63-linked polyubiquitination is involved in the endocytosis of mGluR1. The attachment of Lys63-linked ubiquitin chains facilitates the endocytosis of many integral membrane proteins (46). How Lys63-linked polyubiquitination chain formation leads to the endocytosis of mGluR1 and what role the Lys1112 residue plays in this process would be important to investigate in future. Another important issue that needs to be discussed is the fate of the receptor subsequent to internalization. Our earlier studies have suggested that subsequent to ligand-mediated internalization, group I mGluRs recycle back to the cell surface (15, 16). This implies that ubiquitination of mGluR1 must be a highly dynamic and reversible process. Subsequent to internalization the receptor probably gets deubiquitinated and recycles back to the cell surface. The verification of the above hypotheses, the identity of the deubiquitinase(s), and the specific location where this deubiquitination takes place would be other important areas to study.
Interestingly, we find that a specific E3 ubiquitin ligase Siah-1A that was reported to interact with the group I mGluRs plays a critical role in the ligand-dependent internalization of mGluR1. According to our results, acute knockdown of endogenous Siah-1A leads to the complete inhibition of mGluR1 internalization. E3 ubiquitin ligases consist of diverse members of the protein family, and each recognizes a specific protein substrate to perform specific functions (47). It therefore is important to identify an ubiquitin ligase specific for a key receptor molecule and assign its specific role in regulating its substrate. In this investigation, Siah-1A is revealed to act as an ubiquitin ligase involved in the ligand-mediated internalization of mGluR1. In addition, we have also shown in this study that acute knockdown of Siah-1A resulted in the increased mGluR-mediated AMPAR endocytosis. It has been reported earlier in one study that blocking of ubiquitin system by pharmacological inhibitor UBEI-41/PYR-41 results in the enhanced mGluR-mediated AMPAR endocytosis, as well as mGluR-LTD in hippocampal cells (23). It must be stated that PYR-41 being a drug might have some other effect in addition to the inhibition of E1-activating enzyme. For example, in addition to blocking E1-activating enzyme, PYR-41 has been demonstrated to increase protein SUMOylation (24). Thus, it was important to check for the effect of blocking of ubiquitination in mGluR-mediated AMPAR endocytosis by specifically inhibiting the ubiquitination process. That is why we checked for the effect of acute knockdown of endogenous Siah-1A on the mGluR-mediated AMPAR endocytosis in primary hippocampal neurons. The finding that mGluR-mediated AMPAR internalization was increased by acute knockdown of Siah-1A suggests that ubiquitination may function as a negative feedback to inhibit the activity of group I mGluRs. As stated before, it has been reported that activation of group I mGluRs does not increase the ubiquitination of AMPA receptors (45). Thus, our data also suggest that a ubiquitin-independent mGluR-mediated AMPAR endocytic pathway probably exists.
Our data presented here suggest that ubiquitination plays a critical role in the regulation of group I mGluRs, which in turn regulates the mGluR-dependent AMPAR endocytosis, the cellular correlate for mGluR-dependent synaptic plasticity in hippocampal cells. Inappropriate signaling of group I mGluRs has been suggested to be involved in the pathophysiology of multiple cognitive disorders such as fragile X syndrome, autism, etc. (41). Thus, our results raise the possibility that impairment of the ubiquitin system, as observed in several other brain disorders (48), could contribute to these disorders by reducing negative feedback regulation of group I mGluRs. Therefore further study of the regulation of group I mGluRs by ubiquitin system would be of paramount importance in the near future.
Experimental Procedures
Materials
The myc-mGluR1 and myc-mGluR5 constructs were obtained from Kathrine Roche (National Institute of Health). In these constructs the myc epitope was tagged at the N terminus of the full-length mGluR1 and mGluR5. The FLAG-mGluR1 construct was a generous gift from Johanna Montgomery (University of Auckland, Auckland, New Zealand). All the HA-tagged ubiquitin constructs were kind gifts from Maddika Subba Reddy (Centre for DNA Fingerprinting and Diagnostics, India). HEK293 cells were obtained from the National Centre For Cell Science (Pune, India). Primary hippocampal neurons were obtained from Panacea Biotech (New Delhi, India). Cell culture reagents and Alexa 568-labeled transferrin were purchased from Invitrogen. 5-fluorodeoxyuridine (floxuridine), paraformaldehyde (PFA), poly-d-lysine, and FluoromountTM aqueous mounting medium were purchased from Sigma. R,S-DHPG and PYR-41 were purchased from Tocris. Fine chemicals were obtained from Life Technologies and Merck. The primary antibodies were purchased from following companies: anti-myc antibody from Abcam, anti-FLAG antibody from Sigma, anti-bassoon antibody from Stressgen, anti-HA antibody from Roche, anti-Siah-1A antibody and anti-β-actin antibody from Santa Cruz Biotechnology, and anti-GluA1 antibody from Calbiochem. All secondary antibodies were purchased from Invitrogen.
Dissociated Hippocampal Neuron Cultures
Primary hippocampal neuron cultures were prepared from P0 C57BL/6 mouse pups as described previously with minor changes (15). Briefly, hippocampi were obtained from P0 mouse pups, and subsequently, tissue was dissociated by papain treatment. The cells were then plated on poly-d-lysine in sodium borate-coated coverslips at a density of ∼100,000 cells/12-mm well in a 24-well plate. Cultures were maintained in Neurobasal medium with 0.5 mm glutamine and B27 supplement. Glial growth was inhibited by adding 5-fluorodeoxyuridine (floxuridine) on day 3 of culture.
HEK293 Cell Culture and Transfection
HEK293 cells were cultured in DMEM supplemented with 10% FBS, antibiotic-antimycotic mix at 37 °C and 5% CO2. The cells were transfected on 12-mm coverslips coated with 50 μg/ml poly-d-lysine by mixing a total 2 μg of DNA with 10 μg of Lipofectamine 2000 in 1 ml of OptiMEM. All the experiments in HEK293 cells were done 24 h post-transfection.
Transfection in Primary Hippocampal Neurons and Knockdown Experiments
Primary hippocampal neurons were transfected with mGluR1 or mGluR5 constructs at 8–9 days in vitro using calcium phosphate, and all the experiments were performed when the cells were at 12–14 days in vitro. For knockdown experiments, ON-TARGET plus SMARTpool siRNA against Siah-1A and scrambled siRNA (ThermoScientific Dharmacon) were either transfected alone (for AMPAR endocytosis experiments) or co-transfected with myc-mGluR1 cDNA (for mGluR1 endocytosis experiments) in primary hippocampal neurons at 8–9 days in vitro using Lipofectamine 2000 following the manufacturer's instructions, and experiments were carried out in cells at 12–14 days in vitro.
mGluR and AMPAR Endocytosis Assay
HEK293 cells or primary hippocampal neurons were transfected with either myc-mGluR1 (wild type or various mutants) or myc-mGluR5 cDNA as described above. Live cells were incubated with mouse anti-myc primary antibody (1:500 for HEK293 cells and 1:200 for primary hippocampal neurons) for 15 min at 37 °C. The cells were then washed, and 100 μm R,S-DHPG was applied for indicated times or for 5 min. The cells were then incubated at 37 °C for various times in plain DMEM/Neurobasal medium in the absence of the ligand. Subsequently, the cells were fixed without permeabilization using ice-cold 4% PFA for 15 min on ice. Surface localized receptors were labeled with saturating concentration of goat anti-mouse Alexa 568-conjugated secondary antibody (1:100) for 1 h at 37 °C. The cells were then permeabilized with 0.1% Triton X-100 for 30 min in room temperature. The endocytosed receptors were then labeled by the application of goat anti-mouse Alexa 647-conjugated secondary antibody (1:750) for 1 h at 37 °C. The coverslips were then mounted on glass slides and imaged under the confocal microscope. For experiments involving PYR-41, the cells were pretreated with PYR-41 (50 μm) for 30 min before the application of the primary antibody, and the endocytosis assay was done identically as described above. PYR-41 was present throughout the experiment. The effect of knockdown of the endogenous Siah-1A on the internalization of mGluR1 in primary hippocampal neurons was investigated by co-transfection of cells with myc-mGluR1 and si-Siah-1A. For all experiments two coverslips were used for each condition, and all experiments were repeated at least three times. To ensure that the Alexa 647-conjugated secondary antibody did not label any detectable surface receptors in our assays, we performed control experiments to determine the saturating concentration of the first secondary antibody similar to that we have described in our earlier studies (15, 16, 38, 49).
To assay mGluR-mediated AMPAR endocytosis surface, GluA1 containing AMPARs were labeled in live neurons by 15 min of incubation at 37 °C with a rabbit polyclonal antibody directed against the N terminus of the GluA1 subunit (1:20). After washing, the cells were treated with appropriate mixtures of antagonists (1 μm tetrodotoxin, 20 μm 6,7-dinitroquinoxaline-2,3-dione, and 50 μm 2-amino-5-phosphonopentanoic acid) for 5 min. Subsequently, 100 μm R,S-DHPG was applied for 5 min. The agonist was then washed out, and the cells were further incubated in the presence of antagonists for a total of 15 min at 37 °C. The cells were next fixed in 4% PFA for 15 min on ice without permeabilization, and surface receptors were visualized by using saturating amount of goat anti-rabbit Alexa 568-conjugated secondary antibody (1:100) followed by permeabilization of cells with 0.1% Triton X-100 for 30 min at room temperature and staining of internalized receptors with goat anti-rabbit Alexa 647-conjugated secondary antibody (1:750). Again, to make sure that the Alexa 647-conjugated secondary antibody did not label any detectable surface receptors in our assays, we determine the saturating concentration of the first secondary antibody through a control experiment similar to that we have described in our earlier studies (23, 38, 40). For investigation of the role of Siah-1A in the mGluR-mediated AMPAR endocytosis, primary neurons were transfected with si-Siah-1A or si-control as described before. Subsequently, the mGluR-mediated AMPAR endocytosis assay was performed with identical protocol as described above.
Co-localization Assay
To investigate whether mGluR activation by R,S-DHPG leads to the endocytosis of synaptic AMPARs, synaptic GluA1 puncta were quantified by co-localization of surface GluA1 puncta and presynaptic Bassoon puncta. After live cell staining with the rabbit polyclonal antibody directed against the N terminus of the GluA1, subunit cells were either not treated (control) or treated with 100 μm R,S-DHPG as described above. The cells were then fixed in 4% PFA on ice for 15 min without permeabilization and were incubated with goat anti-rabbit Alexa 568 secondary antibody for 1 h at 37 °C. The cells were subsequently permeabilized in 0.1% Triton X-100 for 30 min at room temperature and were incubated with a monoclonal antibody against Bassoon for overnight at 4 °C. After that, the cells were treated with goat anti-mouse Alexa 488 secondary antibody for Bassoon puncta visualization.
Immunoprecipitation and Western Blotting Analysis
To check for the ubiquitination profile of mGluR1 in the presence of PYR-41, HEK293T cells were transfected with FLAG-tagged mGluR1 (FLAG-mGluR1) and HA-tagged wild type ubiquitin. Subsequently, the cells were incubated in 50 μm PYR-41 and 50 μm MG-132 for 30 min followed by the application of 100 μm R,S-DHPG for 5 min. To investigate the effect of Siah-1A knockdown on the ubiquitination of the receptor, subsequent to the co-transfection of FLAG-mGluR1 and HA-Ub, the cells were transfected with si-Siah-1A or si-control using the protocol described above. 3–4 days after transfection control cells, si-Siah-1A transfected cells and si-control transfected cells were treated with 100 μm R,S-DHPG, and the ubiquitination profile of the receptor was checked by the procedure described below. To check whether K63RUb construct gets incorporated in mGluR1 upon 100 μm R,S-DHPG application, the cells were co-transfected with FLAG-mGluR1 and with either wild type HA-Ub or HA-K63RUb. In all the above experiments, subsequently, the cells were placed on ice and lysed in lysis buffer (150 mm NaCl, 50 mm Tris-HCl, pH 7.4, 5 mm EDTA, 1% Triton X-100, 0.1% SDS, 20 mm N-ethylmaleimide, protease inhibitor mixture). Following lysis of the cells, immunoprecipitation was performed by overnight incubation with anti-FLAG M2 affinity beads (Sigma-Aldrich). Immunoprecipitates were run in SDS-PAGE followed by Western blotting analysis. For immunoblotting, anti-HA antibody was used to check for the ubiquitination profile of the receptor, and anti-FLAG antibody was used to check for the pulled down level of the receptor. Immunoblotting was performed using horseradish peroxidase-conjugated secondary antibodies, and blots were developed using the ECL Western detection kit (Amersham Biosciences). Image acquisition was done using ImageQuant LAS 4000.
Knockdown of endogenous Siah-1A was studied by transfecting primary neurons with either siRNA against Siah-1A (ON-TARGET plus SMARTpool) or scrambled siRNA (si-control) (Dharmacon, ThermoScientific). 72 h post-transfection, neurons were lysed in radioimmune precipitation assay lysis buffer having protease inhibitor mixture. The samples were boiled in Laemmli sample buffer and run in SDS-PAGE by loading an equal amount of protein in each lane. Subsequently, they were transferred to the PVDF membrane and blocked with 5% skimmed milk for 1 h at room temperature. The membrane was then incubated with anti-Siah-1A polyclonal antibody (1:800) or anti-β-actin (1:800) primary antibodies in 4 °C overnight. The membranes were washed and incubated in horseradish peroxidase-conjugated secondary antibodies for 45 min at room temperature. The blots were developed using ECL Western detection kit and imaging was performed in ImageQuant LAS 4000.
Image Acquisition and Analysis
The images were obtained in Zeiss LSM 780 confocal laser scanning microscope using a 63× oil immersion objective with 1.4 numerical aperture. 100–120 HEK293 cells and 40–50 primary hippocampal neurons were imaged, and every experiment was repeated at least three times. In a particular experiment images from all the conditions were obtained using identical parameters. Subsequently raw images were used for all analyses and quantitation in ImageJ software (National Institutes of Health). All the analyses procedures have been described in our earlier studies (15, 16, 49). Briefly, each raw image was maximally projected and thresholded using identical values for a particular experiment. Thresholded areas occupied by the fluorescence of the labeled surface and internalized receptors were quantified. The internalization index was then calculated by dividing the value contributed by the internal fluorescence with the value contributed by the total fluorescence (surface + internal). They were then normalized with that of untreated control cells. To measure the surface receptors in all our assays, surface fluorescence was divided by cell area, which was determined by measuring background fluorescence using a low threshold level. These values were then normalized to the average surface fluorescence of untreated control cells. In case of primary hippocampal neurons, all the data represent dendritic values that were defined by the area that was 10 μm away from the soma. All the quantitation has been represented as combined results for all the repeats of a particular experiment. Raw images were adjusted using equal values of brightness and contrast to obtain the representative images.
Statistical Analysis
The data are presented as means ± S.E. 100–120 HEK293 cells, 40–50 primary hippocampal neurons were imaged, and each experiment was repeated three times. The experimental group results were compared with each other using Student's t test or one-way analysis of variance followed by Tukey's post-test. p > 0.05 was considered as non-significant.
Author Contributions
R. G., R. S., and S. B. designed experiments. R. G. and R. S. performed experiments. R. G., R. S., and S. B analyzed the data and wrote the paper.
Acknowledgments
We thank Dr. Kathrine Roche (National Institutes of Health) for the generous gift of myc-mGluR1 and myc-mGluR5 constructs. We are also thankful to Dr. Johanna Montgomery (University of Auckland, Auckland, New Zealand) for the FLAG-mGluR1 construct. We acknowledge Dr. Maddika Subba Reddy (Centre for DNA Fingerprinting and Diagnostics, Hyderabad, India) for the generous gift of ubiquitin constructs. We also acknowledge members of our laboratory for valuable inputs.
This work was supported by Indian Institute of Science Education and Research Mohali and University Grants Commission, India fellowships (to R. G. and R. S.). The authors declare that they have no conflicts of interest with the contents of this article.
- mGluR
- metabotropic glutamate receptor
- AMPAR
- AMPA receptor
- GPCR
- G protein-coupled receptor
- Ub
- ubiquitin
- PFA
- paraformaldehyde
- DHPG
- dihydroxyphenylglycine.
References
- 1. Bhattacharyya S. (2016) Inside story of group I metabotropic glutamate receptors (mGluRs). Int. J. Biochem. Cell Biol. 77, 205–212 [DOI] [PubMed] [Google Scholar]
- 2. Dhami G. K., and Ferguson S. S. (2006) Regulation of metabotropic glutamate receptor signaling, desensitization and endocytosis. Pharmacol. Ther. 111, 260–271 [DOI] [PubMed] [Google Scholar]
- 3. Pin J. P., and Duvoisin R. (1995) The metabotropic glutamate receptors: structure and functions. Neuropharmacology 34, 1–26 [DOI] [PubMed] [Google Scholar]
- 4. Baude A., Nusser Z., Roberts J. D., Mulvihill E., McIlhinney R. A., and Somogyi P. (1993) The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron 11, 771–787 [DOI] [PubMed] [Google Scholar]
- 5. Shigemoto R., Nomura S., Ohishi H., Sugihara H., Nakanishi S., and Mizuno N. (1993) Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci. Lett. 163, 53–57 [DOI] [PubMed] [Google Scholar]
- 6. Kim C. H., Lee J., Lee J. Y., and Roche K. W. (2008) Metabotropic glutamate receptors: phosphorylation and receptor signaling. J. Neurosci. Res. 86, 1–10 [DOI] [PubMed] [Google Scholar]
- 7. Conn P. J., and Pin J. P. (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 37, 205–237 [DOI] [PubMed] [Google Scholar]
- 8. Citri A., and Malenka R. C. (2008) Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 33, 18–41 [DOI] [PubMed] [Google Scholar]
- 9. Anwyl R. (2006) Induction and expression mechanisms of postsynaptic NMDA receptor-independent homosynaptic long-term depression. Prog. Neurobiol. 78, 17–37 [DOI] [PubMed] [Google Scholar]
- 10. Huber K. M., Gallagher S. M., Warren S. T., and Bear M. F. (2002) Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. U.S.A. 99, 7746–7750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koekkoek S. K., Yamaguchi K., Milojkovic B. A., Dortland B. R., Ruigrok T. J., Maex R., De Graaf W., Smit A. E., VanderWerf F., Bakker C. E., Willemsen R., Ikeda T., Kakizawa S., Onodera K., Nelson D. L., et al. (2005) Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in fragile X syndrome. Neuron 47, 339–352 [DOI] [PubMed] [Google Scholar]
- 12. Dölen G., Osterweil E., Rao B. S., Smith G. B., Auerbach B. D., Chattarji S., and Bear M. F. (2007) Correction of fragile X syndrome in mice. Neuron 56, 955–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Michalon A., Sidorov M., Ballard T. M., Ozmen L., Spooren W., Wettstein J. G., Jaeschke G., Bear M. F., and Lindemann L. (2012) Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron 74, 49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Francesconi A., and Duvoisin R. M. (2000) Opposing effects of protein kinase C and protein kinase A on metabotropic glutamate receptor signaling: selective desensitization of the inositol trisphosphate/Ca2+ pathway by phosphorylation of the receptor-G protein-coupling domain. Proc. Natl. Acad. Sci. U.S.A. 97, 6185–6190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahato P. K., Pandey S., and Bhattacharyya S. (2015) Differential effects of protein phosphatases in the recycling of metabotropic glutamate receptor 5. Neuroscience 306, 138–150 [DOI] [PubMed] [Google Scholar]
- 16. Pandey S., Mahato P. K., and Bhattacharyya S. (2014) Metabotropic glutamate receptor 1 recycles to the cell surface in protein phosphatase 2A-dependent manner in non-neuronal and neuronal cell lines. J. Neurochem. 131, 602–614 [DOI] [PubMed] [Google Scholar]
- 17. Hicke L., and Dunn R. (2003) Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 19, 141–172 [DOI] [PubMed] [Google Scholar]
- 18. Hershko A., and Ciechanover A. (1998) The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 [DOI] [PubMed] [Google Scholar]
- 19. Hicke L., and Riezman H. (1996) Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell 84, 277–287 [DOI] [PubMed] [Google Scholar]
- 20. Terrell J., Shih S., Dunn R., and Hicke L. (1998) A function for monoubiquitination in the internalization of a G protein-coupled receptor. Mol. Cell 1, 193–202 [DOI] [PubMed] [Google Scholar]
- 21. Shenoy S. K., McDonald P. H., Kohout T. A., and Lefkowitz R. J. (2001) Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and β-arrestin. Science 294, 1307–1313 [DOI] [PubMed] [Google Scholar]
- 22. Marchese A., and Benovic J. L. (2001) Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J. Biol. Chem. 276, 45509–45512 [DOI] [PubMed] [Google Scholar]
- 23. Citri A., Soler-Llavina G., Bhattacharyya S., and Malenka R. C. (2009) N-Methyl-d-aspartate receptor- and metabotropic glutamate receptor-dependent long-term depression are differentially regulated by the ubiquitin-proteasome system. Eur. J. Neurosci. 30, 1443–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang Y., Kitagaki J., Dai R. M., Tsai Y. C., Lorick K. L., Ludwig R. L., Pierre S. A., Jensen J. P., Davydov I. V., Oberoi P., Li C. C., Kenten J. H., Beutler J. A., Vousden K. H., and Weissman A. M. (2007) Inhibitors of ubiquitin-activating enzyme (E1), a new class of potential cancer therapeutics. Cancer Res. 67, 9472–9481 [DOI] [PubMed] [Google Scholar]
- 25. Dey A., Tergaonkar V., and Lane D. P. (2008) Double-edged swords as cancer therapeutics: simultaneously targeting p53 and NF-κB pathways. Nat. Rev. Drug Discov. 7, 1031–1040 [DOI] [PubMed] [Google Scholar]
- 26. Satheshkumar P. S., Anton L. C., Sanz P., and Moss B. (2009) Inhibition of the ubiquitin-proteasome system prevents vaccinia virus DNA replication and expression of intermediate and late genes. J. Virol. 83, 2469–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zaarur N., Meriin A. B., Gabai V. L., and Sherman M. Y. (2008) Triggering aggresome formation: dissecting aggresome-targeting and aggregation signals in synphilin 1. J. Biol. Chem. 283, 27575–27584 [DOI] [PubMed] [Google Scholar]
- 28. Alonso V., and Friedman P. A. (2013) Minireview: ubiquitination-regulated G protein-coupled receptor signaling and trafficking. Mol. Endocrinol. 27, 558–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Komander D. (2009) The emerging complexity of protein ubiquitination. Biochem. Soc. Trans. 37, 937–953 [DOI] [PubMed] [Google Scholar]
- 30. Komander D., and Rape M. (2012) The ubiquitin code. Annu. Rev. Biochem. 81, 203–229 [DOI] [PubMed] [Google Scholar]
- 31. Finley D. (2009) Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 78, 477–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen Z. J., and Sun L. J. (2009) Nonproteolytic functions of ubiquitin in cell signaling. Mol. Cell 33, 275–286 [DOI] [PubMed] [Google Scholar]
- 33. Hislop J. N., and von Zastrow M. (2011) Role of ubiquitination in endocytic trafficking of G-protein-coupled receptors. Traffic 12, 137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Della N. G., Senior P. V., and Bowtell D. D. (1993) Isolation and characterisation of murine homologues of the Drosophila seven in absentia gene (sina). Development 117, 1333–1343 [DOI] [PubMed] [Google Scholar]
- 35. Moriyoshi K., Iijima K., Fujii H., Ito H., Cho Y., and Nakanishi S. (2004) Seven in absentia homolog 1A mediates ubiquitination and degradation of group 1 metabotropic glutamate receptors. Proc. Natl. Acad. Sci. U.S.A. 101, 8614–8619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ishikawa K., Nash S. R., Nishimune A., Neki A., Kaneko S., and Nakanishi S. (1999) Competitive interaction of seven in absentia homolog-1A and Ca2+/calmodulin with the cytoplasmic tail of group 1 metabotropic glutamate receptors. Genes Cells 4, 381–390 [DOI] [PubMed] [Google Scholar]
- 37. Kammermeier P. J., and Ikeda S. R. (2001) A role for seven in absentia homolog (Siah1a) in metabotropic glutamate receptor signaling. BMC Neurosci. 2, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bhattacharyya S., Biou V., Xu W., Schlüter O., and Malenka R. C. (2009) A critical role for PSD-95/AKAP interactions in endocytosis of synaptic AMPA receptors. Nat. Neurosci. 12, 172–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Biou V., Bhattacharyya S., and Malenka R. C. (2008) Endocytosis and recycling of AMPA receptors lacking GluR2/3. Proc. Natl. Acad. Sci. U.S.A. 105, 1038–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Citri A., Bhattacharyya S., Ma C., Morishita W., Fang S., Rizo J., and Malenka R. C. (2010) Calcium binding to PICK1 is essential for the intracellular retention of AMPA receptors underlying long-term depression. J. Neurosci. 30, 16437–16452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bhattacharyya S. (2016) Inside story of group I metabotropic glutamate receptors (mGluRs). Int. J. Biochem. Cell Biol. 77, 205–212 [DOI] [PubMed] [Google Scholar]
- 42. Gladding C. M., Fitzjohn S. M., and Molnár E. (2009) Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacol. Rev. 61, 395–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Niswender C. M., and Conn P. J. (2010) Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 50, 295–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Regalado M. P., Terry-Lorenzo R. T., Waites C. L., Garner C. C., and Malenka R. C. (2006) Transsynaptic signaling by postsynaptic synapse-associated protein 97. J. Neurosci. 26, 2343–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Widagdo J., Chai Y. J., Ridder M. C., Chau Y. Q., Johnson R. C., Sah P., Huganir R. L., and Anggono V. (2015) Activity-dependent ubiquitination of GluA1 and GluA2 regulates AMPA receptor intracellular sorting and degradation. Cell Rep. pii, S2211–1247(15)00028-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Galan J. M., and Haguenauer-Tsapis R. (1997) Ubiquitin Lys63 is involved in ubiquitination of a yeast plasma membrane protein. EMBO J. 16, 5847–5854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Glickman M. H., and Ciechanover A. (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 82, 373–428 [DOI] [PubMed] [Google Scholar]
- 48. Ciechanover A., and Brundin P. (2003) The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron 40, 427–446 [DOI] [PubMed] [Google Scholar]
- 49. Trivedi R. R., and Bhattacharyya S. (2012) Constitutive internalization and recycling of metabotropic glutamate receptor 5 (mGluR5). Biochem. Biophys. Res. Commun. 427, 185–190 [DOI] [PubMed] [Google Scholar]