Abstract
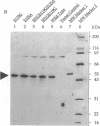
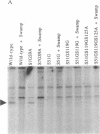
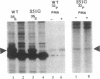
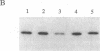
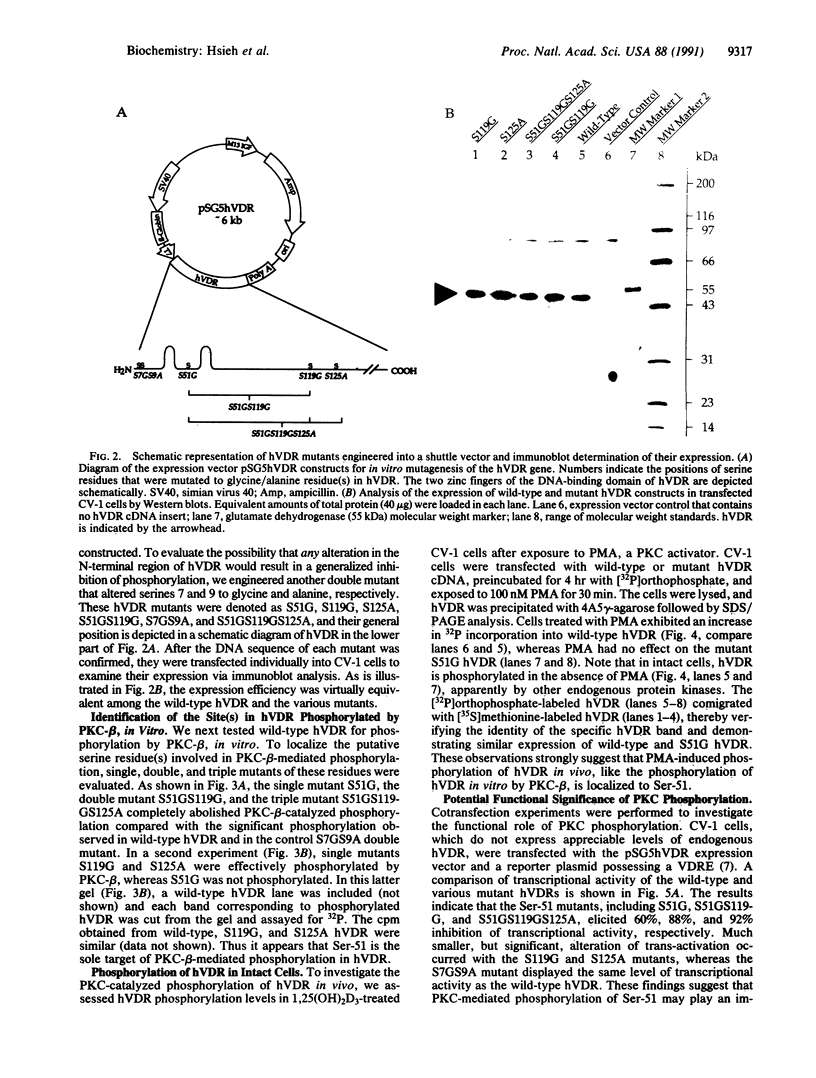
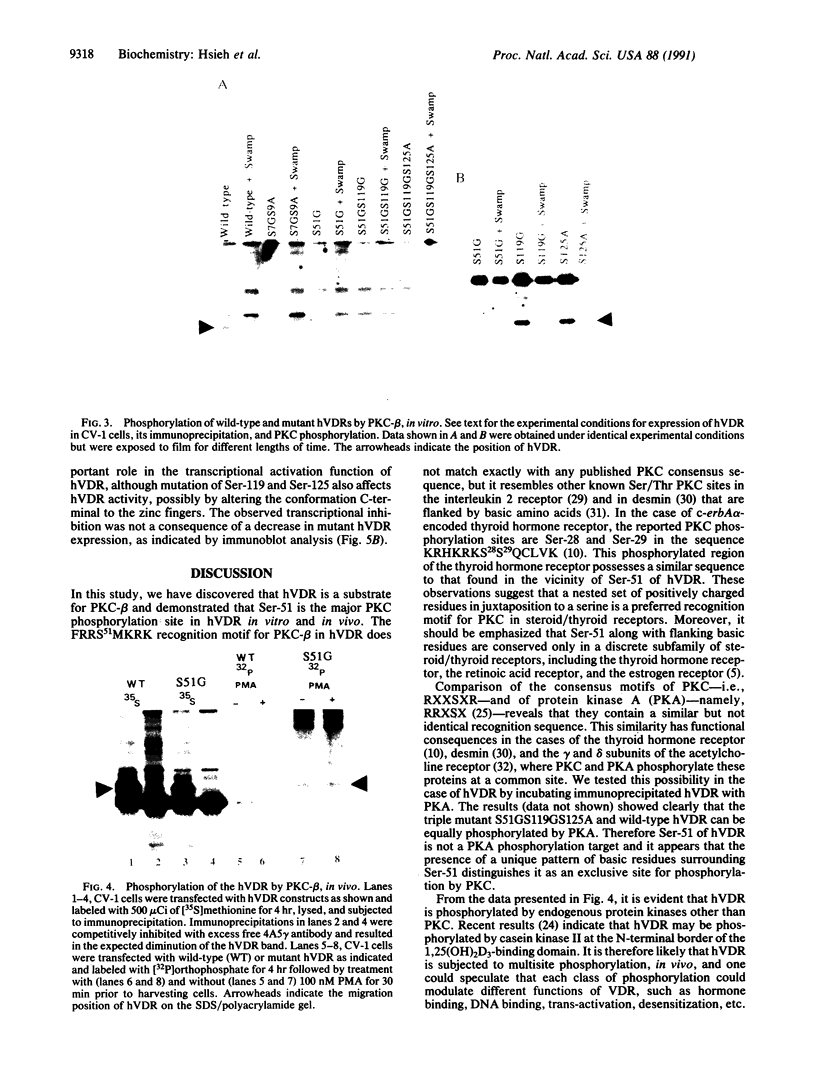
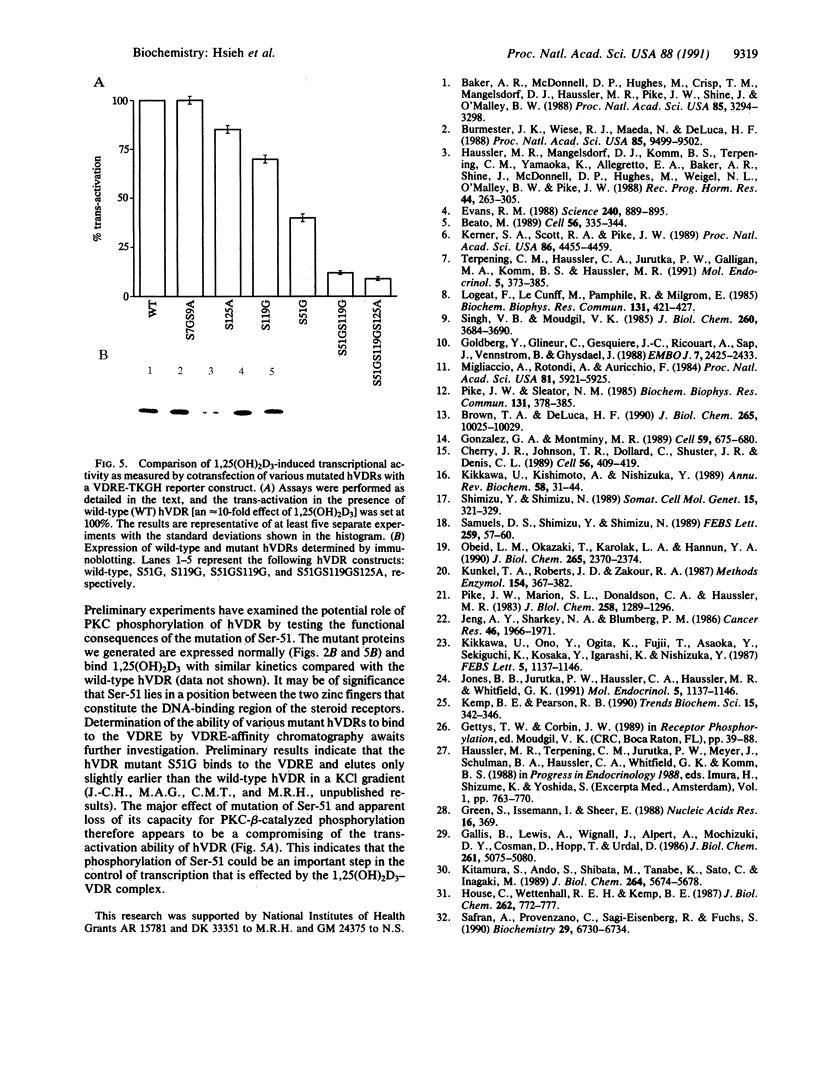
The vitamin D receptor (VDR) is known to be a phosphoprotein and inspection of the deduced amino acid sequence of human VDR (hVDR) reveals the conservation of three potential sites of phosphorylation by protein kinase C (PKC)--namely, Ser-51, Ser-119, and Ser-125. Immunoprecipitated extracts derived from a rat osteoblast-like osteosarcoma cell line that contains the VDR in high copy number were incubated with the alpha, beta, and gamma isozymes of PKC, and VDR proved to be an effective substrate for PKC-beta, in vitro. When hVDR cDNAs containing single, double, and triple mutations of Ser-51, Ser-119, and Ser-125 were expressed in CV-1 monkey kidney cells, immunoprecipitated and phosphorylated by PKC-beta, in vitro, the mutation of Ser-51 selectively abolished phosphorylation. Furthermore, when transfected CV-1 cells were treated with phorbol 12-myristate 13-acetate, a PKC activator, phosphorylation of wild-type hVDR was enhanced, whereas that of the Ser-51 mutant hVDR was unaffected. Therefore, Ser-51 is the site of hVDR phosphorylation by PKC, both in vitro and in vivo. To evaluate the functional role of Ser-51 and its potential phosphorylation, hVDR-mediated transcription was tested using cotransfection with expression plasmids and a reporter gene that contained a vitamin D response element. Mutation of Ser-51 markedly inhibited transcriptional activation by the vitamin D hormone, suggesting that phosphorylation of Ser-51 by PKC could play a significant role in vitamin D-dependent transcriptional activation. Therefore, the present results link the PKC signal transduction pathway of growth regulation and tumor promotion to the phosphorylation and function of VDR.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker A. R., McDonnell D. P., Hughes M., Crisp T. M., Mangelsdorf D. J., Haussler M. R., Pike J. W., Shine J., O'Malley B. W. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci U S A. 1988 May;85(10):3294–3298. doi: 10.1073/pnas.85.10.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Brown T. A., DeLuca H. F. Phosphorylation of the 1,25-dihydroxyvitamin D3 receptor. A primary event in 1,25-dihydroxyvitamin D3 action. J Biol Chem. 1990 Jun 15;265(17):10025–10029. [PubMed] [Google Scholar]
- Burmester J. K., Wiese R. J., Maeda N., DeLuca H. F. Structure and regulation of the rat 1,25-dihydroxyvitamin D3 receptor. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9499–9502. doi: 10.1073/pnas.85.24.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J. R., Johnson T. R., Dollard C., Shuster J. R., Denis C. L. Cyclic AMP-dependent protein kinase phosphorylates and inactivates the yeast transcriptional activator ADR1. Cell. 1989 Feb 10;56(3):409–419. doi: 10.1016/0092-8674(89)90244-4. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallis B., Lewis A., Wignall J., Alpert A., Mochizuki D. Y., Cosman D., Hopp T., Urdal D. Phosphorylation of the human interleukin-2 receptor and a synthetic peptide identical to its C-terminal, cytoplasmic domain. J Biol Chem. 1986 Apr 15;261(11):5075–5080. [PubMed] [Google Scholar]
- Goldberg Y., Glineur C., Gesquière J. C., Ricouart A., Sap J., Vennström B., Ghysdael J. Activation of protein kinase C or cAMP-dependent protein kinase increases phosphorylation of the c-erbA-encoded thyroid hormone receptor and of the v-erbA-encoded protein. EMBO J. 1988 Aug;7(8):2425–2433. doi: 10.1002/j.1460-2075.1988.tb03088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G. A., Montminy M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989 Nov 17;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler M. R., Mangelsdorf D. J., Komm B. S., Terpening C. M., Yamaoka K., Allegretto E. A., Baker A. R., Shine J., McDonnell D. P., Hughes M. Molecular biology of the vitamin D hormone. Recent Prog Horm Res. 1988;44:263–305. doi: 10.1016/b978-0-12-571144-9.50013-2. [DOI] [PubMed] [Google Scholar]
- House C., Wettenhall R. E., Kemp B. E. The influence of basic residues on the substrate specificity of protein kinase C. J Biol Chem. 1987 Jan 15;262(2):772–777. [PubMed] [Google Scholar]
- Jeng A. Y., Sharkey N. A., Blumberg P. M. Purification of stable protein kinase C from mouse brain cytosol by specific ligand elution using fast protein liquid chromatography. Cancer Res. 1986 Apr;46(4 Pt 2):1966–1971. [PubMed] [Google Scholar]
- Jones B. B., Jurutka P. W., Haussler C. A., Haussler M. R., Whitfield G. K. Vitamin D receptor phosphorylation in transfected ROS 17/2.8 cells is localized to the N-terminal region of the hormone-binding domain. Mol Endocrinol. 1991 Aug;5(8):1137–1146. doi: 10.1210/mend-5-8-1137. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990 Sep;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Kerner S. A., Scott R. A., Pike J. W. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4455–4459. doi: 10.1073/pnas.86.12.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Kishimoto A., Nishizuka Y. The protein kinase C family: heterogeneity and its implications. Annu Rev Biochem. 1989;58:31–44. doi: 10.1146/annurev.bi.58.070189.000335. [DOI] [PubMed] [Google Scholar]
- Kitamura S., Ando S., Shibata M., Tanabe K., Sato C., Inagaki M. Protein kinase C phosphorylation of desmin at four serine residues within the non-alpha-helical head domain. J Biol Chem. 1989 Apr 5;264(10):5674–5678. [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Logeat F., Le Cunff M., Pamphile R., Milgrom E. The nuclear-bound form of the progesterone receptor is generated through a hormone-dependent phosphorylation. Biochem Biophys Res Commun. 1985 Aug 30;131(1):421–427. doi: 10.1016/0006-291x(85)91819-4. [DOI] [PubMed] [Google Scholar]
- Migliaccio A., Rotondi A., Auricchio F. Calmodulin-stimulated phosphorylation of 17 beta-estradiol receptor on tyrosine. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5921–5925. doi: 10.1073/pnas.81.19.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid L. M., Okazaki T., Karolak L. A., Hannun Y. A. Transcriptional regulation of protein kinase C by 1,25-dihydroxyvitamin D3 in HL-60 cells. J Biol Chem. 1990 Feb 5;265(4):2370–2374. [PubMed] [Google Scholar]
- Pike J. W., Marion S. L., Donaldson C. A., Haussler M. R. Serum and monoclonal antibodies against the chick intestinal receptor for 1,25-dihydroxyvitamin D3. Generation by a preparation enriched in a 64,000-dalton protein. J Biol Chem. 1983 Jan 25;258(2):1289–1296. [PubMed] [Google Scholar]
- Pike J. W., Sleator N. M. Hormone-dependent phosphorylation of the 1,25-dihydroxyvitamin D3 receptor in mouse fibroblasts. Biochem Biophys Res Commun. 1985 Aug 30;131(1):378–385. doi: 10.1016/0006-291x(85)91813-3. [DOI] [PubMed] [Google Scholar]
- Safran A., Provenzano C., Sagi-Eisenberg R., Fuchs S. Phosphorylation of membrane-bound acetylcholine receptor by protein kinase C: characterization and subunit specificity. Biochemistry. 1990 Jul 17;29(28):6730–6734. doi: 10.1021/bi00480a024. [DOI] [PubMed] [Google Scholar]
- Samuels D. S., Shimizu Y., Shimizu N. Protein kinase C phosphorylates DNA topoisomerase I. FEBS Lett. 1989 Dec 18;259(1):57–60. doi: 10.1016/0014-5793(89)81493-0. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Shimizu N. Cell genetic evidence of correlation of intracellular translocation of protein kinase C (PKC) and PKC-mediated phosphorylation of 80-kDa protein with mitogenic action of tumor promoters. Somat Cell Mol Genet. 1989 Jul;15(4):321–329. doi: 10.1007/BF01534971. [DOI] [PubMed] [Google Scholar]
- Singh V. B., Moudgil V. K. Phosphorylation of rat liver glucocorticoid receptor. J Biol Chem. 1985 Mar 25;260(6):3684–3690. [PubMed] [Google Scholar]
- Terpening C. M., Haussler C. A., Jurutka P. W., Galligan M. A., Komm B. S., Haussler M. R. The vitamin D-responsive element in the rat bone Gla protein gene is an imperfect direct repeat that cooperates with other cis-elements in 1,25-dihydroxyvitamin D3- mediated transcriptional activation. Mol Endocrinol. 1991 Mar;5(3):373–385. doi: 10.1210/mend-5-3-373. [DOI] [PubMed] [Google Scholar]