Abstract
Human chorionic gonadotropin (hCG) is necessary for the maintenance of early pregnancy and promotes normal breast cell differentiation. Administered hCG reduces risk of carcinogen-induced breast cancer in animal models, and higher circulating hCG concentrations were associated with significantly lower long-term risk of breast cancer in a prior nested case-control study. In this study, we investigated early pregnancy hCG concentrations and subsequent breast cancer risk.
We conducted a nested case-control study with 1,191 cases and 2,257 controls (matched on age and date at blood collection) in the Finnish Maternity Cohort (FMC), a cohort with serum samples from 98% of pregnancies registered in Finland since 1983. This study included women with a serum sample collected early (<140 days gestation) in their first pregnancy resulting in a live, term birth. Breast cancer cases were identified via the Finnish Cancer Registry. Age at breast cancer diagnosis ranged from 22–58 years (mean: 41 years). hCG was measured using a solidphase competitive chemiluminescence assay. Odds ratios (ORs) were calculated using conditional logistic regression.
We observed no association between hCG and breast cancer risk, overall (Quartile 4 vs. 1, OR: 1.14 95% confidence interval [0.94–1.39]), by estrogen and progesterone receptor status, or by ages at first term birth or diagnosis. Associations did not differ by time between pregnancy and diagnosis (e.g., <5 years, ORQ4 vs. Q1: 1.10 [0.64–1.89]; ≥ 15 years, ORQ4 vs. Q1: 1.36 [0.86–2.13]; pheterogeneity=0.62).
This large prospective study does not support an inverse relationship between early pregnancy serum hCG concentrations and breast cancer risk.
Keywords: pregnancy, parity, human chorionic gonadotropin, breast cancer
Introduction
A full-term pregnancy before age 25 provides a well-established long-term protective effect against hormone receptor-positive breast cancer, following a transient increase in risk that is evident in the years immediately following pregnancy (1). Human chorionic gonadotropin (hCG), a key hormone in early pregnancy, has been explored as a potential biologic mediator underpinning the long-term protective effect of parity on breast cancer risk (2–5). Circulating hCG concentrations are very low in healthy non-pregnant women, increase rapidly and peak in early pregnancy, during the first 60–90 days of gestation, and then decrease to relatively low concentrations until the end of pregnancy, returning to pre-pregnant concentrations after delivery. In murine models, administered hCG (i.e., mimicking pregnancy) resulted in mammary tissue differentiation similar to that observed after pregnancy (4) and a dose-dependent reduction in the frequency of carcinogen-induced mammary tumors (6–9). hCG is anti-proliferative and pro-apoptotic in breast cancer cells (10–12), and promotes differentiation in normal breast tissue (13).
On this basis, it has been hypothesized that comparatively high circulating concentrations of hCG during human pregnancy may confer greater protection against long-term breast cancer risk. However, given the relative rarity of large maternity cohorts with biospecimens and cancer follow-up data, as required to investigate this hypothesis, circulating early pregnancy hCG and breast cancer risk in the mother has been only minimally explored. Two studies in the Northern Sweden Maternity Cohort (NSMC; n≤242 cases) reported inverse associations between early pregnancy (<20 weeks gestation) serum hCG concentrations and maternal breast cancer risk (2, 3). High serum hCG in a primiparous pregnancy (i.e., first pregnancy resulting in a live or stillborn birth) was associated with significantly lower breast cancer risk among women younger than age 25 at first term birth, or diagnosed at age 40 or older or at least 10 years after first term birth, with suggestive increases in risk among women diagnosed less than 10 years after first term birth (2). However, these previous investigations were relatively small, and, to date, these findings have not been replicated. Further, potential heterogeneity by tumor hormone receptor status (i.e., estrogen (ER) and progesterone (PR) receptors) has not previously been evaluated.
We conducted the first large-scale investigation (n=1,191 cases) on early pregnancy hCG and breast cancer risk, overall and by hormone receptor subtype, in a nested case-control study in the Finnish Maternity Cohort (FMC) to address the hypothesis that higher circulating hCG concentrations in early pregnancy are associated with lower breast cancer risk diagnosed more than 5–10 years post-pregnancy in the mother, and that this association is strongest for ER+/PR+ disease.
Methods
The FMC is a nationwide initiative to store blood samples collected during early pregnancy for research; the cohort includes 98% of all registered pregnancies in Finland since 1983. The FMC (14) and case and control selection for this investigation have been described in detail previously (15, 16). Briefly, early in pregnancy, women enrolled in prenatal care at municipal maternity care centers which provide free prenatal care throughout Finland. Peripheral venous blood samples were collected for routine screening tests in early pregnancy, and remaining serum was stored for research purposes at −25°C, and shipped frozen to a central biorepository located in Oulu, Finland. Follow-up and covariate data were available through linkages with the Finnish Population, Birth, and Cancer Registries. The Finnish Cancer Registry (1952-present) has close to 100% coverage for diagnosed cancers (17). Tumor hormone receptor was not available from the cancer registry, therefore, data were obtained via linkage with hospital pathology records where available (ER and PR status available for 56% of cases).
Women eligible for inclusion in this study had serum sample available from a singleton primiparous pregnancy with term delivery, were age <40 years at time of blood collection, had no prior history of invasive cancer or in situ breast cancer prior to breast cancer diagnosis/selection as a control, and had data available on gestational age (GA) at time of blood collection. Incident invasive breast cancer cases diagnosed between 1988 and 2007 were identified through the Finnish Cancer Registry. Cases were matched to up to 2 controls on age (±6 months) and date (±3 months) at blood collection. The final study population included 1,191 cases and 2,257 controls (1,066 cases with 2 controls; for 125 cases only 1 eligible control was identified). The ethical committee of the National Institute for Health and Welfare, Finland approved the study.
Serum hCG and Estradiol Assays
Serum total hCG was quantified using a solidphase competitive chemiluminescence assay and an Immulite 2000 Siemens analyzer. Serum estradiol concentrations were measured using high performance liquid chromatography-tandem mass spectrometry, performed with an Applied Biosystems API4000 triple stage quadrupole mass spectrometer. Assays were performed at the Department of Clinical Chemistry at Umeå University Sweden. Laboratory personnel were blinded to the case, control, or quality control status of the samples, and case and matched control samples were analyzed in the same laboratory batch. Quality control samples were included in each laboratory batch; mean inter- and intra-batch CVs were ≤9.3% for both hCG and estradiol.
Statistical Analysis
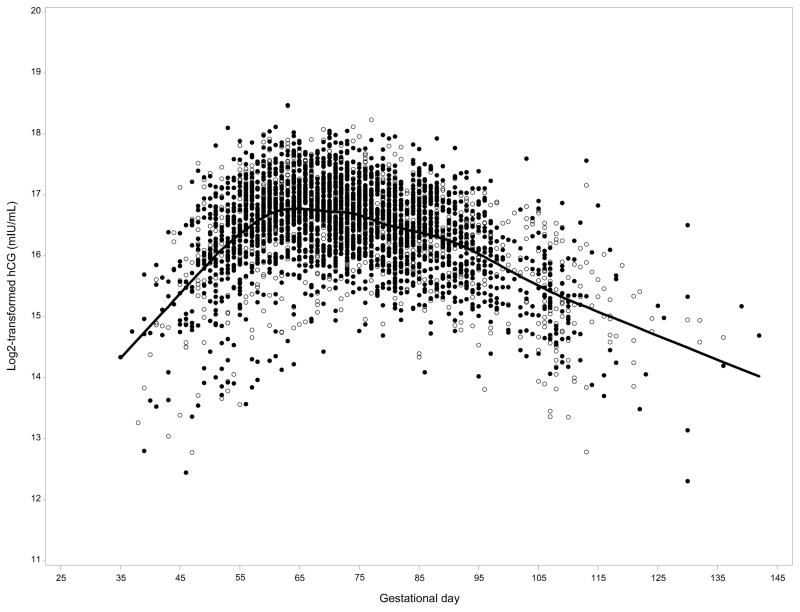
Hormone concentrations were log2 transformed to normalize distributions. Two outliers were identified for hCG (values >3 times the interquartile range), and excluded in sensitivity analyses. GA at blood collection was calculated using the formula: 280-(date of expected delivery minus date of blood draw); 280 days in a 40-week pregnancy. We adjusted for variation in hCG by GA at blood collection by using hCG residuals, as has been done previously (2, 3). The residual (difference) was calculated as: assay value minus gestational day-specific mean value from a local linear regression model. hCG residuals are henceforth referred to as “adjusted hCG”. Results from analyses adjusting for gestational age as a covariate were not meaningfully different from those using adjusted hCG from the residual method. Therefore, we present results from analyses using hCG residuals. We evaluated cross-sectional relationships between maternal and pregnancy-related characteristics and early pregnancy hCG concentrations in controls using generalized linear models. Quartiles were defined using the hCG distribution in controls. We modeled the quartile median concentration to test for trend. Odds ratios (OR) were calculated using conditional logistic regression models. Statistical adjustment for maternal or pregnancy characteristics (e.g. parity at index date, family history of breast cancer, smoking, sex of the neonate) or circulating estradiol changed ORs by less than 5%. Therefore, we present the unadjusted results. We evaluated risk overall, by ER and PR status (ER+/PR+ vs. ER−/PR−), and stratified by age at first term birth (i.e., age at blood collection), age at diagnosis, and time between first term birth and diagnosis. Due to previously observed associations between early pregnancy serum estradiol and breast cancer risk (15), we cross-classified hCG and estradiol concentrations (i.e., hCG/estradiol split at median: low/low, high/low, low/high, high/high). In sensitivity analyses, we restricted the study population to women who were primigravid (i.e., first ever pregnancy) at blood collection (n=794 complete sets) and who provided blood samples between gestational days 60–90 (n=679 complete sets), the period during which hCG peaks and is more stable (Figure 1).
Figure 1.
hCG concentrations by gestational age at blood collection. Solid line represents mean hCG by gestational day and cases are represented by unfilled circles and controls by filled circles: Finnish Maternity Cohort
Heterogeneity (phet) between breast cancer subtypes was assessed using a likelihood ratio test comparing a model assuming the same association between hCG and breast cancer overall (e.g., all hormone receptor subtypes) to one assuming different associations for ER+/PR+ and ER−/PR− disease in a conditional polytomous logistic regression model (18). Interaction (e.g., by age at first term pregnancy) was tested by including a multiplicative interaction term in the models and evaluating the Wald p value. We examined the possibility of non-linearity with restricted cubic splines (19). Tests for non-linearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. We did not observe significant deviation from linearity (p>0.05), with the exception of the association for ER+/PR+ breast cancer among women <30 at first term birth and ≥40 at diagnosis. Given the sample size of 1,191 cases and 2,257 controls, the minimum detectable ORs contrasting extreme quartiles (or for continuous 1-unit increase in hCG) with 80% power and 95% confidence were: overall, 0.74 (0.89); ER+/PR+, 0.57 (0.81); ER−/PR−, 0.40 (0.71); age at first term birth, range: 0.40 (0.71) for <25 years to 0.57 (0.81) for 25–29 years; time between first term birth and diagnosis, range: 0.40 (0.71) for <5years to 0.60 (0.83) for 10–14 years; age at diagnosis, range: 0.31 (0.65) for ≥50 years to 0.65 (0.85) for 40–49 years. The minimum detectable OR for a positive association is the reciprocal of that for inverse association.
Statistical analyses were conducted using SAS 9.3 (Cary, NC). Two-sided p-values <0.05 were considered statistically significant.
Results
Baseline characteristics of the study population have been published previously (15). Briefly, mean age at first birth was 30 years (range: 18–40 years), and the majority were multiparous at diagnosis (64%) or selection as a control (67%). Blood samples were collected at mean 74 days GA in cases and controls (median: 73 days; range: 35–142 days), and mean hCG concentrations were similar in cases and controls (residuals, 0.02 versus 0.01, p=0.83; corresponds to concentrations of 115,717 and 114,962 mIU/mL at median gestational day 73). Cases were diagnosed with breast cancer at mean age 41 years (range: 22–58 years), a mean of 11 years after first pregnancy. In cross-sectional analyses, maternal smoking and male embryo/fetus were associated with lower hCG concentrations (<0.01), whereas maternal age and gravidity did not impact concentrations (Table 1; Supplemental Table 1). Serum hCG concentrations peaked at approximately gestational day 60 and declined thereafter (Figure 1).
Table 1.
Mean* maternal hCG levels by maternal characteristics and child sex in among 2,257 controls: Finnish Maternity Cohort
| Characteristic | N | hCG (mlU/mL) | p |
|---|---|---|---|
| Age at first term birth (years) | 0.08 | ||
| <25 years | 386 | 111,678 | |
| 25–30 years | 694 | 117,132 | |
| 30–35 years | 669 | 118,528 | |
| ≥35 years | 508 | 115,927 | |
| Maternal smoking | <0.01 | ||
| Yes | 292 | 95,301 | |
| No | 1,900 | 119,489 | |
| Gravidity | 0.47 | ||
| Primigravid | 1,606 | 116,641 | |
| Multigravid | 600 | 115,192 | |
| Neonate sex | <0.01 | ||
| Boy | 1,118 | 111,820 | |
| Girl | 1,132 | 120,708 | |
| Time to 2nd birth (years) | 0.55 | ||
| No additional pregnancy | 750 | 114,560 | |
| <2 years | 535 | 116,743 | |
| 2–5 years | 837 | 117,548 | |
| >5 years | 130 | 116,615 |
Calculated using adjusted hCG; mean corresponds to concentrations at gestational day 73
Serum hCG concentration was not associated with breast cancer overall (Quartile 4 vs. 1, OR: 1.14 95% confidence interval [0.94–1.39]) or by hormone receptor subtype (ER+/PR+ vs. ER−/PR−), age at first term birth (<25, 25–29, 30–34, ≥35 years), age at diagnosis (<40, 40–49, ≥50 years), or time between blood collection and diagnosis (<5, 5–9, 10–14, ≥15 years) (Table 2). For example, comparing extreme quartiles, high hCG was associated with an OR of 1.10 [0.64–1.89] among cases diagnosed within 5 years of first term birth (n=143) and an OR of 1.36 [0.86–2.13] for women diagnosed 15 or more years after first term birth (n=254). Higher concentrations of hCG were suggestively associated with increased risk among women <25 years at first term birth (Quartile 4 vs. 1, OR: 1.67 [0.99–2.80]).
Table 2.
Early Pregnancy hCG Concentrations and Breast Cancer Risk: Finnish Maternity Cohort
| Quartiles
|
ptrend | phet | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Overall | ||||||
| Cases/Controls | 282/565 | 317/564 | 272/565 | 320/563 | ||
| OR (95% CI) | ref. | 1.13 (0.92–1.37) | 0.96 (0.78–1.17) | 1.14 (0.94–1.39) | 0.38 | |
| Hormone Receptor Status | 0.31 | |||||
| ER+/PR+ | ||||||
| Cases/Controls | 99/193 | 113/212 | 108/206 | 114/201 | ||
| OR (95% CI) | ref. | 1.04 (0.75–1.45) | 1.01 (0.72–1.42) | 1.12 (0.80–1.57) | 0.54 | |
| ER−/PR− | ||||||
| Cases/Controls | 38/96 | 49/72 | 35/82 | 38/58 | ||
| OR (95% CI) | ref. | 1.74 (1.02–2.98) | 1.07 (0.60–1.88) | 1.62 (0.92–2.83) | 0.21 | |
| Age at first term birth | 0.62 | |||||
| <25 years | ||||||
| Cases/Controls | 41/114 | 55/80 | 52/96 | 47/78 | ||
| OR (95% CI) | ref. | 1.88 (1.14–3.08) | 1.47 (0.89–2.43) | 1.67 (0.99–2.80) | 0.08 | |
| 25–29 years | ||||||
| Cases/Controls | 77/157 | 113/190 | 68/175 | 105/168 | ||
| OR (95% CI) | ref. | 1.19 (0.83–1.70) | 0.76 (0.52–1.13) | 1.25 (0.87–1.79) | 0.55 | |
| 30–34 years | ||||||
| Cases/Controls | 90/166 | 86/151 | 80/158 | 95/183 | ||
| OR (95% CI) | ref. | 1.05 (0.73–1.52) | 0.93 (0.64–1.34) | 0.97 (0.68–1.38) | 0.74 | |
| ≥35 years | ||||||
| Cases/Controls | 72/124 | 62/140 | 71/129 | 72/130 | ||
| OR (95% CI) | ref. | 0.77 (0.51–1.18) | 0.97 (0.64–1.47) | 0.98 (0.65–1.49) | 0.86 | |
| Age at diagnosis | 0.65 | |||||
| <40 years | ||||||
| Cases/Controls | 129/257 | 137/254 | 103/230 | 139/232 | ||
| OR (95% CI) | ref. | 1.06 (0.79–1.43) | 0.89 (0.65–1.22) | 1.19 (0.88–1.61) | 0.42 | |
| 40–49 years | ||||||
| Cases/Controls | 129/270 | 157/264 | 148/292 | 158/293 | ||
| OR (95% CI) | ref. | 1.25 (0.93–1.67) | 1.05 (0.78–1.40) | 1.12 (0.85–1.49) | 0.63 | |
| ≥50 years | ||||||
| Cases/Controls | 24/38 | 23/46 | 21/43 | 23/38 | ||
| OR (95% CI) | ref. | 0.84 (0.41–1.75) | 0.80 (0.38–1.67) | 1.01 (0.47–2.18) | 0.99 | |
| Time between first term birth and diagnosis | 0.62 | |||||
| <5 years | ||||||
| Cases/Controls | 39/73 | 35/62 | 26/66 | 43/74 | ||
| OR (95% CI) | ref. | 1.05 (0.59–1.86) | 0.75 (0.41–1.38) | 1.10 (0.64–1.89) | 0.90 | |
| 5–9 years | ||||||
| Cases/Controls | 98/177 | 93/202 | 86/167 | 93/159 | ||
| OR (95% CI) | ref. | 0.83 (0.59–1.17) | 0.92 (0.64–1.33) | 1.04 (0.73–1.48) | 0.73 | |
| 10–15 years | ||||||
| Cases/Controls | 89/195 | 122/186 | 97/210 | 116/220 | ||
| OR (95% CI) | ref. | 1.47 (1.05–2.07) | 1.00 (0.71–1.43) | 1.15 (0.82–1.62) | 0.81 | |
| ≥15 years | ||||||
| Cases/Controls | 56/120 | 67/114 | 63/122 | 68/110 | ||
| OR (95% CI) | ref. | 1.24 (0.80–1.91) | 1.08 (0.70–1.67) | 1.36 (0.86–2.13) | 0.26 | |
We examined the effect of an 1-unit increase in adjusted hCG concentration and breast cancer risk by ages at first term birth and diagnosis, and time between first term birth and diagnosis, overall and among ER+/PR+ or ER−/PR− cases (Table 3). hCG was not associated with breast cancer risk in any of the examined subgroups, and we observed no heterogeneity between ER+/PR+ and ER−/PR− disease in these analyses (e.g., age at diagnosis <40, ORcont: ER+/PR+: 1.05 [0.76–1.44]; ER−/PR−: 1.19 [0.83–1.70]). Results were similar using alternative cutpoints (i.e., <10 vs. ≥10 years between first term birth and diagnosis; data not shown).
Table 3.
Early Pregnancy hCG Concentrations (residuals and continuous) and Breast Cancer Risk by Age at First Term Birth and Diagnosis, and Time between First Birth and Diagnosis: Finnish Maternity Cohort
| Cases/Controls | OR* | ER+/PR+
|
ER−/PR−
|
phetǂ | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cases/Controls | OR* | phet† | Cases/Controls | OR* | phet† | ||||
| Overall | 1,191/2,257 | 1.01 (0.91–1.12) | 434/812 | 1.09 (0.90–1.34) | 160/308 | 1.09 (0.79–1.50) | 0.31 | ||
| Age at first term birth | 0.52 | 0.84 | |||||||
| < 25 years | 195/368 | 1.24 (0.96 – 1.61) | 45/82 | 1.38 (0.79–2.41) | 37/70 | 1.36 (0.77–2.38) | 0.87 | ||
| 25 – 29 years | 355/651 | 1.01 (0.83 – 1.23) | 128/237 | 0.94 (0.67–1.32) | 60/111 | 1.26 (0.76–2.07) | 0.28 | ||
| 30 – 34 years | 341/619 | 0.92 (0.76 – 1.13) | 144/262 | 0.95 (0.69–1.31) | 35/64 | 0.95 (0.47–1.94) | 0.80 | ||
| ≥ 35 years | 267/494 | 0.95 (0.75 – 1.21) | 102/184 | 0.82 (0.54–1.24) | 25/47 | 0.92 (0.40–2.13) | 0.82 | ||
| Age at diagnosis | 0.41 | 0.80 | |||||||
| < 40 years | 508/973 | 0.96 (0.71–1.29) | 148/280 | 1.05 (0.76 – 1.44) | 96/186 | 1.19 (0.83–1.70) | 0.38 | ||
| 40 – 49 years | 592/1,119 | 0.99 (0.77–1.27) | 244/459 | 1.10 (0.83 – 1.44) | 58/111 | 1.17 (0.68–2.00) | 0.58 | ||
| ≥ 50 years | 91/165 | 1.32 (0.65–2.70) | 42/73 | 1.41 (0.65 – 3.04) | 6/11 | 3.02 (0.17–53.41) | 0.57 | ||
| Time between first term birth and diagnosis | 0.25 | 0.38 | |||||||
| < 5 years | 143/275 | 0.89 (0.66 – 1.20) | 41/76 | 0.82 (0.48–1.41) | 27/52 | 1.31 (0.53–3.26) | 0.38 | ||
| 5 – 9 years | 370/705 | 1.01 (0.84 – 1.22) | 131/247 | 0.94 (0.67–1.31) | 55/108 | 1.04 (0.68–1.60) | 0.70 | ||
| 10 – 14 years | 424/811 | 0.99 (0.83 – 1.18) | 152/292 | 0.94 (0.70–1.27) | 57/109 | 1.32 (0.78–2.23) | 0.27 | ||
| ≥ 15 years | 254/465 | 1.15 (0.89 – 1.49) | 110/197 | 1.34 (0.89–2.03) | 21/39 | 1.54 (0.51–4.62) | 0.82 | ||
per 1-unit increase in hCG residuals
p for heterogeneity across age at blood draw, age at diagnosis, or lag-time categories
p for heterogeneity comparing ER+/PR+ and ER−/PR− subtypes
Overall, results were similar in analyses cross-classifying age at first term birth and age at diagnosis or time between first birth and diagnosis (Table 4). In analyses cross-classifying serum hCG and estradiol concentrations, low hCG in the context of high estradiol was suggestively associated with increased risk of breast cancer diagnosed <5 years after first term birth (hCG/estradiol: low/high vs. low/low, OR: 1.81 [0.98–3.33]), but not in any other subgroup (Table 5). Results were similar in analyses cross-classifying age at diagnosis and time between first birth and diagnosis, restricting the study sample to primigravid women, or to samples collected between 60 and 90 days gestation (data not shown).
Table 4.
Early Pregnancy hCG Concentrations (residuals and continuous) and Breast Cancer Risk: Cross-Classification by Age at First Term Birth and Age at Diagnosis and Time between First Birth and Diagnosis: Finnish Maternity Cohort
| Age at first term birth < 30 years | Age at first term birth ≥ 30 years | phet† | |||||
|---|---|---|---|---|---|---|---|
| Ca/Co | OR | phet* | Ca/Co | OR | phet* | ||
| Age at diagnosis | |||||||
| < 40 years | |||||||
| Overall | 382/734 | 1.05 (0.88–1.26) | 126/239 | 0.97 (0.71–1.32) | 0.68 | ||
| ER+/PR+ | 107/203 | 0.97 (0.69–1.37) | 0.26 | 41/77 | 0.93 (0.52–1.66) | 0.51 | 0.89 |
| ER−/PR− | 76/148 | 1.32 (0.88–1.97) | 20/38 | 0.63 (0.23–1.73) | 0.26 | ||
| ≥ 40 yrs | |||||||
| Overall | 181/342 | 1.12 (0.83–1.51) | 502/942 | 0.95 (0.80–1.13) | 0.39 | ||
| ER+/PR+ | 68/129 | 1.36 (0.83–2.23)ǂ | 0.81 | 218/403 | 0.93 (0.71–1.22) | 0.68 | 0.24 |
| ER−/PR− | 22/42 | 1.56 (0.58–4.16) | 42/80 | 1.08 (0.57–2.05) | 0.79 | ||
| Time between blood collection and diagnosis | |||||||
| < 10 yrs | |||||||
| Overall | 208/398 | 0.96 (0.75–1.22) | 355/678 | 1.15 (0.94–1.40) | 0.79 | ||
| ER+/PR+ | 55/103 | 1.02 (0.61–1.68) | 0.68 | 117/220 | 0.85 (0.60–1.21) | 0.77 | 0.56 |
| ER−/PR− | 45/89 | 1.18 (0.72–1.94) | 37/71 | 0.95 (0.50–1.81) | 0.76 | ||
| ≥ 10 yrs | |||||||
| Overall | 305/582 | 0.99 (0.80–1.22) | 323/599 | 0.92 (0.74–1.14) | 0.15 | ||
| ER+/PR+ | 120/229 | 1.13 (0.80–1.59) | 0.30 | 142/260 | 1.01 (0.72–1.43) | 0.74 | 0.76 |
| ER−/PR− | 53/101 | 1.59 (0.90–2.80) | 25/47 | 0.85 (0.33–2.18) | 0.44 | ||
p for heterogeneity comparing ER+/PR+ and ER−/PR−
p for heterogeneity comparing age at first term birth <30 vs. ≥30 years; no significant heterogeneity by age at diagnosis (p>0.27) or time between first term birth and diagnosis (p>0.26).
Non-linear association (p<0.05). No association observed in spline model.
Table 5.
Early Pregnancy hCG and Estradiol Concentrations and Breast Cancer Risk: Finnish Maternity Cohort
| hCG/Estradiol concentrations
|
||||
|---|---|---|---|---|
| Low/Low | High/Low | Low/High | High/High | |
| Overall | ||||
| Cases/Controls | 350/681 | 230/446 | 248/446 | 361/682 |
| OR (95% CI) | 1.00 | 0.99 (0.81–1.21) | 1.08 (0.88–1.32) | 1.03 (0.86–1.24) |
| Hormone Receptor Status | ||||
| ER+/PR+ | ||||
| Cases/Controls | 125/241 | 95/166 | 88/166 | 125/238 |
| OR (95% CI) | 1.00 | 1.09 (0.78–1.53) | 1.02 (0.72–1.43) | 1.02 (0.74–1.40) |
| ER−/PR− | ||||
| Cases/Controls | 47/101 | 29/49 | 41/65 | 43/93 |
| OR (95% CI) | 1.00 | 1.25 (0.70–2.22) | 1.33 (0.78–2.27) | 0.97 (0.58–1.61) |
| Age at first term birth | ||||
| <25 years | ||||
| Cases/Controls | 53/110 | 24/57 | 46/92 | 77/127 |
| OR (95% CI) | 1.00 | 0.87 (0.50–1.53) | 1.07 (0.66–1.75) | 1.27 (0.81–1.98) |
| 25–29 years | ||||
| Cases/Controls | 105/208 | 61/131 | 80/139 | 116/211 |
| OR (95% CI) | 1.00 | 0.90 (0.61–1.32) | 1.12 (0.78–1.60) | 1.08 (0.78–1.51) |
| 30–34 years | ||||
| Cases/Controls | 104/200 | 78/136 | 72/118 | 97/204 |
| OR (95% CI) | 1.00 | 1.10 (0.77–1.59) | 1.16 (0.80–1.68) | 0.92 (0.65–1.29) |
| ≥35 years | ||||
| Cases/Controls | 88/163 | 67/122 | 50/97 | 71/140 |
| OR (95% CI) | 1.00 | 1.02 (0.68–1.51) | 0.94 (0.61–1.45) | 0.93 (0.63–1.39) |
| Age at diagnosis | ||||
| <40 years | ||||
| Cases/Controls | 148/312 | 86/185 | 118/200 | 156/275 |
| OR (95% CI) | 1.00 | 0.97 (0.71–1.34) | 1.24 (0.91–1.67) | 1.22 (0.91–1.62) |
| 40–49 years | ||||
| Cases/Controls | 169/320 | 128/227 | 114/213 | 179/358 |
| OR (95% CI) | 1.00 | 1.04 (0.78–1.39) | 1.01 (0.75–1.35) | 0.94 (0.72–1.22) |
| ≥50 years | ||||
| Cases/Controls | 33/49 | 16/34 | 16/33 | 26/49 |
| OR (95% CI) | 1.00 | 0.69 (0.32–1.49) | 0.76 (0.37–1.58) | 0.79 (0.40–1.54) |
| Time between first term birth and diagnosis | ||||
| <5 years | ||||
| Cases/Controls | 41/95 | 36/60 | 32/42 | 33/78 |
| OR (95% CI) | 1.00 | 1.39 (0.79–2.42) | 1.81 (0.98–3.33) | 0.99 (0.57–1.69) |
| 5–9 years | ||||
| Cases/Controls | 124/231 | 77/155 | 71/144 | 98/173 |
| OR (95% CI) | 1.00 | 0.90 (0.63–1.28) | 0.89 (0.62–1.28) | 1.05 (0.75–1.47) |
| 10–14 years | ||||
| Cases/Controls | 117/229 | 74/148 | 91/154 | 141/280 |
| OR (95% CI) | 1.00 | 0.97 (0.68–1.38) | 1.15 (0.82–1.63) | 0.97 (0.71–1.31) |
| ≥15 years | ||||
| Cases/Controls | 68/126 | 43/83 | 54/106 | 89/151 |
| OR (95% CI) | 1.00 | 0.94 (0.58–1.52) | 1.00 (0.65–1.53) | 1.13 (0.76–1.69) |
Discussion
This large, prospective investigation does not support an important role for early pregnancy hCG concentrations in the etiology of breast cancer.
The long-term protective effect of an early pregnancy (i.e., <25 years at first birth) on risk of ER+/PR+ breast cancer is well established (1). Pregnancy results in differentiation of the breast terminal ductal lobular unit (TDLU) (20) and gene expression profiling studies have identified pregnancy-related “signatures” in the normal breast tissue of parous pre- (21, 22) and postmenopausal (22–24) women, underscoring the long-term molecular changes in breast tissue decades after pregnancy. hCG administration in virgin rats promotes mammary differentiation and alters mammary gland gene expression (4), and shields the mammary gland from carcinogenic transformation before or after DMBA administration (6–9). This effect is dose-dependent (8, 9), leading to the hypothesis that relatively high concentrations of hCG in early pregnancy would decrease long-term breast cancer risk in women.
Epidemiologic data on hCG and breast cancer risk are sparse (2, 3, 25). A retrospective case-control study reported reduced breast cancer risk among women administered hCG for weight control or fertility treatments, however, this association was limited to nulliparous women with BMI ≤27.4 kg/m2 (ever vs. never hCG use, OR: 0.30 [0.10–0.96]) (25). Two studies, both in the NSMC, have assessed hCG in pregnancy and breast cancer risk. The first investigation included both primiparous and multiparous (i.e., births prior to the index pregnancy) women (3), whereas the second was restricted to primiparous women (2), as in the current study. Both investigations used hCG residuals to account for gestational age at blood collection; we utilized the same approach in this investigation. In the initial investigation, Lukanova et al. (3) observed suggestive associations between hCG and breast cancer risk among women diagnosed 14 or more years after the index pregnancy (n=91 cases; 3rd vs. 1st tertile OR: 0.53 [0.27–1.03]), however, no statistically significant associations were observed. In contrast, Toniolo et al. (3) observed a significant inverse association between circulating hCG and breast cancer risk in the investigation limited to primiparous women (n=242 cases; 3rd vs. 1st tertile OR: 0.67 [0.46–0.99]). The inverse association was strongest among women <25 years at first birth (3rd vs. 1st tertile OR: 0.41 [0.21–0.80]), and observed among women 40 or older at diagnosis or with 10 or more years between pregnancy and diagnosis. We examined risk in the same subgroups as published previously, and observed no significant association between hCG and breast cancer, overall or in any subgroup.
We have previously shown high early pregnancy estradiol concentrations to be inversely associated with breast cancer diagnosed at age 40 or older, and positively associated with ER−/PR− disease among women diagnosed before age 40, in this study population (15). Therefore, we controlled for and stratified by circulating estradiol concentrations in this investigation. Results from these analyses were consistent with the overall results, with the exception of a suggestive positive association among women with low hCG and high estradiol (concentrations below/above median relative to both below median), and diagnosed within 5 years of first term birth. It could be speculated that early pregnancy estradiol increases breast cancer risk in the short-term after pregnancy only in the context of less complete differentiation (i.e., low hCG). However, these results should be interpreted with caution as the association did not reach statistical significance, and there were a limited number of cases in this subgroup (n=142).
Both the NSMC and FMC investigations used samples from well-established biorepositories with similar storage conditions (NSMC: −20°C; FMC: −25°C). Further, the same laboratory conducted the hCG assays for both studies. Considering the NSMC study most similar to the current study, restricted to primiparous women (2), blood samples were collected at similar gestational ages (means, NSMC: 70 days; FMC: 74 days), and both studies used the same matching factors. The inverse relationship between smoking and hCG observed in this study is consistent with a prior investigation in the NSMC (26). hCG concentrations at mean GA at blood collection were somewhat lower in the NSMC (geometric means, among controls, NSMC: 97,416 mIU/mL at 70 days GA vs. FMC: 114,962 mIU/mL at 73 days GA). Further, relative to women in the FMC, women in the NSMC were younger at first term birth (means, NSMC vs. FMC: 27 v. 30 years), cases were older at diagnosis (means, NSMC vs. FMC: 46 vs. 41 years), and a longer time elapsed between age at first birth and diagnosis (means, NSMC vs. FMC: 19 vs. 11 years). However, hCG concentrations were not correlated with storage time in either investigation (current study, Spearman r=0.06; previous study r≤0.07). Finally, women in the FMC had lower parity at diagnosis/selection as control (e.g., in controls, NSMC vs. FMC: 19% vs. 33% with parity of 1). Despite these differences in the study populations, given the size of the current study, we were able to evaluate risk in all subgroups investigated in the NSMC investigation.
Our study has important strengths and limitations. We conducted the largest study to date on early pregnancy hCG and maternal breast cancer risk in a nested case-control study using prospectively collected serum samples from a population with extensive, registry-based follow-up. A limitation of our study is incomplete tumor hormone receptor status, as a total of 56% of cases had ER and PR data available. Therefore, analyses in some subgroups, particularly for ER−/PR− cases, were limited by sample size. Nonetheless, we provide the first data on early pregnancy hCG and breast cancer risk by hormone receptor status. We evaluated hCG in a single blood sample collected during the first 20 weeks of a primiparous pregnancy. The extent to which a single hCG measure more broadly represents early pregnancy exposure is not known. The current study included breast cancer cases diagnosed between ages 22 and 58 years. Therefore, our results may not be generalizable to women diagnosed at older ages. Finally, consistent with the comparable NSMC study (2), we measured total hCG, a composite of intact and free hCG subforms. Total, free β-hCG, and the ratio of total to free β-hCG were similarly associated with risk in a small (n=159 cases), subsequent investigation among a subset of the cases in the NSMC (27) suggesting no meaningful difference by isoform.
We investigated early pregnancy hCG and risk of breast cancer, given strong experimental evidence linking higher doses of hCG to lower incidence of mammary tumors in virgin animal models (i.e. “nulliparous”). Our investigation does not address whether hCG mediates the protective effect of pregnancy on breast cancer risk in women, given that our study population was parous by design. However, in contrast to the experimental literature, and limited epidemiologic data, our large investigation, with more than quadruple the case numbers of prior studies, provides no evidence supporting an association between comparatively high early pregnancy hCG and subsequent breast cancer risk in the mother.
Supplementary Material
Acknowledgments
Funding
This work was supported in part by the National Cancer Institute at the National Institutes of Health (CA114329). RT Fortner was supported by a Marie Curie International Incoming Fellowship of the European Commission’s Seventh Framework Programme (MC-IIF-623984).
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.Schedin P. Pregnancy-associated breast cancer and metastasis. Nature Reviews Cancer. 2006;6:281–91. doi: 10.1038/nrc1839. [DOI] [PubMed] [Google Scholar]
- 2.Toniolo P, Grankvist K, Wulff M, Chen T, Johansson R, Schock H, et al. Human Chorionic Gonadotropin in Pregnancy and Maternal Risk of Breast Cancer. Cancer Research. 2010;70:6779–86. doi: 10.1158/0008-5472.CAN-09-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lukanova A, Andersson R, Wulff M, Zeleniuch-Jacquotte A, Grankvist K, Dossus L, et al. Human chorionic gonadotropin and alpha-fetoprotein concentrations in pregnancy and maternal risk of breast cancer: a nested case-control study. American Journal of Epidemiology. 2008;168:1284–91. doi: 10.1093/aje/kwn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santucci-Pereira J, George C, Armiss D, Russo IH, Vanegas JE, Sheriff F, et al. Mimicking pregnancy as a strategy for breast cancer prevention. Breast cancer management. 2013;2:283–94. doi: 10.2217/bmt.13.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sivaraman L, Medina D. Hormone-induced protection against breast cancer. Journal of mammary gland biology and neoplasia. 2002;7:77–92. doi: 10.1023/a:1015774524076. [DOI] [PubMed] [Google Scholar]
- 6.Russo IH, Koszalka M, Gimotty PA, Russo J. Protective effect of chorionic gonadotropin on DMBA-induced mammary carcinogenesis. Br J Cancer. 1990;62:243–7. doi: 10.1038/bjc.1990.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo IH, Koszalka M, Russo J. Effect of human chorionic gonadotropin on mammary gland differentiation and carcinogenesis. Carcinogenesis. 1990;11:1849–55. doi: 10.1093/carcin/11.10.1849. [DOI] [PubMed] [Google Scholar]
- 8.Russo IH, Koszalka M, Russo J. Human chorionic gonadotropin and rat mammary cancer prevention. J Natl Cancer Inst. 1990;82:1286–9. doi: 10.1093/jnci/82.15.1286. [DOI] [PubMed] [Google Scholar]
- 9.Yuri T, Lai YC, Yoshizawa K, Tsubura A. Human chorionic gonadotropin inhibits N-Methyl-N-nitrosourea-induced mammary carcinoma growth in female Lewis rats. In vivo. 2012;26:361–7. [PubMed] [Google Scholar]
- 10.Liao XH, Wang Y, Wang N, Yan TB, Xing WJ, Zheng L, et al. Human chorionic gonadotropin decreases human breast cancer cell proliferation and promotes differentiation. IUBMB Life. 2014;66:352–60. doi: 10.1002/iub.1269. [DOI] [PubMed] [Google Scholar]
- 11.Lopez D, Sekharam M, Coppola D, Carter WB. Purified human chorionic gonadotropin induces apoptosis in breast cancer. Molecular cancer therapeutics. 2008;7:2837–44. doi: 10.1158/1535-7163.MCT-08-0339. [DOI] [PubMed] [Google Scholar]
- 12.Yuri T, Kinoshita Y, Emoto Y, Yoshizawa K, Tsubura A. Human chorionic gonadotropin suppresses human breast cancer cell growth directly via p53-mediated mitochondrial apoptotic pathway and indirectly via ovarian steroid secretion. Anticancer Res. 2014;34:1347–54. [PubMed] [Google Scholar]
- 13.Janssens JP, Russo J, Russo I, Michiels L, Donders G, Verjans M, et al. Human chorionic gonadotropin (hCG) and prevention of breast cancer. Mol Cell Endocrinol. 2007;269:93–8. doi: 10.1016/j.mce.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Lehtinen M, Koskela P, Ogmundsdottir HM, Bloigu A, Dillner J, Gudnadottir M, et al. Maternal herpesvirus infections and risk of acute lymphoblastic leukemia in the offspring. Am J Epidemiol. 2003;158:207–13. doi: 10.1093/aje/kwg137. [DOI] [PubMed] [Google Scholar]
- 15.Fortner RT, Schock H, Kaaks R, Lehtinen M, Pukkala E, Lakso HA, et al. Early pregnancy sex steroids and maternal breast cancer: a nested case-control study. Cancer Res. 2014;74:6958–67. doi: 10.1158/0008-5472.CAN-14-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukanova A, Surcel H-M, Lundin E, Kaasila M, Lakso H-A, Schock H, et al. Circulating estrogens and progesterone during primiparous pregnancies and risk of maternal breast cancer. International Journal of Cancer. 2012;130:910–20. doi: 10.1002/ijc.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry. Experience in Finland. Acta Oncologica. 1994;33:365–9. doi: 10.3109/02841869409098430. [DOI] [PubMed] [Google Scholar]
- 18.Wang M, Spiegelman D, Kuchiba A, Lochhead P, Kim S, Chan AT, et al. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35:782–800. doi: 10.1002/sim.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durrleman S, Simon R. Flexible regression models with cubic splines. Statistics in Medicine. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 20.Russo J, Mailo D, Hu Y-F, Balogh G, Sheriff F, Russo IH. Breast differentiation and its implication in cancer prevention. Clinical Cancer Research. 2005;11:931s–6s. [PubMed] [Google Scholar]
- 21.Asztalos S, Gann PH, Hayes MK, Nonn L, Beam CA, Dai Y, et al. Gene Expression Patterns in the Human Breast after Pregnancy. Cancer Prevention Research. 2010;3:301–11. doi: 10.1158/1940-6207.CAPR-09-0069. [DOI] [PubMed] [Google Scholar]
- 22.Rotunno M, Sun X, Figueroa J, Sherman ME, Garcia-Closas M, Meltzer P, et al. Parity-related molecular signatures and breast cancer subtypes by estrogen receptor status. Breast Cancer Research. 2014;16:R74. doi: 10.1186/bcr3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belitskaya-Levy I, Zeleniuch-Jacquotte A, Russo J, Russo IH, Bordas P, Ahman J, et al. Characterization of a Genomic Signature of Pregnancy Identified in the Breast. Cancer Prevention Research. 2011;4:1457–64. doi: 10.1158/1940-6207.CAPR-11-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peri S, de Cicco RLp, Santucci-Pereira J, Slifker M, Ross EA, Russo IH, et al. Defining the genomic signature of the parous breast. BMC Medical Genomics. 2012;5:1. doi: 10.1186/1755-8794-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernstein L, Hanisch R, Sullivan-Halley J, Ross RK. Treatment with human chorionic gonadotropin and risk of breast cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1995;4:437–40. [PubMed] [Google Scholar]
- 26.Chen T, Lundin E, Grankvist K, Zeleniuch-Jacquotte A, Wulff M, Afanasyeva Y, et al. Maternal hormones during early pregnancy: a cross-sectional study. Cancer causes & control : CCC. 2010;21:719–27. doi: 10.1007/s10552-009-9500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toriola AT, Tolockiene E, Schock H, Surcel HM, Zeleniuch-Jacquotte A, Wadell G, et al. Free beta-human chorionic gonadotropin, total human chorionic gonadotropin and maternal risk of breast cancer. Future oncology. 2014;10:377–84. doi: 10.2217/fon.13.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.