ABSTRACT
We have previously demonstrated that nestin-expressing hair follicle-associated-pluripotent (HAP) stem cells are located in the bulge area. HAP stem cells have been previously shown to differentiate to neurons, glial cells, keratinocytes, smooth-muscle cells, melanocytes and cardiac-muscle cells in vitro. Subsequently, we demonstrated that HAP stem cells could effect nerve and spinal cord regeneration in mouse models, differentiating to Schwann cells and neurons. In previous studies, we established an efficient protocol for the differentiation of cardiac-muscle cells from mouse HAP stem cells. In the present study, we isolated the upper part of human hair follicles containing human HAP (hHAP) stem cells. The upper parts of human hair follicles were suspended in DMEM containing 10% FBS where they differentiated to cardiac-muscle cells as well as neurons, glial cells, keratinocytes and smooth-muscle cells. This method is appropriate for future use with human hair follicles to produce hHAP stem cells in sufficient quantities for future heart, nerve and spinal cord regeneration in the clinic.
KEYWORDS: cardiac-muscle cell, differentiation, Hair follicle, stem cell
Introduction
Stem cells have great promise for regenerative medicine. Embryonic stem (ES) cells can in principle differentiate to any cell type, but ES cells can form teratomas.1 Induced pluripotent stem cells (iPS) cells, can be established from adult cells via introduction of differentiation-related genes,2,3 but iPS cells can also form tumors.4
Our laboratory discovered hair-follicle-associated pluripotent (HAP) stem cells, located in the bulge area,5 HAP stem cells from mouse expressed nestin and could differentiate to neurons, glia, keratinocytes, smooth-muscle cells, and melanocytes in vitro.6,7 HAP stem cells from mouse could effect nerve repair8,9 and spinal cord regeneration10 in mouse models. We then demonstrated that mouse HAP stem cells differentiate to beating cardiac-muscle cells.11
Isoproterenol stimulated mouse HAP stem cells to differentiate to cardiac-muscle cells in large numbers in culture. The addition of activin A, bone morphogenetic protein 4, and basic fibroblast growth factor, along with isoproternal, stimulated the cardiac-muscle cells to form tissue sheets of beating heart muscle cells.12
Yoshida et al4 showed that hypoxia increased the generation of iPS cells. We also reported that, under hypoxia conditions, mouse HAP stem cells differentiated to troponin-positive cardiac-muscle cells at a higher rate that under normoxia conditions. Hypoxia did not influence the differentiation to other cell types.13 Aging decreased the potential of mouse HAP stem cells to differentiate to cardiac-muscle cells.
We previously showed that human HAP (hHAP) stem cells can also differentiate into neurons, glia, keratinocytes, smooth-muscle cells, and melanocytes in vitro. hHAP stem cells were transplanted in the severed sciatic nerve of the mouse where they differentiated into glial fibrillary-acidic-protein (GFAP)-positive Schwann cells and promoted the recovery of pre-existing axons, leading to nerve generation. The regenerated nerve recovered function and, upon electrical stimulation, contracted the gastrocnemius muscle. hHAP stem cells can be readily isolated from the human scalp, thereby providing an accessible, autologous source of stem cells.9
Yu et al.14 also observed hHAP stem cells the bulge area of human hair follicles. hHAP stem cells gave rise to myogenic, melanocytic, and neuronal cell lineages after in vitro clonal single-cell culture. Neuronal differentiation of hHAP stem cells induced increased expression of neuron-associated genes. The differentiated neuronal cells persisted in mouse brain and retained neuronal differentiation markers.15
In the present study, we demonstrate that hHAP stem cells can differentiate to cardiac-muscle cells as well as neurons, glial cells, keratinocytes and smooth-muscle cells.
Results and discussion
hHAP stem cells from the upper part of hair follicle can differentiate to cardiac-muscle and multiple type of cells
The upper parts of human hair follicles were isolated and cultured in DMEM containing 10% FBS (Fig. 1). After culture, hHAP stem cells differentiated to troponin-positive cardiac-muscle cells, nestin- and βIII-tubulin-positive neurons, GFAP-positive glial cells, K15-positive keratinocytes and smooth-muscle actin (SMA)-positive smooth-muscle cells (Fig. 2, Table 1).
Figure 1.
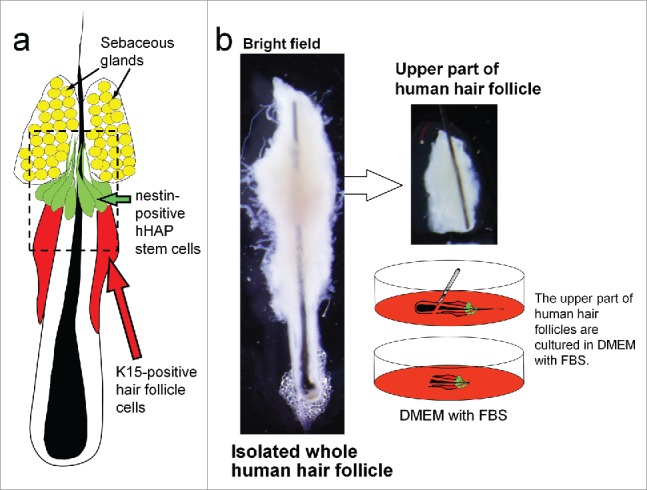
Isolated human hair follicle and culture of the upper follicle. a. Schema of a human scalp hair follicle shows the location of nestin-positive hHAP stem cells. b. The upper parts of human scalp hair follicles were isolated and cultured in DMEM containing 10% FBS.
Figure 2.
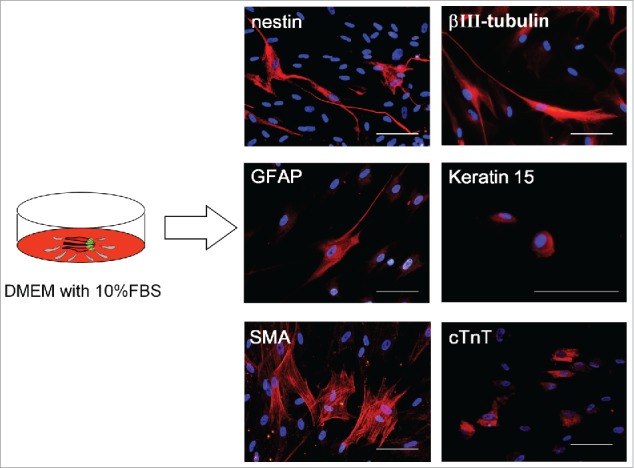
Differentiation of hHAP stem cells. Four weeks after culture in DMEM containing 10% FBS, the upper part of hair follicles differentiated to troponin (cTnT)-positive cardiac-muscle cells, nestin- and βIII-tubulin-positive neurons, GFAP-positive glial cells, K15-positive keratinocytes and smooth-muscle actin (SMA)-positive smooth-muscle cells. Scale bar = 100 µm.
Table 1.
FACS analysis of cells differentiated from the upper part of the hair follicle and hHAP stem-cell colonies.
| Four weeks after culture of the upper part of human hair follicle | Two weeks after culture of hHAP stem-cell colonies | |
|---|---|---|
| Cardiac-muscle cells | 0.4 ± 0.3 % | 0.3 ± 0.2 % |
| Neurons | 39.2 ± 7.3 % | 75.7 ± 13.7 % |
| Glial cells | 34.9 ± 3.8 % | 9.5 ± 4.5 % |
| Keratinocytes | 3.4 ± 1.5 % | 4.5 ± 5.1 % |
| Smooth-muscle cells | 13.9 ± 4.0 % | 6.6 ± 5.6 % |
Upper parts of human hair follicles form hHAP stem cell colonies
Upper parts of human hair follicles were cultured in DMEM containing 10% FBS. Four weeks after culture, the growing cells from the upper parts of the human hair follicles were transferred to DMEM/F12 medium without FBS. One week after culture, the growing cells formed many hHAP stem-cell colonies (Fig. 3a). The hHAP stem-cell colonies were SSEA1-negative and SSEA3-, SSEA4-, Nanog-, Oct3/4-, and nestin-positive (Fig. 4A).
Figure 3.
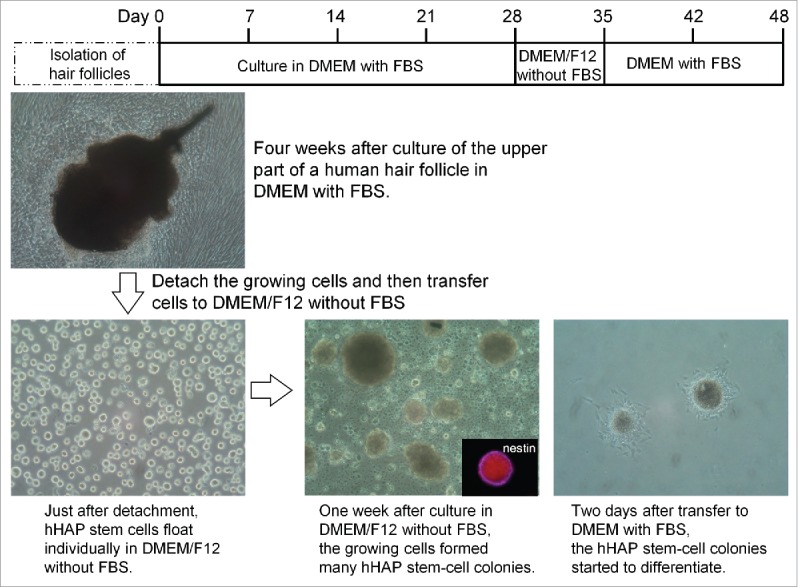
Production of hHAP stem-cell colonies. Human hair follicle culture protocol: isolated upper parts of human hair follicles were cultured in DMEM containing 10% FBS. Four weeks after culture growing cells from the upper parts of human hair follicle were transferred to DMEM/F12 without FBS. One week after culture, the growing cells formed many hHAP stem-cell colonies. Two days after transfer to DMEM containing 10% FBS, hHAP stem-cell colonies started to differentiate.
hHAP stem cell colonies from the upper part of human hair follicles are capable of differentiating into cardiac-muscle and multiple type of cells
The hHAP stem cell colonies were switched to DMEM containing 10% FBS from DMEM/F12 containing B-27, supplemented with bFGF every 2 d. Two days after switching to DMEM containing 10% FBS, differentiating cells migrated away from the nestin-expressing hHAP stem cell colonies (Fig. 3a). The hHAP stem cell colonies differentiated to troponin-positive cardiac-muscle cells, nestin- and βIII-tubulin-positive neurons, GFAP-positive glial cells, K15-positive keratinocytes and SMA-positive smooth-muscle cells (Fig. 4B, Table 1).
Figure 4.
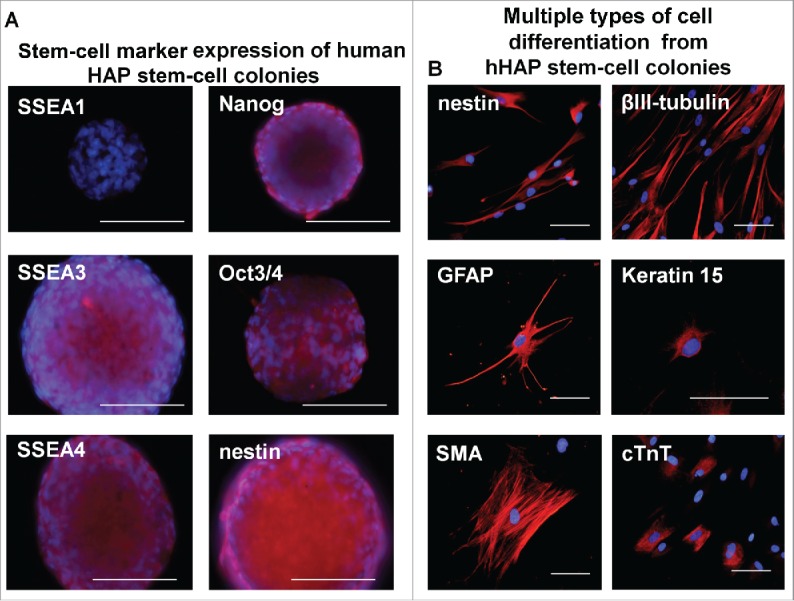
Stem-cell marker expression in hHAP stem-cell colonies and cells differentiated from them. (A) Stem-cell marker expression in hHAP stem-cells colonies. B) Two weeks after transfer to DMEM containing 10% FBS, the nestin-expressing hHAP stem-cell colonies differentiated to troponin (cTnT)-positive cardiac-muscle cells, nestin and βIII-tubulin-positive neurons, GFAP-positive glial cells, K15-positive keratinocytes and smooth-muscle actin (SMA)-positive smooth-muscle cells. Scale bar = 100 μm.
Our new method described here is appropriate for future use with human hair follicles to produce hHAP stem cells in sufficient quantities for regenerative medicine for heart, nerve and spinal-cord disease and injury.
The present study, along with our previous studies of HAP stem cells,5-13,16,17 demonstrate their superiority over ES and iPS cells for regenerative medicine due to HAP stem cells facile accessibility from any patient, their lack of tumorigenicity, lack of the need to insert foreign genes and lack of ethical problems. It is expected in the near future that hHAP stem cells will be the predominant stem cells used for regenerative medicine.
Materials and methods
Isolation and culture of hHAP stem cells
For isolation of hHAP stem cells, specimens were obtained from surgical specimens of normal human scalp from 5 patients, 3 males and 2 females, ranging from 42 to 63 years old (means: 49.8 ± 8.7 years). The surgical specimens of normal human scalp were taken from patients without systemic disease, who had given informed consent at the Kitasato University, School of Medicine. All the experiments were performed according to the Declaration of Helsinki guidelines, in compliance with national regulations for the experimental use of human material.
To isolate whole hair follicles, the scalp hair follicle pad was cut and its inner surface was exposed. The scalp hair follicles were dissected under a binocular microscope and split off from the pad using a surgical knife. The scalp specimen size was approximately 0.5 × 0.5 × 0.5 cm and 80 ± 26 whole hair follicles were isolated per patient. All procedures were performed under sterile conditions.
Efficient generation of hHAP stem cells from the upper part of human hair follicles
In order to induce differentiation, the upper part of hair follicles were suspended in fresh DMEM (Sigma-Aldrich, St. Louis, MO) containing 10% fetal bovine serum (FBS), 50 µg/ml gentamycin (GIBCO, Grand Island, NY), 2 mM L-glutamine (GIBCO) and 10 mM HEPES (MP Biomedicals, Santa Ana, CA) in 6-well flat-bottom cell-culture plates (Corning, Kennebunk, ME). Four weeks after culture of the upper parts of human hair follicles in DMEM with FBS, the growing cells were treated enzymatically with Accumax (Innovative Cell Technologies, Inc., San Diego, CA) to detach. The detached cells were transferred to non-adhesive culture dishes with DMEM/F12 (GIBCO) containing 2% B-27 (GIBCO), 5 ng/ml basic fibroblast growth factor (bFGF) (Millipore, Temecula, CA) without FBS. One week after culture in DMEM/F12 medium without FBS, the growing cells formed many hHAP stem-cell colonies. Two days after transfer to DMEM with FBS, the hHAP stem-cell colonies started to differentiate. Two weeks after switching to DMEM containing FBS, the hHAP stem cell colonies differentiated to multiple types of cells. The cells differentiated from hHAP stem cell colonies were used for immuno-fluorescence staining.
Immuno-fluorescence staining and FACS of hHAP stem-cell colonies and cells differentiated from hHAP stem cells
The hHAP stem-cell colonies were immuno-stained for stem-cell markers. Stem-cell markers tested included anti-SSEA1 mouse monoclonal IgM (1:100, BioVision, Milpitas, CA); anti-SSEA3 rat monoclonal IgM (1:100, Millipore); anti-SSEA4 mouse monoclonal IgG (1:100, BioLegend); anti-Nanog goat polyclonal (1:100, R&D); anti-Oct3/4 goat polyclonal (1:100, R&D); and anti-nestin antibodies. Secondary antibodies used included Alexa Fluor® 594-conjugated goat anti-mouse IgM (1:400, Molecular Probes); Alexa Fluor® 594-conjugated goat anti-rat IgM (1:400, Molecular Probes); Alexa Fluor® 568-conjugated goat anti-mouse IgG; Alexa Fluor® 568-conjugated donkey anti-goat IgG (1:400); and Alexa Fluor® 568-conjugated goat anti-rabbit IgG; along with DAPI (Molecular Probes).
The primary antibodies used for differentiated cells were: anti-nestin rabbit polyclonal (1:50, IBL, Gunma, Japan); anti-βIII-tubulin monoclonal (1:500, TUJ1 clone; Covance, San Leandro, CA); anti-glial fibrillary acidic protein (GFAP) monoclonal (1:200, GA-5, Lab Vision, UK); anti-GFAP chicken polyclonal (1:300, Abcam, UK); anti-keratin 15 (K15) monoclonal (1:200, Lab Vision); anti-smooth-muscle actin (SMA) monoclonal (1:400, Lab Vision); and anti-cardiac troponin T (cTnT) monoclonal (1:500, GeneTex, Taiwan). Secondary antibodies for immunofluorescence were Alexa Fluor® 568-conjugated goat anti-rabbit IgG (1:400, Molecular Probes, Eugene, OR); Alexa Fluor® 568-conjugated goat anti-mouse IgG (1:400, Molecular Probes); along with 4′, 6-diamino-2-phenylindole, dihydrochloride (DAPI) (Molecular Probes). Secondary antibodies for FACS were goat anti-mouse IgG H&L phycoerythrin (1:500, Abcam, Cambridge, UK); goat anti-chicken IgY biotinylated (1:500, R&D, Minneapolis, MN); and Brilliant Violet 421™ streptavidin (1:500, BioLegend, San Diego, CA).
Statistical analysis
The experimental data are expressed as the mean ± SD.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was partially supported in part by grant NS086217 from the National Institute of Neurological Disorders and Stroke and a Grant-in-Aid for Scientific Research (C) 16K10173 from the Ministry of Education, Science, Sports, and Culture of Japan, a grant from the Ministry of Education, Culture, Sports, Science, and Technology of the Japan Government (Assistance for Strategic Creation of Research Basis, 2014–2016), and the Terumo Life Science Foundation (to Y. Amoh).
References
- [1].Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282:1145-7; PMID:9804556; http://dx.doi.org/ 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- [2].Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature 2007; 448:313-7; PMID:17554338; http://dx.doi.org/ 10.1038/nature05934 [DOI] [PubMed] [Google Scholar]
- [3].Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science 2008; 322:949-53; PMID:18845712; http://dx.doi.org/ 10.1126/science.1164270 [DOI] [PubMed] [Google Scholar]
- [4].Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 2009; 5:237-41; PMID:19716359; http://dx.doi.org/ 10.1016/j.stem.2009.08.001 [DOI] [PubMed] [Google Scholar]
- [5].Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G, Hoffman RM. Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci USA 2003; 100:9958-61; PMID:12904579; http://dx.doi.org/ 10.1073/pnas.1733025100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Amoh Y, Li L, Yang M, Moossa AR, Katsuoka K, Penman S, Hoffman RM. Nascent blood vessels in the skin arise from nestin-expressing hair follicle cells. Proc Natl Acad Sci USA 2004; 101:13291-5; PMID:15331785; http://dx.doi.org/ 10.1073/pnas.0405250101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin-negative hair-follicle-bulge stem cells can form neurons. Proc Natl Acad Sci USA 2005; 102:5530-4; PMID:15802470; http://dx.doi.org/ 10.1073/pnas.0501263102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Amoh Y, Li L, Campillo R, Kawahara K, Katsuoka K, Penman S, Hoffman RM. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci USA 2005; 102:17734-8; PMID:16314569; http://dx.doi.org/ 10.1073/pnas.0508440102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Amoh Y, Kanoh M, Niiyama S, Hamada Y, Kawahara K, Sato Y, Hoffman RM, Katsuoka K. Human hair follicle pluripotent stem (hfPS) cells promote regeneration of peripheral-nerve injury: An advantageous alternative to ES and iPS cells. J Cell Biochem 2009; 107:1016-20; PMID:19507228; http://dx.doi.org/ 10.1002/jcb.22204 [DOI] [PubMed] [Google Scholar]
- [10].Amoh Y, Li L, Katsuoka K, Hoffman RM. Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle 2008; 7:1865-9; PMID:18583926; http://dx.doi.org/ 10.4161/cc.7.12.6056 [DOI] [PubMed] [Google Scholar]
- [11].Yashiro M, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, Hoffman RM, Amoh Y. From hair to heart: nestin-expressing hair-follicle-associated pluripotent (HAP) stem cells differentiate to beating cardiac muscle cells. Cell Cycle 2015; 14:2362-6; PMID:25970547; http://dx.doi.org/ 10.1080/15384101.2015.1042633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yamazaki A, Yashiro M, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, Hoffman RM, Amoh Y. Isoproterenol directs hair follicle-associated pluripotent (HAP) stem cells to differentiate in vitro to cardiac muscle cells which can be induced to form beating heart muscle tissue sheets. Cell Cycle 2016; 15:760-5; PMID:27104748; http://dx.doi.org/ 10.1080/15384101.2016.1146837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shirai K, Hamada Y, Arakawa N, Yamazaki A, Tohgi N, Aki R, Mii S, Hoffman RM, Amoh Y. Hypoxia enhances differentiation of hair follicle-associated-pluripotent (HAP) stem cells to cardiac muscle cells. J Cell Biochem 2016; Epub ahead of print; http://dx.doi.org/ 10.1002/jcb.25734 [DOI] [PubMed] [Google Scholar]
- [14].Yu H, Fang D, Kumar SM, Li L, Nguyen TK, Acs G, Herlyn M, Xu X. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am J Pathol 2006; 168:1879-88; PMID:16723703; http://dx.doi.org/ 10.2353/ajpath.2006.051170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yu H, Kumar SM, Kossenkov AV, Showe L, Xu X. Stem cells with neural crest characteristics derived from the bulge region of cultured human hair follicles. J Invest Dermatol 2010; 130:1227-36; PMID:19829300; http://dx.doi.org/ 10.1038/jid.2009.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hoffman RM. Nestin-expressing hair follicle-accessible-pluripotent stem cells for nerve and spinal cord repair. Cells Tissues Organs 2014; 200:42-7; PMID:25766743; http://dx.doi.org/ 10.1159/000366098 [DOI] [PubMed] [Google Scholar]
- [17].Hoffman R.M., editor. Multipotent Stem Cells of the Hair Follicle: Methods and Protocols Methods in Molecular Biology 1453. Walker John M, series editor. Humana Press (Springer Science+Business Media; New York: ), 2016. DOI: 10.1007/978-1-4939-3786-8 [DOI] [Google Scholar]


