Abstract
Background
Novice and light adolescent smokers can develop symptoms of nicotine dependence, which predicts smoking behavior several years into the future. However, little is known about how the association between these early emerging symptoms and later smoker behaviors may change across time from early adolescence into young adulthood.
Methods
Data were drawn from a 7-year longitudinal study of experimental (<100 cigarettes/lifetime; N=594) and light (100+ cigarettes/lifetime, but ≤5 cigarettes/day; N=152) adolescent smokers. Time-varying effect models were used to examine the relationship between baseline nicotine dependence (assessed at age 15 ±2 years) and future smoking frequency through age 24, after controlling for concurrent smoking heaviness. Baseline smoking status, race, and sex were examined as potential moderators of this relationship.
Results
Nicotine dependence symptoms assessed at approximately age 15 significantly predicted smoking frequency through age 24, over and above concurrent smoking heaviness, though it showed declining trends at older ages. Predictive validity was weaker among experimenters at young ages (<16), but stronger at older ages (20-23), relative to light smokers. Additionally, nicotine dependence was a stronger predictor of smoking frequency for White smokers around baseline (ages 14.5-16), relative to non-White smokers.
Conclusions
Nicotine dependence assessed in mid-adolescence predicts smoking frequency well into early adulthood, over and above concurrent smoking heaviness, especially among novice smokers and non-White smokers. Early-emerging nicotine dependence is a promising marker for screening and interventions aimed at preventing smoking progression.
Keywords: adolescents, nicotine dependence, smoking, time-varying effect models
Introduction
Cigarette smoking remains a substantial public health threat, causing approximately one in five deaths in the US in recent years (U.S. Department of Health and Human Services, 2014). Since the majority of smokers initiate smoking in their teenage years (Anderson, Burns, Dodd, & Feuer, 2012) and establish smoking patterns that persist into adulthood (Jefferis, Graham, Manor, & Power, 2003), adolescence is a critical period for research and interventions on smoking. That is, understanding the etiology of chronic smoking behavior among adolescents is crucial for subsequently developing and implementing prevention programs that are aimed at reducing the disease burden of smoking.
Nicotine dependence is central to the progression and persistence of smoking behavior. While nicotine dependence was traditionally thought of as a direct result of long-term, heavy smoking behavior, a growing body of literature has established that adolescents can experience symptoms of nicotine dependence soon after initiation and well before the onset of daily smoking habits (DiFranza et al., 2000; O'Loughlin et al., 2003). This early-emerging nicotine dependence among adolescent smokers predicts future smoking behavior (Dierker & Mermelstein, 2010; DiFranza et al., 2002), and is negatively associated with future cessation (Mercincavage, Branstetter, Muscat, & Horn, 2013). Recently, early-emerging nicotine dependence symptoms among novice adolescent smokers have been shown to predict smoking into young adulthood (Dierker, Hedeker, Rose, Selya, & Mermelstein, 2015).
While this literature establishes that early-emerging nicotine dependence has predictive validity for future smoking behavior, less is known about possible changes in the predictive value of early-emerging symptoms across age. In other words, do early-emerging nicotine dependence symptoms consistently and stably predict smoking behavior into adulthood, or does its predictive value weaken or decline over time, such that it no longer predicts smoking behavior after a certain point? Most previous studies have examined smoking outcomes at only a single time point (Dierker et al., 2015; Dierker & Mermelstein, 2010; DiFranza et al., 2002), and while some comparisons across studies indicate a declining trend in the predictive validity of nicotine dependence (e.g. Dierker et al., 2015; vs. Dierker & Mermelstein, 2010), the question of how this relationship may change across time or age remains unknown.
Since most smokers firmly establish their smoking habits during adolescence (Jefferis et al., 2003), identifying risk factors that reliably predict smoking behavior throughout this age range and beyond can be used to more accurately identify those at highest risk for targeted prevention efforts. Identifying such risk factors with long-lasting predictive validity may be challenging, considering all the transitions that typically occur during this age range that can influence smoking behavior (e.g. legality of purchasing cigarettes, changing peer groups, becoming financially independent). Thus, rigorously examining the predictive validity of early-emerging nicotine dependence across age is crucial in order to evaluate its possible use as a screening tool for prevention efforts.
The current study uses time-varying effect modeling (Tan, Shiyko, Li, Li, & Dierker, 2012), an innovative methodological approach, to estimate how the predictive validity of nicotine dependence for future smoking frequency changes continuously across age through adolescence and into young adulthood. Data were drawn from a longitudinal cohort study that oversampled experimental (<100 cigarettes/life) and light (100+ cigarettes/lifetime but ≤5 cigarettes/day) adolescent smokers at baseline and followed them over 7 years. Time-varying effect models were used to examine the relationship between early-emerging nicotine dependence (assessed at approximately age 15 ±2 years) and past-month smoking frequency into young adulthood (approximately age 22 ±2 years), continuously across the age range. Race and gender were examined as potential moderators, as well as smoking history at baseline (<100 vs. 100+ cigarettes/lifetime), based on previous findings that the predictors (Selya, Dierker, Rose, Hedeker, & Mermelstein, 2012; Selya et al., 2013) and the time-varying correlates of smoking behavior (Selya, Dierker, Rose, Hedeker, Tan, et al., 2012) can differ across groups with different levels of prior smoking experience.
Methods
Sample
The sample was drawn from the Social and Emotional Contexts of Adolescent Smoking Patterns (SECASP) Study, which has been described in detail previously (e.g. Dierker & Mermelstein, 2010). Students in 9th and 10th grade at 16 Chicago-area high schools were given a brief screener survey, and those reporting certain smoking histories, as well as random samples of non-smokers, were invited to participate in the SECASP Study. Of 3654 students invited, 1263 agreed, provided their own or their parent's consent, and completed the baseline survey in 2005-2006. The current analyses include N=746 participants who reported smoking <100 cigarettes/lifetime and smoking within the past 90 days (“experimenters,” N=594) and those who reported smoking >100 cigarettes/lifetime and smoking within the past 30 days, but smoking ≤5 cigarettes/day (“light smokers,” N=152). This criterion of 100 cigarettes/lifetime was used to distinguish these groups because it is a common definition of lifetime smoking status (Center for Disease Control, 2002), and perhaps a point at which dependence is more likely to be manifest (Dierker & Mermelstein, 2010). In addition to the baseline survey, this cohort was given several follow-up surveys over 7 years; the current study includes assessment conducted 6 months, 15 months, 2 years, 5 years, 6 years, and 7 years after baseline.
Retention of the current study sample at the 7-year follow-up was 81.6% (N=609) and was not significantly associated with race/ethnicity, age, past-month smoking frequency, past-week smoking heaviness, or nicotine dependence scores. Males (24.8%), however, were more likely than females (13.3%) to have missing data at the 7-year follow-up (X2(1) =15.5, p<.01). Of the 746 experimenters and light smokers at baseline, 639 (85.7%) completed five or more assessment waves, 579 (77.6%) completed six or more assessment waves, and 485 (65.0%) completed the full seven assessment waves. The current sample was 55.6% female, 76.5% white, and averaged 15.7 years old at baseline. Demographic and smoking characteristics for experimenters and light smokers are shown in Table 1.
Table 1.
Sample Characteristics. Demographic and smoking characteristics are shown separately for experimenters (left column) and light smokers (right column). Categorical variables are reported in % (N), and numeric variables are reported in median (M) and inter-quartile range (IQR).
| Characteristic | Experimenters* (N = 594) | Light Smokers** (N = 152) | |
|---|---|---|---|
| Sex | Female | 57.9% (N = 344) | 46.7% (N = 71) |
| Male | 42.1% (N = 250) | 53.3% (N = 81) | |
| Race | White | 74.4% (N = 442) | 84.9% (N = 129) |
| Non-White | 25.6% (N = 152) | 15.1% (N = 23) | |
| Age, baseline | M = 15.6, IQR = [15.1 – 16.1] | M = 15.9, IQR = [15.3 – 16.4] | |
| Age, 7 year follow-up | M = 22.6, IQR = [22.1 – 23.1] | M = 22.9, IQR = [22.3 – 23.4] | |
| Past-month smoking frequency, baseline | M = 1.0, IQR = [0.0 – 4.5] | M = 25.0, IQR = [6.5 – 30.0] | |
| Past-month smoking frequency, 7 year follow-up | M = 3.0, IQR = [3.0 – 30.0] | M = 28.0, IQR = [0.0 – 30.0] | |
| NDSS score, baseline | M = 1.0, IQR = [0.8 – 1.5] | M = 2.2, IQR = [1.6 – 3.0] | |
| Past-week smoking frequency, baseline | M = 0.0, IQR = [0.0 – 2.0] | M = 13.0, IQR = [3.0 – 27.0] | |
| Past-week smoking frequency, 7 year follow-up | M = 0.0, IQR = [0.0 – 28.0] | M = 35.0, IQR = [0.0 – 70.0] | |
| Other tobacco use, baseline | Any | 32.1% (N = 189) | 46.7% (N = 71) |
| None | 67.9% (N = 400) | 53.3% (N = 81) | |
At baseline, <100 cigarettes/lifetime, smoked within past 90 days.
At baseline, >100 cigarettes/lifetime, smoked within past 30 days, ≤ 5 cigarettes/day.
Measures
Time-varying variables
Past-month frequency of smoking behavior was measured using the question “Now think about the past 30 days. On how many days did you smoke or try cigarettes?” Response categories were 0, 1, 2-3, 4-5, 6-7, 8-10, 11-20, 21-29, and all 30 days. This was coded as a numeric variable using the midpoint of each category. Frequency was chosen as the outcome because higher smoking frequency indicates shorter smoking intervals, a hallmark of nicotine addiction (DiFranza et al., 2011).
Heaviness of smoking was measured as past-week smoking quantity using the question “During the last 7 days, how many cigarettes did you smoke?”
Age was computed at each wave from self-reported birth dates (month and year).
Time-invariant variables assessed at baseline
Nicotine dependence symptoms were measured using a modified version of the Nicotine Dependence Syndrome Scale (NDSS) (Shiffman, Waters, & Hickcox, 2004) which has been psychometrically validated for use in adolescent smokers (Clark et al., 2005; Sterling et al., 2009). The current study assessed 10 items from the adolescent version based on psychometric analyses (Sterling et al., 2009) that mainly reflect the “drive” and “tolerance” subscales; these 10 items have been described in detail elsewhere (Dierker & Mermelstein, 2010). These 10 individual items were averaged into a combined NDSS score that ranged from 1 (low ND) to 4 (high ND); lower scores were possible for those who reported not smoking in the morning because in those cases, one related item was scored as 0. Experimenters had a median baseline ND score of 1 [range 0.6 – 3.6], and light smokers had a median baseline ND score of 2.2 [range 1.6 – 4] (see Table 1).
Past-month other tobacco use was assessed with the questions “During the past 30 days, on how many days did you: a) use chewing tobacco, snuff, or dip; b) smoke cigars, cigarillos, or little cigars; c) smoked bidis, or d) smoked kreteks?” Hookah and electronic nicotine delivery systems were assessed starting in the 5-year follow-up assessment, but were not included in analyses for reasons of consistency between earlier and later assessment waves. Responses were dichotomized into any other vs. no other past-month tobacco use. This was used as a time-invariant covariate, drawn from the responses at the baseline assessment.
Biological sex and race/ethnicity (White vs. non-White) were self-reported, and were used as time-invariant covariates.
Analyses
Time-varying effect models were used to examine how and when nicotine dependence predicts smoking frequency across adolescence and into young adulthood: that is, how baseline measures of these symptoms predict longitudinal, time-varying smoking frequency, while controlling for past-week smoking heaviness (time-varying variable), other tobacco use (time-invariant, baseline value), race (time-invariant, baseline value), and sex (time-invariant, baseline value). Age was used as the “time” variable in the time-varying effect models to examine moderation across the continuous age range of 14-24 (described below as “age-varying effects” of baseline nicotine dependence). Results were interpreted with respect to whether the coefficient of the age-varying effect of nicotine dependence significantly changed across age, whether the coefficient significantly differed from zero, and if so, at what ages. Age-varying effect models were run first on the full sample. Next, the models were stratified by 1) baseline smoking status (experimenters vs. light smokers), 2) race, and 3) sex in order to examine possible moderation of age-varying effects.
Models were run using a publicly available SAS macro, the TVEM Macro (The Methodology Center, 2015), version 3.1.0, developed for analyzing time-varying effects of intensive longitudinal data. This macro offers the advantage of empirically estimating how the trend of the coefficient for the association between nicotine dependence and smoking frequency changes across a continuous moderator (here, age), unlike traditional models which make strong parametric assumptions about the shape of the trend (e.g. linear or quadratic) (Tan et al., 2012). Estimation was performed using P-spline based estimation and a user-specified number of knots (here, 10); that is, the age interval was split up into 10 equal intervals, and a lower-order polynomial function was used to estimate the age-varying effect within each section. Observations with missing data were automatically removed from analysis. Graphing was performed in R version 3.2.0 with the ggplot2 package (Wickham, 2009).
Results
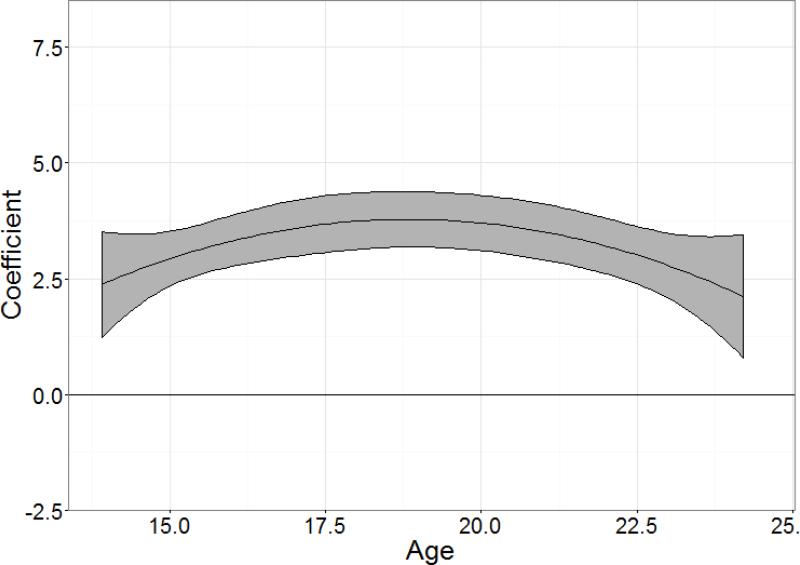
Figure 1 shows the age-varying effects of the baseline NDSS score on smoking frequency for the full study sample (experimenters and light smokers combined). Notably, baseline nicotine dependence assessed around age 15 ±2 years significantly predicts smoking frequency beyond age 24, after controlling for concurrent smoking heaviness, as indicated by the 95% confidence intervals (CI) being above zero throughout the entire age range of this sample. The coefficient for the association between baseline NDSS and time varying smoking frequency peaks near age 19, when the association is strongest. The corresponding coefficient of 3.7 indicates that every 1-unit increase in the NDSS score at age 15 (±2 years) is associated with smoking an additional 3.7 days out of the past month at age 19. The effect of baseline nicotine dependence was stable throughout this age range, as indicated by the overlapping confidence intervals at all ages. While there was a slight declining trend after age 22, the decline itself did not reach significance, indicating a significant and relatively stable effect throughout the age range. Individual nicotine dependence items from the NDSS also show similar age-varying effects (not presented) to those of the average NDSS score presented in Figure 1.
Figure 1. Age-varying effects of baseline NDSS score on smoking frequency among the full study sample.
The coefficient of (y-axis) indicates the strength of the baseline NDSS score in predicting smoking frequency across age (x-axis). The central line shows the point estimate of the coefficient, and the gray ribbon shows the 95% confidence interval. The horizontal line indicates a coefficient of 0 (no predictive validity).
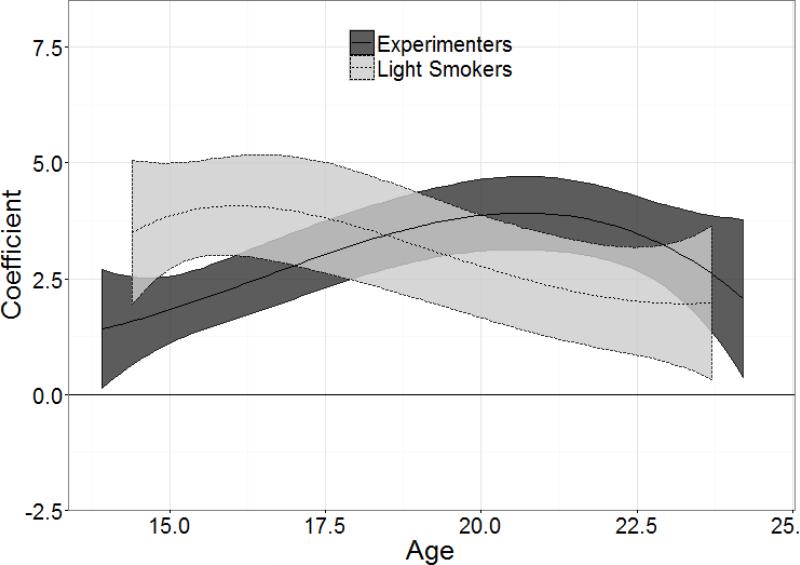
Figure 2 shows the age-varying effects of baseline NDSS on smoking frequency separately for experimenters and light smokers, after controlling for concurrent smoking heaviness and personal characteristics. Initially, below age 16, the age-varying effects are substantially stronger for light smokers relative to experimenters, as shown by the non-overlapping confidence intervals. The age-varying effects are similar between ages 18 and 20 between groups: the coefficient measuring the association between baseline nicotine dependence and smoking frequency is significant (i.e. both CI's differ from 0) and similar across baseline smoking groups (i.e. their CI's overlap each other) during this age range, indicating that the predictive validity of nicotine dependence for smoking frequency is positive and approximately equal in strength between experimenters (those entering the study smoking <100 cigarettes/lifetime) and light smokers (those who already smoked 100+ cigarettes/lifetime at baseline). Above age 20, the age-varying effects again diverge, with the predictive validity of nicotine dependence being significantly higher among experimenters than light smokers. Since the confidence bands overlap, this group difference was verified in a follow-up test by examining the age-varying effects of the group difference (experimenters vs. light smokers). The resulting CI for the group difference was positive and excluded 0 between ages 20 and 23 (data not shown), indicating that nicotine dependence more strongly predicts smoking frequency for experimenters than for light smokers at these ages. Above age 23, the age-varying effects were once again similar in magnitude (i.e. the group comparison overlapped zero) for both experimenters and light smokers, and remained significant throughout the remaining age range (i.e. the CI's did not overlap zero).
Figure 2. Age-varying effects of baseline NDSS score on smoking frequency, stratified by baseline smoking status.
The coefficient of (y-axis) indicates the strength of the baseline NDSS score in predicting smoking frequency across age (x-axis) for experimenters (<100 cigarettes/lifetime at baseline; dark gray) and for light smokers (≥100 cigarettes/lifetime at baseline; light gray). The central line shows the point estimate of the coefficient, and the gray ribbon shows the 95% confidence interval. The horizontal line indicates a coefficient of 0 (no predictive validity).
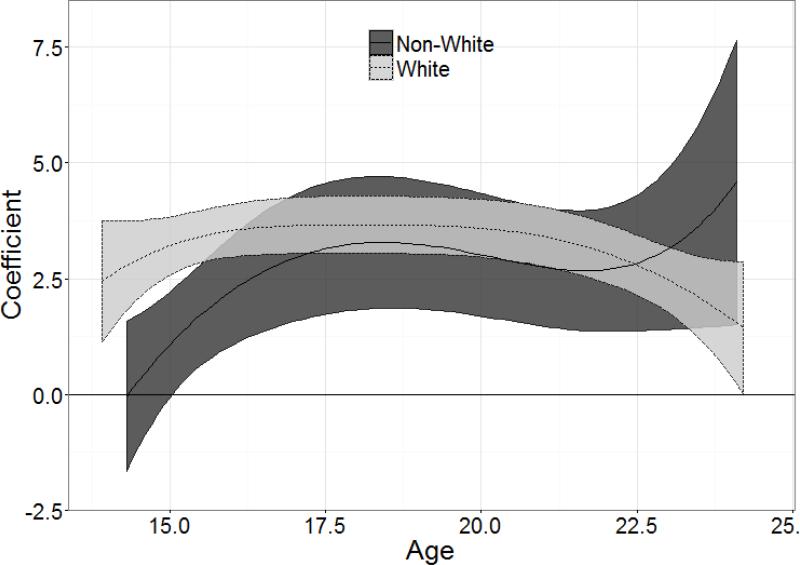
Figure 3 shows the age-varying effects stratified by race (White vs. non-White), after controlling for concurrent smoking heaviness and personal characteristics. The coefficients are significant (i.e. CI's do not overlap zero) and largely similar in magnitude between groups after age 16, indicating that baseline NDSS predicts smoking frequency similarly for White and non-White smokers during mid-adolescence and young adulthood. However, at younger ages, approximately 14.5 to 16, baseline nicotine dependence is more strongly predictive of smoking frequency for White relative to non-White smokers, as shown by the higher coefficient of White smokers and the non-overlapping CI's. Nicotine dependence has stronger predictive validity for White smokers only during this brief range, due to the earlier plateauing of its age-varying effect relative to non-White smokers (see Figure 3). Due to the lack of data at very young ages (approximately age 14) for non-White smokers, it is unclear at what age this difference emerges. After approximately age 16, the predictive validity of NDSS peaks for both groups at a coefficient of approximately 3.5.
Figure 3. Age-varying effects of baseline NDSS score on smoking frequency, stratified by white race.
The coefficient of (y-axis) indicates the strength of the baseline NDSS score in predicting smoking frequency across age (x-axis) for non-white (dark gray) and white (light gray). The central line shows the point estimate of the coefficient, and the gray ribbon shows the 95% confidence interval. The horizontal line indicates a coefficient of 0 (no predictive validity).
Finally, exploratory analyses stratifying by sex showed no significant differences between males and females in the age-varying associations between baseline nicotine dependence and smoking frequency measured from adolescence through young adulthood (data not shown).
Discussion
The current study examined the validity of nicotine dependence assessed at age 15 (±2 years) in predicting smoking frequency across time, through approximately age 24, and how this association varies across age. Nicotine dependence symptoms measured at the mid-adolescent, baseline assessment was significantly and positively associated with smoking frequency into young adulthood (beyond age 24), over and above concurrent smoking heaviness. Moreover, the predictive validity of adolescent nicotine dependence was moderated by race (such that nicotine dependence predicted smoking frequency more strongly for Whites under age 16) and by smoking status (such that, for those who had smoked <100 cigarettes/lifetime at baseline, nicotine dependence was a weaker predictor of smoking frequency below age 16, but a stronger predictor between ages 20-23, relative to those who entered the study already having smoked 100+ cigarettes/lifetime).
The main finding that nicotine dependence has strong predictive validity for smoking behavior throughout adolescence and into young adulthood confirms and extends existing literature. Like the current study, previous studies based on this sample have shown that adolescent-appropriate measures of nicotine dependence predict smoking behavior several years into the future (Dierker et al., 2015; Dierker & Mermelstein, 2010; DiFranza et al., 2002). The current study extends the evidence for the value of early emerging nicotine dependence symptoms in predicting future smoking behavior, due to the longer follow-up period and the evaluation of continuous changes in this association across age.
Previous studies based on this sample have suggested that nicotine dependence predicts smoking 2–6 years after baseline for experimenters (>100 cigarettes/lifetime at baseline) but not for more experienced smokers (>100 cigarettes/lifetime), above and beyond previous smoking behavior (Dierker et al., 2015; Dierker & Mermelstein, 2010). The current study's use of time-varying effect models allowed a more in-depth examination of this group difference, and showed that nicotine dependence predicts smoking behavior among both groups of smokers, but this predictive validity was significantly weaker during early adulthood among the more experienced light smokers. This difference in how long nicotine dependence predicts smoking frequency highlights the importance of early-emerging nicotine dependence symptoms in the etiology of regular smoking behavior: despite lower average nicotine dependence in the group of experimenters, those who reported nicotine-dependence symptoms at this very early stage of smoking were at high risk for more regular smoking in the future, even after conservatively accounting for concurrent smoking heaviness. For light smokers, who had already smoked more than 100 cigarettes at baseline, the weaker ability of nicotine dependence to predict smoking behavior into the future is consistent with previous findings of this sample that prior smoking behavior is the best predictor of future smoking behavior (Selya, Dierker, Rose, Hedeker, & Mermelstein, 2012; Selya et al., 2013).
One potential explanation for these observed differences between experimenters and light smokers is that there is a “stage-varying” relationship between nicotine dependence and smoking frequency (Selya, Dierker, Rose, Hedeker, Tan, et al., 2012). That is, the relationship between nicotine dependence and smoking frequency may be moderated by the stage of smoking, and not strictly time or age per se, with nicotine dependence being most predictive of long-term future smoking behavior when assessed early among very novice smokers. Once regular smoking patterns are established, however, prior smoking behavior becomes such a strong predictor of future smoking behavior (Selya, Dierker, Rose, Hedeker, & Mermelstein, 2012; Selya et al., 2013) that nicotine dependence becomes a weak or insignificant predictor. Early-emerging nicotine dependence assessed from novice smokers, then, may provide a special insight into one's initial sensitivity or susceptibility to nicotine dependence, while nicotine dependence assessed from more experienced smokers is likely to be conflated with the subsequent effects of smoking on nicotine dependence (Hu, Griesler, Wall, & Kandel, 2014). Put another way, nicotine dependence and smoking have a bi-directional relationship (Doubeni, Reed, & Difranza, 2010), and early-emerging nicotine dependence assessed from novices may more precisely measure one's susceptibility to future chronic smoking. In contrast, nicotine dependence that develops or is assessed after more smoking experience is also impacted by prior smoking behavior, which would mask this measure of initial sensitivity by conflating it with nicotine dependence that results from prior smoking behavior.
Differences across race groups in the association between nicotine dependence measured during adolescence and smoking frequency showed that the predictive validity of nicotine dependence plateaus at an earlier age among White smokers relative to non-White (i.e. Black and Hispanic) smokers during early-adolescence. It is unclear whether one or more specific non-White racial or ethnic groups are driving these differences, due to the limitations of sample size in the current study. This finding seems consistent with the little that is known about race/ethnic differences in smoking and nicotine dependence. The earlier acquisition of smoking behavior and nicotine dependence among White smokers likely contributes to the current findings that the predictive validity of nicotine dependence peaks sooner for Whites relative to non-Whites. In the “stage-varying effect” explanation described above, White smokers are at a more advanced smoking stage than non-White smokers at a given age; thus early-emerging nicotine dependence (which requires having initiated smoking) is expected to emerge at a younger age for Whites, and the time course of its predictive validity is expected to be shifted to the left (i.e. to younger ages), relative to non-Whites. This is consistent with the earlier plateau among Whites, and with the similar coefficients for Whites and non-Whites once the plateau is reached.
A novel finding in this study is that, for some groups of smokers, predictive validity significantly increases at intermediate ages relative to early adolescence, before showing a declining trend in young adulthood. These trends indicate that nicotine dependence is more strongly associated with future smoking behavior than with concurrent smoking behavior in these cases. Consistent with the “stage-varying effects” explanation above, early-emerging nicotine dependence may be a stronger indicator of one's susceptibility to nicotine addiction when assessed from more novice smokers, and thus may be especially informative about future smoking behavior. In contrast, nicotine dependence may be a weaker predictor of concurrent smoking among novice smokers because they have not yet developed regular smoking patterns. That is, early-emerging nicotine dependence is a weaker driver of low cigarette use among novices, whose light and infrequent smoking patterns are still in flux and for whom other factors such as social pressure and access to cigarettes may play a stronger role.
At a broader level, the current study's focus on the changing relationship between nicotine dependence and smoking frequency across time extends the smoking etiology literature using an innovative methodology. Previous studies have shown that the cross-sectional association between nicotine dependence and smoking frequency increases in strength over time (Selya, Dierker, Rose, Hedeker, Tan, et al., 2012), and that nicotine dependence and smoking behavior have strong reciprocal relationships based on cross-lagged analyses (Doubeni et al., 2010), with smoking behavior being a stronger predictor of nicotine dependence symptoms than vice versa (Hu et al., 2014). The current study is consistent with this literature in that it shows the importance of early-emerging nicotine dependence in establishing regular and long-term smoking patterns, and extends it by showing that ND assessed within 2 years of age 15 predicts smoking frequency beyond age 24, over and above concurrent past-week smoking frequency. Additionally, the current use of time-varying effects models is advantageous in that it shows how this predictive validity changes smoothly across the range of age from 14 to 24, in contrast to previous studies which were limited to analyzing discrete changes across disjointed assessment waves.
Study limitations should be taken into account when interpreting these findings. First, the current sample is drawn from novice and light adolescent smokers in the metropolitan Chicago area, and the findings may not generalize to other populations. Second, there was substantial age variation (around 3 years) within each assessment wave, which constrains the interpretation of the current findings in a couple of ways. Specifically, nicotine dependence was not assessed at the same age for everyone, and it is unclear whether certain ages are more optimal than others with respect to maximizing the time duration for which nicotine dependence predicts smoking behavior. Third, while the full age range across participants is approximately 10 years (14 – 24), it is important to note that for a given subject, data on smoking behavior were collected for a maximum of 7 years (i.e. from baseline to the 7-year follow-up). Thus, these findings should not be interpreted as ND being predictive of smoking behavior 10 years into the future. Finally, since this study uses observational data, interpretation is limited to associations, rather than causality, between nicotine dependence measured in mid-adolescence and smoking frequency measured across adolescence and into young adulthood.
This study also has a number of strengths. The sample is a large, diverse cohort of relatively novice smokers who were followed over 7 years, and is ideal to study the etiology of smoking behavior during the period of highest risk for the establishment of regular and chronic smoking. The use of an innovative version of time-varying effect modeling allows an analysis of how well, and at what ages, nicotine dependence symptoms measured around age 15 predict smoking behavior across adolescence and into young adulthood. This research advances existing literature on the role of early-emerging nicotine dependence on the development of smoking in important ways: by extending the age range at which the predictive validity of nicotine dependence symptoms are evaluated, by describing differences by race and baseline smoking, and by using time-varying effects methods to examine nuanced and continuous changes in predictive validity across age.
These findings have important implications for anti-tobacco interventions that target adolescents who have already initiated smoking, but aim to prevent the progression to more regular smoking behavior. Assessing nicotine dependence in mid-adolescence can be used to predict smoking frequency into young adulthood, over and above concurrent smoking heaviness, especially for those who have just recently initiated smoking and for non-White smokers. This consistent predictive validity of early-emerging nicotine dependence is notable in the face of many social, economic, and legal changes throughout adolescence and young adulthood, many of which can impact smoking behavior. This brief window after smoking initiation provides a promising target for such interventions, given that the vast majority of smokers initiates smoking in adolescence (Anderson et al., 2012; Rath, Villanti, Abrams, & Vallone, 2012) and develop smoking habits that are likely to continue throughout adulthood (Jefferis et al., 2003). Thus, nicotine dependence measures can identify those novice and light adolescent smokers who are at a heightened risk for smoking into young adulthood, and future research should explore appropriate intervention strategies for this group.
Acknowledgments
Funding
This research was funded by Project Grant P01 CA098262 from the National Cancer Institute; R01 DA022313 and R21 DA029834 from the National Institute on Drug Abuse; and Center Grant P50 DA010075 awarded to Penn State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, NCI, or NIDA.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in this study.
References
- Anderson CM, Burns DM, Dodd KW, Feuer EJ. Birth-cohort-specific estimates of smoking behaviors for the U.S. population. Risk Analysis. 2012;32(Suppl 1):S14–24. doi: 10.1111/j.1539-6924.2011.01703.x. Chapter 2, doi: 10.1111/j.1539-6924.2011.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control Cigarette smoking among adults- United States. 2000 Morbidity and Mortality Weekly Report. 2002;51:642–645. [PubMed] [Google Scholar]
- Clark DB, Wood DS, Martin CS, Cornelius JR, Lynch KG, Shiffman S. Multidimensional assessment of nicotine dependence in adolescents. Drug & Alcohol Dependence. 2005;77(3):235–242. doi: 10.1016/j.drugalcdep.2004.08.019. doi: 10.1016/j.drugalcdep.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Dierker L, Hedeker D, Rose J, Selya A, Mermelstein R. Early emerging nicotine dependence symptoms in adolescence predict daily smoking in young adulthood. Drug & Alcohol Dependence. 2015;151:267–271. doi: 10.1016/j.drugalcdep.2015.03.009. doi: 10.1016/j.drugalcdep.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker L, Mermelstein R. Early emerging nicotine-dependence symptoms: a signal of propensity for chronic smoking behavior in adolescents. The Journal of Pediatrics. 2010;156(5):818–822. doi: 10.1016/j.jpeds.2009.11.044. doi: 10.1016/j.jpeds.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Rigotti NA, McNeill AD, Ockene JK, Savageau JA, St Cyr D, Coleman M. Initial symptoms of nicotine dependence in adolescents. Tobacco Control. 2000;9(3):313–319. doi: 10.1136/tc.9.3.313. doi: 10.1136/tc.9.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Rigotti NA, Fletcher K, Ockene JK, McNeill AD, Wood C. Development of symptoms of tobacco dependence in youths: 30 month follow up data from the DANDY study. Tobacco Control. 2002;11(3):228–235. doi: 10.1136/tc.11.3.228. doi: 10.1136/tc.11.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Wellman RJ, Mermelstein R, Pbert L, Klein JD, Sargent JD, Winickoff JP. The natural history and diagnosis of nicotine addiction. Current Pediatric Reviews. 2011;7:88–96. doi: 10.2174/1573396311666150501002703. [DOI] [PubMed] [Google Scholar]
- Doubeni CA, Reed G, Difranza JR. Early course of nicotine dependence in adolescent smokers. Pediatrics. 2010;125(6):1127–1133. doi: 10.1542/peds.2009-0238. doi: 10.1542/peds.2009-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Griesler PC, Wall MM, Kandel DB. Reciprocal associations between cigarette consumption and DSM-IV nicotine dependence criteria in adolescent smokers. Addiction. 2014;109(9):1518–1528. doi: 10.1111/add.12619. doi: 10.1111/add.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis B, Graham H, Manor O, Power C. Cigarette consumption and socioeconomic circumstances in adolescence as predictors of adult smoking. Addiction. 2003;98(12):1765–1772. doi: 10.1111/j.1360-0443.2003.00552.x. [DOI] [PubMed] [Google Scholar]
- Mercincavage M, Branstetter SA, Muscat JE, Horn KA. Time to first cigarette predicts cessation outcomes in adolescent smokers. Nicotine & Tobacco Research. 2013;15(12):1996–2004. doi: 10.1093/ntr/ntt087. doi: 10.1093/ntr/ntt087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Loughlin J, DiFranza J, Tyndale RF, Meshefedjian G, McMillan-Davey E, Clarke PB, Paradis G. Nicotine-dependence symptoms are associated with smoking frequency in adolescents. American Journal of Preventive Medicine. 2003;25(3):219–225. doi: 10.1016/s0749-3797(03)00198-3. doi: S0749379703001983. [DOI] [PubMed] [Google Scholar]
- Rath JM, Villanti AC, Abrams DB, Vallone DM. Patterns of tobacco use and dual use in US young adults: the missing link between youth prevention and adult cessation. Journal of Environmental and Public Health. 2012;2012:679134. doi: 10.1155/2012/679134. doi: 10.1155/2012/679134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selya AS, Dierker LC, Rose JS, Hedeker D, Mermelstein RJ. Risk factors for adolescent smoking: Parental smoking and the mediating role of nicotine dependence. Drug & Alcohol Dependence. 2012 doi: 10.1016/j.drugalcdep.2012.02.004. doi: 10.1016/j.drugalcdep.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selya AS, Dierker LC, Rose JS, Hedeker D, Tan X, Li R, Mermelstein RJ. Time-varying effects of smoking quantity and nicotine dependence on adolescent smoking regularity. Drug & Alcohol Dependence. 2012 doi: 10.1016/j.drugalcdep.2012.08.026. doi: 10.1016/j.drugalcdep.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selya AS, Wakschlag LS, Dierker LC, Rose JS, Hedeker D, Mermelstein RJ. Exploring alternate processes contributing to the association between maternal smoking and the smoking behavior among young adult offspring. Nicotine & Tobacco Research. 2013;15(11):1873–1882. doi: 10.1093/ntr/ntt072. doi: 10.1093/ntr/ntt072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Waters A, Hickcox M. The nicotine dependence syndrome scale: a multidimensional measure of nicotine dependence. Nicotine & Tobacco Research. 2004;6(2):327–348. doi: 10.1080/1462220042000202481. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Sterling KL, Mermelstein R, Turner L, Diviak K, Flay B, Shiffman S. Examining the psychometric properties and predictive validity of a youth-specific version of the Nicotine Dependence Syndrome Scale (NDSS) among teens with varying levels of smoking. Addictive Behaviors. 2009;34(6-7):616–619. doi: 10.1016/j.addbeh.2009.03.016. doi: 10.1016/j.addbeh.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Shiyko MP, Li R, Li Y, Dierker L. A time-varying effect model for intensive longitudinal data. Psychological Methods. 2012;17(1):61–77. doi: 10.1037/a0025814. doi: 10.1037/a0025814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Methodology Center . TVEM SAS Macro Suite (Version 3.1.0) [Software] The Methodology Center, Penn State; University Park: 2015. Retrieved from http://methodology.psu.edu. [Google Scholar]
- U.S. Department of Health and Human Services . The Health Consequences of Smoking - 50 Years of Progress. A Report of the Surgeon General. U.S Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. [Google Scholar]
- Wickham H. ggplot2: elegant graphics for data analysis. Springer; New York: 2009. [Google Scholar]