Abstract
To cope with heterogeneous environments and resource distributions, filamentous fungi have evolved a spatially extensive growth enabling their hyphae to penetrate air–water interfaces and pass through air-filled pores. Such mycelia are also known to act as dispersal networks for the mobilisation of bacteria (‘fungal highways') and connection of microbial microhabitats. Hitherto, however, nothing is known about the effect of mycelia-based dispersal on interactions between bacterial predators and their prey and concomitant effects on biomass formation. We here hypothesise that mycelia enable the contact between predators and their prey and shape a prey's population. We investigated the impact of predation by Bdellovibrio bacteriovorus 109J on the growth of its potential prey Pseudomonas fluorescens LP6a in the presence of mycelia. Our data give evidence that hyphae increase the accessibility of the prey to B. bacteriovorus 109J and, hence, allow for efficient foraging and shaping of prey populations not seen in the absence of mycelia. To test our hypothesis tailored microbial landscapes were used for better reduction of emerging properties in complex systems. Our data suggest that mycelia have substantial influence on prey–predator relationship and hereby may promote the structure of prey and predator populations and, hence, may be a determinant for biomass formation in heterogeneous environments.
Introduction
How can a water-bound predator reach a prey item, which is separated by an air-filled soil pore? Whereas various aspects of soil heterogeneity, such as the transport and distribution of resources as controls of microbial activity and biomass formation, have been dealt with in detail (Bosma et al., 1997; Johnsen et al., 2005; Harms and Wick, 2006; Semple et al., 2007), restrictions to the physical interaction between micro-predators and their prey in poorly permeable environments have only scarcely been addressed (Yair et al., 2003). Recently, it was discovered that fungal mycelia act as networks (‘fungal highways'), along which bacteria can disperse and even pass through air-filled parts of water-unsaturated environments (Kohlmeier et al., 2005; Wick et al., 2007b). Several studies demonstrated a positive effect of bacteria–fungi interactions on ecosystem services such as increased transformation of anthropogenic and natural compounds in soil (Boonchan et al., 2000; Warmink et al., 2011; Banitz et al., 2011b; Pion et al., 2013a). However, bacterial dispersal along mycelia could also evoke antagonistic effects such as the mutual inhibition or competition between bacteria and fungi (Rousk et al., 2008; Pion et al., 2013b).
In the present study, we tested whether bacterial predators can use mycelia as means to reach their prey and whether there are consequences for the prey population. As environmental conditions (for example, the availability of carbon sources) are known to trigger tactic bacterial dispersal (Furuno et al., 2010; Krell et al., 2012; McDougald et al., 2012; Son et al., 2015), we further approximated if a predator's dispersal may be directed by the presence of bacteria. Bdellovibrio-and-like-organisms (BALOs) are the best studied bacterial predators. The biphasic life cycle of BALOs consists of a motile free-living phase, in which the cells are searching and attacking the prey, and a growth phase within the periplasm of their Gram-negative prey (bdelloplast), during which multiple progeny are produced before they escape after the host has been consumed (Stolp and Starr, 1963, 1965; Sockett, 2009). Because of their exceptional motility and unique life cycle, BALOs can control prey populations so effectively that they even hold promise as antibacterial agents in food industry, agriculture or medicine (Markelova, 2010; Dwidar et al., 2012; Johnke et al., 2014). From medical studies, for instance, it is known that Bdellovibrio bacteriovorus is able to attack and destroy oral multilayer biofilms (Dashiff and Kadouri, 2011; Dashiff et al., 2011). The role of predatory bacteria in the microbial ecology of heterogeneous subsurface environments, including the control of population size and structure of Gram-negative bacterial populations, which often live and grow in association with surfaces, is less clear (Markelova and Colwell, 1999; Dashiff et al., 2011). For our study, a controlled microcosm system as a mimic for an air-filled porous system was tailored. Mycelia were allowed to bridge physically separated predator and prey populations, and the impact of predation on the prey population was assessed. Our data show that BALOs disperse along mycelia and that this movement is consequential for the prey populations.
Materials and methods
Bacteria strains, media and growth conditions
Pseudomonas fluorescens LP6a was used as the prey bacterium in dispersal and chemotaxis experiments (Foght et al., 1998). It was grown at 28 °C on a rotary shaker at 150 rpm in Erlenmeyer flasks containing 200 ml of 1:10 diluted nutrient broth (1:10 dNB). For microcosm experiments, 24 h old cultures were harvested in the exponential phase, washed twice in 50 mm potassium phosphate buffer (PBS) of pH 7.2 and centrifuged at 1000 g for 10 min. The pellet was re-suspended in PBS containing ≈109 cells per ml. Cell numbers were counted with a MoFlo flow cytometer (DakoCytomation, Fort Collins, CO, USA) as described earlier (Hübschmann et al., 2007). The instrument was adjusted using fluorescent 1-μm beads and cell counting was performed with 488 nm light of 400 mW to analyse the forward light scatter and the side light scatter.
Escherichia coli ML-35 was used as the prey bacterium for plaque-forming units (PFU) quantification as well as in chemotaxis experiments. It was grown over night at 30 °C on a rotary shaker at 150 rpm in Erlenmeyer flasks containing 200 ml of 1:10 dNB. B. bacteriovorus 109J was routinely grown as plaques in double-layered 1:10 diluted nutrient broth (1:10 dNB) agar, amended with 3 mmol l−1 MgCl2 × 6H2O and 2 mmol l−1 CaCl2 × 2H2O (pH 7.2) and agar (0.7% w/v agar in the top layer). To initiate a lysate, a plug of agar containing B. bacteriovorus 109J was added to washed prey cells of P. fluorescens LP6a in Erlenmeyer flasks containing 200 ml 1:10 dNB and incubated at 30 °C on a rotary shaker at 150 rpm for 72 h. To harvest the predators, co-cultures were prepared, in which 3 ml of washed host cells were incubated with 600 μl of stock lysate of B. bacteriovorus 109J in 150 ml of 1:10 dNB. The co-cultures were incubated for 48 h to reach a final concentration of ≈108 cells per ml of the predator and the suspension was passed three times through a 0.45 μm Millex pore-size filter (Millipore, Billerica, MA, USA) to remove residual prey and cell debris (Dashiff et al., 2011). Prior to the experiments the initial cell densities of B. bacteriovorus 109J were quantified by microscopic counting of DAPI-stained cells. For that, sodium azide (10% (v/v)) fixed aliquots were filtered onto 0.2 μm Millipore filters (type GTTP) and DAPI-stained (final concentration 12.5 μg ml−1, diluted with Citifluor AF1). Cell densities were determined by counting five randomly selected fields with the Nikon macroscope (AZ 100 Multizoom) equipped with an Hg vapor lamp and a black-and-white camera (Nikon, Amsterdam, Netherlands) and using the GFP-filter settings. Mycelia of the oomycete Pythium ultimum (Maurhofer et al., 1992) were used as model dispersal networks. They were pre-grown at room temperature on potato dextrose agar (PDA; 1.5% w/v; pH 6.8, Difco Laboratories, Franklin Lakes, NJ, USA) (Schamfuss et al., 2013).
Dispersal experiments
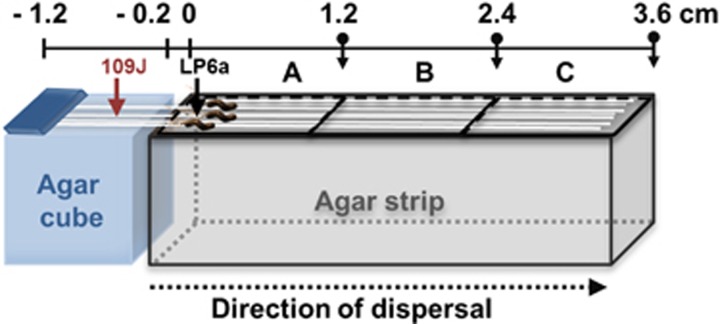
Dispersal experiments were conducted at least in triplicate in a microcosm mimicking surfaces of soil constituents under predation pressure and in the presence of a dispersal network (Figure 1). The basic body of a microcosm consisted of an agar strip of 1:10 dNB (length: 3.6 cm, height: 1.0 cm, width: 1.0 cm and agar 1.5% w/v). As illustrated in Figure 1, the agar strip was divided in three zones of interest (A, B and C, length: 1.2 cm, each). An agar cube (1 cm3, minimal medium agar, 2% w/v) was placed next to the agar strip as a starting point for the growth of the P. ultimum mycelium and dispersal of the predator B. bacteriovorus 109J. The microcosms were inoculated with P. ultimum and incubated for 18 h to allow it to grow over the air gap and into the initial zone of the agar strip. Then, the left-hand third of zone A of the microcosms was inoculated with 2 μl (containing 2 × 106 cells) of suspended P. fluorescens LP6a. The microcosms were again incubated for 24 h to allow dispersal of prey cells along the mycelia prior to the inoculation with 2 μl (containing 8 × 103 cells) predator suspensions on the agar cube and incubation for additional 72 h. All microcosm setups were placed in standard glass petri dishes and stored at 25 °C in a 30-l desiccator.
Figure 1.
Microcosms used to study the effect of predation of Bdellovibrio bacteriovorus 109J on dispersal of Pseudomonas fluorescens LP6a along mycelia of P. ultimum that was inoculated on an agar block separated by an air gap of 0.2 cm from the agar strip. Prey (black arrow) and predator (grey arrow) bacteria were spot-inoculated consecutively after 24 h on the top of the agar strip and an agar block separated by an air gap of 0.2 cm from the agar strip. P. ultimum provided dispersal networks for both bacterial strains. It was inoculated as a freshly overgrown 1:10 dNB agar piece top-side down on the left-hand third of the agar cube. Identical setups without bacterial predator and without prey bacteria, respectively, served as controls. A full colour version of this figure is available at the ISME journal online.
After 24, 48 and 72 h, the zones A–C of individual microcosms were harvested separately and the number of predator and prey bacteria quantified. For that the agar piece was cut with a sterile spatula and the agar pieces successively suspended in 3 ml PBS, vortexed for 1 min, sonicated (2 × 30 s with a break of 1 min), and their bacterial prey count analysed by colony-forming units. To quantify the predator, a double-layer procedure was used and PFU counted: a suspension of E. coli ML-35 cells in soft agar serving as indicator prey was poured on top of agar on which predator cells had been spread. Within 5–7 days incubation, PFU of 2 mm diameter developed, which were counted to quantify predator concentrations. Controls were performed without mycelia as dispersal network (in order to correct for potential air-borne transport of the predator; control I), without predator (in order to assess dispersal and growth of the prey bacteria in the absence of predation; control II), and without the prey (in order to estimate dispersal and growth of the predator in the absence of the prey; control III). To rule out possible negative biotic effects of mycelia, experiments with glass fibres (~300 pcs, length: 5 cm with a diameter of ≈8 μm) as abiotic dispersal networks were tested in presence (control IV) and absence of prey cells (control V). To evaluate dispersal of the predator along the agar surface in the absence of mycelia, B. bacteriovorus 109J cells were inoculated at the left-hand third of zone A (Figure 1) and LP6a prey cells were placed at a distance of ca. 1.2 cm in zone B of the microcosms (control VI). The setups were incubated for 72 h at 26 °C and the number of predator cells quantified as PFU on zone B and C after 48 h and 72 h. All controls were performed at least in triplicate using (where applicable) 2 μl of a B. bacteriovorus 109J suspension (1 × 104 cells) and 2 μl of P. fluorescens LP6a (2 × 106 cells) as inocula.
Assessment of directed dispersal of predator towards prey cells
A modified capillary test described by Adler (1973) was used to assess possible directed dispersal of B. bacteriovorus 109J towards P. fluorescens LP6a (Supplementary Figure SI1; Adler, 1973; Straley and Conti, 1974). In short, a culture of B. bacteriovorus 109J exponentially growing on P. fluorescens LP6a was harvested, centrifuged at 1000 g for 10 min, filtered through a 0.45 μm pore-size Millex filter and re-suspended in HEPES metals (HM) buffer (Ferguson et al., 2014). About 0.3 ml of this suspension was placed in a small chamber formed by a U-shaped capillary placed on a microscope slide and closed with a glass coverslip in order to prevent the formation of air bubbles within the chamber. Two capillary tubes with volumes of 2 μl (BRAND GmbH & Co KG, Wertheim, Germany), heat-sealed at one end, containing either living prey cells from the late exponential phase, lysed prey cells (heated in a microwave oven at 600–700 W for 60 s) or a mixture of both, were exposed to the predator suspension with their open ends. Furthermore, to determine the predator's response to the supernatant of P. fluorescens LP6a, late exponential prey cells of strain LP6a were removed by centrifugation (1000 g for 20 min) and the supernatant was filter sterilised. Identical setups with capillaries containing HM buffer and 1:10 dNB medium served as controls. The chambers were incubated for 20 min (that is, below reported generation times of 2–3 h (Sockett, 2009)) at room temperature and the number of predator cells immigrated in test capillaries was quantified as PFU. All assays were installed in triplicate using two capillaries per setup.
Predation impact
An adaptation of the unitless predation impact (PI) approach by Fox (2002) was used to approximate predation effect of B. bacteriovorus 109J on the population size of P. fluorescens LP6a:
 |
Here Kbac is the prey cell number at a given time within the system in the absence of predation and R is the corresponding prey cell number remaining under predation pressure. The PI varies between 0 (no effect of predation) and 1 (complete extinction). In case of predation leading to increased abundance of the prey, the value of PI becomes negative.
Statistical analysis
Unless otherwise stated, results of the replicate series for microcosms were compared with each other using a two-sided Student's t-test to test for significant differences (*P-value=0.05; **P-value=0.005; ***P-value=0.001). The variance of absolute quantitative measures is given by the s.d. as depicted in all figures by vertical whiskers. In contrast, the level of uncertainty of relative measures is expressed by reporting 90% confidence intervals (CI) as calculated according to Fieller's method (Fieller, 1940) using the free online calculator of GraphPad (GraphPad Software, San Diego, CA, USA).
Results
Effect of mycelia on dispersal and abundance of B. bacteriovorus 109J
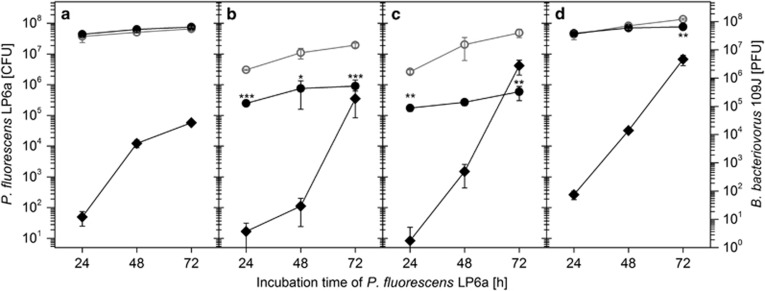
Dispersal of B. bacteriovorus 109J along mycelia and its influence on the prey organism P. fluorescens LP6a were analysed in microcosms (Figure 1) in presence and absence of mycelia of P. ultimum serving as bridging networks. In the absence of mycelia (yet in presence of bacteria; control I) no predator cells (0 PFU) were detected beyond the air gap after 24, 48 and 72 h. In presence of mycelia by contrast, 75±25 PFU (≈1% of the inoculum; Figure 2d) were detected on the agar strip beyond the air gap after 24 h of co-incubation with 17±14 PFU and 8±14 PFU in zones B and C (Figures 2a–d). After 72 h and as a result of growth 4.6 × 106±1.8 × 106 PFU (that is, a 6 × 104-fold increase (CI: 2.9 × 104–1.2 × 105)) of the predator (Figure 2d) were seen. If mycelia yet not bacteria were present (control III), no predator cells were detected at any time beyond the air gap neither by PFU counting (0 PFU) nor by microscopic screening of zone A. In order to rule out biotic effects of mycelia, transport experiments with abiotic dispersal networks were tested in presence (control IV) and absence of prey cells (control V). As with mycelia high abundance of the predator in zone A in presence (control IV) yet not in the absence of prey cells (that is, 0 PFU; control V) was detected.
Figure 2.
Population sizes of Pseudomonas fluorescens LP6a (colony-forming units) over time in zones (a–c) and in the overall setup (d) in the presence (filled circles) and absence (open circles) of predation pressure exerted by Bdellovibrio bacteriovorus 109J. Data represent averages and s.d. of triplicate experiments. Abundances of the predator as quantified by PFU are reflected by rhomboids. The mean measured colony-forming units at a distinct zone showed a statistically significant difference from the system without the predator. The asterisks refer to the statistical significance: *P=0.05; **P=0.01; ***P=0.001.
Effect of predation on abundance of P. fluorescens LP6a
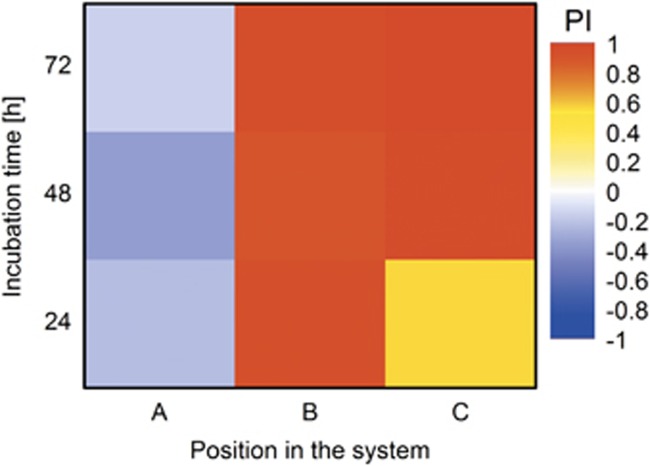
The effect of dispersal and abundance of B. bacteriovorus 109J along mycelia was evidenced by quantifying the number of prey P. fluorescens LP6a cells in zones A–C in presence and absence of predators (Figure 2). In order to avoid starvation of the prey, a mildly nutrient-enriched (that is, 1:10 dNB) environment was provided. Regardless of the presence of active predators dispersing along mycelia, LP6a cells were detected in all three zones with declining cell densities from zone A to C at all times (Figure 2). In zone A, despite constantly rising predator numbers, predation resulted in a statistically not significant increased prey biomass (Figure 2a) as reflected by a PI<0 (Figure 3). In the other zones, by contrast, prey populations decreased statistically significant by 12–22-fold (zone B) and 15–80-fold (zone C) relative to a predator-free control during the experiment (Figures 2b and c). This also led to a 43% (CI: 34–52%) decrease of the overall prey's population size (zone A–C) and simultaneous shift of the prey-to-predator ratio from 1.3 × 103 (CI: 106–1555) to 0.15 (CI: 0.01–0.4) from zone A–C within 72 h (Supplementary Figure SI2). In the experiments with glass fibres, likely due to the absence of P. ultimum competing for the dNB nutrients, significant growth of the LP6a cells and poor predation effects on LP6a abundances were observed (Supplementary Figure SI3).
Figure 3.
Effects of mycelia on the mean spatio-temporal predation impact (PI, cf. equation 1; n=3) by Bdellovibrio bacteriovorus 109J on Pseudomonas fluorescens LP6a. The value of PI varies between 1 (complete extinction) and 0 (no effect of predation). In case of an improved prey growth under predation pressure, the value of PI becomes negative.
Effect of P. fluorescens LP6a on directed dispersal of B. bacteriovorus 109J
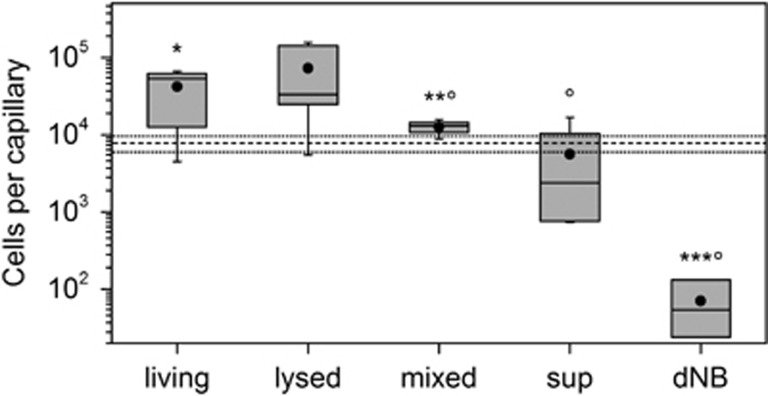
The possibility of directed movement of B. bacteriovorus 109J to prey cells was tested in a modified Adler capillary test (Supplementary Figure SI1) using either living LP6a cells, lysed LP6a cells, a 1:1 mixture of living and dead LP6a cells, a supernatant of LP6a cells, as well as either 1:10 dNB (control) or HM buffer (control) as possible attractants. The capillary tests suggested directed movement of the predators towards the LP6a cells (Figure 4) as statistically significant 5.7-fold (CI: 3.3–8.8) and 596-fold (CI: 251–4754) more predator cells were detected in capillaries filled with living cells than in a cell-free HM buffer and the 1:10 dNB, respectively (Figure 4). Increased attraction was also observed in the presence of a 1:1 mixture of living and dead LP6a cells (HM buffer: 1.7-fold (CI: 1.3–2.3); 1:10 dNB: 180-fold (CI: 88–2.5 × 103). Interestingly, suspensions of lysed LP6a cells showed no statistically significant accumulation of the predator either as compared with the HM buffer or 1:10 dNB (Figure 4). Avoidance of the predator was observed relative to HM buffer when capillaries were filled with 1:10 dNB or the supernatant of LP6a cell cultures, respectively (Figure 4).
Figure 4.
Number of PFU of Bdellovibrio bacteriovorus 109J cells in chemotaxis capillaries filled with Pseudomonas fluorescens LP6a (‘living'), 1:1 mixtures of living and dead LP6a prey cells (‘mixed'), the supernatant LP6a cells in the late exponential growth phase (‘sup') and the 1:10 dNB (‘dNB'). The horizontal solid and dashed lines represent the average and s.d. of the number of B. bacteriovorus 109J cells in capillaries filled with HM buffers only. Number of inoculated predator cells within the pond was 4 × 107 PFU per capillary. The plot shows data from six capillaries (except for data marked by the superscript ° where n=4 capillaries were analyzed). The asterisks refer to the statistically significant difference from control capillaries filled with HM buffer: *P=0.05; **P=0.01; ***P=0.001.
Discussion
Effect of mycelia on predator dispersal and prey population dynamics
Mycelia have been found to facilitate dispersal of bacteria thereby improving ecological functions such as biodegradation and nutrient cycling (Wick et al., 2007a; Banitz et al., 2011a, 2011b). Through their general effect on bacterial mobility, mycelia will also increase the probability of encounters between antagonistic bacteria such as predators and prey. We thus tested the hypothesis that the highly motile bacterial predator B. bacteriovorus 109J is able to use mycelia for dispersal with consequences for its predation success and the population dynamics of its prey, respectively. B. bacteriovorus 109J was chosen for the experiments because of its ubiquitous presence in the environment and its ability to attack a wide range of Gram-negative bacteria including Pseudomonads, which have key functions in soil including biodegradation of organic pollutants (Abbasnezhad et al., 2011; Adebusuyi et al., 2012). Pre-grown mycelia of P. ultimum were used to minimise theoretically possible passive spreading of predators attached to growing hyphae. Surprisingly, no dispersal of the predator along mycelia (and glass fibres as abiotic model dispersal networks) was observed in the absence of prey cells (controls III and V). As at least random dispersal of the predator along mycelia (and glass fibres) would be expected, this observation may be explained by the detection limit of our approach: given an inoculum of 8 × 103 B. bacteriovorus 109J cells and assuming that the predator is able to move along mycelia only (that is, not on the agar surface; cf. Supplementary Figure SI4; control VI), a one-dimensional diffusion coefficient of Deff=5.8 × 10−6 cm2 s−1 along hyphae (Kohlmeier et al., 2005), and an effective hyphae coverage of 10% of the total area, one would calculate that approximately six predators would disperse over 1 cm to zone A within 24 h (Lawler and Limic, 2010). In presence of prey cells, by contrast, dispersal and growth of B. bacteriovorus 109J along hyphae of P. ultimum was manifested by the presence of clearly detectable numbers of predator cells in habitats accessible by the ‘fungal highway' after 24 h (zones A–C). This reflects the importance of the presence of prey cells as carbon and energy sources for efficient long-distance colonisation of the mycelia by the predator. The predator's presence also clearly reduced prey populations (Figure 2). Thereby most significant effects on prey populations were observed in the least colonised zones B and C, where the relative predator impact rose along with drastically decreasing numbers of prey cells (Figure 2 and Supplementary Figure SI2) within the first 24 h after predator inoculation. Predation effects are in good agreement with previous studies on B. bacteriovorus predation on planktonic (Liu et al., 2014) and biofilm bacteria (Markelova and Colwell, 1999; Kadouri and O'Toole, 2005). Between 24 h and 72 h increased growth of the LP6a cells was observed in presence of the predator (zone B: ≈0.03 h−1; zone C: ≈0.02 h−1) as compared with its absence (zone B: ≈0.04 h−1; zone C: ≈0.06 h−1). No quantitative predation effect on LP6a cells was seen in the densely colonised zone A. Assuming that six to eight predator cells would be produced by one prey cell (Sockett, 2009) ~8 × 105 prey cells would have been killed in order to allow for growth of 5 × 105 B. bacteriovorus 190J cells in zone A and, hence, would be in the range of the s.d. of our replicate data. More interestingly, the high cell density in zone A did not inhibit dispersal of B. bacteriovorus 109J to zones B and C (Joubert et al., 2006; McDougald et al., 2012). Clear foraging effects by B. bacteriovorus 109J, however, became evident in the less populated zones B and C (Figures 2 and 3), suggesting that predation may be influenced by the density and the distribution of the prey. To corroborate the role of mycelia as physical dispersal infrastructures, we also installed parallel control experiments using glass fibres as artificial dispersal networks, whereby similar positive effects on the dispersal of predator cells were observed (Supplementary Figure SI3). However, due to the absence of competition for substrates between prey bacteria and P. ultimum, high prey cell numbers and, hence, poor apparent predation effects were observed.
Previous studies have shown that B. bacteriovorus responds tactically to yeast extract (Straley and Conti, 1974), amino acids (Lamarre et al., 1977), acetate or propionate (Straley et al., 1979; Rendulic et al., 2004; Chauhan and Williams, 2006) and potentially also towards its prey. We hence further tested in a modified capillary test whether dispersal of the B. bacteriovorus 109J would be directed by the presence of LP6a cells. Data from capillary experiments containing living LP6a cells pointed at a directed movement of the predators towards prey bacteria as evidenced by six and 596 times higher cell numbers in the capillaries filled with living LP6a cells as compared with capillaries filled with HM buffer and 1:10 dNB as used in the dispersal experiments, respectively (Figure 4). Although the effect on directed dispersal may appear small, it is statistically significant and also reproducible with a 1:1 mixtures of dead and living LP6a cells or E.coli ML-35 cells (ca. five-fold increase relative to HM buffer cf. Supplementary Figure SI5). It is also in good agreement with an earlier study assessing targeted predator–E.coli interactions in bioluminescent assays (Lambert et al., 2003). No directed dispersal towards the supernatant of prey cells was observed thus underpinning earlier studies (Straley and Conti, 1974). It rather seems that for presently unknown reasons intact bacteria effectively triggered directed movement, whereas no or unclear movement towards supernatants of prey cultures or cell lysates was found. A possible explanation still awaiting experimental verification would be that initial infection events are needed to trigger the attraction of further predators (Wadhams and Armitage, 2004). Data from our capillary experiments, hence, suggest that LP6a cells may act as attractant for predatory dispersal along mycelia, although this could not be underpinned unequivocally in our mycelia-based experiments.
Ecological relevance of predation along dispersal networks
Our data give evidence that hyphae increase the accessibility of prey bacteria to B. bacteriovorus 109J and, hence, allow for efficient foraging and shaping of prey populations not seen in the absence of mycelia. The mere existence of mycelia, however, seems not sufficient to enable efficient long-distance migration of the predator; likely due to the absence of the prey as carbon and energy source or its role as attractant for the predator, respectively. To test our hypothesis well-controlled, tailored microbial landscapes were used for better reduction of emerging properties in complex systems. Due to the novelty of our hypotheses, it does not come as a surprise that little is known about the prevalence and ecological consequences of predator–mycelium–prey interactions in soil systems. Quantitative application of our results to complex natural environments has to be performed with great care due to the often high heterogeneity of environmental (for example, soil) habitats. Our findings and their dimension, however, suggest that mycelial networks in natural habitats are locations of similar interactions. This is corroborated by the fact that dispersal networks in soil and other habitats influence multifarious functions relying on bacterial mobility such as biodegradation (Wick et al., 2007a; Otto et al., 2016; Worrich et al., 2016), oxalate turnover (Martin et al., 2012) or food spoilage (Lee et al., 2014). Hence, it is most likely that BALOs would use available mycelia for their dispersal also in natural settings. It also can be speculated that this may increase the fitness (for example, by increased nutrient cycling) of both predator and prey communities and their ecosystem functions. The detailed consequences for the size and functioning of prey populations will be highly circumstantial and difficult to predict. Further studies including functional end points such as biodegradation rates would be needed to judge the overall effect of increased predator mobility (Varon and Shilo, 1981). At present, the effects of (possibly prey-directed) predation on ecological or functional services in soil still remain unclear. Our findings, however, pave the way to research into the prevalence of predator–mycelium–prey interactions, the underlying mechanisms and their influence on ecosystem functions and services.
Acknowledgments
This project has been performed in the frame of the Helmholtz Association Research Programme ‘Terrestrial Environment' and contributes to the research topic Chemicals in the Environment (CITE). It also was supported by the research program Chemical Active Transport (CAT) and Networking Fund through the Helmholtz Interdisciplinary Graduate School for Environmental Research (HIGRADE). We thank Jana Reichenbach, Rita Remer and Manuel Trost for skilled technical help and advice.
Author contributions
SO and LYW designed the experiments; SO and EPB performed the experiments; SO analysed the data; SO, LYW and HH wrote the manuscript and Supplementary Information. All authors revised the manuscript and Supplementary Information.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Abbasnezhad H, Foght JM, Gray MR. (2011). Adhesion to the hydrocarbon phase increases phenanthrene degradation by Pseudomonas fluorescens LP6a. Biodegradation 22: 485–496. [DOI] [PubMed] [Google Scholar]
- Adebusuyi AA, Smith AY, Gray MR, Foght JM. (2012). The EmhABC efflux pump decreases the efficiency of phenanthrene biodegradation by Pseudomonas fluorescens strain LP6a. Appl Microbiol Biotechnol 95: 757–766. [DOI] [PubMed] [Google Scholar]
- Adler J. (1973). A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol 74: 77–91. [DOI] [PubMed] [Google Scholar]
- Banitz T, Fetzer I, Johst K, Wick LY, Harms H, Frank K. (2011a). Assessing biodegradation benefits from dispersal networks. Ecol Model 222: 2552–2560. [Google Scholar]
- Banitz T, Wick LY, Fetzer I, Frank K, Harms H, Johst K. (2011b). Dispersal networks for enhancing bacterial degradation in heterogeneous environments. Environ Pollut 159: 2781–2788. [DOI] [PubMed] [Google Scholar]
- Boonchan S, Britz ML, Stanley GA. (2000). Degradation and mineralization of high-molecular-weight polycyclic aromatic hydrocarbons by defined fungal-bacterial cocultures. Appl Environ Microbiol 66: 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma TNP, Middeldorp PJM, Schraa G, Zehnder AJB. (1997). Mass transfer limitation of biotransformation: quantifying bioavailability. Environ Sci Technol 31: 248–252. [Google Scholar]
- Chauhan A, Williams HN. (2006). Response of Bdellovibrio and like organisms (BALOs) to the migration of naturally occurring bacteria to chemoattractants. Curr Microbiol 53: 516–522. [DOI] [PubMed] [Google Scholar]
- Dashiff A, Junka RA, Libera M, Kadouri DE. (2011). Predation of human pathogens by the predatory bacteria Micavibrio aeruginosavorus and Bdellovibrio bacteriovorus. J Appl Microbiol 110: 431–444. [DOI] [PubMed] [Google Scholar]
- Dashiff A, Kadouri DE. (2011). Predation of oral pathogens by Bdellovibrio bacteriovorus 109J. Mol Oral Microbiol 26: 19–34. [DOI] [PubMed] [Google Scholar]
- Dwidar M, Monnappa AK, Mitchell RJ. (2012). The dual probiotic and antibiotic nature of Bdellovibrio bacteriovorus. BMB Rep 45: 71–78. [DOI] [PubMed] [Google Scholar]
- Ferguson MA, Nunez ME, Kim HJ, Goffredi S, Shamskhou E, Faudree L et al. (2014). Spatially organized films from Bdellovibrio bacteriovorus prey lysates. Appl Environ Microbiol 80: 7405–7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieller EC. (1940). The biological standardization of insulin. J R Stat Soc 7: 1–64. [Google Scholar]
- Foght J, Semple K, Westlake DWS, Blenkinsopp S, Sergy G, Wang Z et al. (1998). Development of a standard bacterial consortium for laboratory efficacy testing of commercial freshwater oil spill bioremediation agents. J Ind Microbiol Biotechnol 21: 322–330. [Google Scholar]
- Fox JW. (2002). Testing a simple rule for dominance in resource competition. Am Nat 159: 305–319. [DOI] [PubMed] [Google Scholar]
- Furuno S, Pazolt K, Rabe C, Neu TR, Harms H, Wick LY. (2010). Fungal mycelia allow chemotactic dispersal of polycyclic aromatic hydrocarbon-degrading bacteria in water-unsaturated systems. Environ Microbiol 12: 1391–1398. [DOI] [PubMed] [Google Scholar]
- Harms H, Wick LY. (2006). Dispersing pollutant-degrading bacteria in contaminated soil without touching it. Eng Life Sci 6: 252–260. [Google Scholar]
- Hübschmann T, Vogt C, Till S, Rohwerder T, Sand W, Harms H et al. (2007). Detection of sulfur microparticles in bacterial cultures by flow cytometry. Eng Life Sci 7: 403–407. [Google Scholar]
- Johnke J, Cohen Y, de Leeuw M, Kushmaro A, Jurkevitch E, Chatzinotas A. (2014). Multiple micro-predators controlling bacterial communities in the environment. Curr Opin Biotechnol 27: 185–190. [DOI] [PubMed] [Google Scholar]
- Johnsen AR, Wick LY, Harms H. (2005). Principles of microbial PAH-degradation in soil. Environ Pollut 133: 71–84. [DOI] [PubMed] [Google Scholar]
- Joubert LM, Wolfaardt GM, Botha A. (2006). Microbial exopolymers link predator and prey in a model yeast biofilm system. Microb Ecol 52: 187–197. [DOI] [PubMed] [Google Scholar]
- Kadouri D, O'Toole GA. (2005). Susceptibility of biofilms to Bdellovibrio bacteriovorus attack. Appl Environ Microbiol 71: 4044–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier S, Smits THM, Ford RM, Keel C, Harms H, Wick LY. (2005). Taking the fungal highway: mobilization of pollutant-degrading bacteria by fungi. Environ Sci Technol 39: 4640–4646. [DOI] [PubMed] [Google Scholar]
- Krell T, Lacal J, Reyes-Darias JA, Jimenez-Sanchez C, Sungthong R, Ortega-Calvo JJ. (2012). Bioavailability of pollutants and chemotaxis. Curr Opin Biotechnol 24: 451–456. [DOI] [PubMed] [Google Scholar]
- Lamarre AG, Straley SC, Conti SF. (1977). Chemotaxis toward amino-acids by Bdellovibrio-bacteriovorus. J Bacteriol 131: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert C, Smith MCM, Sockett RE. (2003). A novel assay to monitor predator-prey interactions for Bdellovibrio bacteriovorus 109 J reveals a role for methyl-accepting chemotaxis proteins in predation. Environ Microbiol 5: 127–132. [DOI] [PubMed] [Google Scholar]
- Lawler GF, Limic V. (2010) Random Walk: A Modern Introduction. Cambridge University Press: New York, NY, USA. [Google Scholar]
- Lee K, Kobayashi N, Watanabe M, Sugita-Konishi Y, Tsubone H, Kumagai S et al. (2014). Spread and change in stress resistance of Shiga toxin-producing Escherichia coli O157 on fungal colonies. Microb Biotechnol 7: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu S, Sun K, Sheng YH, Gu YJ, Gao YZ. (2014). Colonization on root surface by a phenanthrene-degrading endophytic bacterium and its application for reducing plant phenanthrene contamination. PLoS One 9: e108249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markelova NY. (2010). Predacious bacteria, Bdellovibrio with potential for biocontrol. Int J Hyg Environ Health 213: 428–431. [DOI] [PubMed] [Google Scholar]
- Markelova NY, Colwell RR. (1999). Two-component bacterial predator-prey system immobilized on the surface of a transparent plastic substrate is a promising model for investigation of the role of bacterial predators in ecosystems. Microbiology 68: 328–331. [Google Scholar]
- Martin G, Guggiari M, Bravo D, Zopfi J, Cailleau G, Aragno M et al. (2012). Fungi, bacteria and soil pH: the oxalate-carbonate pathway as a model for metabolic interaction. Environ Microbiol 14: 2960–2970. [DOI] [PubMed] [Google Scholar]
- Maurhofer M, Keel C, Schnider U, Voisard C, Haas D, Defago G. (1992). Influence of enhanced antibiotic production in Pseudomonas Fluorescens strain-Cha0 on its disease suppressive capacity. Phytopathology 82: 190–195. [Google Scholar]
- McDougald D, Rice SA, Barraud N, Steinberg PD, Kjelleberg S. (2012). Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal. Nat Rev Microbiol 10: 39–50. [DOI] [PubMed] [Google Scholar]
- Otto S, Banitz T, Thullner M, Harms H, Wick LY. (2016). Effects of facilitated bacterial dispersal on the degradation and emission of a desorbing contaminant. Environ Sci Technol 50: 6320–6326. [DOI] [PubMed] [Google Scholar]
- Pion M, Bshary R, Bindschedler S, Filippidou S, Wick LY, Job D et al. (2013a). Gains of bacterial flagellar motility in a fungal world. Appl Environ Microbiol 79: 6862–6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pion M, Spangenberg JE, Simon A, Bindschedler S, Flury C, Chatelain A et al. (2013b). Bacterial farming by the fungus Morchella crassipes. Proc Biol Sci 280: 2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendulic S, Jagtap P, Rosinus A, Eppinger M, Baar C, Lanz C et al. (2004). A predator unmasked: life cycle of Bdellovibrio bacteriovorus from a genomic perspective. Science 303: 689–692. [DOI] [PubMed] [Google Scholar]
- Rousk J, Demoling LA, Bahr A, Baath E. (2008). Examining the fungal and bacterial niche overlap using selective inhibitors in soil. FEMS Microbiol Ecol 63: 350–358. [DOI] [PubMed] [Google Scholar]
- Schamfuss S, Neu TR, van der Meer JR, Tecon R, Harms H, Wick LY. (2013). Impact of mycelia on the accessibility of fluorene to PAH-degrading bacteria. Environ Sci Technol 47: 6908–6915. [DOI] [PubMed] [Google Scholar]
- Semple KT, Doick KJ, Wick LY, Harms H. (2007). Microbial interactions with organic contaminants in soil: definitions, processes and measurement. Environ Pollut 150: 166–176. [DOI] [PubMed] [Google Scholar]
- Sockett RE. (2009). Predatory lifestyle of Bdellovibrio bacteriovorus. Annu Rev Microbiol 63: 523–539. [DOI] [PubMed] [Google Scholar]
- Son K, Brumley DR, Stocker R. (2015). Live from under the lens: exploring microbial motility with dynamic imaging and microfluidics. Nat Rev 13: 761–775. [DOI] [PubMed] [Google Scholar]
- Stolp H, Starr MP. (1963). Bdellovibrio bacteriovorus gen. Et Sp. N., a predatory, ectoparasitic, and bacteriolytic microorganism. Antonie Van Leeuwenhoek 29: 217–248. [DOI] [PubMed] [Google Scholar]
- Stolp H, Starr MP. (1965). Bacteriolysis. Annu Rev Microbiol 19: 79–104. [DOI] [PubMed] [Google Scholar]
- Straley SC, Conti SF. (1974). Chemotaxis in Bdellovibrio-Bacteriovorus. J Bacteriol 120: 549–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley SC, Lamarre AG, Lawrence LJ, Conti SF. (1979). Chemotaxis of Bdellovibrio-Bacteriovorus toward pure compounds. J Bacteriol 140: 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon M, Shilo M. (1981). Inhibition of the predatory activity of Bdellovibrio by various environmental-pollutants. Microb Ecol 7: 107–111. [DOI] [PubMed] [Google Scholar]
- Wadhams GH, Armitage JP. (2004). Making sense of it all: bacterial chemotaxis. Nat Rev Mol Cell Biol 5: 1024–1037. [DOI] [PubMed] [Google Scholar]
- Warmink JA, Nazir R, Corten B, van Elsas JD. (2011). Hitchhikers on the fungal highway: the helper effect for bacterial migration via fungal hyphae. Soil Biol Biochem 43: 760–765. [Google Scholar]
- Wick LY, Remer R, Wurz B, Reichenbach J, Braun S, Scharfer F et al. (2007a). Effect of fungal hyphae on the access of bacteria to phenanthrene in soil. Environ Sci Technol 41: 500–505. [DOI] [PubMed] [Google Scholar]
- Wick LY, Remer R, Würz B, Reichenbach J, Braun S, Scharfer F et al. (2007b). Effect of fungal hyphae on the access of bacteria to phenanthrene in soil. Environ Sci Technol 41: 500–505. [DOI] [PubMed] [Google Scholar]
- Worrich A, König S, Miltner A, Banitz T, Centler F, Frank K et al. (2016). Mycelia-like networks increase bacterial dispersal, growth and biodegradation in a model ecosystem at varying water potentials. Appl Environ Microbiol 82: 2902–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yair S, Yaacov D, Susan K, Jurkevitch E. (2003). Small eats big: ecology and diversity of Bdellovibrio and like organisms, and their dynamics in predator-prey interactions. Agronomie 23: 433–439. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.