Abstract
Purpose
All-trans retinoic acid (RA) supplementation was investigated as a method of enhancing the differentiation of human adipose-derived stem cells (ASCs) to corneal keratocytes in vitro, in combination with a chemically defined serum-free medium.
Methods
Adipose-derived stem cells were cultured in monolayer and supplemented with 0.1, 1, or 10 μM RA for 14 days. The effects of RA on cell proliferation, migration, and extracellular matrix (ECM) accumulation were evaluated. In addition, the expression of phenotypic keratocyte markers was examined by reverse transcription polymerase chain reaction (PCR), immunocytochemistry, and Western blotting.
Results
Adipose-derived stem cells cultured with RA showed improved cell proliferation and ECM production. In addition, RA enhanced the expression of keratocyte-specific markers, keratocan, aldehyde dehydrogenase 3A1, lumican, and decorin, when compared to serum-free media alone. Furthermore, the presence of RA increased the amount of collagen type I while reducing the expression of fibrotic marker, α-smooth muscle actin.
Conclusions
These findings indicate that RA is a useful supplement for promoting a keratocyte phenotype in ASC.
Translational Relevance
This study is particularly important for the generation of biological corneal substitutes and next generation cell based therapies for corneal conditions.
Keywords: retinoic acid, stem cells, keratocytes, differentiation
Introduction
Blindness caused by trauma and disease represents a significant global health challenge and the only viable treatment option in many cases is a keratoplasty.1,2 However, a worldwide shortage of donor corneas has prompted a need for alternative therapies. Cell-based therapies and tissue-engineered strategies have promising potential as alternatives to donor allografts to treat corneal blindness.3,4 Many studies have investigated the use of stromal keratocytes for this purpose. To attain the large number of cells required for tissue engineering, keratocytes must be expanded in serum-supplemented media before being redirected toward a keratocyte phenotype in vitro by culture in serum-free media. Culture in serum induces an in vivo wound healing process leading to corneal fibroblast (CF) and myofibroblast differentiation. However, previous studies have demonstrated only partial restoration of a keratocyte phenotype in CFs when serum was removed.5–10 Recently, stem cells have garnered considerable interest as a replacement cell source for tissue engineering.
Adipose-derived stem cells (ASCs) are a group of cells that can be readily isolated in high numbers from adipose-tissue with a minimally invasive procedure.11 These cells have multilineage potential, possess immunomodulatory properties, and have the capacity for self-renewal.12–15 Previous studies have demonstrated the potential of ASCs to undergo keratocyte differentiation in vitro and in vivo.12,16–19 However, there still is considerable scope to optimize the media conditions for this process. Retinoic acid (RA) is a metabolic derivative of vitamin A that has an essential role in developmental and signaling processes of the eye.20 It has been demonstrated previously that RA can influence the expression of keratocyte markers, including proteoglycans keratocan, decorin, and lumican, and aldehyde dehydrogenase 3A1 (ALDH3A1) in human keratocytes.21,22 In this study, the effect of an RA supplemented serum-free medium on ASCs was investigated to determine its ability to promote cell differentiation towards a keratocyte phenotype and enhance the expression of corneal specific proteoglycans.
Materials and Methods
All chemicals and reagents were purchased from Sigma-Aldrich Corp. (Arklow, Ireland), unless otherwise stated.
Isolation and Expansion of ASCs
Human subcutaneous adipose tissue was obtained following patient consent. This study received ethical approval from St. James's Hospital Institutional Review Board. Adipose-derived stem cells were isolated by collagenase digestion as described previously.19 Isolated ASCs were seeded at a density of 5000 cells/cm2 and cultured in high glucose Dulbecco's modified Eagle's medium (hgDMEM; Hyclone; Thermo Fisher Scientific, Dublin, Ireland) supplemented with 10% (vol/vol) fetal bovine serum (FBS; Hyclone), 100 U/mL penicillin (Gibco, Biosciences, Dublin, Ireland); 100 μg/mL streptomycin (Gibco), 250 ng/mL amphotericin-B, and 10 ng/mL fibroblast growth factor (FGF-2; R&D Systems, Abingdon, UK), hereafter called HGM. Cells were expanded to passage 2 and were cultured at 37°C with 5% CO2. Passage 3 (P3) ASCs were used in subsequent experiments.
Cell Culture Conditions
To promote keratocyte differentiation cells were cultured in keratocyte differentiation media23 (KM) consisting of advanced DMEM (Gibco) supplemented with 10 ng/mL FGF-2, 0.1 mM/mL L-ascorbic-acid-2-phosphate, and 2 mM/mL GlutaMax (Gibco) with the addition of penicillin/streptomycin and amphotericin B. Adipose-derived stem cells were cultured for 14 days in the following conditions: (1) KM; (2) KM supplemented with 0.1, 1, or 10 μM RA solubilized in DMSO, and (3) KM supplemented with DMSO. Cells were seeded at a density of 1 × 104 cells/cm2 for all experiments and media was changed every 2 days.
Cell Proliferation and Cell Motility
For cell proliferation, cells were incubated with PrestoBlue reagent (Molecular Probes; Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Cell viability was assessed at days 0, 3, 7, and 14. Results were calculated relative to viability at day 0. The effect of RA on cell migration was examined by a scratch assay as described previously.22 Migration was evaluated by imaging on day 0 and every day until the cell-free area was confluent using an inverted microscope (CKX41; Olympus Corporation, Tokyo, Japan) coupled to a digital camera (QImaging MicroPublisher 5.0 RTV; QImaging, Surrey, Canada). Each image was converted to binary format and migration was calculated using the tracing tool in ImageJ v1.46r (National Institutes of Health [NIH], Bethesda, MD). The rate of migration was calculated relative to the cell-free area at day 0.
Extracellular Matrix (ECM) Characterization
Quantitative Biochemical Analysis
Cells were digested with 125 μg/mL papain on day 14. Acid sulfated glycosaminoglycans (sGAG) content was measured using a dimethylmethylene blue (DMMB) dye binding assay (Blyscan; Biocolor Ltd, Antrim, UK) with bovine chondroitin sulfate as a standard. DNA was quantified using a Bisbenzimide Hoechst 33258 DNA assay with calf thymus DNA as a standard.24
Immunocytochemistry
Cells were cultured on μ-slides (Ibidi GmbH, Martinsried, Germany). Wells were washed briefly in phosphate buffered saline (PBS) and fixed in 4% (wt/vol) paraformaldehyde (PFA) for 15 minutes at room temperature (RT). Cells were incubated with blocking buffer consisting of 2% (wt/vol) bovine serum albumin in PBS for 30 minutes at RT, and incubated with anti-keratocan 1:50 (sc-66941; Santa Cruz, Heidelberg, Germany), anti-ALDH3A1 1:50 (ab76976; Abcam, Cambridge, United Kingdom), and anti-α-smooth muscle actin (αSMA) 1:50 (ab7817; Abcam) at 4°C for 18 hours. Cells were rinsed in PBS and incubated with fluorescently labelled antibodies for 1 hour at RT in the dark. Keratocan and ALDH3A1 were detected with donkey anti-rabbit AlexaFluor 488 (ab150073; Abcam) and αSMA was detected with goat anti-mouse biotin followed by ExtrAvidin-FITC (B7151 and E2761). Cells were rinsed in PBS and incubated with 1 mg/mL 4′, 6′-diamidino-2-phenylindole 1:500 (DAPI) for nuclear staining. Filamentous-actin (f-actin) staining was performed using Phalloidin-TRITC 1:50. Samples were photographed using an inverted epifluorescent microscope coupled to a digital camera (Olympus IX71; Olympus).
Real-Time Reverse Transcription Polymerase Chain Reaction (rtPCR)
Real-time reverse transcription PCR was performed to quantify relative gene expression as described previously.19 The primers examined were glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Hs02758991_g1), keratocan (KERA; Hs00559942_m1), ALDH3A1 (Hs00964880_m1), lumican (LUM; Hs00929860_m1), decorin (DCN; Hs00754870_s1), αSMA (Hs00426835_g1), collagen type I (COL1A1; Hs00164004_m1), and collagen type III (COL3A1; Hs00943809_m1; all from Applied Biosystems, Biosciences, Dublin, Ireland). Each gene of interest was normalized against GAPDH using the ΔΔCt method. Calculated values were expressed as a power of 2−ΔΔCt. All values then were normalized to KM at day 7.
Western Blotting
Protein was isolated on day 14 by lysis with ice-cold RIPA lysis buffer (Merck Millipore, Cork, Ireland) supplemented with ×1 protease inhibitor cocktail (Roche, West Sussex, UK) for 60 minutes. Samples were centrifuged and the supernatant was collected for analysis. Protein quantification was determined by bicinchoninic acid (BCA) assay (Thermo Fisher Scientific) according to the manufacturer's instructions. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed using 10% Mini Protean gels (Bio-Rad, Fannin Ltd., Dublin, Ireland). Protein was combined with ×2 Laemmli sample buffer (Bio-Rad) and loaded into wells of the gel. Gels were run at 100 V for 1.5 hours. Protein was transferred to a polyvinylidine fluoride (PVDF) membrane (Merck Millipore) via wet-transfer method at 100 V for 1 hour. For protein detection, membranes were incubated with ×1 blocking buffer (Molecular Devices, Sunnyvale, CA) for 1 hour at RT and incubated with primary antibodies against keratocan 1:200, ALDH3A1 1:1000, and αSMA 1:500 (same as immunocytochemistry) for 18 hours at 4°C. Membranes were incubated with Eu-labelled secondary antibodies (Molecular Devices) for 1 hour at RT in the dark. Mouse anti-GAPDH (ab9484; Abcam) was used as a protein loading control. Blots were resolved with ScanLater Western Blot Assay Kit using a SpectraMax i3 Multi-Mode Detection Platform (both Molecular Devices).
Statistical Analysis
All samples were examined in triplicate (n = 3) and statistical analysis was performed using GraphPad Prism 7 software (GraphPad Software, Inc., La Jolla, CA). All data are represented as a mean ± standard deviation (SD). The statistical differences were evaluated by 1-way or 2-way analysis of variance (ANOVA) followed by Bonferroni's post hoc multiple comparison test. Significance was accepted at a level of P < 0.05.
Results
Effect of RA on Cell Proliferation and Migration
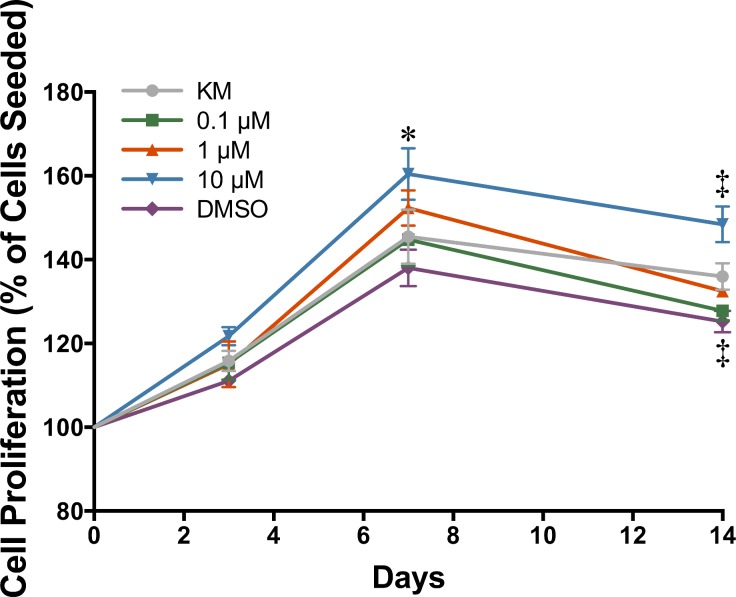
Cell proliferation was examined by PrestoBlue assay. All groups showed cell proliferation over the first 7 days of culture with no significant change thereafter (Fig. 1). Media containing 10 μM RA demonstrated a significant increase in cell proliferation at day 7 (160.43% ± 6.17%) and day 14 (148.44% ± 4.24%) compared to KM at the same time points, 145.47% ± 6.46% and 135.98% ± 3.15%, respectively. The addition of DMSO significantly reduced the rate of proliferation, 125.21% ± 2.55%, compared to KM at day 14. Cells cultured in 0.1 and 1 μM RA demonstrated no significant change compared to KM at any time point.
Figure 1.
Cell proliferation as determined by Presto Blue assay (n = 3 ± SD; significant difference compared to KM at *day 7 and ‡day 14, P < 0.05).
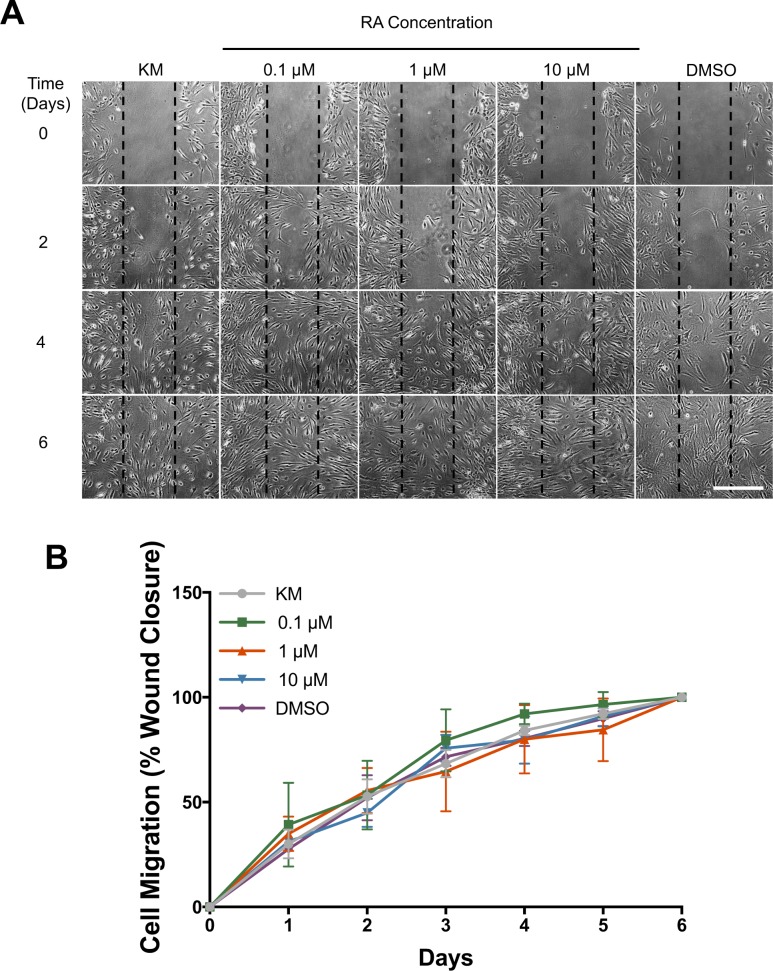
Cell migration was evaluated using a migration assay (Figs. 2A, 2B). There was no significant difference compared to KM alone for each group at any time point. At day 5 percentage cell migration for each group was as follows; KM 92.11% ± 2.50%, 0.1 μM 96.55% ± 5.97%, 1 μM 84.51% ± 14.96%, 10 μM 90.94% ± 4.80%, and DMSO 89.79% ± 3.51%, while all groups displayed 100% cell infiltration by day 6.
Figure 2.
(A) Cell migration over 6 days after scratch at day 0. Scale bar: 500 μm. (B) Quantification of cell migration (n = 3 ± SD).
Effect of RA on Total ECM Accumulation
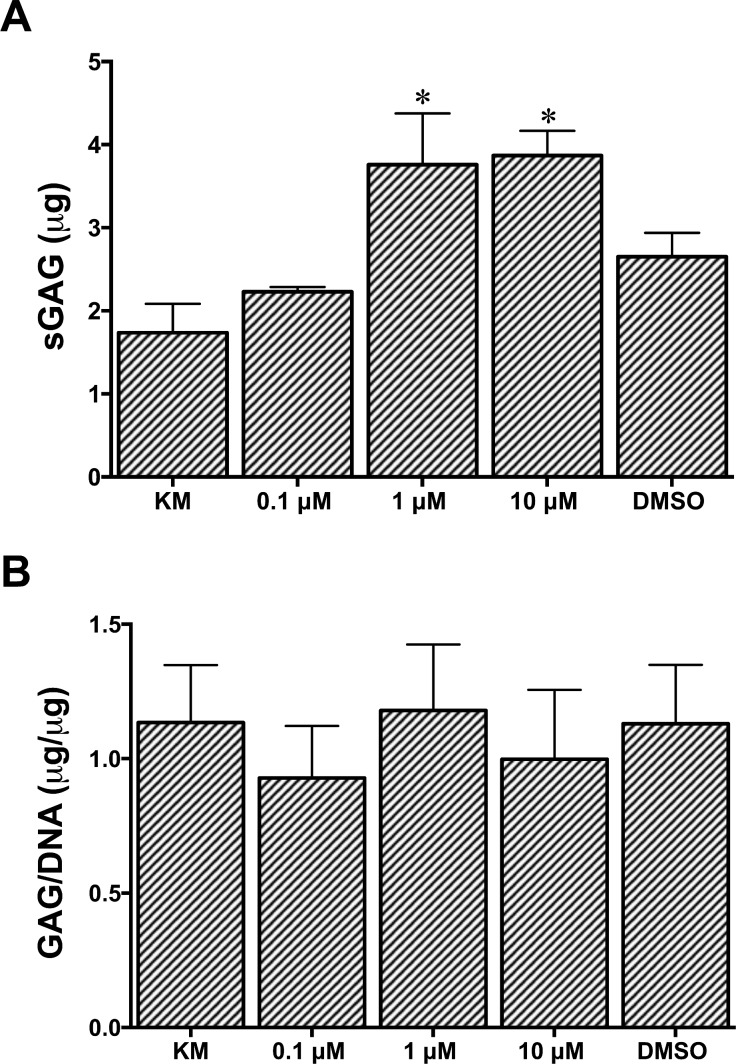
The synthesis of sGAG was examined by DMMB assay (Figs. 3A, 3B). Assay with 1 μM (3.76 ± 0.62 μg) and 10 μM (3.87 ± 0.30 μg) RA demonstrated significantly more sGAG was present when compared to KM (1.74 ± 0.35 μg) at day 14 (Fig. 3A). Cells treated with 0.1 μM RA (2.23 ± 0.06 μg) and DMSO (2.65 ± 0.29 μg) were not significantly different from KM. When the data were normalized to the DNA present at day 14, there was no significant difference between any of the groups (Fig. 3B).
Figure 3.
(A) sGAG as quantified by DMMB (n = 3 ± SD; *significant difference compared to KM at day 14, P < 0.05). (B) sGAG/DNA (n = 3 ± SD).
Expression of Keratocyte Markers
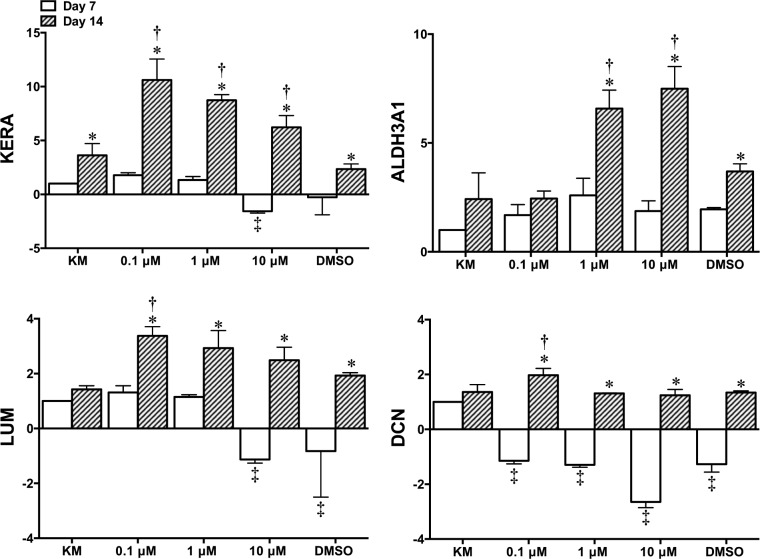
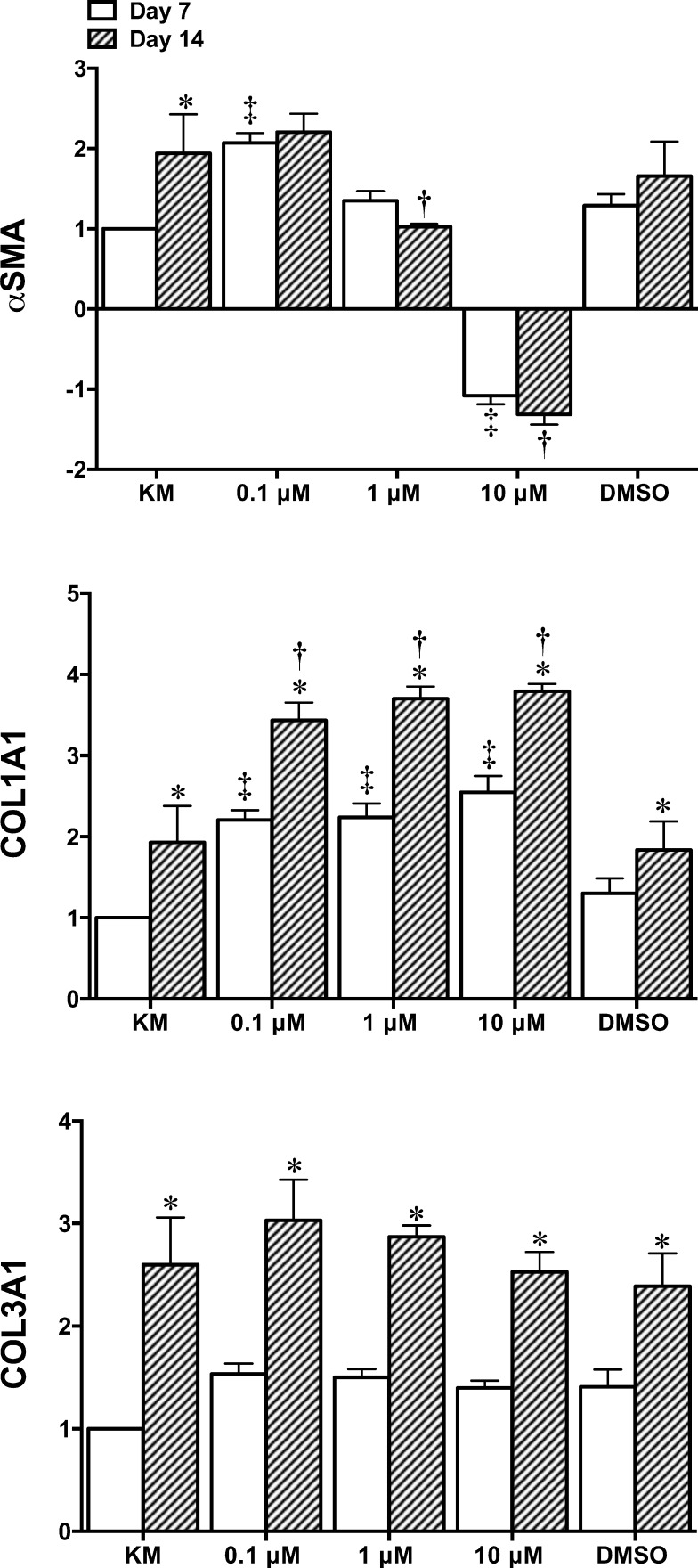
Expression level of keratocytes markers was evaluated using rtPCR after 7 and 14 days in culture. Results revealed that, in general, the addition of RA significantly increased the expression of keratocan, ALDH3A1, lumican, and decorin by day 14 (Fig. 4). Keratocan expression was significantly increased for 0.1, 1, and 10 μM RA (10.62 ± 1.94–, 8.73 ± 0.53– and 6.23 ± 1.09–fold increase, respectively) when compared to KM alone at day 14 (3.63 ± 1.09–fold increase, relative to KM at day 7). A significant increase in ALDH3A1 was demonstrated for 1 and 10 μM RA (6.58 ± 0.85– and 7.49 ± 1.02–fold increase, respectively) compared to KM alone (2.42 ± 1.21–fold increase). However, 0.1 μM RA was the only group to show a significant increase for lumican and decorin (3.37 ± 0.34– and 1.97 ± 0.25–fold increase, respectively) compared to KM at day 14 (1.43 ± 0.13– and 1.36 ± 0.27–fold increase, respectively). Genes coding for fibrotic/myofibroblastic markers αSMA and collagen type III and the main collagen in the cornea, type I were examined (Fig. 5). Results for αSMA revealed that 1 μM (1.03 ± 0.03–fold increase, relative to KM at day 7) and 10 μM (1.32 ± 0.18–fold decrease) RA displayed significantly lower expression than KM at day 14 (1.94 ± 0.23–fold increase). Results for collagen type III showed that although there was a significant increase for each culture condition from days 7 to 14 there was no difference compared to KM at day 14 (2.60 ± 0.46–fold increase). Collagen type I was significantly enhanced by day 14 for 0.1 μM (3.44 ± 0.22–fold increase), 1 μM (3.70 ± 0.15–fold increase), and 10 μM (3.79 ± 0.09–fold increase) RA compared to 1.99 ± 0.45–fold increase for KM.
Figure 4.
Fold change gene expression analysis of corneal-specific markers KERA, ALDH3A1, LUM, and DCN as quantified by rtPCR (n = 3 ± SD; *significant difference comparing days 7 to 14; ‡significant difference compared to KM at day 7; †significant difference compared to KM at day 14, P < 0.05).
Figure 5.
Fold change gene expression analysis of ECM markers αSMA, COL1A1, and COL3A1 as quantified by rtPCR (n = 3 ± SD; *significant difference comparing days 7 to 14l; ‡significant difference compared to KM at day 7; †significant difference compared to KM at day 14, P < 0.05).
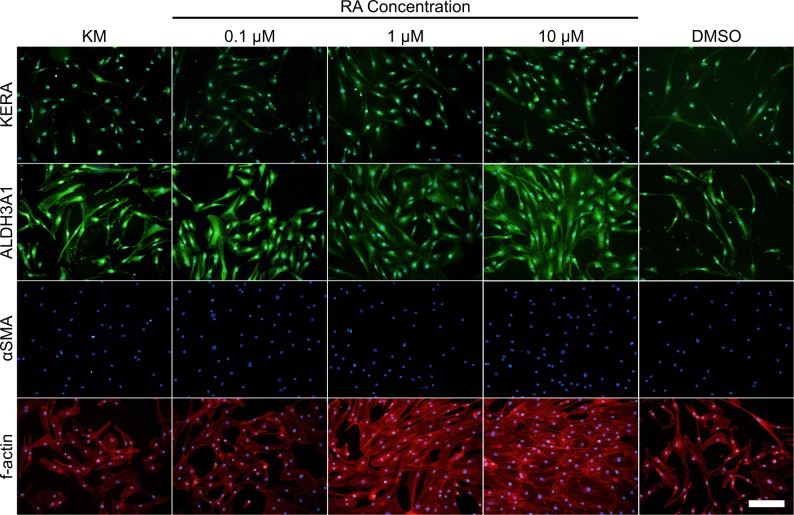
Protein expression was evaluated by immunofluorescent staining (Fig. 6) and Western blotting (Fig. 7). Keratocan was observed in all culture conditions with the staining intensity appearing to be enhanced in the presence of 1 and 10 μM RA. ALDH3A1 was shown to be present in all culture conditions when examined by immunofluorescent staining; however, 10 μM RA displayed enhanced expression. Western blot analysis demonstrated expression of keratocan and ALDH3A1; however, there was not any noticeable difference between groups. Fibrotic marker, αSMA was undetected for all groups by immunofluorescent staining; however, Western blot staining demonstrated that some αSMA was present in all RA containing groups. Morphology and actin filament organization of the cells also was examined using f-actin staining, results showed minimal differences in morphology between culture conditions (Fig. 6).
Figure 6.
Immunofluorescent staining of protein markers keratocan, ALDH3A1, αSMA (all green) and f-actin (red) after 14 days in culture. Nuclei were stained with DAPI (blue). Scale bar: 200 μm.
Figure 7.
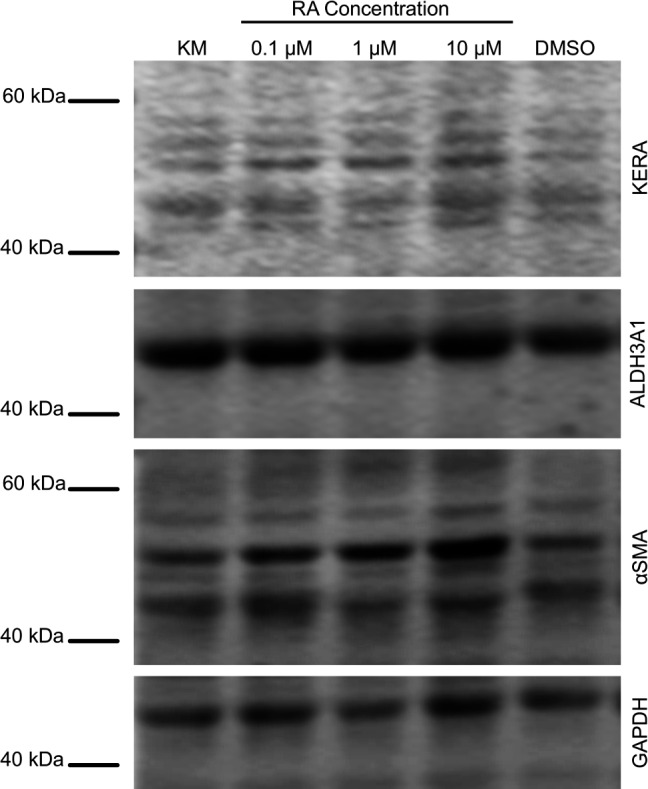
Expression of keratocyte and fibrotic markers keratocan, ALDH3A1, and αSMA at the protein level. Lysates from serum-free KM, RA supplemented a,nd DMSO control cells extracted at day 14 and analyzed by Western blotting.
Discussion
In this study, the addition of RA to serum-free media has been shown to enhance the differentiation of ASCs towards a keratocyte phenotype. While previous studies have demonstrated that RA can assist in maintaining the keratocyte-like characteristics of human corneal stromal keratocytes cultured in vitro,21 this is the first study we are aware of that demonstrates its ability to support stem cells isolated from other tissues towards a keratocyte lineage. Adipose-derived stem cells are a particularly attractive source of stem cells due to their ease of isolation, the high number of cells in the tissue and the high quantity of tissues available from which the cells can be obtained. In this study, multipotent ASCs were used instead of keratocytes or CFs as they represent an ideal autologous cell source for corneal tissue engineering.12 The multilineage potential of the cells was demonstrated (See Supplementary Figs. S1, S2) and when cultured in serum-free media alone, ASCs could be differentiated toward a keratocyte lineage similar to results reported previously.12,16,19 In this study, differentiated ASCs displayed significant upregulation of keratocan, a marker associated with a keratocyte phenotype and collagen type I, a marker associated with structurally appropriate corneal ECM synthesis.25 Here, RA supplementation was investigated to determine if it could modulate the differentiation of ASCs to significantly enhance the expression of corneal keratocyte markers, including keratocan, ALDH3A1, decorin, and lumican. Retinoic acid acts via either a nuclear receptor mediated pathway or by nonnuclear receptor mediated mechanisms to induce differentiation. Retinoic acid acts primarily by binding to RA receptors (RAR).26 These RARs act as transcription factors binding to RA response elements (RARE) in the nucleus or associate with retinoic X receptors (RXR) leading to transcriptional activation of target genes or repression of retinoid-controlled genes.27,28 To achieve differentiation to a keratocyte phenotype ASCs were cultured in a chemically defined serum-free medium that was supplemented with 0.1 to 10 μM RA for 14 days.
There was a significant increase in cell proliferation when a concentration of 10 μM RA was used. This concentration of RA has been shown previously to successfully stimulate proliferation of human keratocytes22 and DNA synthesis in rabbit stromal fibroblasts after 24 hours of exposure.29 However, differentiated ASCs displayed a slight reduction in cell number between days 7 and 14 for all conditions, mostly likely due to the use of serum-free media. Alternative media formulations may be more suited for prolonged culture periods. For example, it has been demonstrated that low glucose conditions allow human corneal stromal cells to survive for long periods in culture without compromising viability.30 Our serum-free medium has high levels of glucose compared to that observed in the corneal stroma,31 so future studies could examine this effect by transferring cells into lower glucose medium after initial keratocyte induction. Retinoic acid also has been shown to affect keratocyte migratory capacity. While cell migration and motility are not specific indicators of ASC differentiation, they have been used previously as an indicator of a quiescent phenotype normally associated with keratocytes in vivo.30,32 Increases in cell migration are associated with a stromal wound healing phenotype.33 Gouveia and Connon22 demonstrated a delay in cell migration rate in corneal stromal keratocytes supplemented with 1 to 10 μM RA, indicating a quiescent keratocyte phenotype. Other studies also have examined cell migration as a marker of cell quiescence in a variety of cell types.34–36 However, in our study although proliferation was increased in some conditions changes in cell motility were not observed. This may be due to limitations in the specificity of the assay used. However, the cell migration data would be of interest to those developing corneal stromal implants or tissue-engineered corneas since migration would be required to initiate tissue regeneration.
To generate corneal tissue, cells are required to synthesize and secrete corneal specific ECM. Retinoic acid supplementation significantly enhanced expression of corneal stromal ECM components, including proteoglycans keratocan, lumican, and decorin, corneal crystallin ALDH3A1, and collagen type I compared to the basal differentiation medium. Studies with human keratocytes demonstrated similar results with RA supplementation showing that gene and protein expression for keratocyte phenotypic markers was significantly increased.21,22 In addition, RA at 0.1 to 1 μM stimulated cell proliferation and release of hyaluronic acid, a GAG that has a role in corneal wound healing,37 from cultured rabbit epithelial cells and keratocytes.38 Furthermore, RA has been shown to have a beneficial effect on human corneal limbal epithelial cell differentiation inducing a nonkeratinized stratified epithelium with normal appearance.39 The upregulation of keratocytes markers by RA is important for their potential application in regenerative therapies since in vivo keratocyte phenotype is essential to the proper functioning of the cornea including the maintenance of corneal transparency.22 Furthermore, the addition of RA did not enhance the expression of fibrotic markers, αSMA, and collagen type III, and in some cases lead to significantly reduced expression of αSMA at the transcript level. However, it was observed by Western blotting that there was little change in protein expression of αSMA. A study investigating the effect of RA on the expression of αSMA in ASCs in a serum-reduced media demonstrated that 1 μM RA had limited effect on the expression of this fibrotic marker when examining protein abundance.40 However, in the same study they demonstrated a synergistic effect between RA and dibutyryl-cyclic adenosine monophosphate (d-cAMP) that led to a significant downregulation of αSMA expression. The mechanism behind this effect is not yet fully understood; however, it would be of interest to elucidate whether d-cAMP would have a similar effect in our study. In this study, the presence of 1 μM RA increased the expression of corneal specific markers, keratocan, ALDH3A1, and lumican, while upregulating collagen type I expression and downregulating αSMA, at the transcript level, compared to the serum-free media alone. This suggests that this concentration of RA might be optimal for ASC differentiation to keratocytes. Furthermore, it has been demonstrated that RARE sequences are absent in the promoter regions of genes coding for keratocyte markers, including collagen type-I and keratocyte-characteristic proteoglycans keratocan, lumican, and decorin suggesting that RA acts via indirect mechanisms through other signaling pathways to stimulate synthesis.22 This could include binding to extranuclear retinoid receptors, activation of or interaction with other signaling molecules and the mediation of effects via its metabolites.41
The addition of RA to ASCs could be combined with other methods to optimize the process of generating transplantable corneal tissue in vitro. For example, 3-dimensional (3D) culture systems have proved beneficial in the differentiation of ASCs to keratocytes, namely spheroidal and hydrogel culture.12,17 In addition, substrate/scaffold alignment has been associated with enhanced keratocyte phenotype in CFs and corneal stromal stem cells enabling cells to generate tissue that mimics the normal corneal stroma structure and ECM composition.9,10,25,42,43 Future directions could incorporate the use of 3D culture systems and RA supplementation to examine if ASCs can be guided to synthesize structurally appropriate ECM that would be essential to constructing biomimetic equivalents for corneal tissue engineering.
In conclusion, these results have demonstrated that the addition of RA can enhance the ability of ASCs to undergo keratocyte differentiation. Retinoic acid improved the expression of keratocyte specific markers, keratocan, ALDH3A1, lumican, and decorin, when compared to serum-free media alone. Furthermore, the presence of RA increased the amount of collagen type I. These findings indicated that RA is a useful supplement for promoting a keratocyte phenotype in ASCs and is particularly important for the generation of biological corneal substitutes and next generation cell based therapies for corneal conditions.
Supplementary Material
Acknowledgments
The authors thank Dr. Joanne Lysaght, Trinity College Dublin, and the staff at St. James's Hospital for their assistance in securing adipose tissue for cell isolation.
Supported by the Science Foundation Ireland & the Marie-Curie Action COFUND under Grant Number 11/SIRG/B2104.
Disclosure: A.P. Lynch, None; M. Ahearne, None
References
- 1. Pascolini D,, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012. ; 96: 614–618. [DOI] [PubMed] [Google Scholar]
- 2. Resnikoff S,, Pascolini D,, Mariotti SP,, Pokharel GP. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull World Health Organ. 2008. ; 86: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu Y,, Gan L,, Carlsson DJ,, et al. A simple, cross-linked collagen tissue substitute for corneal implantation. Invest Ophthalmol Vis Sci. 2006. ; 47: 1869–1875. [DOI] [PubMed] [Google Scholar]
- 4. Whitcher JP,, Srinivasan M,, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001. ; 79: 214–221. [PMC free article] [PubMed] [Google Scholar]
- 5. Berryhill BL,, Kader R,, Kane B,, Birk DE,, Feng J,, Hassell JR. Partial restoration of the keratocyte phenotype to bovine keratocytes made fibroblastic by serum. Invest Ophthalmol Vis Sci. 2002. ; 43: 3416–3421. [PubMed] [Google Scholar]
- 6. Scott SG,, Jun AS,, Chakravarti S. Sphere formation from corneal keratocytes and phenotype specific markers. Exp Eye Res. 2011. ; 93: 898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beales MP,, Funderburgh JL,, Jester JV,, Hassell JR. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: maintenance of the keratocyte phenotype in culture. Invest Ophthalmol Vis Sci. 1999. ; 40: 1658–1663. [PubMed] [Google Scholar]
- 8. Musselmann K,, Alexandrou B,, Kane B,, Hassell JR. Maintenance of the keratocyte phenotype during cell proliferation stimulated by insulin. J Biol Chem. 2005. ; 280: 32634–32639. [DOI] [PubMed] [Google Scholar]
- 9. Wu J,, Du Y,, Mann MM,, Funderburgh JL,, Wagner WR. Corneal stromal stem cells versus corneal fibroblasts in generating structurally appropriate corneal stromal tissue. Exp Eye Res. 2014. ; 120: 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson SL,, Wimpenny I,, Ahearne M,, Rauz S,, El Haj AJ,, Yang Y. Chemical and topographical effects on cell differentiation and matrix elasticity in a corneal stromal layer model. Adv Funct Mater. 2012. ; 22: 3641–3649. [Google Scholar]
- 11. Tsuji W,, Rubin JP,, Marra KG. Adipose-derived stem cells: implications in tissue regeneration. World J Stem Cells. 2014. ; 6: 312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Du Y,, Roh DS,, Funderburgh ML,, et al. Adipose-derived stem cells differentiate to keratocytes in vitro. Mol. 2010. ; 16: 2680–2689. [PMC free article] [PubMed] [Google Scholar]
- 13. Du Y,, Funderburgh ML,, Mann MM,, SundarRaj N,, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells. 2005. ; 23: 1266–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McIntosh Ambrose W,, Schein O,, Elisseeff J. A tale of two tissues: stem cells in cartilage and corneal tissue engineering. Curr Stem Cell Res Ther. 2010. ; 5: 37–48. [DOI] [PubMed] [Google Scholar]
- 15. Pittenger MF,, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004. ; 95: 9–20. [DOI] [PubMed] [Google Scholar]
- 16. Zhang S,, Espandar L,, Imhof KM,, Bunnell BA. Differentiation of human adipose-derived stem cells along the keratocyte lineage. J Clin Exp Ophthalmol. 2013. ; 4. [DOI] [PMC free article] [PubMed]
- 17. Espandar L,, Bunnell B,, Wang GY,, Gregory P,, McBride C,, Moshirfar M. Adipose-derived stem cells on hyaluronic acid-derived scaffold: a new horizon in bioengineered cornea. Arch Ophthalmol. 2012. ; 130: 202–208. [DOI] [PubMed] [Google Scholar]
- 18. Arnalich-Montiel F,, Pastor S,, Blazquez-Martinez A,, et al. Adipose-derived stem cells are a source for cell therapy of the corneal stroma. Stem Cells. 2008. ; 26: 570–579. [DOI] [PubMed] [Google Scholar]
- 19. Ahearne M,, Lysaght J,, Lynch A. Combined influence of basal media and fibroblast growth factor on the expansion and differentiation capabilities of adipose-derived stem cells. Cell Regen. 2014. ; 3: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duester G. Keeping an eye on retinoic acid signaling during eye development. Chem Biol Interact. 2009. ; 178: 178–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abidin FZ,, Gouveia RM,, Connon CJ. Application of retinoic acid improves form and function of tissue engineered corneal construct. Organogenesis. 2015. ; 11: 122–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gouveia RM,, Connon CJ. The effects of retinoic acid on human corneal stromal keratocytes cultured in vitro under serum-free conditions. Invest Ophthalmol Vis Sci. 2013. ; 54: 7483–7491. [DOI] [PubMed] [Google Scholar]
- 23. Du Y,, Sundarraj N,, Funderburgh ML,, Harvey SA,, Birk DE,, Funderburgh JL. Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma. Invest Ophthalmol Vis Sci. 2007. ; 48: 5038–5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim YJ,, Sah RL,, Doong JY,, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988. ; 174: 168–176. [DOI] [PubMed] [Google Scholar]
- 25. Karamichos D,, Funderburgh ML,, Hutcheon AEK,, et al. A role for topographic cues in the organization of collagenous matrix by corneal fibroblasts and stem cells. PLoS One. 2014. ; 9: e86260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gudas LJ,, Wagner JA. Retinoids regulate stem cell differentiation. J Cell Physiol. 2011. ; 226: 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Balmer JE,, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002. ; 43: 1773–1808. [DOI] [PubMed] [Google Scholar]
- 28. Bossenbroek NM,, Sulahian TH,, Ubels JL. Expression of nuclear retinoic acid receptor and retinoid X receptor mRNA in the cornea and conjunctiva. Curr Eye Res. 1998. ; 17: 462–469. [DOI] [PubMed] [Google Scholar]
- 29. Kirschner SE,, Ciaccia A,, Ubels JL. The effect of retinoic acid on thymidine incorporation and morphology of corneal stromal fibroblasts. Curr Eye Res. 1990. ; 9: 1121–1125. [DOI] [PubMed] [Google Scholar]
- 30. Foster JW,, Gouveia RM,, Connon CJ. Low-glucose enhances keratocyte-characteristic phenotype from corneal stromal cells in serum-free conditions. Sci Rep. 2015. ; 5: 10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCarey BE,, Schmidt FH. Modeling glucose distribution in the cornea. Curr Eye Res. 1990. ; 9: 1025–1039. [DOI] [PubMed] [Google Scholar]
- 32. Kim A,, Lakshman N,, Karamichos D,, Petroll WM. Growth factor regulation of corneal keratocyte differentiation and migration in compressed collagen matrices. Invest Ophthalmol Vis Sci. 2010. ; 51: 864–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sloniecka M,, Le Roux S,, Zhou Q,, Danielson P,, Substance P. Enhances keratocyte migration and neutrophil recruitment through interleukin-8. Mol Pharmacol. 2016. ; 89: 215–225. [DOI] [PubMed] [Google Scholar]
- 34. Guan J,, Zhang H,, Wen Z,, et al. Retinoic acid inhibits pancreatic cancer cell migration and EMT through the downregulation of IL-6 in cancer associated fibroblast cells. Cancer Lett. 2014. ; 345: 132–139. [DOI] [PubMed] [Google Scholar]
- 35. Flamini MI,, Gauna GV,, Sottile ML,, Nadin BS,, Sanchez AM,, Vargas-Roig LM. Retinoic acid reduces migration of human breast cancer cells: role of retinoic acid receptor beta. J Cell Mol Med. 2014. ; 18: 1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Phillips PA,, Wu MJ,, Kumar RK,, et al. Cell migration: a novel aspect of pancreatic stellate cell biology. Gut. 2003. ; 52: 677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gronkiewicz KM,, Giuliano EA,, Sharma A,, Mohan RR. Effects of topical hyaluronic acid on corneal wound healing in dogs: a pilot study (published online ahead of print April 7, 2016). Vet Ophthalmol. [DOI] [PubMed]
- 38. Toshida H,, Tabuchi N,, Koike D,, et al. The effects of vitamin A compounds on hyaluronic acid released from cultured rabbit corneal epithelial cells and keratocytes. J Nutr Sci Vitaminol (Tokyo). 2012. ; 58: 223–229. [DOI] [PubMed] [Google Scholar]
- 39. Kim SW,, Seo KY,, Rhim T,, Kim EK. Effect of retinoic acid on epithelial differentiation and mucin expression in primary human corneal limbal epithelial cells. Curr Eye Res. 2012. ; 37: 33–42. [DOI] [PubMed] [Google Scholar]
- 40. Lee WC,, Rubin JP,, Marra KG. Regulation of alpha-smooth muscle actin protein expression in adipose-derived stem cells. Cells Tissues Organs. 2006. ; 183: 80–86. [DOI] [PubMed] [Google Scholar]
- 41. Samarawickrama C,, Chew S,, Watson S. Retinoic acid and the ocular surface. Surv Ophthalmol. 2015. ; 60: 183–195. [DOI] [PubMed] [Google Scholar]
- 42. Phu D,, Wray LS,, Warren RV,, Haskell RC,, Orwin EJ. Effect of substrate composition and alignment on corneal cell phenotype. Tissue Eng Part A. 2011. ; 17: 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Torbet J,, Malbouyres M,, Builles N,, et al. Tissue engineering of the cornea: orthogonal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction. Conf Proc IEEE Eng Med Biol Soc. 2007. ; 2007: 6400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.