Abstract
Importance
Little is known about the benefits of antenatal corticosteroids on extremely preterm multiples.
Objective
To examine in extremely preterm multiples if use of antenatal corticosteroids is associated with improvement in major outcomes.
Design, Setting, and Participants
Infants with gestational age 22–28 weeks born at an NICHD Neonatal Research Network center (1998–2013) were studied. Generalized estimating equation models were used to generate adjusted relative risks (aRR) controlling for important maternal and neonatal variables.
Main Outcome Measures
In-hospital mortality, the composite outcome of neurodevelopmental impairment at 18–22 months’ corrected age or death before assessment.
Results
Of 6925 multiple-birth infants, 6094 (88%) were born to women who received antenatal corticosteroids. In-hospital mortality was lower among infants with exposure to antenatal corticosteroids vs no exposure (aRR=0.87, 95% CI 0.78–0.96). Neurodevelopmental impairment or death was not significantly lower among those exposed to antenatal corticosteroids vs no exposure (aRR=0.93, 95% CI 0.84–1.03). Other adverse outcomes that occurred less frequently among infants of women receiving antenatal corticosteroids included severe intraventricular hemorrhage (aRR=0.68, 95% CI 0.58–0.78) and the combined outcomes of necrotizing enterocolitis or death and severe intraventricular hemorrhage or death. Subgroup analyses indicated that exposure to antenatal corticosteroids was associated with a lower risk of mortality and the composite of neurodevelopmental impairment or mortality among non-small for gestational age multiples (aRR=0.82, 95% CI 0.74–0.92 and aRR=0.89, 95% CI 0.80–0.98, respectively) and a higher risk among small for gestational age multiples (aRR=1.40, 95% CI 1.02–1.93 and aRR=1.62, 95% CI 1.22–2.16, respectively). Antenatal corticosteroids were associated with higher neurodevelopmental impairment or mortality among multiple-birth infants of mothers with diabetes (aRR=1.55, 95% CI 1.00–2.38) but not among infants of mothers without diabetes (aRR=0.91, 95% CI 0.83–1.01).
Conclusion
In extremely preterm multiples, exposure to antenatal corticosteroids compared with no exposure was associated with a lower risk of mortality with no significant differences for the composite of neurodevelopmental impairment or death. Future research should investigate the increased risks of mortality and the composite of neurodevelopmental impairment or death associated with exposure to corticosteroids among small for gestational age multiples.
INTRODUCTION
Multiple gestation increases the risk of preterm delivery and the associated morbidities of prematurity.1 Among singleton pregnancies, a single course of antenatal corticosteroids (ANS) administered 24 hours to 7 days before birth to women in labor before 34 weeks’ gestation reduces early mortality, respiratory distress syndrome, intraventricular hemorrhage, and necrotizing enterocolitis.2 Data on ANS effects on multiples, including extremely preterm multiples have been limited and conflicting. Such contradictory findings have been reflected in the 1995 National Institutes of Health Consensus Conference recommendations, which state that evidence is insufficient to assess the effectiveness of ANS use among women with multiple gestations.3 The Cochrane review on ANS also called for further information to examine ANS effects in multiple pregnancies.2
We used data from the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) to examine whether ANS exposure in extremely preterm multiples at 22–28 weeks’ gestation is associated with a lower incidence of in-hospital mortality, the composite outcome of neurodevelopmental impairment or death, and other important morbidities.
METHODS
We analyzed data on multiples born at one of 28 NICHD NRN centers between January 1, 1998 and December 31, 2013 with gestational age (GA) 22–28 weeks included in a prospective registry. From 1998–2007, all inborn very low birth weight (VLBW, 401–1500 g) infants were included in the registry. In 2008, eligibility criteria changed to infants with GA 22–28 weeks or birth weight (BW) 401–1000 g or preterm infants enrolled in another NRN study. Trained research nurses prospectively entered maternal demographic, pregnancy and delivery information, and infant data collected from birth to discharge, transfer, death, or 120 days. The institutional review board at each center approved the registry.
Infants with a major congenital anomaly (n=211), infants with missing data on ANS administration (n=11), and twin infants where at least one of the set of twins died due to twin-to-twin-transfusion syndrome (n=26) were excluded from the analyses. Twin-to-twin-transfusion syndrome was only recorded if it was a cause of death. In our primary analyses, we also excluded 435 infants (251 at 22 weeks, 156 at 23 weeks, 21 at 24 weeks, and 7 at 25–28 weeks) who died within the first 12 hours after birth without receiving any intensive care (no ventilation, intubation, or medications) to ensure that postnatal care restriction did not affect our outcome estimates. In a sensitivity analysis, analyses were repeated with these infants included. Exposure to ANS was defined if the mother received ≥1 dose of betamethasone or dexamethasone. Data on whether the ANS course was full or partial were collected, but data on timing and dose were not available. Type of ANS was collected starting in 2002.
In-Hospital Outcomes
Primary in-hospital outcome included in-hospital death, defined as death of an infant before discharge or by 120 days for infants still hospitalized at that age. In-hospital secondary outcomes were morbidities diagnosed during hospital stay and recorded for infants surviving >12 hours including: respiratory distress syndrome (RDS), necrotizing enterocolitis (NEC) stage ≥IIA,4 severe intraventricular hemorrhage (IVH) (grade 3 or 4)5 or periventricular leukomalacia (PVL), retinopathy of prematurity (ROP) defined for infants still hospitalized at 28 days with severe ROP defined as ≥stage 3, bronchopulmonary dysplasia (BPD) defined as continuous use of supplemental oxygen at 36 weeks’ postmenstrual age, and the composite outcome of each morbidity or death. The composite outcome of morbidity or death was defined as present if the infant had the morbidity or died before the outcome was evaluated (for NEC, BPD, and IVH or PVL, death before 36 weeks PMA; for ROP, death before discharge) and absent if the infant survived until evaluation and did not have the morbidity.
Follow-Up Outcomes
The primary follow-up outcome included the composite of severe neurodevelopmental impairment (NDI) or death before assessment at 18 to 22 months’ corrected age (CA). Secondary follow-up outcomes at 18 to 22 months’ CA included death, NDI, and the individual components of NDI. Neurodevelopmental assessment was conducted by certified examiners for surviving infants if the infant weighed 401–1000 grams (those whose follow-up window opened before Jan. 1, 2008) at birth or was born at ≤26 weeks GA or was enrolled in an NRN study with follow-up (those whose follow-up window opened on or after Jan. 1, 2008). The definition of NDI at 18 to 22 months’ CA changed during the study period. From 1994–2005, NDI was defined as ≥1 of: Bayley-II Mental Developmental Index score <70, Bayley-II Psychomotor Developmental Index score <70, moderate to severe cerebral palsy, bilateral blindness, hearing impairment requiring hearing aids in both ears. From 2006–2009, NDI was defined as ≥1 of: Bayley-III cognitive composite score <70, gross motor function level ≥2, blindness (little or no useful vision in either eye), deafness (functional hearing impairment in both ears). From 2010–2013, the Bayley-III motor score was collected and a motor composite score <70 was added to the 2006–2009 NDI definition.
Statistical Analysis
Maternal and infant characteristics were compared between infants of mothers exposed to any ANS and infants of mothers not exposed to ANS using standard descriptive statistics. Chi-square test or t-test determined statistical significance for unadjusted comparisons. Generalized estimation equation (GEE) models6 were used to explore the association between exposure to ANS and outcomes, adjusting for maternal variables (age, marital status, race, diabetes, hypertension, rupture of membranes >24 hours), study center, and infant variables (sex, GA, small for gestational age (SGA)) while accounting for correlation due to clustering of multiple births within a mother. SGA was defined as <10th percentile using the Alexander et al. norms.7 For models examining outcomes at 18–22 months’ CA, we also adjusted for maternal education and study period to indicate Bayley-II vs Bayley-III assessments. In separate models, we examined potential effect modifiers of the primary outcomes by assessing the significance of the interaction term between ANS and each of: week of GA, diabetes vs no diabetes, hypertension vs no hypertension, chorioamnionitis vs no chorioamnionitis, vaginal vs cesarean delivery, SGA vs non-SGA, multiple vs singleton, twin vs higher-order multiple, and among twins, sex concordant vs sex discordant, and birth weight concordant vs birthweight discordant (where discordance was defined as a difference of >30% in BW between the twins, calculated by dividing the difference in BWs within a twin pair by the BW of the larger twin). Models with a significant interaction term were stratified by each subgroup accordingly. We also examined the associations between exposure to full or partial ANS and the primary outcomes. Adjusted relative risks (aRR) and 95% confidence intervals (CI) are reported based on robust GEE standard error estimates. A p-value <0.05 indicates statistical significance. P-values were not adjusted for multiple comparisons. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
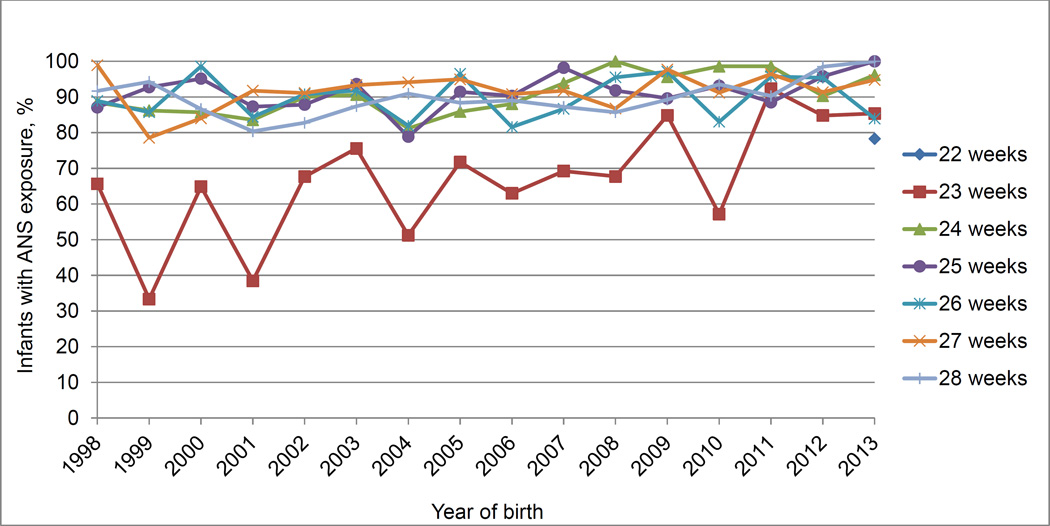
The study population for the primary analysis consisted of 6925 multiples (83.4% twins and 16.6% higher-order multiples) of whom 6094 (88%) were born to mothers who received ANS. Women who did not receive ANS were more likely to be black, unmarried, 19 years old or younger, and to have less than high school education (Table 1). Infants of mothers who received ANS were more likely to be born by cesarean delivery and to have a higher BW and GA. The follow-up rate for NDI assessment was slightly higher for those exposed to ANS (85%) than for those not exposed (80%) (p=0.03) (Table 1). The percentage of multiples whose mothers received ANS across the years is shown in Figure 1. The usage of ANS did not differ significantly by birth year or GA.
Table 1.
Infant and maternal characteristics among multiples by exposure to ANS (N=6925)
| Characteristic | No. (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 22 wk | 23 wk | 24 wk | 25 wk | Total 22–28 wk | p-valuea | ||||||
| ANS (n=68) |
No ANS (n=68) |
ANS (n=394) |
No ANS (n=181) |
ANS (n=893) |
No ANS (n=93) |
ANS (n=885) |
No ANS (n=89) |
ANS (n=6094) |
No ANS (n=831) |
||
| Infant | |||||||||||
| BW, mean (SD), g |
511 (75) |
502 (60) |
567 (81) | 574 (87) |
635 (100) |
656 (113) |
746 (118) |
749 (145) |
866 (235) |
794 (251) | <0.001 |
| SGA | 2 (3%) |
1 (1%) |
20 (5%) | 8 (4%) |
67 (8%) | 7 (8%) |
45/883 (5%) |
6 (7%) |
390/6091 (6%) |
50 (6%) |
0.71 |
| Male sex | 33(49%) | 35 (51%) |
213 (54%) |
94 (52%) |
469 (53%) |
53 (57%) |
508/884 (57%) |
45 (51%) |
3238/6092 (53%) |
420 (51%) |
0.16 |
| Twins | 57 (84%) |
61 (90%) |
346 (88%) |
144 (80%) |
751 (84%) |
83 (89%) |
734 (83%) |
82 (92%) |
5034 (83%) |
741 (89%) |
<0.001 |
| Higher orderb | 11 (16%) |
7 (10%) | 48 (12%) |
37 (20%) |
142 (16%) |
10 (11%) |
151 (17%) |
7 (8%) | 1060 (17%) |
90 (11%) | |
| Apgar score ≤3 at 5 min |
21 (31%) |
30/66 (45%) |
104/390 (27%) |
65/175 (37%) |
140 (16%) |
23 (25%) |
81/882 (9%) |
17/88 (19%) |
566 (9%) | 165/820 (20%) |
<0.001 |
| Intubation | 63 (93%) |
45/67 (67%) |
365/393 (93%) |
166/180 (92%) |
816/891 (92%) |
90 (97%) |
719 (81%) |
84 (94%) |
4228/6089 (69%) |
665/829 (80%) |
<0.001 |
| Delivery room resuscitation |
68 (100%) |
67/67 (100%) |
388/393 (99%) |
180/180 (100%) |
892/892 100%) |
93 (100%) |
884 (100%) |
89 (100%) |
6045/6090 (99%) |
828/829 (100%) |
0.04 |
| Surfactant use |
61 (90%) |
41 (60%) |
360 (91%) |
159 (88%) |
843 (94%) |
87 (94%) |
811/884 (92%) |
85 (96%) |
5105/6081 (84%) |
722 (87%) |
0.03 |
| Maternal | |||||||||||
| Age ≤19 y | 4 (6%) |
14 (21%) |
40 (10%) |
12 (7%) |
70 (8%) | 16 (17%) |
51 (6%) | 16 (18%) |
447 (7%) | 98 (12%) | <0.001 |
| Unmarried | 27/66 (41%) |
39/67 (58%) |
159/393 (40%) |
80/180 (44%) |
347/889 (39%) |
55 (59%) |
316/882 (36%) |
62/87 (71%) |
2232/6052 (37%) |
442/820 (54%) |
<0.001 |
| Race/ethnicity | <0.001 | ||||||||||
| Black | 23 (34%) |
41 (60%) |
142/392 (36%) |
73/177 (41%) |
284/877 (32%) |
38 (41%) |
281/880 (32%) |
56 (63%) |
1835/6039 (30%) |
384/825 (47%) |
|
| White | 45 (66%) |
27 (40%) |
231/392 (59%) |
91/177 (51%) |
539/877 (61%) |
55 (59%) |
563/880 (64%) |
32 (36%) |
3868 (64%) |
408 (49%) |
|
| Other | 0 (0%) |
0 (0%) |
19/392 (5%) |
13/177 (7%) |
54/877 (6%) |
0 (0%) |
36/880 (4%) |
1 (1%) |
336 (6%) |
33 (4%) |
|
| Cesarean delivery |
11 (16%) |
12 (18%) |
150 (38%) |
63/180 (35%) |
611/892 (69%) |
60 (65%) |
707/884 (80%) |
61 (69%) |
4661/6092 (77%) |
492/830 (59%) |
<0.001 |
| Diabetes | 0 (0%) |
0/67 (0%) |
7 (2%) |
7 (4%) |
30 (3%) | 3 (3%) |
26/884 (3%) |
3 (3%) |
246/6093 (4%) |
37/830 (4%) |
0.57 |
| Hypertension | 3 (4%) |
4/67 (6%) |
34 (9%) | 21 (12%) |
88 (10%) |
3 (3%) |
116/884 (13%) |
10 (11%) |
870/6091 (14%) |
99/830 (12%) |
0.07 |
| Less than high school degreec |
9/45 (20%) |
3/31 (10%) |
50/273 (18%) |
31/105 (30%) |
104/690 (15%) |
26/70 (37%) |
119/757 (16%) |
27/65 (42%) |
729/4578 (16%) |
164/545 (30%) |
<0.001 |
| Income <$20,000d |
4/7 (57%) |
0/1 (0%) |
13/75 (17%) |
13/28 (46%) |
80/345 (23%) |
16/32 (50%) |
129/473 (27%) |
26/39 (67%) |
576/2021 (29%) |
109/221 (49%) |
<0.001 |
| Medicaidc | 30/52 (58%) |
35/53 (66%) |
148/345 (43%) |
70/129 (54%) |
320/784 (41%) |
50/75 (67%) |
317/825 (38%) |
45/77 (58%) |
2169/5333 (41%) |
369/664 (56%) |
<0.001 |
| Non-speaking Englishe |
0/9 (0%) |
0/6 (0%) |
11/103 (11%) |
3/27 (11%) |
80/426 (19%) |
2/27 (7%) |
65/535 (12%) |
8/46 (17%) |
287/2265 (13%) |
33/230 (14%) |
0.47 |
| Follow-up rate (NDI)f |
9/9 (100%) |
6/7 (86%) |
102/113 (90%) |
23/32 (72%) |
400/464 (86%) |
24/37 (65%) |
508/596 (85%) |
45/48 (94%) |
2134/2509 (85%) |
210/263 (80%) |
0.03 |
Abbreviations: ANS, antenatal corticosteroids; BW, birth weight; GA, gestational age; NDI, neurodevelopmental impairment; SGA, small for gestational age.
P-value comparing total 22–28 wk ANS vs no ANS obtained from Fisher’s exact test for categorical variables and from non-parametric Wilcoxon 2-sample tests for BW and GA
Higher order multiples include: 937 triplets (92% with ANS), 162 quadruplets (96% with ANS), 34 quintuplets (85% with ANS), and 17 sextuplets (100% with ANS).
For infants born from 1998 to 2001, maternal education and Medicaid are only available if provided during follow-up. In 2002, both variables were collected for all infants irrespective of follow-up.
For infants born from 1998 to 2011, income is only available if provided during follow-up. Income was removed entirely in 2012.
Primary language of mother is only available if provided during follow-up.
Follow-up rate based on non missing NDI for 2772 infants, with an overall follow-up rate of 85%. Follow-up is only given for infants born between 1998 and June 2012.
Figure 1.
Frequency of Exposure to Antenatal Corticosteroids by Gestational Age and Year of Birth Among Multiples.
Cells with fewer than 20 infants per gestational age per year of birth are not reported.
Overall, 5361 (77.6%) infants survived to discharge and 1,548 (22.4%) died. Among infants at 22–28 weeks’ gestation, in-hospital mortality (20% with exposure vs 39% without exposure to ANS, aRR=0.87, 95% CI 0.78–0.96), IVH grade 3–4 and/or PVL (17% with exposure vs 29% without exposure to ANS, aRR=0.68, 95% CI 0.58–0.78), and the composite outcomes NEC and/or death and IVH grade 3–4 and/or PVL and/or death were significantly lower for multiples exposed to ANS compared to those without exposure (Table 2). There were no differences in the risks of RDS, BPD, NEC, and ROP between the two groups.
Table 2.
Hospital outcomes at 22 to 28 weeks’ gestation for multiples by exposure to ANS
| Outcome | ANS (n=6094) |
No ANS (n=831) |
No. missing |
|---|---|---|---|
| Death | |||
| No. (%) | 1220/6078 (20%) | 328 (39%) | 16 |
| RR (95% CI) | |||
| Unadjusted | 0.52 (0.46–0.59) | 16 | |
| Adjusteda | 0.87 (0.78–0.96) | 256 | |
| RDS | |||
| No. (%) | 5767/5892 (98%) | 708/722 (98%) | 311 |
| aRR (95% CI)a | 1.00 (0.99–1.02) | 523 | |
| BPDb | |||
| No. (%) | 2206/4947 (45%) | 222/514 (43%) | 1464 |
| aRR (95% CI)a | 1.08 (0.97–1.21) | 1618 | |
| NEC | |||
| No. (%) | 549/5895 (9%) | 62/723 (9%) | 307 |
| aRR (95% CI)a | 1.30 (0.98–1.72)c | 519 | |
| ROP | |||
| No. (%) | 2840/4875 (58%) | 307/517 (59%) | 1533 |
| aRR (95% CI)a | 1.05 (0.97–1.14) | 1683 | |
| Severe ROP | |||
| No. (%) | 814/4865 (17%) | 115/516 (22%) | 1544 |
| aRR (95% CI)a | 0.96 (0.80–1.15) | 1693 | |
| IVH grade 3–4 and/or PVL | |||
| No. (%) | 948/5725 (17%) | 192/672 (29%) | 528 |
| aRR (95% CI)a | 0.68 (0.58–0.78) | 727 | |
| BPDb or death | |||
| No. (%) | 3322/6063 (55%) | 536/828 (65%) | 34 |
| aRR (95% CI)a | 1.07 (1.01–1.14) | 272 | |
| NEC and/or death | |||
| No. (%) | 1512/6090 (25%) | 362 (44%) | 4 |
| aRR (95% CI)a | 0.91 (0.83–0.996) | 244 | |
| ROP and/or death | |||
| No. (%) | 1992/5934 (34%) | 432/824 (52%) | 167 |
| aRR (95% CI)a | 1.04 (0.96–1.13) | 398 | |
| IVH grade 3–4 and/or PVL and/or death | |||
| No. (%) | 1739/6061 (29%) | 416/828 (50%) | 36 |
| aRR (95% CI)a | 0.83 (0.76–0.90) | 273 | |
Abbreviations: ANS, antenatal corticosteroids; aRR, adjusted relative risk; BPD, bronchopulmonary dysplasia; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; PVL, periventricular leukomalacia; RDS, respiratory distress syndrome; ROP, retinopathy of prematurity; RR, relative risk.
Generalized estimation equation models adjusted for center, year of birth, maternal age (≤19 vs. ≥20 years), sex, race/ethnicity (black, white, or other), marital status, diabetes, hypertension, rupture of membrane more than 24 hours, gestational age, and small for gestational age. Composite outcomes include death by the age of diagnosis of morbidity (36 weeks for BPD, NEC, and IVH/PVL; discharge for severe ROP).
BPD defined as supplemental oxygen at 36 weeks, or discharged/transferred on oxygen prior to 36 weeks’ postmenstrual age and after 28 days of life.
Not adjusted for center; model did not converge with center included.
At the 18–22 month follow-up for eligible infants, mortality (30% vs 54%, aRR=0.71, 95% CI 0.60–0.84) was lower among those exposed to ANS compared to those not exposed to ANS. There were no differences in the risks of the composite outcome of NDI or death (54% vs 75%, aRR=0.93, 95% CI 0.84–1.03), NDI, or the individual components of NDI (Table 3).
Table 3.
Outcomes by 18 to 22 months’ corrected age for eligible multiples by exposure to ANS
| Outcome | ANS (n=3588) |
No ANS (n=575) |
No. missing |
|---|---|---|---|
| Death by follow-up | |||
| No. (%) | 1076/3586 (30%) | 312 (54%) | 2 |
| RR (95% CI) | |||
| Unadjusted | 0.57 (0.51–0.63) | 2 | |
| Adjusteda | 0.71 (0.60–0.84)c | 870 | |
| NDI or death | |||
| No. (%) | 1723/3210 (54%) | 392/522 (75%) | 431 |
| aRR (95% CI)a | 0.93 (0.84–1.03) | 1209 | |
| Among survivors | N=2510 | N=263 | |
| NDI | |||
| No. (%) | 647/2134 (30%) | 80/210 (38%) | 429 |
| aRR (95% CI)a | 0.97 (0.79–1.19)c | 521 | |
| MDI <70 | |||
| No. (%) | 419/1202 (35%) | 56/128 (44%) | 1443 |
| aRR (95% CI)a | 0.95 (0.76–1.20) | 1481 | |
| PDI <70 | |||
| No. (%) | 277/1180 (23%) | 40/127 (32%) | 1466 |
| aRR (95% CI)a | 0.76 (0.57–1.02) | 1505 | |
| Bayley III cognitive score <70 | |||
| No. (%) | 79/953 (8%) | 9/83 (11%) | 1737 |
| aRR (95% CI)a | 1.14 (0.52–2.51)c | 1789 | |
| Moderate to severe cerebral palsy | |||
| No. (%) | 155/2241 (7%) | 21/231 (9%) | 301 |
| aRR (95% CI)a | 0.98 (0.59–1.64)c | 400 | |
| Blindnessb | |||
| No. (%) | 29/2248 (1%) | 2/230 (1%) | 295 |
| aRR (95% CI)a | Model did not converge | ||
| Deafnessb | |||
| No. (%) | 59/2245 (3%) | 7/229 (3%) | 299 |
| aRR (95% CI)a | 0.69 (0.31–1.53)c | 398 | |
Abbreviations: ANS, antenatal corticosteroids; aRR, adjusted relative risk; MDI, mental development index; NDI, neurodevelopmental impairment; PDI, psychomotor development index; RR, relative risk.
Generalized estimation equation models adjusted for center, year of birth, maternal age (≤19 vs. ≥20 years), sex, race/ethnicity (black, white, or other), marital status, diabetes, hypertension, rupture of membrane more than 24 hours, gestational age, small for gestational age, and maternal education. Model for NDI or death and model for NDI also adjust for epoch.
From 1998 to 2005, blindness was defined as no functional vision (both eyes); deafness was defined as hearing impairment with aids in both ears. From 2006 to 2013, blindness was defined as no/some functional vision (both eyes); deafness was defined as hearing impairment (regardless of amplification).
Not adjusted for center; model did not converge with center included.
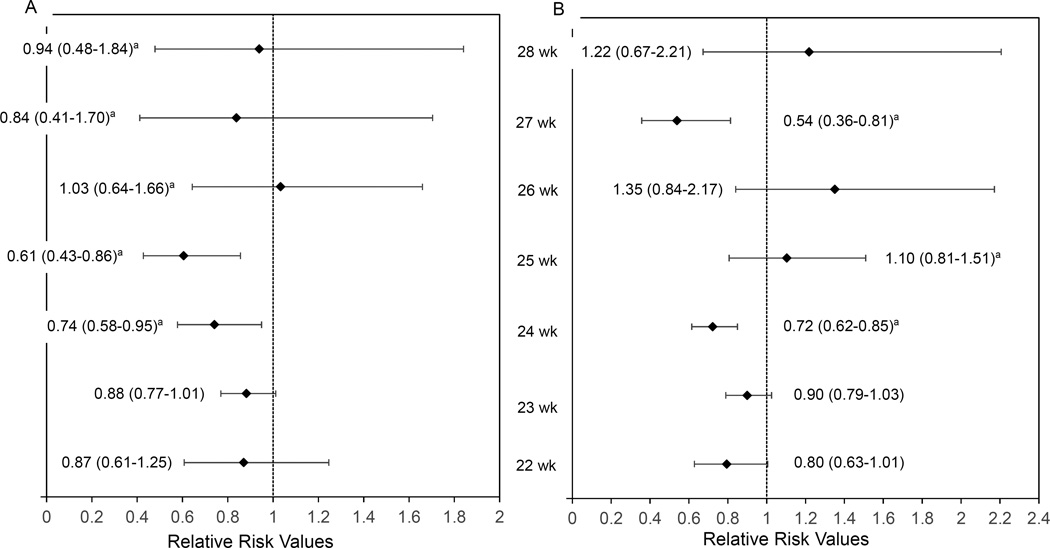
Interaction analyses performed for the primary outcomes in-hospital mortality and NDI or death showed that the association of ANS with outcomes did not differ for maternal hypertension vs no hypertension, chorioamnionitis vs no chorioamnionitis, vaginal vs cesarean delivery, multiple vs singleton, and for twins, sex concordant vs sex discordant and BW concordant vs BW discordant. The association of ANS with both outcomes, mortality and NDI or death, differed across the GA groups and SGA vs non-SGA. Infants born at 24, and 25 weeks’ gestation had a lower mortality with exposure to ANS compared to no exposure while there was no significant difference in mortality between the two groups for those born at 22, 23, 26, 27, and 28 weeks (Figure 2A). The composite outcome of NDI or death was only lower with exposure to ANS for those born at 24 and 27 weeks (Figure 2B). The relative risks of both in-hospital mortality and the composite outcome of NDI or death were lower by 18% and 11%, respectively, among non-SGA infants with exposure to ANS. Among SGA infants, however, the risks of mortality and the composite of NDI or death were significantly increased by 40% and 62%, respectively, among infants whose mothers were exposed to ANS compared to those not exposed (aRR=1.40, 95% CI 1.02–1.93; aRR=1.62, 95% CI 1.22–2.16) (eTable 1 in the Supplement). We explored further the risk for NDI or death and found that SGA multiples also had a higher NDI risk with exposure to ANS (aRR=2.40, 95% CI 1.24–4.65) (data not shown). ANS exposure was associated with increased NDI or death among multiple-birth infants of mothers with diabetes (aRR=1.55, 95% CI 1.00–2.38) but not among infants of mothers with diabetes (aRR=0.91, 95% CI 0.83–1.01) (eTable 1). For the in-hospital mortality outcome only, higher-order multiples had a higher reduction in mortality risk than twins with exposure to ANS (aRR= 0.66, 95% CI 0.53–0.82 vs aRR=0.90, 95% CI 0.81–1.01, respectively). Subgroup analysis showed that exposure to only full ANS treatment was associated with reduced risks of hospital mortality and NDI or death compared to no exposure, while exposure to partial ANS treatment was not associated with either outcome (eTable 1). When data collected after 2002 were analyzed by type of ANS, estimates of the main outcomes for dexamethasone vs no ANS exposure did not change but in-hospital mortality for betamethasone vs no exposure lost statistical significance (eTable 1). When we added the 435 early deaths that occurred during the first 12 hours after birth without receiving delivery room resuscitation, we observed very similar relative risks for the association of ANS with in-hospital mortality (data not shown). However, the addition of these infants resulted in a significant association of ANS on NDI or death (aRR=0.90; 95% CI 0.83–0.98), whereas previously, this result had not reached statistical significance (aRR=0.93; 95% CI 0.84–1.03).
Figure 2.
A. Adjusted relative risks by gestational age at 22–28 weeks for in-hospital mortality; B. Adjusted relative risks by gestational age at 22–28 weeks for neurodevelopmental impairment (NDI) or death at 18–22 months’ corrected age.
Models adjusted for center, year of birth, maternal age (≤19 vs. ≥20 years), sex, race/ethnicity (black, white, or other), marital status, diabetes, hypertension, small for gestational age, and rupture of membrane more than 24 hours. Regression for NDI or death also adjusts for epoch and maternal education.
aNot adjusted for center; model did not converge with center included.
The error bars indicate 95% CIs.
DISCUSSION
In this large cohort of extremely preterm multiples after adjustment for several potential confounders, exposure to ANS was associated with lower in-hospital mortality and reduced, albeit non-significantly, NDI or death risk. Subgroup analyses showed that exposure to ANS was associated with lower mortality at 24 and 25 weeks’ gestation. For infants born at 22, 23, 27, and 28 weeks, while the aRRs were lower with exposure to ANS, the results were not statistically significant; however, the 95% confidence intervals were wide and the power was inadequate. Only infants born at 24 and 27 weeks had lower NDI or death risk. Among SGA infants, exposure to ANS was associated with a 40% higher in-hospital mortality risk and a 62% higher NDI or death risk. Only the secondary outcome of severe IVH and/or PVL was significantly reduced with exposure to ANS.
Previous studies examining exposure to ANS and morbidities in multiples are limited. While there are no stand-alone clinical trials examining the impact of ANS in multiples, a recent meta-analysis of clinical trials with subgroup analysis in multiples reported no significant differences between multiples treated with ANS and controls for neonatal death (RR=0.79, 95% CI 0.39–1.61, two studies, 236 infants), RDS (RR=0.85, 95% CI 0.60–1.20, four studies, 320 infants), and cerebroventricular hemorrhage (RR=0.39, 95% CI 0.07–2.06, one study, 137 infants).2 Data from observational studies in multiples have been conflicting. Three studies, with sample sizes ranging between 168 and 1038 twin infants, showed that ANS had no association with the odds of RDS.8–10 However, in another report (n=416), premature twins treated with ANS compared to those without treatment had significantly lower odds of RDS (OR=0.49, 95% CI 0.27–0.91) and surfactant use (OR=0.58, 95% CI 0.34–0.99), while no significant differences were reported for mortality (OR=0.53, 95% CI 0.26–1.08) and severe IVH.11 A study of 259 twin infants showed a lower incidence of RDS only among those born at 28–32 weeks’ gestation.12 Similarly, in another study with a subgroup analysis of 88 twin infants, RDS was significantly reduced among those exposed to ANS.13
The efficacy of ANS therapy in multiples is a controversial topic. A study comparing the pharmacokinetics of betamethasone in singleton and twin pregnancies showed a shorter half-life and a greater clearance of the drug in twin pregnancies, suggesting that the dosage used in singleton pregnancies might be inadequate to yield a therapeutic level in multiple pregnancies.14 Another small twin study (n=18) suggested that the current regimen of ANS used in singleton pregnancies is adequate in preterm twin pregnancies as it results in similar fetal physiologic and behavioral changes.15 We did not find any evidence that the association between ANS and the main outcomes differed between singletons and multiples.
The higher mortality and NDI risk among SGA multiples exposed to ANS in our cohort is difficult to explain. Few studies limited to singletons have examined the association between ANS and SGA, and their results have been conflicting.16 In one study of 1931 SGA VLBW infants born between 22 and 33 weeks in Japan, there were no significant differences in hospital morbidities and neurodevelopmental outcomes assessed at 3 years between the ANS group and the non-ANS group.17 Similar results were found in another study of 220 SGA infants weighing ≤1750 g that examined only in-hospital morbidities without examining whether baseline differences in GA existed between the two groups.18 The same study, however, found improved in-hospital outcomes among appropriate for gestational age infants exposed to ANS compared to those not exposed.18 In a recent study of singletons and multiples born at 22 to 25 weeks using data from the NICHD NRN, Carlo et al. showed that exposure to ANS compared with no exposure was associated with a lower rate of NDI or death among non-SGA infants only; no significant results were seen among SGA infants.19 A review of cohort studies with 1067 SGA preterm infants found no evidence that ANS affected RDS, IVH, NEC, or death.20 However, data from a large cohort of 19,759 VLBW infants from the Vermont Oxford Network showed that ANS reduced RDS, IVH, and death in SGA infants born between 25 and 30 weeks.21 A matched case-control study of 62 growth restricted pairs due to placental insufficiency reported higher survival without disability at 2 years’ CA in the ANS group but a negative effect on physical growth at school age.22 Growth restricted infants are known to be stressed and have high levels of endogenous steroids, which might alter their response to ANS.23 The etiology of growth restriction in multiples is different from that in singletons, and the response to ANS might vary based on the cause of growth restriction. We did not have data on the etiology of SGA, a proxy for growth restriction, to explore this further, but future studies should reassess the benefits of ANS among SGA multiples.
Aside from SGA, higher-order multiples had a lower in-hospital mortality risk than twins only but no difference in NDI or death. Infants of mothers with diabetes had a higher NDI or death risk (aRR=1.55, 95% CI 1.00–2.38) while there was no difference for infants of mothers without diabetes. It is difficult to speculate on the reasons for this finding; maternal diabetes might impact how newborns respond to ANS. Data on the benefits of ANS among mothers with diabetes are limited as randomized controlled trials have usually excluded women with insulin-dependent or gestational diabetes.2
Strengths of this study are the large number of infants and the rigorous prospective data collection. Other strengths include the high follow-up rate for the assessment of neurodevelopmental outcomes (85%) and the adjustment for several maternal and neonatal variables. The inherent weaknesses of a cohort study design, however, must be acknowledged. While we adjusted for several factors, the baseline differences between the groups might indicate a number of co-interventions during and after delivery that could have resulted in better neonatal outcomes in the ANS group. We attempted to address this by doing a sensitivity analysis excluding infants who died during the first 12 hours without receiving delivery room resuscitation and showed similar outcome estimates. And while hospitals differ in providing active treatment for infants born at 22, 23, and 24 weeks’ gestation, this is less likely to impact infants born at 25 weeks’ gestation,24 a group for which we still showed lower mortality with ANS exposure. We did not have data on the timing and number of ANS doses; it is not known if and how these factors impact the ANS treatment effect.
CONCLUSIONS
Exposure to ANS among multiples was associated with lower overall mortality and lower NDI or death risk in some but not all GA groups. SGA multiples who survived had a higher mortality and NDI risk with exposure to ANS, calling for future studies to reassess the benefits of ANS in this subgroup of infants.
Supplementary Material
Acknowledgments
Funding Source: The National Institutes of Health (NIH), the Eunice Kennedy Shriver NICHD, the National Center for Research Resources, and the National Center for Advancing Translational Sciences provided grant support for the NRN Generic Database and Follow-Up Studies through cooperative agreements.
Role of the Sponsor: Dr. Rosemary Higgins, an NICHD employee and an author of this study, had input into the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication.
Appendix
Group Information: We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chairs: Alan H. Jobe, MD PhD, University of Cincinnati (2003–2006); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006–2011); Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University, (2011-present).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904) – William Oh, MD; Martin Keszler, MD; Betty R. Vohr, MD; Robert T. Burke, MD MPH; Bonnie E. Stephens, MD; Yvette Yatchmink, MD; Barbara Alksninis, RNC PNP; Angelita M. Hensman, MS RNC-NIC;; Kristin Basso, RN MaT; Elisa Vieira, RN BSN; Lenore Keszler, MD; Teresa M. Leach, MEd CAES; Martha R. Leonard, BA BS; Lucy Noel; Rachel A. Vogt, MD; Victoria E. Watson, MS CAS.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80) – Michele C. Walsh, MD; Avroy A. Fanaroff, MD; Deanne E. Wilson-Costello, MD; Nancy S. Newman, RN; Bonnie S. Siner, RN; Harriet G. Friedman, MA.
Children’s Mercy Hospital, University of Missouri Kansas City School of Medicine (U10 HD68284) – William E. Truog, MD; Eugenia K. Pallotto, MD MSCE; Howard W. Kilbride MD; Cheri Gauldin, RN BS CCRC; Anne Holmes, RN MSN MBA-HCM CCRC; Kathy Johnson, RN CCRC; Allison Knutson, RN BSN.
Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital (U10 HD27853, M01 RR8084) – Kurt Schibler, MD; Edward F. Donovan, MD; Kate Bridges, MD; Jean J. Steichen, MD; Kimberly Yolton, PhD; Barbara Alexander, RN; Estelle E. Fischer, MHSA MBA; Cathy Grisby, BSN CCRC; Marcia Worley Mersmann, RN; Holly L. Mincey, RN BSN; Jody Hessling, RN; Teresa L. Gratton, PA; Lenora D. Jackson, CRC; Kristin Kirker, CRC; Greg Muthig, BS.
Duke University School of Medicine, University Hospital, Duke Regional Hospital, and the University of North Carolina (U10 HD40492, M01 RR30) – Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Ricki F. Goldstein, MD; Kathy J. Auten, MSHS; Joanne Finkle, RN JD; Kimberley A. Fisher, PhD FNP-BC IBCLC; Katherine A. Foy, RN; Sandra Grimes, RN BSN; Kathryn E. Gustafson, PhD; Melody B. Lohmeyer, RN MSN; Matthew M. Laughon, MD MPH; Carl L. Bose, MD; Janice Bernhardt, MS RN; Gennie Bose, RN; Cynthia L. Clark, RN.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, M01 RR39) – David P. Carlton, MD; Ira Adams-Chapman, MD; Ellen C. Hale, RN BS CCRC; Yvonne C. Loggins, RN BSN; Diane I. Bottcher, RN MSN; Maureen Mulligan LaRossa, RN; Sheena L. Carter, PhD.
Eunice Kennedy Shriver National Institute of Child Health and Human Development–Linda L. Wright, MD; Elizabeth M. McClure, MEd; Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750) – Gregory M. Sokol, MD; Brenda B. Poindexter, MD MS; James A. Lemons, MD; Anna M. Dusick, MD; Carolyn Lytle, MD MPH; Lon G. Bohnke, MS; Greg Eaken, PhD; Faithe Hamer, BS; Dianne E. Herron, RN; Abbey Hines, PsyD; Lucy C. Miller, RN BSN CCRC; Heike M. Minnich, PsyD HSPP; Lu-Ann Papile, MD; Leslie Richard, RN; Leslie Dawn Wilson, BSN CCRC.
Nationwide Children’s Hospital and the Ohio State University Medical Center (U10 HD68278) – Pablo J. Sánchez, MD; Leif D. Nelin, MD; Sudarshan R. Jadcherla, MD; Patricia Luzader, RN; Christine A. Fortney, PhD RN; Gail E. Besner; Nehal A. Parikh, MD.
RTI International (U10 HD36790) – W. Kenneth Poole, PhD (deceased); Dennis Wallace, PhD; Jamie E. Newman, PhD MPH; Jeanette O’Donnell Auman, BS; Margaret M. Crawford, BS CCRP; Betty K. Hastings; Elizabeth M. McClure, MEd; Carolyn M. Petrie Huitema, MS CCRP; Kristin M. Zaterka-Baxter, RN BSN CCRP.
Stanford University, California Pacific Medical Center, Dominican Hospital, El Camino Hospital, and Lucile Packard Children’s Hospital (U10 HD27880, M01 RR70) – Krisa P. Van Meurs, MD; David K. Stevenson, MD; Susan R. Hintz, MD MS Epi; M. Bethany Ball, BSc CCRC; Marian M. Adams, MD; Charles E. Ahlfors, MD; Joan M. Baran, PhD; Barbara Bentley, PhD; Lori E. Bond, PhD; Ginger K. Brudos, PhD; Alexis S. Davis, MD MS; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN PNP; Barry E. Fleisher, MD; Lynne C. Huffman, MD; Jean G. Kohn, MD MPH; Casey Krueger, PhD; Julie C. Lee-Ancajas, PhD; Andrew W. Palmquist, RN; Melinda S. Proud, RCP; Renee P. Pyle, PhD; Dharshi Sivakumar, MD; Robert D. Stebbins, MD; Nicholas H. St. John, PhD; Halie E. Weiss, MD.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54) – Ivan D. Frantz III, MD; Elisabeth C. McGowan, MD; Brenda L. MacKinnon, RNC; Ellen Nylen, RN BSN; Anne Furey, MPH; Ana Brussa, MS OTR/L; Cecelia Sibley, PT MHA.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32) – Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD MPH; Kathleen G. Nelson, MD; Kirstin J. Bailey, PhD; Fred J. Biasini, PhD; Stephanie A. Chopko, PhD; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Mary Beth Moses, PT MS PCS; Vivien A. Phillips, RN BSN; Julie Preskitt, MSOT MPH; Richard V. Rector, PhD; Sally Whitley, MA OTR-L FAOTA.
University of California - Los Angeles, Mattel Children’s Hospital, Santa Monica Hospital, Los Robles Hospital and Medical Center, and Olive View Medical Center (U10 HD68270) – Uday Devaskar, MD; Meena Garg, MD; Isabell B. Purdy, PhD CPNP; Teresa Chanlaw, MPH; Rachel Geller, RN BSN.
University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10 HD40461) – Neil N. Finer, MD; Maynard R. Rasmussen MD; Yvonne E. Vaucher, MD MPH; Paul R. Wozniak, MD; Kathy Arnell, RNC; Renee Bridge, RN; Clarence Demetrio, RN; Martha G. Fuller, RN MSN; Wade Rich, BSHS RRT.
University of Iowa and Mercy Medical Center (U10 HD53109, M01 RR59) – John A. Widness, MD; Dan L. Ellsbury, MD; Tarah T. Colaizy, MD MPH; Michael J. Acarregui, MD; Karen J. Johnson, RN BSN; Donia B. Campbell, RNC-NIC; Diane L. Eastman, RN CPNP MA.
University of Miami, Holtz Children’s Hospital (U10 HD21397, M01 RR16587) – Shahnaz Duara, MD; Charles R. Bauer, MD; Ruth Everett-Thomas, RN MSN; Amy Mur Worth, RN MS; Mary Allison, RN; Alexis N. Diaz, BA; Elaine E. Mathews, RN; Kasey Hamlin-Smith, PhD; Lissa Jean-Gilles, BA; Maria Calejo, MS; Silvia M. Frade Eguaras, BA; Silvia Fajardo-Hiriart, MD; Yamiley C. Gideon, BA; Michelle Harwood Berkovits, PhD; Alexandra Stoerger, BA; Andrea Garcia, MA; Helena Pierre, BA; Georgette Roder, BSW; Arielle Riguad, MD.
University of New Mexico Health Sciences Center (U10 HD27881, U10 HD53089, M01 RR997) – Kristi L. Watterberg, MD; Andrea Freeman Duncan, MD MScr; Janell Fuller, MD; Robin K. Ohls, MD; Lu-Ann Papile, MD; Conra Backstrom Lacy, RN; Sandra Brown, RN BSN; Jean R. Lowe, PhD; Rebecca Montman, BSN.
University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, and Children’s Hospital of Philadelphia (U10 HD68244) – Barbara Schmidt, MD MSc; Haresh Kirpalani, MB MSc; Sara B. DeMauro, MD MSCE; Aasma S. Chaudhary, BS RRT; Soraya Abbasi, MD; Toni Mancini, RN BSN CCRC; Dara M. Cucinotta, RN; Judy C. Bernbaum, MD; Marsha Gerdes, PhD; Hallam Hurt, MD.
University of Rochester Medical Center, Golisano Children’s Hospital, and the University of Buffalo Women’s and Children’s Hospital of Buffalo (U10 HD68263, U10 HD40521, M01 RR44, UL1 TR42) – Carl T. D’Angio, MD; Dale L. Phelps, MD; Ronnie Guillet, MD PhD; Satyan Lakshminrusimha, MD; Gary J. Myers, MD; Erica Burnell, RN; Stephanie Guilford, BS; Cassandra A. Horihan, MS; Diane Hust, MS RN CS; Rosemary L. Jensen; Julie Babish Johnson, MSW; Emily Kushner, MA; Deanna Maffett, RN; Joan Merzbach, LMSW; Linda J. Reubens, RN CCRC; Ann Marie Reynolds, MD; Mary Rowan, RN; Holly I.M. Wadkins; Ashley Williams, MS Ed; Karen Wynn, NNP RN; Kelly Yost, PhD; Lauren Zwetsch, RN MS PNP.
University of Tennessee Health Science Center (U10 HD21415) – Sheldon B. Korones, MD; Henrietta S. Bada, MD; Tina Hudson, RN BSN; Marilyn Williams, LCSW; Kimberly Yolton, PhD.
University of Texas Southwestern Medical Center, Parkland Health & Hospital System and Children’s Medical Center Dallas (U10 HD40689, M01 RR633) – Myra Wyckoff, MD; Luc P. Brion, MD; Pablo J. Sánchez, MD; Abbot R. Laptook, MD; Charles R. Rosenfeld, MD; Walid A. Salhab, MD; R. Sue Broyles, MD; Roy J. Heyne, MD; Merle Ipson, MD; Sally S. Adams, MS RN CPNP; P. Jeannette Burchfield, RN BSN; Lijun Chen, PhD RN; Cristin Dooley, PhD LSSP; Alicia Guzman; Gaynelle Hensley, RN; Elizabeth T. Heyne, MS MA PA-C PsyD; Jackie F. Hickman, RN; Melissa H. Leps, RN; Linda A. Madden, BSN RN CPNP; Susie Madison, RN; Nancy A. Miller, RN; Janet S. Morgan, RN; Lizette E. Torres, RN; Cathy Twell Boatman, MS CIMI; Diana M. Vasil, RNC-NIC.
University of Texas Health Science Center at Houston Medical School, Children’s Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373) – Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Patrick M. Jones, MD;
Patricia W. Evans, MD; Esther G. Akpa, RN BSN; Nora I. Alaniz, BS; Julie Arldt-McAlister, RN BSN; Katrina Burson, RN BSN; Magda Cedillo Guajardo, RB BSN FAACM; Susan E. Dieterich, PhD; Beverly Foley Harris, RN BSN; Claudia I. Franco, RNC MSN; Carmen Garcia, RN CCRP; Charles Green, PhD; Margarita Jiminez, MD MPH; Janice John, CPNP; Anna E. Lis, RN BSN; Terri Major-Kincade, MD MPH; Karen Martin, RN; Sara C. Martin, RN BSN; Georgia E. McDavid, RN; Brenda H. Morris, MD; Patricia Ann Orekoya, RN BSN; Patti L. Pierce Tate, RCP; M. Layne Poundstone, RN BSN; Stacey Reddoch, BA; Shawna Rodgers, RN; Saba Khan Siddiki, MD; Maegan C. Simmons, RN; Daniel Sperry, RN; Laura L. Whitely, MD; Sharon L. Wright, MT.
University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (U10 HD53124, M01 RR64) – Roger G. Faix, MD; Bradley A. Yoder, MD; Michael Steffen, MS CPM; Shawna Baker, RN; Karie Bird, RN; Jill Burnett, RN; Jennifer J. Jensen, RN BSN; Karen A. Osborne, RN BSN CCRC; Cynthia Spencer, RNC; Kimberlee Weaver-Lewis, RN MS; Sarah Winter, MD; Karen Zanetti, RN.
Wake Forest University Baptist Medical Center, Brenner Children’s Hospital, and Forsyth Medical Center (U10 HD40498, M01 RR7122) – T. Michael O’Shea, MD MPH; Robert G. Dillard, MD; Nancy J. Peters, RN CCRP; Korinne Chiu, MA; Deborah Evans Allred, MA LPA; Donald J. Goldstein, PhD; Raquel Halfond, MA; Barbara G. Jackson, RN BSN; Carroll Peterson, MA; Dia Roberts, BSN PNP; Ellen L. Waldrep, MS; Melissa Whalen Morris, MA; Gail Wiley Hounshell, PhD; Lisa K. Washburn, MD; Cherrie D. Welch, MD MPH.
Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385) – Seetha Shankaran, MD; Beena G. Sood, MD MS; Athina Pappas, MD; John Barks, MD; Yvette R. Johnson, MD MPH; Rebecca Bara, RN BSN; Laura Goldston, MA; Mary E. Johnson, RN BSN; Deborah Kennedy, RN BSN; Geraldine Muran, RN BSN; Elizabeth Billian, RN MBA; Laura Sumner, RN BSN; Kara Sawaya, RN BSN; Mary K. Christensen, BA RRT; Stephanie A. Wiggins, MS.
Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, UL1 TR142, M01 RR125, M01 RR6022) – Richard A. Ehrenkranz, MD; Christine Butler, MD; Harris Jacobs, MD; Patricia Cervone, RN; Nancy Close, PhD; Patricia Gettner, RN; Walter Gilliam, PhD; Sheila Greisman, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Elaine Romano, MSN; Janet Taft, RN BSN; Joanne Williams, RN BSN.
Footnotes
Trial Registration: clinicaltrials.gov identifiers: NCT00063063 (Generic Database) and NCT00009633 (Follow-Up Study).
Author Contributions: Dr. Das and Mr. McDonald had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Boghossian, Bell, Carlo.
Acquisition of data: Bell, Carlo, Stoll, Laptook, Shankaran, Walsh.
Analysis and interpretation of data: Boghossian, Bell, McDonald, Carlo, Das, Higgins.
Drafting of the manuscript: Boghossian, Bell.
Critical revision of the manuscript for important intellectual content: Boghossian, McDonald, Bell, Carlo, Brumbaugh, Stoll, Laptook, Shankaran, Walsh, Das, Higgins.
Statistical analysis: McDonald, Das.
Obtained funding: Bell, Carlo, Stoll, Laptook, Shankaran, Walsh, Das.
Study supervision: Boghossian, Bell, Carlo, Stoll, Laptook, Shankaran, Walsh, Higgins.
Conflict of Interest Disclosures: All authors have no conflicts of interest and no financial relationships relevant to this article to disclose.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Warner BB, Kiely JL, Donovan EF. Multiple births and outcome. Clin Perinatol. 2000;27(2):347–361. doi: 10.1016/s0095-5108(05)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Gilstrap LC, Christensen R, Clewell WH, et al. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273(5):413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 4.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33(1):179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 6.Diggle PJ, Heagerty PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. 2nd. Oxford, UK: Oxford University Press; 2002. [Google Scholar]
- 7.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 8.Murphy DJ, Caukwell S, Joels LA, Wardle P. Cohort study of the neonatal outcome of twin pregnancies that were treated with prophylactic or rescue antenatal corticosteroids. Am J Obstet Gynecol. 2002;187(2):483–488. doi: 10.1067/mob.2002.123891. [DOI] [PubMed] [Google Scholar]
- 9.Turrentine MA, Wilson PD, Wilkins IA. A retrospective analysis of the effect of antenatal steroid administration on the incidence of respiratory distress syndrome in preterm twin pregnancies. Am J Perinatol. 1996;13(6):351–354. doi: 10.1055/s-2007-994355. [DOI] [PubMed] [Google Scholar]
- 10.Choi SJ, Song SE, Seo ES, Oh SY, Kim JH, Roh CR. The effect of single or multiple courses of antenatal corticosteroid therapy on neonatal respiratory distress syndrome in singleton versus twin pregnancies. Aust N Z J Obstet Gynaecol. 2009;49(2):173–179. doi: 10.1111/j.1479-828X.2009.00970.x. [DOI] [PubMed] [Google Scholar]
- 11.Hacking D, Watkins A, Fraser S, Wolfe R, Nolan T. Respiratory distress syndrome and antenatal corticosteroid treatment in premature twins. Arch Dis Child Fetal Neonatal Ed. 2001;85(1):F77–F78. doi: 10.1136/fn.85.1.F75g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ardila J, Le Guennec JC, Papageorgiou A. Influence of antenatal betamethasone and gender cohabitation on outcome of twin pregnancies 24 to 34 weeks of gestation. Semin Perinatol. 1994;18(1):15–18. [PubMed] [Google Scholar]
- 13.Spinillo A, Capuzzo E, Ometto A, Stronati M, Baltaro F, Iasci A. Value of antenatal corticosteroid therapy in preterm birth. Early Hum Dev. 1995;42(1):37–47. doi: 10.1016/0378-3782(95)01638-j. [DOI] [PubMed] [Google Scholar]
- 14.Ballabh P, Lo ES, Kumari J, et al. Pharmacokinetics of betamethasone in twin and singleton pregnancy. Clin Pharmacol Ther. 2002;71(1):39–45. doi: 10.1067/mcp.2002.120250. [DOI] [PubMed] [Google Scholar]
- 15.Mulder EJ, Derks JB, Visser GH. Effects of antenatal betamethasone administration on fetal heart rate and behavior in twin pregnancy. Pediatr Res. 2004;56(1):35–39. doi: 10.1203/01.PDR.0000130476.97700.2B. [DOI] [PubMed] [Google Scholar]
- 16.Morrison JL, Orgeig S. Antenatal glucocorticoid treatment of the growth-restricted fetus: benefit or cost? Reprod Sci. 2009;16(6):527–538. doi: 10.1177/1933719109332821. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa H, Miyazaki K, Ikeda T, et al. The Effects of Antenatal Corticosteroids on Short-and Long-Term Outcomes in Small-for-Gestational-Age Infants. Int J Med Sci. 2015;12(4):295–300. doi: 10.7150/ijms.11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elimian A, Verma U, Canterino J, Shah J, Visintainer P, Tejani N. Effectiveness of antenatal steroids in obstetric subgroups. Obstet Gynecol. 1999;93(2):174–179. doi: 10.1016/s0029-7844(98)00400-1. [DOI] [PubMed] [Google Scholar]
- 19.Carlo WA, McDonald SA, Fanaroff AA, et al. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks’ gestation. JAMA. 2011;306(21):2348–2358. doi: 10.1001/jama.2011.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torrance HL, Derks JB, Scherjon SA, Wijnberger LD, Visser GH. Is antenatal steroid treatment effective in preterm IUGR fetuses? Acta Obstet Gynecol Scand. 2009;88(10):1068–1073. doi: 10.1080/00016340903176784. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol. 2000;182(1 pt 1):198–206. doi: 10.1016/s0002-9378(00)70513-8. [DOI] [PubMed] [Google Scholar]
- 22.Schaap AH, Wolf H, Bruinse HW, Smolders-De Haas H, Van EI, Treffers PE. Effects of antenatal corticosteroid administration on mortality and long-term morbidity in early preterm, growth-restricted infants. Obstet Gynecol. 2001;97(6):954–960. doi: 10.1016/s0029-7844(01)01343-6. [DOI] [PubMed] [Google Scholar]
- 23.Economides DL, Nicolaides KH, Linton EA, Perry LA, Chard T. Plasma cortisol and adrenocorticotropin in appropriate and small for gestational age fetuses. Fetal Ther. 1988;3:158–164. doi: 10.1159/000263348. [DOI] [PubMed] [Google Scholar]
- 24.Rysavy MA, Li L, Bell EF, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372(19):1801–1811. doi: 10.1056/NEJMoa1410689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.