Abstract
Exaggerated anxious responding to unpredictable threat (U-threat) is a core feature of panic disorder (PD). However, it is unknown whether this abnormality is specific to the diagnosis of PD or would manifest along a continuum of panic symptomatology (PS). Additionally, little is known about the neural processes underlying this abnormality among those high in PS. Finally, no studies have tested whether startle potentiation and limbic neural reactivity – commonly used indices of U-threat responsivity – are associated and therefore reflect common abnormalities. These questions were investigated in 42 adults with a range of PS. U-threat responding was measured twice during threat-of-shock – once with startle and once with functional magnetic resonance imaging (fMRI). As hypothesized, PS positively predicted startle potentiation and dACC reactivity to U-threat. Startle potentiation and dACC activation to U-threat were positively associated. These results suggest a biobehavioral profile of aberrant responding to U-threat associated with PS.
Keywords: anterior cingulate cortex, anticipatory anxiety, panic disorder, unpredictable threat
Panic disorder (PD) is characterized by periods of acute fear (i.e., panic attacks) and prolonged anxiety and worry between panic attacks (American Psychiatric Association [APA], 2013). Several theoretical models of PD suggest that exaggerated anticipatory anxiety regarding the temporal uncertainty of the next panic attack increases the likelihood of additional attacks, resulting in a positive feedback loop that precipitates disorder onset (Başoğlu et al., 1994; Bouton, Mineka, & Barlow, 2001). Indeed, among individuals who have experienced a panic attack, greater self-reported intolerance of uncertainty (IU) is positively associated with severity of PD symptoms (Carleton, Fetzner, Hackl, & McEvoy,, 2013; Dugas, Buhr & Ladouceur, 2004). As such, exaggerated anticipatory anxiety in response to unpredictable aversive events is considered a core dysfunction of PD (Carleton et al., 2012; Gorka, Nelson, Phan, & Shankman, 2014).
Consistent with this, several studies have demonstrated that relative to healthy controls, individuals with PD display greater startle potentiation to unpredictable, but not predictable, threat (Grillon et al., 2008; Shankman et al., 2013). Heightened startle potentiation to unpredictable threat (U-threat) has also been shown to be mediated by individual differences in IU (Nelson, Hajcak, & Shankman, 2015) and within adults, predict family history of PD independent of ones’ own anxiety psychopathology (Nelson et al., 2013). Moreover, benzodiazepines, an efficacious treatment for PD, have been shown to selectively reduce startle potentiation to U-threat (Grillon et al., 2006). Therefore, laboratory studies have corroborated the central role of exaggerated anticipatory anxiety in PD and indicate that heightened startle potentiation to U-threat may be a behavioral marker of this dysfunction.
In addition to behavioral responses such as startle, examining neural reactivity to U-threat among individuals with PD and panic symptoms (PS) may be useful as it can provide insights into the mechanisms underlying this affective deficit. The broader affective neuroscience literature suggests that across species, a frontolimbic circuit is engaged during the processing of unpredictable aversive events and this circuit may contribute to exaggerated anxious responding to U-threat in PD. This frontolimbic circuit, referred to as the anticipatory anxiety network (AAN), consists of affect-generating limbic regions, such as the amygdala, anterior insula (aINS), and bed nucleus of the stria terminalis, which have bidirectional connections with affect-modulating prefrontal regions, such as the prefrontal cortex (PFC), orbitofrontal cortex, and anterior cingulate cortex (ACC; Aupperle, Melrose, Stein, & Paulus, 2012; Grupe & Nitschke, 2013; Shankman et al., 2014; Walker & Davis, 1997). Of these regions, the aINS and ACC appear to be especially relevant to anxious responding during the anticipation of U-threat. The aINS is involved interoceptive awareness and the generation of anticipatory emotional responses to future events (Binks, Evans, Reed, Moosavi, & Banzett, 2014; Craig, 2009; Wilson & Gilbert, 2003; Shankman et al., 2014). Akin to the aINS, the ACC has also been implicated in interoceptive awareness, as well as the modulation of autonomic arousal and responding (Binks et al. 2014; Etkin, Egner & Kalisch, 2011; Holtz, Pané-Farré, Wendt, Lotze, & Hamm 2012; Milad et al., 2007; Wager et al., 2009).
Notably, hyperactivity of the aINS during the anticipation of U-threat has been observed in both social anxiety disorder (SAD) and generalized anxiety disorder (GAD) (Klumpp, Angstadt & Phan, 2012; Simmons et al., 2011) – two disorders that, like PD, are characterized by heightened IU (Boelen & Reijntjes, 2009; Dugas, Buhr & Ladoucer, 2004). In addition, self-reported individual differences in IU have been shown to be positively associated with aINS activation during the processing of ambiguous stimuli (Simmons, Matthews, Paulus, Stein, 2008) and the anticipation of U-threat (Shankman et al., 2014). Similarly, heightened activity in the ACC has been observed during the anticipation of phobic images among individuals with specific phobia (Straube, Mentzel & Miltner, 2007), and correlates with subjective ratings during the anticipation of threat (Straube, Schmidt, Weiss, Mentzel, & Miltner, 2009). There is also a well-established association between anxiety and heightened error-related negativity (ERN), an event-related component that is thought to be generated by the ACC and reflect individual differences in defensive reactivity to errors/threats (e.g., Hajcak & Foti, 2008; Olvet & Hajcak, 2008; Weinberg, Olvet & Hajcak, 2010; Xiao et al., 2011).
Taken together, prior studies indicate that there is a positive association between activation of the aINS and ACC (two key nodes of the AAN) during the processing of threatening stimuli. Thus, there are reasons to predict that individuals with PD would exhibit exaggerated reactivity to U-threat and AAN dysfunction, particularly hyperactivity of the aINS and ACC. Despite the evidence in support of this hypothesis, only one prior study to our knowledge has investigated the neural correlates of reactivity to U-threat within individuals with PD (Gorka et al., 2014). This study limited the analyses to the insula and all PD participants had a current, comorbid diagnosis of major depressive disorder (MDD) but found that as expected, PD-MDD individuals displayed greater bilateral aINS activation during the anticipation of unpredictable aversive images relative to MDD-only and healthy controls.
Given the unique design of Gorka et al. (2014), and the broader paucity of research on this topic, several key questions remain. First, no study to date has examined whether the association between PD and reactivity to U-threat is specific to the categorical diagnosis of PD as defined by the Diagnostic and Statistical Manual of Mental Disorders (5th ed; DSM; APA, 2013), or extends to the broader continuum of panic symptoms (PS). This is an important distinction given that PD even at subthreshold levels is associated with substantial economic cost and impairment (Batelaan et al., 2007), and using only individuals who are full syndromal for PD ignores this important subgroup and the broader PS spectrum (Shankman & Gorka, 2015). Second, no study has directly examined the association between PS and nodes of the AAN network other than the insula despite accumulating evidence linking this circuit to anticipatory anxiety (Grupe & Nitschke, 2013). As is noted above, other regions besides the insula may be relevant to threat processing in PD and PS; particularly the ACC as prior studies have found hyperactivity of the ACC among individuals with PD during the anticipation of interoceptive threat (e.g., heart palpitations) (Boshuisen, Ter Horst, Paans, Reinders, & den Boer, 2002; Holtz et al., 2012). Third, and most importantly, despite the fact that several studies have shown that PD is associated with behavioral reactivity (i.e., startle potentiation) to U-threat, it is unknown whether this behavioral response is related to functioning of the AAN network as would be expected. In other words, it is often assumed that startle potentiation and AAN activity during U-threat are related; however, this has yet to be directly tested.
Research addressing these questions is critically needed in order to clarify the role of U-threat responding in panic pathophysiology and elucidate potential mechanisms and targets for PD and PS prevention and intervention. This question is also important for NIMH’s Research Domain Criteria (RDoC) initiative, which argues for the importance of examining constructs such as reactivity to U-Threat, with multiple converging units of analysis (Patrick et al., 2013) Importantly, these questions are relevant not just for PD, but for the other anxiety disorders that are characterized by high IU, such as GAD and SAD (Boelen & Reijntjes, 2009; Dugas, Buhr & Ladoucer, 2004).
The present study therefore has several aims. We examined the association between PS and startle potentiation during the anticipation of U-threat within a well-validated threat of shock paradigm, the No-Predictable-Unpredictable (NPU) – threat task (Schmitz & Grillon, 2012) to assess whether prior findings generalize beyond the categorical PD diagnosis to the PS dimension across individuals with internalizing psychopathologies (e.g., depression, anxiety disorders). We also tested whether PS relates to AAN reactivity during an analogous NPU task performed during functional magnetic resonance imaging (fMRI). Lastly, we evaluated whether startle potentiation, and thus behavioral responding to U-threat, is associated with AAN reactivity to U-threat to clarify the extent to which these two separate measures capture a shared dysfunction. As noted above, PS will be defined dimensionally rather than categorically, as this will allow us to include the full panic spectrum. We will also control for broad negative affectively in our analyses to ensure that the results are specific to panic and are not better accounted for by a more general propensity to experience negative emotion. We hypothesized that PS will be associated with greater startle potentiation to U-threat, and that PS will be associated with greater neural reactivity of the AAN reactor nodes (particularly the aINS and ACC) during U-threat. Lastly, we hypothesize that these two separate indicators of U-threat dysfunction will be significantly associated with one another.
Methods
Participants
Participants were recruited from the community as a part of a larger investigation on affective and physiological abnormalities across internalizing psychopathology. A variety of advertisements were used to recruit different treatment-seeking populations (e.g., depression and/or anxiety disorder patients, healthy controls) in an effort to obtain a sample with a broad range of symptom severity and overall functioning. In line with the aims of the larger study, participants were included if they either 1) had anxiety or depressive symptoms severe enough to warrant treatment (as assessed via trained clinicians) and consented to treatment with pharmacotherapy (selective serotonin reuptake inhibitors/SSRIs) or cognitive behavioral therapy (CBT) (i.e., patients), or 2) had no lifetime history of psychopathology (i.e., healthy controls). Participants were required to be between the ages of 18 and 65 years. Exclusion criteria included an inability to provide consent and read and write in English, a major active medical or neurological problem that could impact psychophysiological and brain function, lifetime history of mania or psychosis, any contraindication to receiving SSRIs, being already engaged in any form of psychiatric treatment, history of traumatic brain injury, left-handedness, and being pregnant.
The study took place at the University of Illinois-Chicago and was approved by the university Institutional Review Board. All participants provided written informed consent after review of the protocol. Participants completed a set of laboratory tasks and battery of questionnaires, which were administered in a counterbalance order to eliminate potential order effects. Participants received cash as payment for participation.
A total of 51 individuals met inclusionary criteria; however, 9 were excluded due to missing/poor quality raw startle data (n = 6), poor quality fMRI data (i.e., excessive motion and/or artifact; n = 2), or missing self-report data (n= 1). The final sample included 42 individuals, 62% of whom were Caucasian, 12% were African-American, 21% were Asian, 2% were American Indian, and 2% reported ‘Other.’ Of these individuals, 14% were Hispanic and 74% were female. The mean age of the sample was 25.26 (SD = 7.60). Of these 42 individuals, 15 were enrolled in the larger investigation as healthy controls and 27 as patients to-be-treated with SSRI or CBT treatment. Notably, all data used in the current study was assessed prior to treatment (i.e., baseline assessment) and none of the participants were taking psychoactive medications at the time of study entry. Patients endorsed moderate to high levels of overall depressive (M = 19.26, SD = 8.31) and anxiety (M = 12.78, SD = 4.39) symptoms, as indexed by the HAM-D (Miller, Bishop, Norman, & Maddever, 1985) and HAM-A scales (Shear et al., 2001), respectively. Of the 27 patients included in the current sample, 37% had a lifetime diagnosis of PD. During clinical interview, all participants reported there total number of panic attacks within the past 30-days and the within the current sample, the mean number of panic attacks was 1.2±4.0.
Procedure
Data from the present study was collected across multiple study visits. First, participants attended a baseline-screening visit in which semi-structured diagnostic interviews were conducted by trained clinicians, and a battery of questionnaires was administered. Participants then completed the NPU-threat startle task (Schmitz & Grillon, 2012). During their third study visit, participants completed the fMRI scan and the analogous NPU-threat task.
Prior to completing all laboratory procedures (startle and fMRI), individuals were assessed for acute alcohol intoxication via a breathalyzer and tested negative. Additionally, prior to each of these tasks a shock work-up procedure was completed in which participants received increasing levels of shock intensity until they reached a level that they described as “highly annoying but not painful.” Ideographic shock levels were used to ensure equality in perceived shock aversiveness (Rollman and Harris, 1987) and to be consistent with prior studies (e.g., Grillon et al., 2006; Shankman et al., 2013). The maximum shock level a participant could achieve was 5 mA. For the startle task, shock electrodes were placed on the wrist of the participants’ left hand. In order to minimize movement and potential scan artifact, for the fMRI task, shock electrodes were placed on participants’ left foot.
Measures
Measures of PS
Panic symptoms were assessed using two separate measures, the Inventory for Depression and Anxiety symptoms-II (IDAS-II; Watson et al., 2012) and the Panic Disorder Severity Scale (PDSS; Shear et al., 1997). The IDAS-II is 99-item self-report measure of symptoms of anxiety and depression during the previous two weeks. Participants are asked to respond to each item using a 5-point Likert Scale ranging from 1 (not at all) to 5 (extremely). The IDAS-II yields 10 factor analytically derived symptom scales, including a PS dimension (the panic subscale) and a dysphoria subscale. There are also two broader scales, general depression and dysphoria, which overlap with some of the above symptom scales. The dysphoria subscale, in particular, captures broad negative affectively and was therefore included as a covariate in the present study (see more on this in the data analysis plan below). Prior research has demonstrated that the IDAS-II has excellent psychometric properties including internal consistency, test–retest reliability, and convergent and discriminant validity (Watson et al., 2012). In the present study, the panic (M = 11.90, SD = 5.50) and dysphoria (M = 23.95, SD = 10.46) subscales of the IDAS demonstrated strong internal consistency (α = .89 and .94, respectively).
The PDSS is a seven-item clinician-administered interview that is designed to assess the severity of PS. PDSS items were derived from the DSM-IV (Shear et al., 1997), and so the PDSS was used in the present study in order to obtain an additional index of panic symptomology that is more based in the diagnostic criteria for PD than the factor analytically derived IDAS-II. The PDSS items specifically evaluate: (1) frequency of panic attacks; (2) distress during panic attacks; (3) anticipatory anxiety (worry about future panic attacks); (4) agoraphobic fear and avoidance; (5) interoceptive fear and avoidance (i.e., apprehension and avoidance of bodily sensations); (6) impairment of or interference in work functioning; and (7) impairment of or interference in social functioning. Based on the patient’s response to each question, the clinician rates the response on a scale of zero (none) to four (extreme). Like the IDAS-II, the PDSS has been demonstrated to possess excellent psychometric properties (Keough et al., 2012; Shear et al., 1997), and had strong internal consistency in the present study (α = .92; M = 4.93, SD = 5.81).
Startle Task and Data Collection
Participants completed a modified version of the original NPU-threat task that was developed by Grillon and colleagues (Schmitz, & Grillon, 2012), and used repeatedly in our laboratory (e.g., Gorka, Lieberman, Phan, & Shankman,., 2016). This version of the task includes three within-subject conditions – no shock (N), predictable shock (P), and unpredictable shock (U). Text at the bottom of the computer monitor informs participants of the current threat condition by displaying: “no shock” (N), “shock at 1” (P), or “shock at anytime” (U). Each condition lasted 145-s, during which a 4-s visual countdown (CD) was presented six times. The interstimulus intervals (ISIs; i.e., time between CDs) ranged from 15 to 21-s (M = 18.0-s) during which only the text describing the condition was on the screen. During the N condition, no shocks were delivered. During the P condition, participants received a shock every time the CD reached 1. During the U condition, shocks were administered at any time (i.e., during the CD or ISI). Startle probes were presented both during the CD (1–2-s following CD onset) and ISI (4–13-s following ISI onset). The time interval between a shock (and a startle probe) and the following startle probe was always greater than 10-s to ensure that the subsequent startle response was not significantly affected by an immediately preceding stimulus. Each condition was presented two times in a randomized order (counterbalanced across participants). All participants received 24 total electric shocks (12 in P and 12 in U) and 60 total startle probes (20 in N, 20 in P, and 20 in U).
In order to facilitate accurate comparison across studies, it is important to highlight that the current version of the NPU-threat task differs from the original (described in Schmitz, & Grillon, 2012) in several ways. Most notably, the original NPU includes an N (control) condition between every threat condition, whereas the current version includes an equal number of threat (P and U) and no-threat (N) conditions (e.g., P N U N P U). In addition, the cues between the two tasks differ such that the original uses geometric shapes whereas the current version uses a 4-s countdown (CD). Lastly, in the current version, every CD ends with a shock in the predictable condition, making the threat fully predictable. This was not the case in the original version of the task, as every geometric cue in the predictable condition did not result in a shock/threat. Despite these differences, both tasks have been shown to successfully elicit individual differences in reactivity to threat in prior studies (see Grillon et al., 2008; Grillon et al., 2009, and also Gorka et al., 2016; Shankman et al., 2013).
EMG startle data was acquired using BioSemi Active Two system (BioSemi, Amsterdam, The Netherlands), and stimuli were administered using Presentation (Albany, CA). Acoustic startle probes were 40-ms duration, 103-dB bursts of white noise with near-instantaneous rise time presented binaurally through headphones. Electric shocks lasted 400-ms. Startle responses were recorded from two 4-mm Ag/AgCl electrodes placed over the orbicularis oculi muscle below the left eye. The ground electrode was located at the frontal pole (FPZ) of an electroencephalography (EEG) cap that participants were wearing as part of the larger studies. One startle electrode was placed 1-cm below the pupil and the other was placed 1-cm lateral of that electrode. Data were collected using a bandpass filter of DC-500-Hz at a sampling rate of 2000-Hz.
fMRI Task and Data Collection
The threat task used in the scanner was designed to be analogous to the modified version of the NPU startle task described above. Specifically, this task consists of three within-subjects conditions – safe, predictable, and unpredictable. Rather than text at the bottom of the screen indicating the condition, three discrete images of different rooms were used to signal each condition (counterbalanced across participants). Within each room image, there was a lamp corresponding to the NPU countdown that ‘turned on’ indicating the potential for shock. In the N condition, participants never received a shock. In the P condition, participants were only shocked when the lamp was turned on. In the U condition, participants could be shocked at any time. Each condition lasted 6-s, during which the lamp was turned on once, for a duration of 2-s. The task consisted of 90 trials, with 30 trials in each condition. Light onset occurred at either 0-s (for 1/3rd of trials), 2-s (for 1/3rd of trials), or 4-sec (for 1/3rd of trials) across all conditions. A fixation cross was presented in between trials to represent a ‘jittered’ event-related design to maximize efficiency to approximate the time course of fMRI signal and ranged from 1 to 8-s (M = 4.3-s).
Functional MRI was performed on a 3.0 Tesla GE MR 750 scanner (General Electric Healthcare; Waukesha, WI) using an 8-channel phased-array radio frequency head coil. A standard T2-sensitive gradient-echo echoplanar imaging (EPI) sequence was used (2s TR; 22.2ms TE; 90° flip; 64×64 matrix; 22cm FOV; 44 axial slices; 3.44 × 3.44 × 3.0 mm voxels; 336 volumes).
Processing of EMG Startle and fMRI Data
Blinks were processed and scored according to published guidelines (Blumenthal et al., 2005). Data processing included applying a 28 Hz high-pass filter, rectifying, and then smoothing using a 40 Hz low-pass filter. Peak amplitude of the blink reflex was defined within 20–150-ms following the startle probe onset relative to baseline (i.e., average baseline EMG level for the 50-ms preceding the startle probe onset). Each peak was identified by software but examined by hand to ensure acceptability. Blinks were scored as non-responses if EMG activity during the 20–150-ms post-stimulus time frame did not produce a blink peak that is visually differentiated from baseline activity. Blinks were scored as missing if the baseline period was contaminated with noise, movement artifact, or if a spontaneous or voluntary blink began before minimal onset latency and thus interfered with the startle probe-elicited blink response. Blink magnitude values (i.e., condition averages include values of 0 for non-responses) were used in all analyses.
All fMRI data met criteria for high quality and scan stability with minimum motion correction (i.e., < 2mm displacement in any direction). Statistical Parametric Mapping software (SPM8, Wellcome Department of Imaging Neuro-Science, London, UK) was used to perform conventional preprocessing steps. In brief, images were spatially realigned to correct for head motion, warped to standardized Montreal Neurological Institute (MNI) space using the participants’ mean functional image, resampled to 2 mm3 voxels, and smoothed with an 8 mm3 kernel to minimize noise and residual differences in gyral anatomy. The general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128-s high-pass filter. Condition effects (U, P and N) were separately estimated at each voxel for each subject. U- and P-threat trials in which participants received an electric shock were excluded from the first-level models to minimize the influence of motion and artifact. Notably, an equivalent random number of N trials were also excluded to ensure that data across the threat and no-threat trials was balanced. Movement parameters obtained during realignment were included in the model as regressors of no interest to account for motion-related effects on BOLD. Individual contrast maps for U-threat > no-threat (and P-threat > no-threat) were created for each subject.
Data Analysis Plan
As a manipulation check, for our startle task, we first conducted a 3 (Condition: N, P, U) × 2 (Cue: CD, ISI) repeated measures ANOVA to confirm that the behavioral task elicited startle to P- and U-threat as designed. Next, to examine whether our fMRI task elicited activity within nodes of the AAN, we conducted second-level one-sample t-tests for U-threat > no-threat and P-threat > no-threat. Within these models, we specifically assessed for activity within the key regions of the AAN including the bilateral insula, amygdala, and dorsal ACC (dACC) by applying an anatomically-derived partial brain mask of these anatomical regions to the data. We considered activations that survived small-volume, family-wise error (FWE) correction at p < 0.05, with a cluster size greater than 10 contiguous voxels (volume >80mm3), as significant.
After exploring the main effects of the startle and fMRI tasks, we next created startle potentiation scores for P- and U-threat, consistent with our prior studies (e.g., Gorka et al., 2016; Shankman et al., 2013). Specifically, for P-threat, we subtracted startle magnitude during NCD from PCD, and for U-threat, we subtracted average startle magnitude during NCD and NISI from average startle magnitude during UCD and UISI because both phases of the U conditions (and N conditions) had the same meaning during the task. The U-threat (and P-threat) startle potentiation scores were used as dependent variables in subsequent analyses. Of note, prior to creating the potentiation scores, we confirmed that panic symptoms were not significantly associated with startle magnitude during either no-threat condition (i.e., NCD and NISI; all ps > .21) as anticipated.
To test our primary hypotheses, we then assessed whether panic symptoms were associated with behavioral reactivity to U-threat in the present sample, as this relation has previously been reported by others (e.g., Shankman et al., 2013). To do so, we conducted a hierarchical linear regression analysis in which panic symptoms were specified as the independent variable and startle potentiation to U-threat as the dependent variable. Age and biological sex were entered as covariates in Step 1. To ensure that the effects were specific to panic symptoms, and were not better accounted for by broad negative emotionality, we also entered IDAS dysphoria scores as a covariate in Step 1. Panic symptoms were entered in Step 2. To probe the validity of the startle finding we notably included both IDAS panic and PDSS scores as separate predictors.
Next, we examined the association between panic symptoms and neural activation during U-threat by entering individual U-threat > no-threat contrast maps into a second-level one-sample t-test with panic symptoms included as a regressor. Analogous to the startle analyses, we included age, biological sex, and IDAS dysphoria as covariates, and examined both IDAS panic and PDSS scores. Given that this aim was relatively exploratory, we set the significance threshold at p <0.05 FWE-corrected for multiple comparisons across the entire brain, with a cluster size greater than 10 contiguous voxels. For visualization purposes and to clarify direction of effects, we extracted BOLD signal responses (arbitrary units) from 10mm (radius) spheres surrounding significant peak activations.
Lastly, in order to test whether there was indeed a link between the behavioral and neural data, we tested whether extracted BOLD signal values from peak effects identified as significant in the analyses above were associated with startle potentiation to U-threat using an additional hierarchical linear regression analysis. Age, gender and IDAS dysphoria scores were included as covariates in Step 1. Extracted BOLD values were entered in Step 2 and startle potentiation to U-threat was specified as the dependent variable.
Results
Manipulation Checks
Analysis of startle during the NPU-threat task indicated main effects for Condition, F(2, 40) = 9.04, p < .05, and Cue, F(1, 41) = 41.41, p < .05, that were qualified by a Condition × Cue interaction, F(2, 40) = 6.03, p < .05. The Condition × Cue interaction was followed-up by conducting separate repeated measures ANOVAs for each level of Cue (i.e., CD and ISI). During the ISI, startle magnitude differed among the conditions such that startle was significantly greater during UISI relative to PISI, F(1, 40) = 9.46, p < .05, and NISI, F(1, 40) = 14.62, p < .05. However, as expected, startle during PISI did not significantly differ from startle during NISI, F(1, 40) = 3.44, ns. (NISI = PISI < UISI) Startle magnitude during the CD also differed among the conditions, due to greater startle during PCD, F(1, 40) = 16.71, p < .05, and UCD, F(1, 40) = 9.06, p < .05, relative to the NCD. Startle magnitude did not differ between PCD and UCD, F(1, 40) = .25, ns (NCD < PCD = UCD). This pattern of results indicates that startle was greater during threat relative to no-threat, as designed.
Examination of the fMRI task conditions indicated that across all participants, U-threat significantly activated the right insula (MNI peak [50, 12, −4], Z=3.66, k=4440mm3, p<.05corrected) and right amygdala (MNI peak [32, −4, −12], Z=2.89, k=544mm3, p<.05corrected). Activation of the dACC was considered a trend (MNI peak [6, 8, 44], Z=2.05, k=9416mm3, p = 0.09corrected) and close inspection of this effect revealed significant variability of dACC activation across participants. For P-threat, results indicated significant activation of the right insula (MNI peak [48, 12, −2], Z=3.21, k=14160mm3, p<.05corrected) and a trend for the left amygdala (MNI peak [−38, −2, −22], Z=2.11, k=2544mm3, p = .06corrected). There were no other significant effects within the specified mask.
Symptom Associations with Behavioral Reactivity
Results from the hierarchical linear regression analyses are presented in Table 2. IDAS panic scores positively predicted startle potentiation during U-threat. To examine the specificity of the U-threat – panic symptoms association, we also ran parallel regression analyses examining the association between P-threat and panic symptoms. Results indicated no significant association between startle potentiation to P-threat and IDAS panic scores, β = .15, t(38) = .76, ns.
Table 2.
Results from Linear Regression Analyses assessing the impact of panic symptoms and brain activation on startle potentiation to unpredictable threat
| B | t-score | R (ΔR2) | |
|---|---|---|---|
| Impact of Panic Symptoms on Startle Potentiation | |||
| Step 1 | .28 (.08) | ||
| Age | −.23 | −1.48 | |
| Gender | .01 | .05 | |
| IDAS Dysph | .14 | .90 | |
| Step 2 | .51 (.19*) | ||
| IDAS PD | *.51 | 3.05 | |
| Step 2 | .48 (.11*) | ||
| PDSS | .44* | 2.28 | |
| Impact of Brain Activation on Startle Potentiation | |||
| Step 2 | .48* (.16) | ||
| dACC | .42* | 2.75 |
Note. IDAS PD = Inventory for Depression and Anxiety Symptoms-II, Panic Disorder Subscale; PDSS = Panic Disorder Severity Scale.
p < .05.
To further evaluate the validity of the association between startle potentiation to U-threat and panic symptoms, a separate model was run to test the association between startle potentiation to U-threat and a DSM based index of panic symptoms, the PDSS. Consistent with the above, PDSS scores positively predicted startle potentiation to U-threat (see Table 2), but not P-threat, β = .29, t(38) = 1.39, ns.
Symptom Associations with Neural Reactivity
As for the association with neural reactivity, whole-brain analyses indicated that greater IDAS panic scores were associated with greater dACC activation during U-threat (MNI peak [2, 16, 42], Z=4.80, k=252mm3, p<0.05corrected; see Figure 1). IDAS panic scores were not associated with any other node of the AAN. For completeness, all whole-brain results are presented in Table 1. Notably, consistent with the IDAS panic scores, PDSS scores were significantly correlated with dACC activation (r=0.42, p<0.05).
Fig. 1.
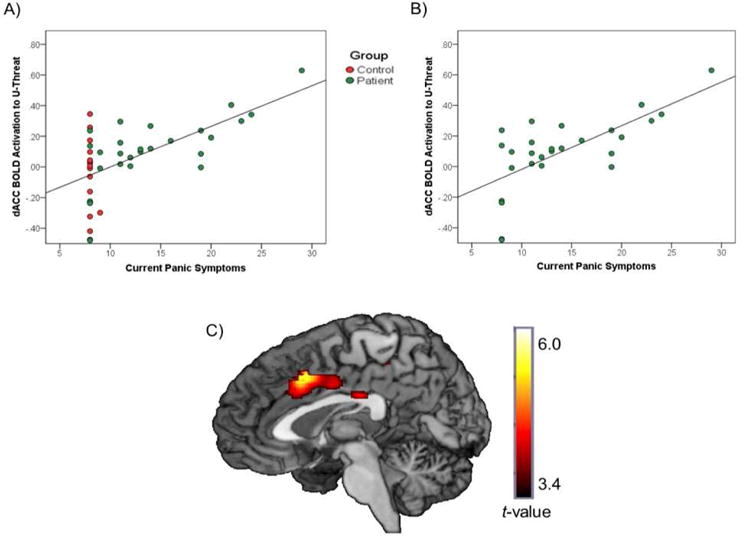
A) Association between IDAS-II panic scores and dACC activation during U-threat relative to No-threat across participants with and without current psychopathology. B) Association between IDAS-II panic scores and dACC activation during U-threat relative to No-threat among participants with current psychopathology. C) dACC activation during U-threat relative to No-threat.
Table 1.
Whole-brain regression between brain activity to U-threat (> No-threat) and panic symptoms (IDAS)
| Region | MNI Coordinates | Voxels (mm3) | Z-score | ||
|---|---|---|---|---|---|
|
| |||||
| X | Y | Z | |||
| L Inferior Parietal Lobe | −60 | −46 | 38 | 984 | 4.94 |
| L Precuneus | −22 | −56 | 46 | 432 | 4.86 |
| Anterior Cingulate Cortex | 2 | 16 | 42 | 672 | 4.80 |
| R Precuneus | 14 | −68 | 44 | 1512 | 4.77 |
Note. Reporting of all significant clusters at p < 0.05 (corrected) with a cluster extent threshold of k (number of contiguous voxels) >20. All associations are positive. L =Left; R =Right; MNI = Montreal Neurologic Institute.
Parallel to above we tested the specificity of the current finding by regressing IDAS panic scores onto the fMRI contrast P-threat > no-threat. Whole brain analyses indicated no significant findings at p<0.05 FEW-corrected. Even when the threshold was lowered to p<0.001, uncorrected, the above finding was not observed.
Association between Behavioral Reactivity and Neural Reactivity
In order to examine whether dACC activity during U-threat was associated with behavioral reactivity to U-threat, we regressed the extracted dACC BOLD values onto startle potentiation to U-threat. Results are presented in Table 2 and Figure 2. As expected, greater dACC activity to U-threat was significantly associated with greater startle potentiation to U-threat.
Fig. 2.
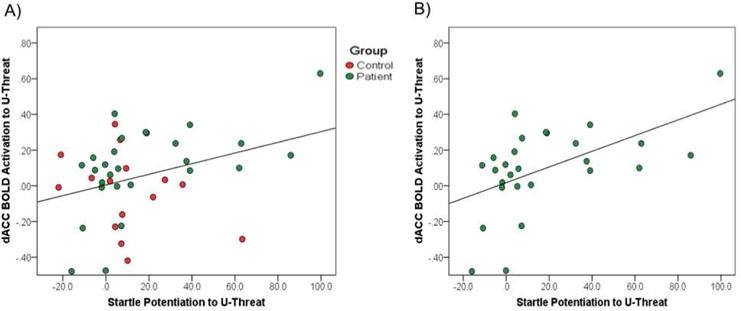
A) Association between startle potentiation to U-threat and dACC activation during U-threat relative to No-threat across participants with and without current psychopathology. B) Association between startle potentiation to U-threat and dACC activation during U-threat relative to No-threat among participants with current psychopathology.
Discussion
Converging lines of evidence indicate that exaggerated anticipatory anxiety in response to U-threat is a core dysfunction in PD. However, several critical gaps within this existing literature remain. First, it is presently unclear whether reactivity to U-threat is associated with a continuum of PS, cutting across PD and other internalizing psychopathologies (depression and anxiety disorders). Second, although there has been a broader literature focused on the neural correlates of threat reactivity, few studies have investigated the neural processes associated with heightened reactivity to U-threat within individuals with panic symptoms. Lastly, parallel literatures have utilized behavioral measures (e.g., startle eyeblink potentiation) and neural measures (e.g., fMRI) of reactivity to U-threat and have often assumed that these indices converge and capture the same affective dysfunction across individuals; however, no study to date has directly tested this key assumption. The aim of the current study was to therefore address each of these three questions in a sample of adult volunteers with a range of internalizing psychopathology and PS. Results indicated that as hypothesized, greater PS were associated with greater startle potentiation to U-threat. In addition, greater PS were associated with greater dACC activation during U-threat. These findings were specific to U-threat, relative to P-threat. Finally, greater dACC activation during U-threat was significantly associated with greater startle potentiation to U-threat.
The current results indicate that across two separate dimensional measures of PS, greater panic symptomology was associated with greater startle potentiation to U-threat, which notably expands upon the extant literature on threat sensitivity in PD. Specifically, although several studies have reported that individuals with a diagnosis of PD exhibit heightened startle potentiation to U-threat, relative to healthy controls (Grillon et al., 2008, Shankman et al., 2013), our findings imply that the relation between panic symptomology and sensitivity to U-threat extends beyond the DSM-defined diagnosis of PD to the broader dimension of PS across individuals with PD and other anxiety and/or depression disorders. This distinction is important in the context of the National Institute of Mental Health’s Research Domain Criteria Initiative (RDoC; Insel et al., 2010), which aims to identify transdiagnostic affective abnormalities that may contribute to symptoms of psychological conditions rather than specific categorical diagnoses. Additionally, the association with PS and startle potentiation to U-threat suggests that heightened anxious responding to U-threat may be relevant to full- and sub-syndromal for PD, and may therefore be a treatment target for the full spectrum of panic severity.
In addition to behavioral responding, the current results suggest that PS are also associated with neural response to U-threat – particularly, increased reactivity of the dACC. The dACC is a region within the broader frontolimbic network, or AAN, that is engaged by U-threat (Aupperle et al., 2012; Grupe & Nitschke, 2013; Shankman et al., 2014; Walker & Davis, 1997), and has been implicated in the self-awareness of physiological sensations (Critchley, Weins, Rotshtein, Öhman, & Dolan, 2004). Specifically, dACC activity has been observed during the self-monitoring of heartbeats, and individuals high in anxiety sensitivity have been found to exhibit hyperactivity of the dACC during the anticipation of hyperventilation (Holtz et al., 2012). Relatedly, enhanced dACC activity has been observed during experimentally induced worrying (Paulesu et al., 2010), and has therefore been posited to be involved in the cognitive appraisal of unpredictable aversive events, such as panic attacks (Kalisch & Gerlicher, 2014). Taken together, hyperactivity of the dACC during U-threat may enhance interoceptive awareness and cognitive appraisal of U-threat, leading to exaggerated anxious responding and overall panic symptomology (Binks et al., 2014; Holtz et al., 2012; Kalisch & Gerlicher, 2014; McNally, 2002).
It is also possible that greater dACC activity reflects increased physiological responding during U-threat. Interestingly, previous studies have shown that dACC activity is associated with increased skin conductance responding to conditioned threat cues (Milad et al., 2007) and greater heart rate during the threat of social evaluation (Wager et al., 2009). Along these lines, hyperactivity of the dACC during U-threat may contribute to the pathogenesis of PD by generating exaggerated autonomic arousal in anticipation of unpredictable panic attacks, which is known to ultimately increase the likelihood of future panic attacks and precipitate disorder onset (Başoğlu et al., 1994; Bouton, Mineka, & Barlow, 2001).
It is important to highlight that the association between PS and dACC activation during U-threat is consistent with our hypotheses and the existing threat and anxiety literatures. In addition to the ACC, however, we also hypothesized that symptoms would be associated with aINS activation during U-threat as the aINS is considered another key node involved in interoceptive awareness and generating emotional responses for future-oriented aversive events (Binks et al., 2014; Craig, 2009, 2011). We did not find evidence to support this specific hypothesis, though the dACC and aINS are highly interconnected, share several processes, and work together as a part of a larger affective salience network (Grupe & Nitschke, 2013), which may suggest an indirect association between PS and aINS functioning. Alternatively, the specificity of this association to the dACC, and not the aINS, may suggest that the relation between PS and dACC reactivity to U-threat is driven by processes that are unique to the dACC, relative to the aINS which may uniformly engage U-threat but not be sensitive to individual differences in panic severity. Also as noted above, the dACC has been particularly implicated in the generation of physiological responding to U-threat (Milad et al., 2007; Wager at al., 2009), suggesting that the association between PD and dACC reactivity to U-threat may be due to the dACC’s unique role in generating and representing individual differences in physiological hyperarousal. However, this is highly speculative and so future studies should explore this hypothesis further.
Adding to the importance of the role of the dACC, an especially noteworthy finding of the present study is the positive association between dACC reactivity to U-threat and behavioral reactivity to U-threat. Across disciplines, it is often assumed that behavioral, psychophysiological and neural indices of affective responding are related and thus capture shared processes and mechanisms; however, this assumption is rarely empirically tested. In other words, to date, it had been unclear whether individuals that display heightened startle potentiation to U-threat also exhibit heightened AAN network reactivity to U-threat as would be expected. The current results help to bridge these two literatures and suggest that within the same subjects, PS are associated with both greater startle potentiation and dACC reactivity to U-threat and importantly, these two separate indices converge and are positively associated with one another. This suggests that heightened startle potentiation and hyperactivity of the dACC to U-threat may constitute a profile (or dimension) of aberrant affective responses within panic pathophysiology that could one day be used as multi-unit, multi-layered targets for prevention and/or intervention techniques, consistent with the goal of NIMH’s RDoC Initiative (Insel et al., 2010).
Although the current study had numerous strengths including the use of multiple measures of reactivity to U-threat and PS, and inclusion of a clinically-representative treatment-seeking sample, there are several limitations worth noting. First, the sample size was modest for an examination of individual differences in panic symptomatology. Future studies utilizing larger clinical samples are therefore necessary in order to better define a panic biobehavioral dimension. Second, startle potentiation and neural reactivity to U-threat were not assessed simultaneously and although the measures were correlated, studies are still needed to determine whether they converge when measured in response to the same exact stimuli. Relatedly, the startle and fMRI tasks were designed to be similar though there were minor differences in stimuli and timing, which may have impacted the current results. Therefore, additional studies should continue to probe the neural and behavioral processes that mediate reactivity to U-threat within individuals with PD and PS, and potentially other fear-based anxiety disorders, to continue to elucidate and define a behavioral-brain phenotype for fear and anxiety psychopathology.
Acknowledgments
Research reported in this publication was supported by the National Institute of Mental Health (NIMH) of the National Institutes of Health (NIH) under Award Number R01MH101497 (PI: KLP). Other support for this work was provided by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS) award number UL1RR029879 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding sources.
Footnotes
Author Contributions
KLP was the principal investigator of the study; KLP and SAS contributed to the conceptual design. LL and SMG conducted the statistical analyses, interpreted the data and wrote the initial draft of the manuscript. SAS and KLP made important contributions to the editing of the manuscript and assisted in data interpretation.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Aupperle RL, Melrose AJ, Stein MB, Paulus MP. Executive function and PTSD: disengaging from trauma. Neuropharmacology. 2012;62(2):686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Başoğlu M, Marks IM, Swinson RP, Noshirvani H, O’Sullivan G, Kuch K. Pre-treatment predictors of treatment outcome in panic disorder and agoraphobia treated with alprazolam and exposure. Journal of Affective Disorders. 1994;30(2):123–132. doi: 10.1016/0165-0327(94)90040-X. [DOI] [PubMed] [Google Scholar]
- Batelaan N, Smit F, de Graaf R, van Balkom A, Vollebergh W, Beekman A. Economic costs of full-blown and subthreshold panic disorder. Journal of Affective Disorders. 2007;104(1):127–136. doi: 10.1016/j.jad.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Binks AP, Evans KC, Reed JD, Moosavi SH, Banzett RB. The time-course of cortico-limbic neural responses to air hunger. Respiratory Physiology & Neurobiology. 2014;204:78–85. doi: 10.1016/j.resp.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, Van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42(1):1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Boelen PA, Reijntjes A. Intolerance of uncertainty and social anxiety. Journal of Anxiety Disorders. 2009;23(1):130–135. doi: 10.1016/j.janxdis.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Boshuisen ML, Ter Horst GJ, Paans AM, Reinders AS, den Boer JA. rCBF differences between panic disorder patients and control subjects during anticipatory anxiety and rest. Biological Psychiatry. 2002;52(2):126–135. doi: 10.1016/S0006-3223(02)013550. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychological Review. 2001;108(1):4–32. doi: 10.1037/0033-295X.108.1.4. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Fetzner MG, Hackl JL, McEvoy P. Intolerance of uncertainty as a contributor to fear and avoidance symptoms of panic attacks. Cognitive Behaviour Therapy. 2013;42(4):328–341. doi: 10.1080/16506073.2013.792100. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Mulvogue MK, Thibodeau MA, McCabe RE, Antony MM, Asmundson GJ. Increasingly certain about uncertainty: Intolerance of uncertainty across anxiety and depression. Journal of Anxiety Disorders. 2012;26(3):468–479. doi: 10.1016/j.janxdis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? the anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Dugas MJ, Buhr K, Ladouceur R. The role of intolerance of uncertainty in etiology and maintenance. In: Heimberg RG, Turk CL, Mennin DS, editors. Generalized anxiety disorder: advances in research and practice. New York, NY: Guilford Press; 2004. pp. 143–163. [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Phan KL, Shankman SA. Association between problematic alcohol use and reactivity to uncertain threat in two independent samples. Drug and Alcohol Dependence. 2016;164:89–96. doi: 10.1016/j.drugalcdep.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Nelson BD, Phan KL, Shankman SA. Insula response to unpredictable and predictable aversiveness in individuals with panic disorder and comorbid depression. Biology of Mood & Anxiety Disorders. 2014;4:9. doi: 10.1186/2045-5380-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biological Psychiatry. 2006;60(7):760–766. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. American Journal of Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biological Psychiatry. 2009;66(1):47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews Neuroscience. 2013;14(7):488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Foti D. Errors are aversive defensive motivation and the error-related negativity. Psychological Science. 2008;19(2):103–108. doi: 10.1111/j.1467-9280.2008.02053.x. [DOI] [PubMed] [Google Scholar]
- Holtz K, Pané-Farré CA, Wendt J, Lotze M, Hamm AO. Brain activation during anticipation of interoceptive threat. Neuroimage. 2012;61(4):857–865. doi: 10.1016/j.neuroimage.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. http://dx.doi.org/10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Gerlicher AM. Making a mountain out of a molehill: On the role of the rostral dorsal anterior cingulate and dorsomedial prefrontal cortex in conscious threat appraisal, catastrophizing, and worrying. Neuroscience & Biobehavioral Reviews. 2014;42:1–8. doi: 10.1016/j.neubiorev.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Keough ME, Porter E, Kredlow MA, Worthington JJ, Hoge EA, Pollack MH, Simon NM. Anchoring the panic disorder severity scale. Assessment. 2012;19(2):257–259. doi: 10.1177/1073191112436668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biological Psychology. 2012;89(1):273–276. doi: 10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ. Anxiety sensitivity and panic disorder. Biological Psychiatry. 2002;52(10):938–946. doi: 10.1016/S0006-3223(02)01475-0. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biological Psychiatry. 2007;62(10):1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Miller IW, Bishop S, Norman WH, Maddever H. The modified Hamilton rating scale for depression: reliability and validity. Psychiatry Research. 1985;14(2):131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- Nelson BD, Hajcak G, Shankman SA. Event-related potentials to acoustic startle probes during the anticipation of predictable and unpredictable threat. Psychophysiology. 2015;52(7):887–894. doi: 10.1111/psyp.12418. [DOI] [PubMed] [Google Scholar]
- Nelson BD, McGowan SK, Sarapas C, Robison-Andrew EJ, Altman SE, Campbell ML, Shankman SA. Biomarkers of threat and reward sensitivity demonstrate unique associations with risk for psychopathology. Journal of Abnormal Psychology. 2013;122(3):662. doi: 10.1037/a0033982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clinical Psychology Review. 2008;28(8):1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, Kramer MD. A construct-network approach to bridging diagnostic and physiological domains: application to assessment of externalizing psychopathology. Journal of Abnormal Psychology. 2013;122(3):902. doi: 10.1037/a0032807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Sambugaro E, Torti T, Danelli L, Ferri F, Scialfa G, Sassaroli S. Neural correlates of worry in generalized anxiety disorder and in normal controls: a functional MRI study. Psychological Medicine. 2010;40:117–124. doi: 10.1017/S0033291709005649. [DOI] [PubMed] [Google Scholar]
- Rollman GB, Harris G. The detectability, discriminability, and perceived magnitude of painful electrical shock. Perception & Psychophysics. 1987;42(3):257–268. doi: 10.3758/bf03203077. doi: N/A. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nature Protocols. 2012;7(3):527–532. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Gorka SM, Nelson BD, Fitzgerald DA, Phan KL, O’Daly O. Anterior insula responds to temporally unpredictable aversiveness: an fMRI study. Neuroreport. 2014;25(8):596. doi: 10.1097/WNR.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Gorka SM. Psychopathology research in the RDoC era: Unanswered questions and the importance of the psychophysiological unit of analysis. International Journal of Psychophysiology. 2015;98(2):330–337. doi: 10.1016/j.ijpsycho.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, Gorka SM. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology. 2013;122(2):322. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear MK, Brown TA, Barlow DH, Money R, Sholomskas DE, Woods SW, Papp LA. Multicenter collaborative panic disorder severity scale. American Journal of Psychiatry. 1997;154(11):1571–1575. doi: 10.1176/ajp.154.11.1571. doi: http://dx.doi.org/10.1176/ajp.154.11.1571. [DOI] [PubMed] [Google Scholar]
- Shear MK, Vander Bilt J, Rucci P, Endicott J, Lydiard B, Otto MW, Frank DM. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A) Depression and Anxiety. 2001;13(4):166–178. doi: 10.1002/da.1033. [DOI] [PubMed] [Google Scholar]
- Simmons A, Matthews SC, Paulus MP, Stein MB. Intolerance of uncertainty correlates with insula activation during affective ambiguity. Neuroscience letters. 2008;430(2):92–97. doi: 10.1016/j.neulet.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons AN, Stein MB, Strigo IA, Arce E, Hitchcock C, Paulus MP. Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli. Human Brain Mapping. 2011;32(11):1836–1846. doi: 10.1002/hbm.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37(4):1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Dynamic activation of the anterior cingulate cortex during anticipatory anxiety. Neuroimage. 2009;44(3):975–981. doi: 10.1016/j.neuroimage.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009;47(3):821–835. doi: 10.1126/science.1093065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. The Journal of Neuroscience. 1997;17(23):9375–9383. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, Ruggero CJ. Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II) Assessment. 2012;19(4):399–420. doi: 10.1177/1073191112449857. doi: http://dx.doi.org/10.1176/ajp.154.11.1571. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Olvet DM, Hajcak G. Increased error-related brain activity in generalized anxiety disorder. Biological Psychology. 2010;85(3):472–480. doi: 10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Wilson TD, Gilbert DT. Affective forecasting. Advances in Experimental Social Psychology. 2003;35:345–411. doi: 10.1016/S0065-2601(03)01006-2. [DOI] [Google Scholar]
- Xiao Z, Wang J, Zhang M, Li H, Tang Y, Wang Y, Fromson JA. Error related negativity abnormalities in generalized anxiety disorder and obsessive compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35(1):265–272. doi: 10.1016/j.pnpbp.2010.11.022. [DOI] [PubMed] [Google Scholar]


