Abstract
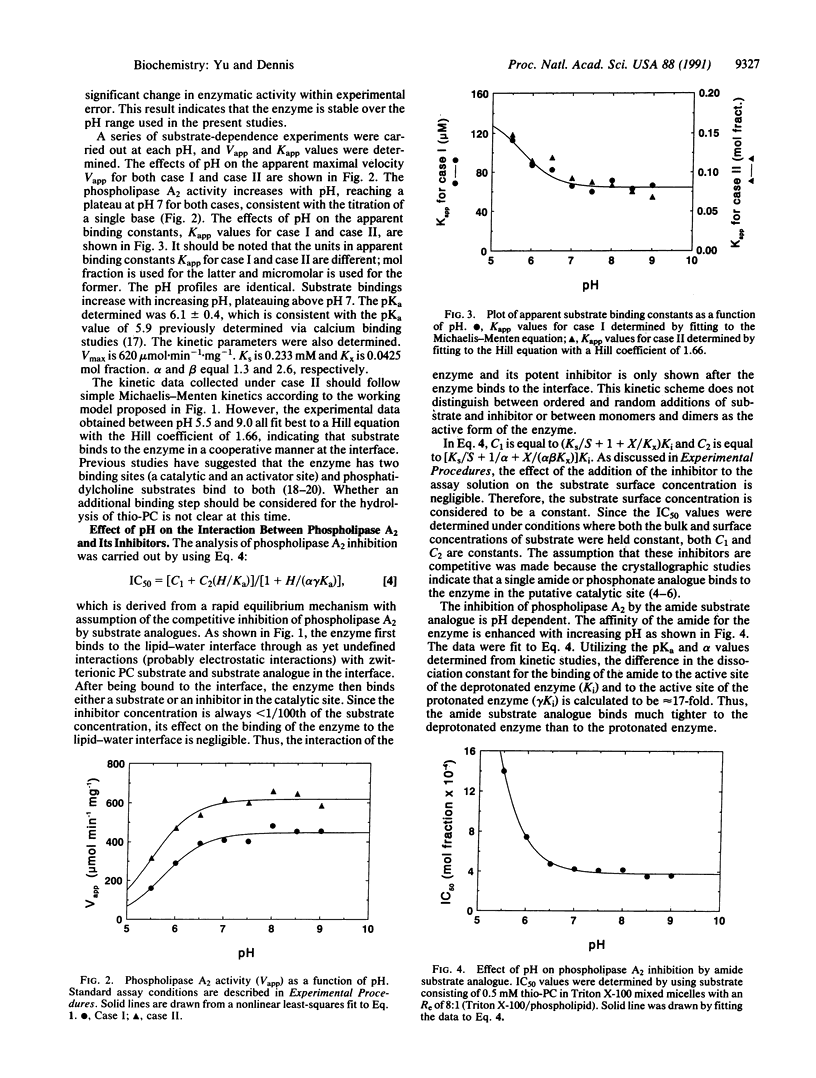
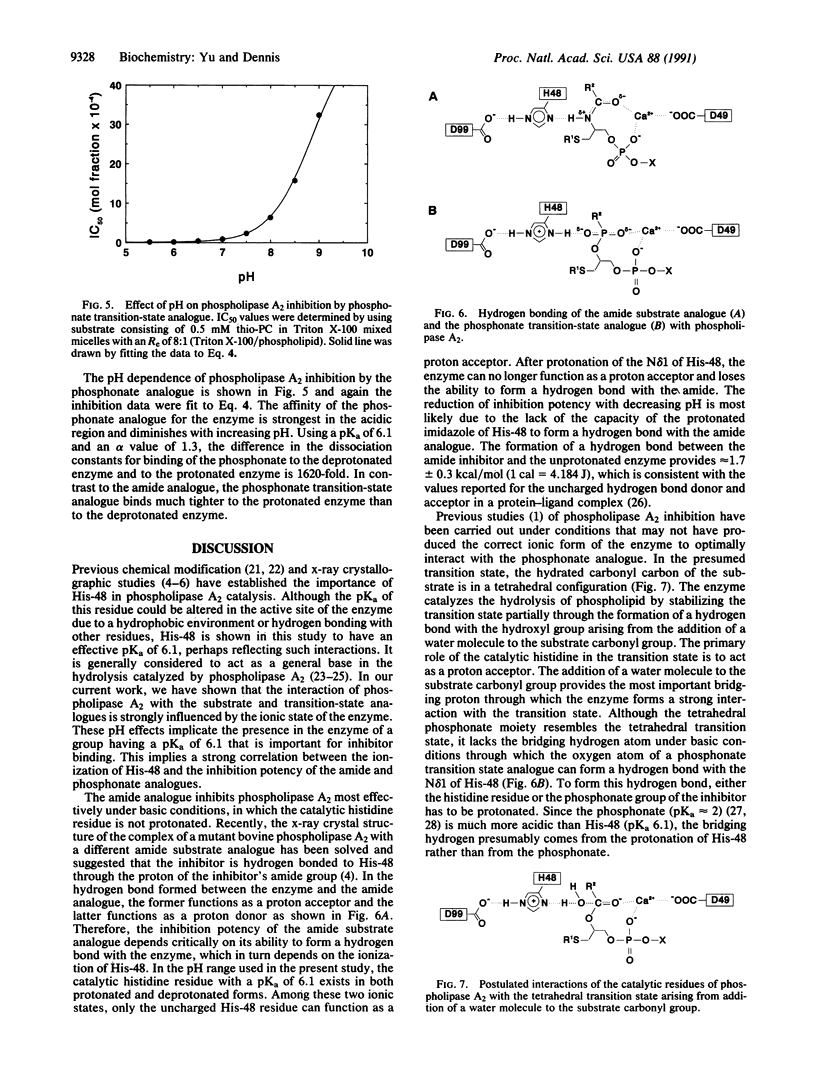
The inhibition of phospholipase A2 by an amide substrate analogue, 1-hexadecylthio-2-hexadecanoyl-amino-1,2-dideoxy-sn-glycero-3-phos phocholine, and a phosphonate transition-state analogue, 1-hexadecylthio-1-deoxy-2-hexadecylphosphono-sn-glycero-3-ph osphocholine, is dramatically influenced by pH. However, these two inhibitors show opposite pH dependencies. The amide analogue acts more potently under basic conditions, whereas the phosphonate acts more potently under acidic conditions. In both cases, ligand binding is perturbed by protonation of an enzyme functional group with an apparent pKa of 6.1, which corresponds to that of a histidine residue. Thus, His-48, which has previously been implicated in catalysis, appears to be critically involved in the hydrogen bond interactions between the enzyme and these two inhibitors. The amide analogue binds most effectively to the enzyme when His-48 is deprotonated. Upon protonation of the histidine residue, the amide cannot form a critical hydrogen bond and loses its ability to interact effectively with the enzyme. In contrast, the phosphonate analogue binds much tighter to the protonated form of the enzyme than to the deprotonated form. The phosphonate analogue needs a bridging hydrogen between the oxygen on its phosphorus atom and the N delta 1 of His-48 to form a strong hydrogen bond. At optimal pH values for inhibitor binding, both the amide and the phosphonate analogues are potent competitive inhibitors of cobra (Naja naja naja) venom phospholipase A2. The IC50 for the amide was 4.4 x 10(-4) mol fraction and for the phosphonate was 1.6 x 10(-5) mol fraction. Under the experimental conditions used, this corresponds to a bulk concentration of 2 microM and 70 nM, respectively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamich M., Roberts M. F., Dennis E. A. Phospholipid activation of cobra venom phospholipase A2. 2. Characterization of the phospholipid--enzyme interaction. Biochemistry. 1979 Jul 24;18(15):3308–3314. doi: 10.1021/bi00582a017. [DOI] [PubMed] [Google Scholar]
- Bartlett P. A., Marlowe C. K. Evaluation of intrinsic binding energy from a hydrogen bonding group in an enzyme inhibitor. Science. 1987 Jan 30;235(4788):569–571. doi: 10.1126/science.3810155. [DOI] [PubMed] [Google Scholar]
- Davidson F. F., Hajdu J., Dennis E. A. 1-Stearyl,2-stearoylaminodeoxy phosphatidylcholine, a potent reversible inhibitor of phospholipase A2. Biochem Biophys Res Commun. 1986 Jun 13;137(2):587–592. doi: 10.1016/0006-291x(86)91118-6. [DOI] [PubMed] [Google Scholar]
- Deems R. A., Eaton B. R., Dennis E. A. Kinetic analysis of phospholipase A2 activity toward mixed micelles and its implications for the study of lipolytic enzymes. J Biol Chem. 1975 Dec 10;250(23):9013–9020. [PubMed] [Google Scholar]
- Dennis E. A. Phospholipase A2 activity towards phosphatidylcholine in mixed micelles: surface dilution kinetics and the effect of thermotropic phase transitions. Arch Biochem Biophys. 1973 Oct;158(2):485–493. doi: 10.1016/0003-9861(73)90540-7. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Shi J. P., Knill-Jones J., Lowe D. M., Wilkinson A. J., Blow D. M., Brick P., Carter P., Waye M. M., Winter G. Hydrogen bonding and biological specificity analysed by protein engineering. Nature. 1985 Mar 21;314(6008):235–238. doi: 10.1038/314235a0. [DOI] [PubMed] [Google Scholar]
- Hazlett T. L., Dennis E. A. Affinity chromatography of phospholipase A2 from Naja naja naja (Indian cobra) venom. Toxicon. 1985;23(3):457–466. doi: 10.1016/0041-0101(85)90029-7. [DOI] [PubMed] [Google Scholar]
- Hendrickson H. S., Dennis E. A. Kinetic analysis of the dual phospholipid model for phospholipase A2 action. J Biol Chem. 1984 May 10;259(9):5734–5739. [PubMed] [Google Scholar]
- Lichtenberg D., Robson R. J., Dennis E. A. Solubilization of phospholipids by detergents. Structural and kinetic aspects. Biochim Biophys Acta. 1983 May 24;737(2):285–304. doi: 10.1016/0304-4157(83)90004-7. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E. Enzymatic catalysis and transition-state theory. Science. 1973 Apr 15;180(4082):149–154. doi: 10.1126/science.180.4082.149. [DOI] [PubMed] [Google Scholar]
- Plückthun A., Rohlfs R., Davidson F. F., Dennis E. A. Short-chain phosphatidylethanolamines: physical properties and susceptibility of the monomers to phospholipase A2 action. Biochemistry. 1985 Jul 16;24(15):4201–4208. doi: 10.1021/bi00336a058. [DOI] [PubMed] [Google Scholar]
- Roberts M. F., Adamich M., Robson R. J., Dennis E. A. Phospholipid activation of cobra venom phospholipase A2. 1. Lipid--lipid or lipid--enzyme interaction. Biochemistry. 1979 Jul 24;18(15):3301–3308. doi: 10.1021/bi00582a016. [DOI] [PubMed] [Google Scholar]
- Roberts M. F., Deems R. A., Dennis E. A. Spectral perturbations of the histidine and tryptophan in cobra venom phospholipase A2 upon metal ion and mixed micelle binding. J Biol Chem. 1977 Sep 10;252(17):6011–6017. [PubMed] [Google Scholar]
- Roberts M. F., Deems R. A., Mincey T. C., Dennis E. A. Chemical modification of the histidine residue in phospholipase A2 (Naja naja naja). A case of half-site reactivity. J Biol Chem. 1977 Apr 10;252(7):2405–2411. [PubMed] [Google Scholar]
- Scott D. L., Otwinowski Z., Gelb M. H., Sigler P. B. Crystal structure of bee-venom phospholipase A2 in a complex with a transition-state analogue. Science. 1990 Dec 14;250(4987):1563–1566. doi: 10.1126/science.2274788. [DOI] [PubMed] [Google Scholar]
- Scott D. L., White S. P., Otwinowski Z., Yuan W., Gelb M. H., Sigler P. B. Interfacial catalysis: the mechanism of phospholipase A2. Science. 1990 Dec 14;250(4987):1541–1546. doi: 10.1126/science.2274785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thunnissen M. M., Ab E., Kalk K. H., Drenth J., Dijkstra B. W., Kuipers O. P., Dijkman R., de Haas G. H., Verheij H. M. X-ray structure of phospholipase A2 complexed with a substrate-derived inhibitor. Nature. 1990 Oct 18;347(6294):689–691. doi: 10.1038/347689a0. [DOI] [PubMed] [Google Scholar]
- Verheij H. M., Volwerk J. J., Jansen E. H., Puyk W. C., Dijkstra B. W., Drenth J., de Haas G. H. Methylation of histidine-48 in pancreatic phospholipase A2. Role of histidine and calcium ion in the catalytic mechanism. Biochemistry. 1980 Feb 19;19(4):743–750. doi: 10.1021/bi00545a021. [DOI] [PubMed] [Google Scholar]
- Volwerk J. J., Pieterson W. A., de Haas G. H. Histidine at the active site of phospholipase A2. Biochemistry. 1974 Mar 26;13(7):1446–1454. doi: 10.1021/bi00704a020. [DOI] [PubMed] [Google Scholar]
- White S. P., Scott D. L., Otwinowski Z., Gelb M. H., Sigler P. B. Crystal structure of cobra-venom phospholipase A2 in a complex with a transition-state analogue. Science. 1990 Dec 14;250(4987):1560–1563. doi: 10.1126/science.2274787. [DOI] [PubMed] [Google Scholar]
- Wolfenden R. Transition state analog inhibitors and enzyme catalysis. Annu Rev Biophys Bioeng. 1976;5:271–306. doi: 10.1146/annurev.bb.05.060176.001415. [DOI] [PubMed] [Google Scholar]
- Yu L., Deems R. A., Hajdu J., Dennis E. A. The interaction of phospholipase A2 with phospholipid analogues and inhibitors. J Biol Chem. 1990 Feb 15;265(5):2657–2664. [PubMed] [Google Scholar]
- Yu L., Dennis E. A. Thio-based phospholipase assay. Methods Enzymol. 1991;197:65–75. doi: 10.1016/0076-6879(91)97133-j. [DOI] [PubMed] [Google Scholar]



