Biological CO2 fixation is a crucial process carried out by plants and a number of microorganisms, which can be harnessed for both agriculture and sustainable, bio‐based production of fuels and chemicals. Fixation of CO2 by plants enables the production of food, feed, fuels and chemicals. Additionally, fixation of CO2 by autotrophic microorganisms such as cyanobacteria and microalgae can be employed for converting CO2 into value‐added products, such as commodity chemicals or fuels. However, a major challenge to fully realize sustainable autotrophic production of chemicals and fuels is the low growth rate, productivity and energy conversion efficiency of autotrophs. Excluding the energy loss photoautotrophs experience due to inefficient light‐harvesting (Blankenship et al., 2011), another major energy loss occurs during the fixation of CO2 in both photoautotrophs and chemolithoautotrophs (Zhu et al., 2010). The large majority of autotrophs employ the relatively inefficient Calvin–Benson–Bassham cycle for CO2 fixation (Fig. 1A). After having a dominant role in nature for billions of years, in the coming years I expect synthetic biologists will replace the Calvin cycle in biotechnology with potentially better and more efficient alternatives.
Figure 1.
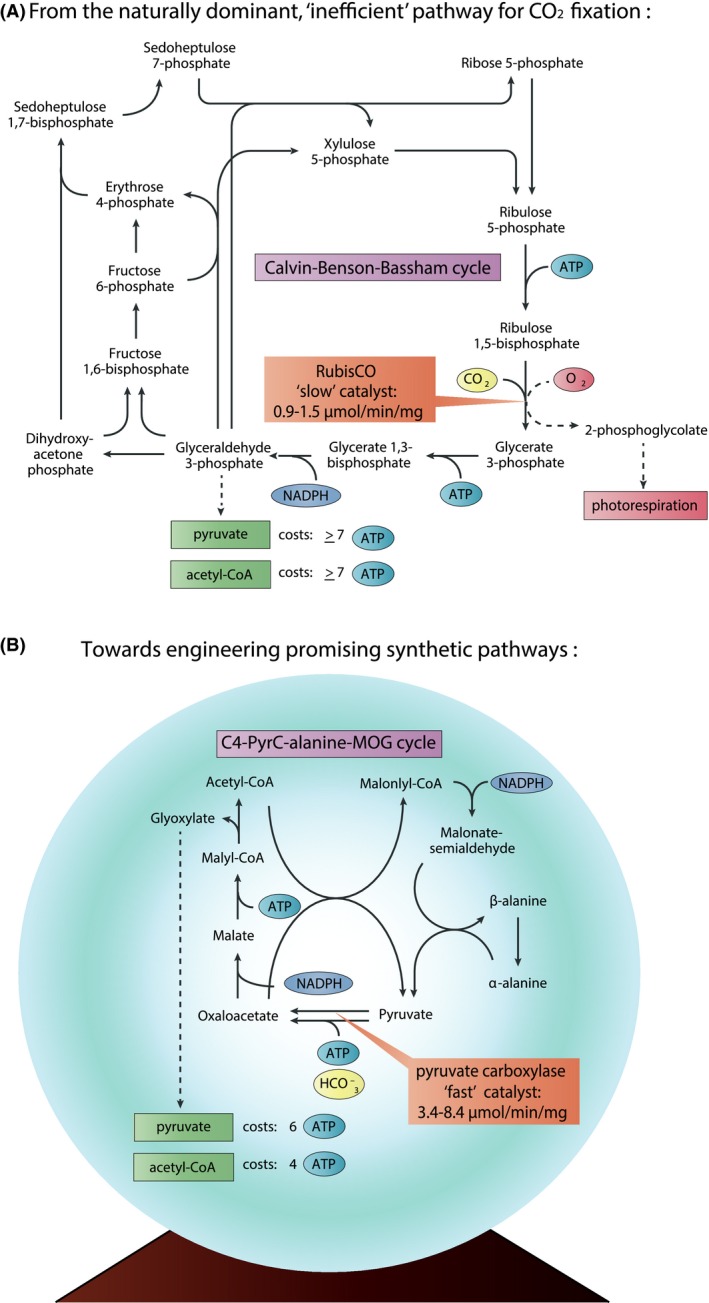
(A) The ‘inefficient’ Calvin–Benson–Bassham cycle with the ‘slow’ carboxylase RubisCO as key enzyme, the cycle has a consumption of seven ATP per pyruvate or acetyl‐CoA produced, which does not include additional costs for photorespiration in aerobic conditions (Bar‐Even et al., 2010). (B) An example of a proposed, promising synthetic MOG‐pathway, based on the ‘faster’ carboxylase pyruvate carboxylase (Bar‐Even et al., 2010), estimates for its ATP consumption are based on assimilation via the bacterial glycerate pathway for pyruvate and the reverse glyoxylate shunt for acetyl‐CoA. Catalytic turnover rates indicated for both carboxylases are based on ambient CO 2 concentrations (Bar‐Even et al., 2010).
But what is the limiting factor of the Calvin cycle? The main issue is the key enzyme ribulose‐bisphosphate carboxylase/oxygenase (RubisCO). RubisCO has a low catalytic rate, and therefore is generally expressed in high levels, which requires a considerable amount of cellular resources. This resource demand comes on top of the existing high ATP‐demand of the Calvin cycle required to produce common metabolic precursors such as acetyl‐CoA and pyruvate (Claassens et al., 2016). Furthermore, RubisCO has a major wasteful side‐activity with O2 in atmospheric conditions. This side‐activity results in the formation of 2‐phoshpoglycoylate that has to be re‐assimilated to the Calvin cycle through photorespiration pathways. These photorespiration pathways partly counteract CO2 fixation by releasing some CO2 and typically add ~40–50% extra NADPH and ATP to the costs of CO2 fixation (Bar‐Even et al., 2010).
In recent years, many ideas have been put forward and attempts have been made to increase the efficiency of the Calvin cycle including; improving catalytic properties of RubisCO by protein engineering (e.g. Parikh et al., 2006; Durão et al., 2015), introducing more efficient photorespiration pathways (e.g. Shih et al., 2014) and introducing CO2 concentrating mechanisms to increase the carboxylating activity of RubisCO (e.g. Bonacci et al., 2012; Kamennaya et al., 2015). Some of these attempts have slightly improved the performance of autotrophs employing the Calvin cycle. However, apart from fixing such inefficiencies of the Calvin cycle, future research should also seriously address the options to completely replace both the Calvin cycle and accompanying enzyme RubisCO by potentially more efficient alternatives.
Fortunately, nature has evolved many carboxylases with more promising properties than RubisCO and more attractive novel carboxylases may be created by protein engineering (Erb, 2011). Some examples of attractive natural carboxylases are employed in alternative natural carbon fixation pathways, thus far five alternative autotrophic CO2 fixation pathways have been discovered (Berg, 2011). Moreover, attractive carboxylases can be embedded in synthetic CO2 fixation pathways, which have been extensively explored by computational analyses (e.g. Bar‐Even et al., 2010; Volpers et al., 2016). Several of the alternative natural and synthetic CO2 fixation pathways have lower ATP costs than the Calvin cycle, however, some trade‐offs exist. For example, the natural Wood–Ljungdahl pathway and the natural reductive tricarboxylic acid cycle have very low ATP costs. However, these pathways can have major drawbacks, as these pathways are mainly limited to anaerobic settings, due to oxygen‐sensitive enzymes, and they require high CO2 concentrations to be thermodynamically feasible (Berg, 2011). Alternative to natural pathways, computational analysis identified several oxygen‐tolerant synthetic pathways that while taking multiple criteria into account, are predicted to have an overall better performance than the Calvin cycle. As an example, a promising group of synthetic pathways are the malonyl‐CoA–oxaloacetate–glyoxylate (MOG) pathways (Fig. 1B) (Bar‐Even et al., 2010). These pathways include the catalytically fast phosphoenol pyruvate carboxylase or pyruvate carboxylase and are expected to have fast overall pathway kinetics. In addition, these MOG pathways involve only oxygen‐tolerant enzymes and have predicted lower ATP costs than the Calvin cycle. In addition, synthetic pathways based on crotonyl‐CoA carboxylases, the fastest known carboxylases, may be highly promising to apply in synthetic CO2 fixation pathways (Erb et al., 2009).
Attractive natural pathways are only found in a few, limitedly characterized microorganisms, which are generally less suitable for biotechnology. The alternative synthetic pathways are not (yet) known to occur in natural microorganisms. Therefore, to realize the potential of alternative CO2 fixation pathways they should be introduced into suitable microbial hosts via metabolic engineering. Hereto, in recent years a few attempts have been made, such as the introduction of the complete natural 3‐hydroxypropionate bi‐cycle in Escherichia coli by the Silver laboratory, which did not yet yield a completely functional pathway (Mattozzi et al., 2013). In the Adams laboratory, a section of the natural 3‐hydroxypropionate/4‐hydroxybutyrate cycle was successfully introduced in the heterotrophic thermophile Pyrococcus furiosus; however, this short section is insufficient to support complete autotrophic growth (Keller et al., 2013).
These partly successful examples demonstrate that introduction of a full functional CO2 fixation pathway is a major challenge. To allow for more efficient autotrophic growth, the engineered CO2 fixation pathway has to become a key functional component of central metabolism and subsequently the alternative pathways should be properly integrated with native metabolism. This will involve reregulation of native metabolism, for which a rational engineering approach will probably be insufficient. However, the power of adaptive laboratory evolution will be very helpful to optimize pathways and re‐regulate native metabolism. CO2 fixation pathways are a perfect case for adaptive laboratory evolution, as their functionality can often be easily coupled to a growth phenotype. This has been exemplified very recently by the Milo laboratory for the engineering of the Calvin cycle in E. coli, for which they coupled pathway activity to growth of E. coli. After about 150 generations in a chemostat, laboratory evolution led to the first completely functional, engineered CO2 pathway in a heterotroph (Antonovsky et al., 2016). Even though this major achievement was shown for the ‘inefficient’ Calvin cycle, which requires only three heterologous genes in E. coli, this approach opens up the door for future engineering of more promising, extensive CO2 fixation pathways in heterologous hosts.
A general challenge for engineering CO2 fixation pathways in E. coli and other model heterotrophs is the lack of autotrophic energy systems to regenerate ATP and reducing equivalents such as NADPH. Therefore, autotrophs already harbouring these systems for ATP and NADPH regeneration are promising hosts for engineering alternative CO2 fixation pathways. Compared to model heterotrophs, such as E. coli and yeast, the available genetic toolboxes for autotrophs are more limited. However, given the ongoing tool development in some biotechnologically relevant autotrophs, these strains should be considered for engineering CO2 fixation pathways (Claassens et al., 2016). Promising autotrophs include photoautotrophs, such as the cyanobacterium Synechocystis PCC6803 and anoxygenic phototroph Rhodobacter sphaeroides, and some chemolithoautotrophs, such as Cupriavidus necator (formerly Ralstonia eutropha), the latter uses hydrogen and CO2 for autotrophic growth. All mentioned promising biotechnological autotrophs contain a native Calvin cycle, which could be replaced by potentially more efficient pathways. By knocking out RubisCO in these autotrophs, the activity of introduced alternative CO2 fixation pathways can be coupled to their autotrophic growth, providing a proper basis for adaptive laboratory evolution.
Some laboratories are already undertaking approaches to engineer and evolve synthetic CO2 fixation in both microbial heterotrophs and autotrophs. In addition, some laboratories are testing promising synthetic CO2 fixation pathways in vitro, by combining purified enzymes for sections or complete pathways in a tube. These in vitro approaches will be helpful to test pathway functionality, especially for synthetic pathways that involve insufficiently characterized natural enzymes or engineered enzymes. For example, issues regarding undesired side‐activities can be identified more easily in vitro. Recently, the Erb laboratory successfully used the in vitro approach for the first‐demonstrated synthetic pathway: the CETCH‐cycle, which involves the kinetically fast carboxylase crotonyl‐CoA carboxylase (Schwander and Erb, 2016). However, after in vitro pathway debugging and a proof‐of‐principle for functionality, subsequent in vivo engineering and testing should be the key approach for engineering novel pathways. Only an in vitro approach will allow for proper assessment of the performance of alternative pathways in a physiological context and the realization of more efficient autotrophic cell factories.
I expect that engineering alternative CO2 fixation pathways in autotrophs will pave the road for more efficient autotrophic production strains. In addition, lessons learnt by engineering synthetic CO2 fixation will be important for the even larger challenge of engineering improved, synthetic CO2 fixation pathways in plants (Ort et al., 2015). Endeavours in this direction may enable realization of the large promise of more efficient agricultural crops. However, these undertakings will be even more challenging than for microbes, given the more limited genetic tools and longer generation times, hampering evolutionary approaches in plants. Moreover, one should not underestimate the large societal resistance against genetically modified plants. Concluding, for autotrophic, biotechnologically relevant microbes that got ‘stuck in evolution’ with the ‘inefficient’ Calvin cycle, we now have knowledge and tools to aim to replace this pathway by more efficient alternatives. It is now up to synthetic biologists to pick up this exciting and promising challenge to realize more efficient autotrophic production platforms for a sustainable bio‐economy.
Acknowledgements
I thank Arren Bar‐Even and Willem M. de Vos for inspiring discussions and critical reading of this manuscript. I also thank the organizers and participants of the Lorentz Workshop ‘Photosynthesis ‐Plug and Play’ 2016 for great discussions. I thank Rebecca McKenzie for proof‐reading of this manuscript. I acknowledge support from the IP/OP Systems Biology program at Wageningen University (grant KB‐17‐003.02‐024).
Microbial Biotechnology (2017) 10(1), 31–34
References
- Antonovsky, N. , Gleizer, S. , Noor, E. , Zohar, Y. , Herz, E. , Barenholz, U. , et al (2016) Sugar synthesis from CO2 in Escherichia coli . Cell 166: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar‐Even, A. , Noor, E. , Lewis, N.E.N.N.E. , and Milo, R. (2010) Design and analysis of synthetic carbon fixation pathways. Proc Natl Acad Sci USA 107: 8889–8894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg, I.A. (2011) Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl Environ Microbiol 77: 1925–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship, R.E. , Tiede, D.M. , Barber, J. , Brudvig, G.W. , Fleming, G. , Ghirardi, M. , et al (2011) Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 332: 805–809. [DOI] [PubMed] [Google Scholar]
- Bonacci, W. , Teng, P.K. , Afonso, B. , Niederholtmeyer, H. , Grob, P. , Silver, P.A. , and Savage, D.F. (2012) Modularity of a carbon‐fixing protein organelle. Proc Natl Acad Sci USA 109: 478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassens, N.J. , Sousa, D.Z. , dos Santos, V.A.P.M. , de Vos, W.M. , and van der Oost, J. (2016) Harnessing the power of microbial autotrophy. Nat Rev Microbiol 14: 692–706. [DOI] [PubMed] [Google Scholar]
- Durão, P. , Aigner, H. , Nagy, P. , Mueller‐Cajar, O. , Hartl, F.U. , and Hayer‐Hartl, M. (2015) Opposing effects of folding and assembly chaperones on evolvability of Rubisco. Nat Chem Biol 11: 148–155. [DOI] [PubMed] [Google Scholar]
- Erb, T.J. (2011) Carboxylases in natural and synthetic microbial pathways. Appl Environ Microbiol 77: 8466–8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb, T.J. , Brecht, V. , Fuchs, G. , Müller, M. , and Alber, B.E. (2009) Carboxylation mechanism and stereochemistry of crotonyl‐CoA carboxylase/reductase, a carboxylating enoyl‐thioester reductase. Proc Natl Acad Sci USA 106: 8871–8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamennaya, N.A. , Ahn, S. , Park, H. , Bartal, R. , Sasaki, K.A. , Holman, H.Y. , and Jansson, C. (2015) Installing extra bicarbonate transporters in the cyanobacterium Synechocystis sp. PCC6803 enhances biomass production. Metab Eng 29: 76–85. [DOI] [PubMed] [Google Scholar]
- Keller, M.W. , Schut, G.J. , Lipscomb, G.L. , Menon, A.L. , Iwuchukwu, I.J. , Leuko, T.T. , et al (2013) Exploiting microbial hyperthermophilicity to produce an industrial chemical, using hydrogen and carbon dioxide. Proc Natl Acad Sci USA 110: 5840–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattozzi, M.D. , Ziesack, M. , Voges, M.J. , Silver, P.A. , and Way, J.C. (2013) Expression of the sub‐pathways of the Chloroflexus aurantiacus 3‐hydroxypropionate carbon fixation bicycle in E. coli: toward horizontal transfer of autotrophic growth. Metab Eng 16: 130–139. [DOI] [PubMed] [Google Scholar]
- Ort, D.R. , Merchant, S.S. , Alric, J. , Barkan, A. , Blankenship, R.E. , Bock, R. , et al (2015) Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc Natl Acad Sci USA 112: 8529–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh, M.R. , Greene, D.N. , Woods, K.K. , and Matsumura, I. (2006) Directed evolution of RuBisCO hypermorphs through genetic selection in engineered E. coli . Protein Eng Des Sel 19: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander, T. , and Erb, T.J. (2016) Do it your (path)way –synthetische Wege zur CO2‐Fixierung. BIOspektrum 22: 590–592. [Google Scholar]
- Shih, P.M. , Zarzycki, J. , Niyogi, K.K. , and Kerfeld, C.A. (2014) Introduction of a synthetic CO2‐fixing photorespiratory bypass into a cyanobacterium. J Biol Chem 289: 9493–9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpers, M. , Claassens, N.J. , Noor, E. , van der Oost, J. , de Vos, W.M. , Kengen, S.W.M. , and dos Santos, V.A.P.M. (2016) Integrated in silico analysis of pathway designs supporting synthetic photo‐electro‐autotrophy. PLoS ONE 11: e0157851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X.G. , Long, S.P. , and Ort, D.R. (2010) Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol 61: 235–261. [DOI] [PubMed] [Google Scholar]


