Abstract Abstract
A molecular phylogeny of the genus Scobura based on the mitochondrial COI and the nuclear EF-1α genes using maximum likelihood and Bayesian inference is proposed. The analyses include 19 specimens from nine ingroup species. The monophyly of Scobura is not strongly supported, but two strongly supported monophyletic groups within the genus are recognized: the Scobura coniata group and the Scobura woolletti group. Judging from combination of the molecular evidence and morphological features, the former consists of six species, including Scobura masutaroi, while four species belong to the latter. Scobura mouchai Krajcik, 2013 is confirmed to be a syn. n. of Scobura masutaroi Sugiyama, 1996. The key to the species of the genus Scobura is modified to reflect these results.
Keywords: COI, EF-1α, Scobura masutaroi, Scobura mouchai
Introduction
The skipper genus Scobura Elwes & Edwards, 1897 was recently revised by Fan et al. (2010), who recognized 14 species. The genus Scobura, however, includes another species, Scobura masutaroi, Sugiyama 1996. Fan et al. (2010) overlooked the existence of this taxon and did not include it in their revisional work, which resulted in Krajcik (2013) proposing a new taxon, Scobura mouchai, from Shaanxi.
Although a comprehensive morphological revision of the genus has been completed, no phylogenetic analysis has been performed to infer relationships within the genus. In the present study, we present a preliminary phylogeny of Scobura, based on molecular evidence. By comparing molecular and morphological evidence, we examine whether Scobura mouchai is a synonym of Scobura masutaroi.
Methods
Morphological examination
See Fan et al. (2010) for materials for the morphological study. In order to examine the wing venation, wings were removed from thorax, cleaned with 95% ethanol, and dyed red with acetocarmine (Wang et al. 2011).
Taxon sampling
Twenty-three specimens including nine of the 15 valid species of Scobura and four outgroup species were included in the phylogenetic reconstruction. Detailed information on the specimens is provided in Table 1. Specimens used in this study were mainly deposited in the (SCAU), except for some specimens in (KU) and Mr. Hiroaki Onodera’s private collection.
Table 1.
Voucher information and GenBank accession numbers for the specimens in this study.
| Species | Locality | Latitude | Longitude | Voucher Number | COI | EF-1α |
|---|---|---|---|---|---|---|
| Scobura cephaloides kinkaEvans, 1949 | China: Hainan | 19.02N | 109.53E | SCAU He102 | KY049936 | KY049958 |
| Scobura cephaloides kinkaEvans, 1949 | Laos: Luang Prabang | 19.93N | 102.07E | Onodera He553 | KY049937 | KY049959 |
| Scobura coniata Hering, 1918 | China: Guangdong | 24.91N | 113.04E | SCAU He073 | KY049938 | KY049960 |
| Scobura coniata Hering, 1918 | China: Guangdong | 24.87N | 113.03E | SCAU He472 | KY049939 | KY049961 |
| Scobura hainana (Gu & Wang, 1997) | China: Guangdong | 24.87N | 113.04E | SCAU He471 | KY049940 | KY049962 |
| Scobura hainana (Gu & Wang, 1997) | China: Guangdong | 24.87N | 113.04E | SCAU He487 | KY049941 | KY049963 |
| Scobura hainana (Gu & Wang, 1997) | China: Guangdong | 24.87N | 113.04E | SCAU He488 | KY049942 | KY049964 |
| Scobura isota (Swinhoe, 1893) | Thailand: Kanchanaburi | 14.08N | 99.36E | SCAU He538 | KY049943 | KY049965 |
| Scobura isota (Swinhoe, 1893) | Thailand: Mae Hong Son | 19.35N | 98.14E | SCAU He468 | KY049944 | KY049966 |
| Scobura lyso (Evans, 1939) | China: Zhejiang | 30.15N | 119.25E | SCAU He465 | KY049945 | — |
| Scobura lyso (Evans, 1939) | China: Zhejiang | 30.15N | 119.25E | SCAU He475 | KY049946 | — |
| Scobura masutaroi Sugiyama, 1996 | China: Sichuan | 29.94N | 102.48E | SCAU He300 | KY049947 | KY049967 |
| Scobura masutaroi Sugiyama, 1996 | China: Sichuan | 29.94N | 102.48E | SCAU He301 | KY049948 | KY049968 |
| Scobura masutaroi Sugiyama, 1996 (=mouchai) | China: Shaanxi | 31.91N | 106.34E | SCAU He303 | KY049949 | KY049969 |
| Scobura parawoolletti Fan et al., 2010 | China: Hainan | 19.03N | 109.53E | SCAU He116 | KY049950 | KY049970 |
| Scobura stellata Fan et al., 2010 | China: Guangdong | 24.92N | 113.01E | SCAU He036 | KY049951 | KY049971 |
| Scobura woolletti (Riley, 1923) | Indonesia: Kabandungan | 6.77 S | 106.60E | KU He535 | KY049952 | KY049972 |
| Scobura woolletti (Riley, 1923) | Indonesia: Kabandungan | 6.77 S | 106.60E | KU He536 | KY049953 | KY049973 |
| Scobura woolletti (Riley, 1923) | Indonesia: Kabandungan | 6.77 S | 106.60E | KU He537 | KY049954 | KY049974 |
| Suastus gremius (Fabricius, 1798) | China: Guangdong | 23.15N | 113.34E | SCAU He157 | KY049955 | KY049975 |
| Suada swerga (deNicéville, 1884) | Thailand: Chiang Mai | 18.80N | 98.92E | SCAU He495 | KY049956 | KY049976 |
| Hyarotis quinquepunctatus Fan & Chiba, 2008 | China: Hainan | 19.03N | 109.54E | SCAU He114 | — | KY049977 |
| Zographetus satwa (deNicéville, 1884) | China: Guangdong | 24.88N | 113.03E | SCAU He442 | KY049957 | KY049978 |
Laboratory protocols
Genomic DNA was extracted from the thorax of specimens preserved in ethanol, or from legs of dried specimens, using Magen’s Blood/cell/tissue DNA extraction kit. One mitochondrial gene cytochrome c oxidase I (COI) and one nuclear gene elongation factor 1-α (EF-1α) were used as molecular phylogenetic markers. The following primers were used for amplification and sequencing in this study: for COI – primers LCO1490 and HCO2198 (Folmer et al. 1994); for EF-1α – primers ef44 and efrcM4 (Monteiro and Pierce 2001). Ploymerase Chain Reaction (PCR) were performed in 20 µl volumes containing 1 µl template DNA, 2 µl 10× buffer, 1.6 µl dNTPs (containing 2.5 mM of each dNTP), 0.8 µl of each primer (10 uM), 0.2 µl Taq Polymerase (2 U/µl), and 13.6 µl ddH2O. The PCR Products were amplified using initial denaturation at 94 °C for 4 min, 35 cycles of denaturation at 94 °C for 30 s, annealing at 47 °C (COI) for 45 s, 55 °C (EF-1α) for 1 min, elongation at 72 °C for 1.5 min, and final elongation at 72 °C for 5 min.
Amplified DNA products were purified using an Agarose Gel Extraction kit (Magen Biotech), and directly sequenced, or cloned with pMD18-T vector (Takara Inc), and then sequenced. Sequencing was performed using the ABI 3730 automated sequencer. All sequences were submitted to the Genbank database (accession numbers are given in Table 1).
Phylogenetic analyses
Alignment of the DNA sequences were performed in Clustal X (Thompson et al. 1997) and edited manually in MEGA 6.0 (Tamura et al. 2013). All base frequencies and molecular character statistics were calculated in MEGA 6.0. Phylogenetic trees were constructed under (ML) and (BI) criteria. For ML analysis, RAxML version 8 (Stamatakis et al. 2014) was used on a concatenated data set of two genes, with 1000 rapid bootstrap replicates using GTR+G substitution model on the CIPRES Science Gateway (Miller et al. 2010). BI was carried out using (MCMC) randomization in MrBayes v3.2.3 (Ronquist et al. 2012). We used reversible-jump MCMC to allow for sampling across the entire substitution rate models. Four Markov chains (three heated chains, one cold) were run for 500, 000 generations, with the first 25% of sampled trees discarded as burn-in. The two independent runs were considered to have converged when the standard deviation of split frequencies value was <0.01. The convergence of the analysis was determined in Tracer v1.6 (Rambaut et al. 2014). Bayesian (PP) and ML (BP) were used to evaluate branch support.
Results
Sequence data
From a total of 23 samples, 22 sequences for COI and 21 for EF-1α were obtained. The alignment of the combined sequences consisted of a total of 1724 bp (658 bp of COI and 1066 bp of EF-1α genes, respectively), including 277 variable and 200 informative sites.
The pairwise P2K distances among the sequences were variable between genes. The ranges of sequence divergences for two loci and ingroup taxa are: COI (0–12.4%), EF-1α (0–5.0%). For COI, sequence divergence between conspecific individuals ranged from 0 to 0.6%; inter-specific genetic distances ranged from 3.6% to 12.4% with divergences among species averaging 7.9% (Table 2).
Table 2.
Uncorrected pairwise genetic distances (Kimura 2-parameter) for the COI sequences of the genus Scobura species.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Scobura cephaloides 102 | ||||||||||||||||||
| 2 | Scobura cephaloides 553 | 0.003 | |||||||||||||||||
| 3 | Scobura coniata 73 | 0.096 | 0.098 | ||||||||||||||||
| 4 | Scobura coniata 472 | 0.096 | 0.098 | 0.000 | |||||||||||||||
| 5 | Scobura hainana 471 | 0.084 | 0.085 | 0.059 | 0.059 | ||||||||||||||
| 6 | Scobura hainana 487 | 0.084 | 0.085 | 0.059 | 0.059 | 0.000 | |||||||||||||
| 7 | Scobura hainana 488 | 0.084 | 0.085 | 0.061 | 0.061 | 0.002 | 0.002 | ||||||||||||
| 8 | Scobura isota 468 | 0.115 | 0.117 | 0.122 | 0.122 | 0.099 | 0.099 | 0.099 | |||||||||||
| 9 | Scobura isota 538 | 0.113 | 0.115 | 0.118 | 0.118 | 0.096 | 0.096 | 0.096 | 0.006 | ||||||||||
| 10 | Scobura lyso 465 | 0.087 | 0.089 | 0.061 | 0.061 | 0.039 | 0.039 | 0.039 | 0.105 | 0.101 | |||||||||
| 11 | Scobura lyso 475 | 0.085 | 0.087 | 0.061 | 0.061 | 0.036 | 0.036 | 0.036 | 0.101 | 0.098 | 0.003 | ||||||||
| 12 | Scobura masutaroi 300 | 0.092 | 0.096 | 0.059 | 0.059 | 0.056 | 0.056 | 0.056 | 0.107 | 0.103 | 0.052 | 0.052 | |||||||
| 13 | Scobura masutaroi 301 | 0.092 | 0.096 | 0.059 | 0.059 | 0.056 | 0.056 | 0.056 | 0.107 | 0.103 | 0.052 | 0.052 | 0.000 | ||||||
| 14 | Scobura masutaroi 303 | 0.092 | 0.096 | 0.059 | 0.059 | 0.056 | 0.056 | 0.056 | 0.109 | 0.105 | 0.052 | 0.052 | 0.002 | 0.002 | |||||
| 15 | Scobura parawoolletti 116 | 0.094 | 0.097 | 0.089 | 0.089 | 0.087 | 0.087 | 0.087 | 0.112 | 0.108 | 0.084 | 0.084 | 0.084 | 0.084 | 0.084 | ||||
| 16 | Scobura stellata 36 | 0.101 | 0.104 | 0.104 | 0.104 | 0.101 | 0.101 | 0.099 | 0.108 | 0.105 | 0.099 | 0.099 | 0.092 | 0.092 | 0.094 | 0.070 | |||
| 17 | Scobura woolletti 535 | 0.094 | 0.097 | 0.096 | 0.096 | 0.085 | 0.085 | 0.085 | 0.124 | 0.121 | 0.092 | 0.092 | 0.099 | 0.099 | 0.099 | 0.039 | 0.074 | ||
| 18 | Scobura woolletti 536 | 0.092 | 0.096 | 0.094 | 0.094 | 0.084 | 0.084 | 0.084 | 0.123 | 0.119 | 0.090 | 0.090 | 0.097 | 0.097 | 0.098 | 0.038 | 0.072 | 0.002 | |
| 19 | Scobura woolletti 537 | 0.094 | 0.097 | 0.096 | 0.096 | 0.085 | 0.085 | 0.085 | 0.124 | 0.121 | 0.092 | 0.092 | 0.099 | 0.099 | 0.099 | 0.039 | 0.074 | 0.000 | 0.002 |
Phylogenetic analyses
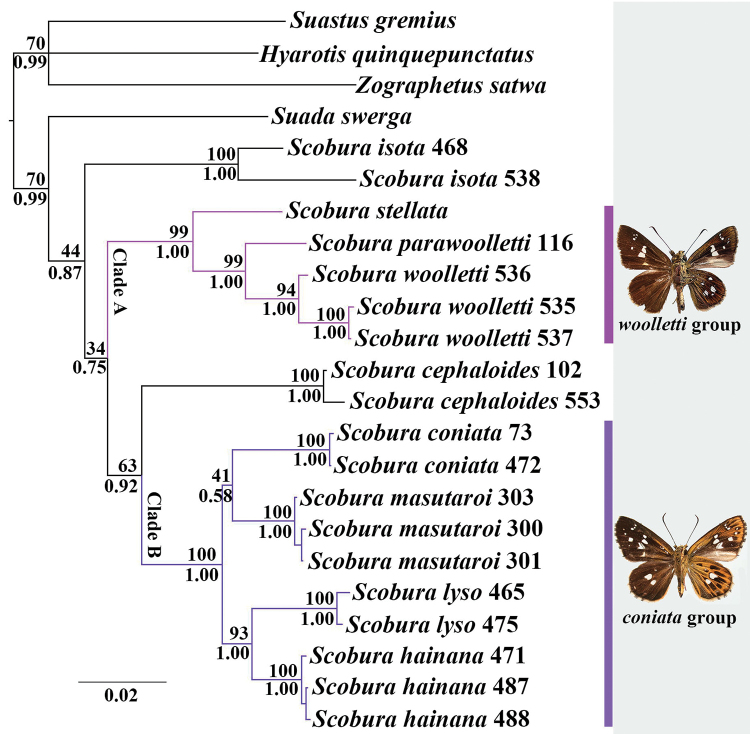
The two model-based analyses (BI and ML) revealed nearly identical topologies, differing mainly in branch support (Fig. 1). In both analyses, the monophyly of the genus Scobura is weakly supported (BP = 44, PP = 0.87). Within the genus, although support for the basal clades was low, the Scobura species included here are clearly distinguished from each other, and formed four clades: the Scobura isota clade (which only included two representative specimens), Clade A, the Scobura cephaloides clade (only with two representative specimens), and Clade B. Clade A is comprised by Scobura stellata + (Scobura parawoolletti + Scobura woolletti) and receive high bootstrap support and posterior probability (BP = 99, PP = 1.00). We hereafter called the clade Scobura woolletti group.
Figure 1.
Majority-rule consensus tree from the (BI) of the concatenated COI and EF-1α sequences. Values at nodes represent the (BS) values of the (ML) and the (PP) of BI analyses, respectively (BP/PP).
Clade B is comprised by Scobura masutaroi and the representatives of Scobura coniata group (Devyatkin 2004): Scobura coniata, Scobura lyso and Scobura hainana, and the latter two are sister species with strong support (BP = 93, PP = 1.00). The monophyly of Scobura coniata group including Scobura masutaroi is strongly supported (BP = 100, PP = 1.00).
In all the analyses, Scobura cephaloides is sister to Clade B, with moderate support (BP = 63, PP = 0.92), whereas the relationships between Scobura isota and the other clades (Clade A, Scobura cephaloides and Clade B) remain unresolved.
Discussion
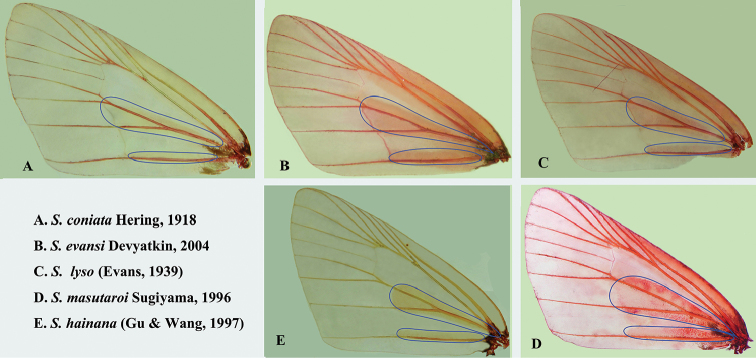
Although our phylogenetic analyses do not strongly support the monophyly of the genus Scobura, two strongly supported monophyletic groups within the genus are recognized: the Scobura coniata group and the Scobura woolletti group. The members of the coniata group share the following four morphological characters: 1) male band of scent scales on both sides of veins CuA1 and CuA2 and above 2A on the forewing (Fig. 2); 2) juxta U-shaped with two spine bearing arms, flat at base; 3) tegumen without socius; and 4) uncus thin and long. Scobura masutaroi is nested within this group. In our present analyses, two individuals (He 300, 301) of masutaroi from Nibashan, Sichuan (close to Dujiangyan, Sichuan, the type locality of Scobura masutaroi) and an individual (He303) from Jialingjiang, Fengxian, Shaanxi (the type locality of Scobura mouchai) are clearly grouped together with strong support values (BP = 100, PP = 1.00). Moreover, the pairwise P2K distances in COI between the species in the Scobura coniata group range from 3.3% to 6.1% with divergences between species averaging 4.5%, while divergence between individuals of Scobura masutaroi from Sichuan and Shaanxi province was 0.2%.
Figure 2.
Male band of scent scales in the Scobura coniata group species.
Based on the original description, distribution data, and the illustrations provided by Krajcik (2013), as well as our phylogenetic inferences, we conclude that Scobura mouchai is identical to Scobura masutaroi and should be considered a junior synonym. The male genitalia are illustrated herein, and the female genitalia are described for the first time. On the basis of morphological study (Devyatkin, 2004), two other species, Scobura phuongi and Scobura evani, which are not included in the present study, likely also belong to this group.
A well-support clade comprised by Scobura stellata, Scobura parawoolletti and Scobura woolletti was recovered in all analyses. These species share the following three characters: 1) hindwing with white spots on underside but not on upperside; 2) socius slender and pointed at tip; and 3) juxta funnel-like, thin and long basally. The generic name Mimambrix Riley, 1923 was proposed with Mimambrix woolletti as the type species, but later synonymized by Evans (1949). We follow Evans’ treatment and consider this clade as a species group within the genus Scobura. Based on morphological characters, the group also includes Scobura tytleri (Evans, 1914).
Taxonomic account
The key given by Fan et al. (2010) is modified to include Scobura masutaroi. The couplets leading to Scobura masuataroi only are included here. Couplets beyond 11 in the original increase their number by one.
| 3 | Forewing upper side without spots in spaces M3 or M1 and M2 | 4 |
| – | Forewing upper side with spots in spaces M1, M2 and M3 | 6 |
| 4 | Forewing upper side without spots in spaces M1 and M3, hindwing under side: basal half yellow, distally ferruginous, with five small spots | Scobura cephaloides |
| – | Forewing upper side without spot in space M3 | 5 |
| 5 | Hindwing under side with a conspicuous rectangular white spot in space CuA2 | Scobura cephala |
| – | Hindwing under side without a conspicuous rectangular white spot in space CuA2 | Scobura isota |
| 6 | Hindwing upper side without spot in space CuA1, under side with small white spots in spaces Sc+R1, M1-2, M3 and cell | Scobura eximia |
| – | Hindwing upper side with the spot in space CuA1 | 7 |
| 7 | Forewing cell spots conjoined, subequal | 8 |
| – | Forewing cell spots separated, if conjoined, the lower spot much larger | 9 |
| 8 | Hindwing upper side hyaline spots white | Scobura evansi |
| – | Hindwing upper side hyaline spots yellow | Scobura masutaroi |
| 9 | Forewing upper side the spot in space CuA2 triangular, and with a linear stigma crossing the spots in spaces CuA1 and CuA2 | Scobura coniata |
| – | Forewing upper side the spot in space CuA2 not as above | 10 |
| 10 | Forewing upper side the spot in space CuA1 narrow, hindwing upper side without spot in space | Scobura lyso |
| – | Forewing upper side the spot in space CuA1 broad | 11 |
| 11 | Hindwing upper side spot in space M3 tiny dot, forewing upper side cell spots cell spots conjoined | Scobura hainana |
| – | Hindwing upper side spot in space M3 significant, forewing upper side cell spots cell spots separated | Scobura phuongi |
Scobura masutaroi
Sugiyama, 1996
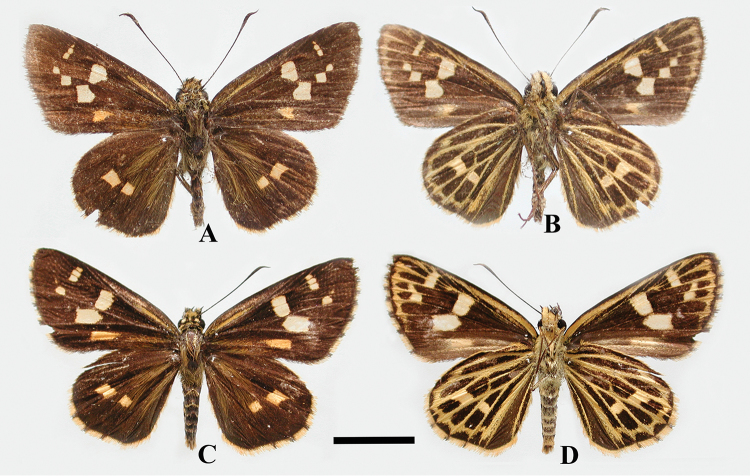
Figure 3.
Scobura masutaroi Sugiyama, 1996 (Sichuan): A, B male C, D female; scale bar 10 mm.
Scobura masutaroi Sugiyama, 1996: 9 (Type locality: Dujiangyan, Sichuan, China)
Scobura mouchai Krajcik, 2013: 2, syn. n. (Type locality: Fengxian, Shaanxi, China)
Material examined.
1♂, 1♀, Nibashan, Rongjing, Sichuan, 26.VII.2009, Min Wang; 1♂, Jialingjiang, Fengxian, Shaanxi, 15.VII.2010, Min Wang.
Diagnosis.
Forewing length 17–18 mm. This species is different from other species of Scobura coniata group in the appearance of the wing upper side: forewing with yellow streak in subcosta space basally, a big cell spots solid across cell, the spot in space CuA2 yellow; hindwing with spots in spaces CuA1 and M1-M2 yellow. Wing under side: forewing costal and submarginal spots yellow; hindwing all veins and submarginal spots from spaces Sc+R1 to CuA2 yellow; and all yellow submarginal spots conjoined both forewing and hindwing.
Description.
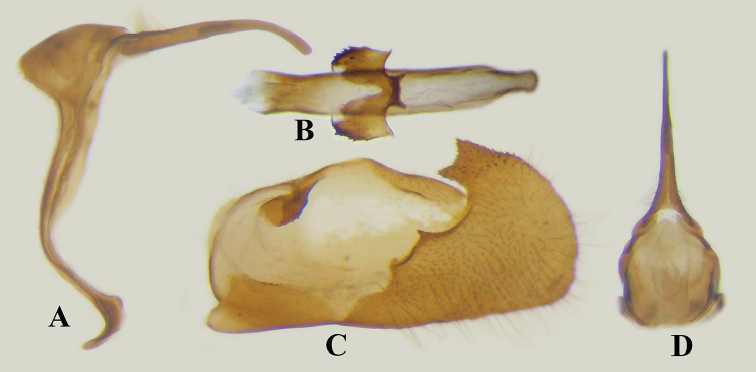
Male genitalia (Fig. 4): Tegumen without socius, weakly rounded from lateral view; uncus slender and much longer than tegumen; valva with transtilla rounded and sclerotized with small spines, ventro-distal process irregularly shaped with outer edge rounded, inner edge uneven, and distal part rectangular with densely small spines; saccus short and broad; gnathos absent; juxta U-shaped with two arms with densely spines.
Figure 4.
Male genitalia of Scobura masutaroi Sugiyama, 1996. (Sichuan). A Genitalia ring, lateral view; B aedeagus and juxta. C valva, inner view; D tegument, dorsal view.
Female genitalia (Fig. 5): Papillae anales rectangular, covered with setae; anterior lamella U-shaped with sclerotization; posterior lamella triangular with upper margin arched; ductus bursae membranous and short; copulatrix bursa elongate, membranous.
Figure 5.

Female genitalia of Scobura masutaroi Sugiyama, 1996 (Sichuan)
Distribution.
China (Sichuan, Shaanxi).
Supplementary Material
Acknowledgements
We are grateful to Drs Liu-Sheng Chen (Shihezi University, Xinjiang, China), Hou-shuai Wang and Hai-ming Xu (SCAU) for collecting the specimens. Materials of some species were provided by the Kyushu University museum, Dr Osamu Yata and Mr Hiroaki Onodera. The work is supported by the Natural Science Foundation of China (NSFC- 31172136 and -31471984).
Citation
Huang Z-F, Fei W, Wang M, Chiba H, Fan X-L (2016) A preliminary molecular phylogeny of the genus Scobura, with a synonym of Scobura masutaroi (Lepidoptera, Hesperiidae). ZooKeys 638: 33–44. https://doi.org/10.3897/zookeys.638.10026
References
- Devyatkin AL. (2004) Taxonomic studies on Oriental Hesperiidae, 1. A revision of the Scobura coniata Hering, 1918-group. Atalanta: 35(1/2): 57–66. [Google Scholar]
- Evans WH. (1949) A catalogue of the Hesperiidae from Europe, Asia & Australia in the British Museum (Natural History). The British Museum, London, England, United Kingdom, 502 pp https://doi.org/10.5962/bhl.title.105941 [Google Scholar]
- Fan XL, Chiba H, Wang M. (2010) The genus Scobura Elwes & Edwards, 1897 from China, with descriptions of two new species (Lepidoptera: Hesperiidae). Zootaxa 2490: 1–15. [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular marine biology and biotechnology 3: 294–299. [PubMed] [Google Scholar]
- Gu MB, Chen PZ. (1997) Butterflies in Hainan Island. China Forestry Publishing House. Beijing, China, 337 pp. [Google Scholar]
- Krajcik M. (2013) Description new Scobura Elwes and Edwards from Shaanxi Province and notes on the genus Ampittia Moore in China (Lepidoptera, Hesperioidea, hesperiinae). ANMMA 10(57): 1–3. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2010) Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. In: SC10 Workshop on Gateway Computing Environments (GCE10). https://doi.org/10.1109/GCE.2010.5676129
- Monteiro A, Pierce NE. (2001) Phylogeny of Bicyclus (Lepidoptera: Nymphalidae) Inferred from COI, COII, and EF-1α Gene Sequences. Molecular Phylogentic Evolution 18(2): 264–281. https://doi.org/10.1006/mpev.2000.0872 [DOI] [PubMed] [Google Scholar]
- Rambaut A, Suchard MA, Xie D, Drummond AJ. (2014) Tracer v1.6. http://beast.bio.ed.ac.uk/Tracer
- Riley ND. (1923) New Rhopalocera from Borneo. Entomologist 56: 35–38. [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Systematic Biology 61: 539–542. https://doi.org/10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. (2014) RAxML Version 8: A tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics. https://doi.org/10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed]
- Sugiyama H. (1996) New Butterflies from Western China (IV). Pallarge 5: 1–11. [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30(12): 2725–2729. https://doi.org/10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. (1997) The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 24: 4876–4882. https://doi.org/10.1093/nar/25.24.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Xiong W, Wang M. (2011) Two new species of the genus Longipenis (Lepidoptera: Lecithoceridae) from China. Florida Entomology 93: 352–356. https://doi.org/10.1653/024.093.0305 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.