Abstract
The polycyclic hydrocarbons (PAHs), pyrene, 1-hydroxypyrene, 1-nitropyrene, and 1-acetylpyrene, were found to induce Type I binding spectra with human cytochrome P450 (P450) 2A13 and were converted to various mono- and di-oxygenated products by this enzyme.
Pyrene was first oxidized by P450 2A13 to 1-hydroxypyrene which was further oxidized to di-oxygenated products, i.e. 1,8- and 1,6-dihydroxypyrene. Of five other human P450s examined, P450 1B1 catalyzed pyrene oxidation to 1-hydroxypyrene at a similar rate to P450 2A13 but was less efficient in forming dihydroxypyrenes. P450 2A6, a related human P450 enzyme, which did not show any spectral changes with these four PAHs, showed lower activities in oxidation of these compounds than P450 2A13.
1-Nitropyrene and 1-acetylpyrene were also found to be efficiently oxidized by P450 2A13 to several oxygenated products, based on mass spectrometry analysis.
Molecular docking analysis supported preferred orientations of pyrene and its derivatives in the active site of P450 2A13, with lower interaction energies (U values) than observed for P450 2A6 and that several amino acid residues (including Ala-301, Asn-297, and Ala-117) play important roles in directing the orientation of these PAHs in the P450 2A13 active site. In addition, Phe-231 and Gly-329 were found to interact with pyrene to orient this compound in the active site of P450 1B1.
These results suggest that P450 2A13 is one of the important enzymes that oxidizes these PAH compounds and may determine how these chemicals are detoxicated and bioactivated in humans.
Keywords: Pyrene, 1-hydroxypyrene, P450 2A13, oxidation, 1-nitropyrene, 1-acetylpyrene
Introduction
A variety of chemicals including polycyclic aromatic hydrocarbons (PAHs), aryl- and heterocyclic amines, and tobacco-related nitrosamines cause toxic and/or carcinogenic responses in human respiratory organs (Anttila et al., 2011; Nebert et al., 2004; Conney 1982; Rendic and Guengerich, 2012; Shimada and Fujii-Kuriyama, 2004). Most of these chemicals require metabolic activation by xenobiotic-metabolizing enzymes to form reactive products that bind covalently to DNA to evoke their toxic and/or carcinogenic effects (Nebert et al., 2004; Shimada, 2006). Cytochromes P450 (P450s) have been shown to play important roles in these bioactivation reactions, and levels of expression of individual forms of P450 are of great concern in determining how these chemicals induce toxicity and/or carcinogenesis in different human organs (Nebert et al., 2004; Rendic and Guengerich, 2012; Shimada and Fujii-Kuriyama, 2004).
Human P450s 2A13 and 2A6 are known to catalyze the activation and detoxification of environmental carcinogens such as tobacco-related nitrosamines (e.g. 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), N-nitrosonornicotine) and to metabolize different kinds of chemicals including coumarin, nicotine, phenacetin, naphthalene, 4-aminobiphenyl, and styrene (Su et al., 2000; Chiang et al., 2011; Fukami et al., 2008; Wong et al., 2005a; Wong et al., 2005b). P450 2A13 is mainly expressed in the respiratory tract and P450 2A6 is primarily found in the liver (Su et al., 2000; Chiang et al., 2012; Zhu et al., 2006). Recent studies have shown that P450 2A13 is more active than P450 2A6 in activating NNK, N-nitrosonornicotine, and other environmental carcinogens that cause cancer in the respiratory organs (Wong et al., 2005a; Wong et al., 2005b; Chiang et al., 2011). We have recently reported that P450 2A13 plays a more significant role than P450 2A6 in interacting with and metabolizing diverse environmental chemicals including PAHs (Shimada et al., 2011; 2013a). The results indicated that P450 2A13 catalyzes the activation of several PAH-diols to products that cause DNA damage in the tester strain Salmonella typhimurium NM2009 much more efficiently than P450 2A6 (Shimada et al., 2013b). Although much has been learned about the metabolism of various PAH compounds by P450 1 family enzymes, little is known about how these PAHs are metabolized by P450 2A family enzymes, (Shimada et al., 1996; 2001; Nebert et al., 2004; Rendic and Guengerich, 2012; Shimada and Fujii-Kuriyama, 2004).
In this study, we selected pyrene, 1-OHP, 1-NP, and 1-AcP as model compounds to examine the oxidative metabolism of PAHs by human P450 2A13 enzyme through analysis with HPLC and LC-MS. The catalytic activities of P450 2A13 were compared with those of P450 2A6 and other human P450s including P450s 1B1, 1A2, 2C9, and 3A4. The results of molecular docking simulations of the interactions of these PAH chemicals with active sites of human P450 enzymes are also reported.
Materials and methods
Chemicals
Pyrene, 1-OHP, 1-NP, and 1-AcP (Figure 1) were obtained from Sigma-Aldrich (St. Louis, MO) or Wako Pure Chemicals (Osaka). Pyrene was recrystallized from hot C2H5OH (mp 149.5–150.5°C, uncorr). Commercial 1-AcP (purity, 97%) was purified by silica gel column chromatography using petroleum ether/ethyl acetate (5:1. v/v) as eluent, and 1-AcP was recrystallized from C2H5OH (mp 86.5–88.0°C). Commercial 1-OHP (Wako) was >97% pure and was used without further purification. Although these PAH chemicals used as substrates in this study had some impurities (HPLC), these contaminants did not significantly interfere with product formation and the results of HPLC analysis. Other chemicals and reagents used in this study were obtained from the sources described previously or were of the highest quality commercially available (Shimada et al., 2011, 2013a; 2013b).
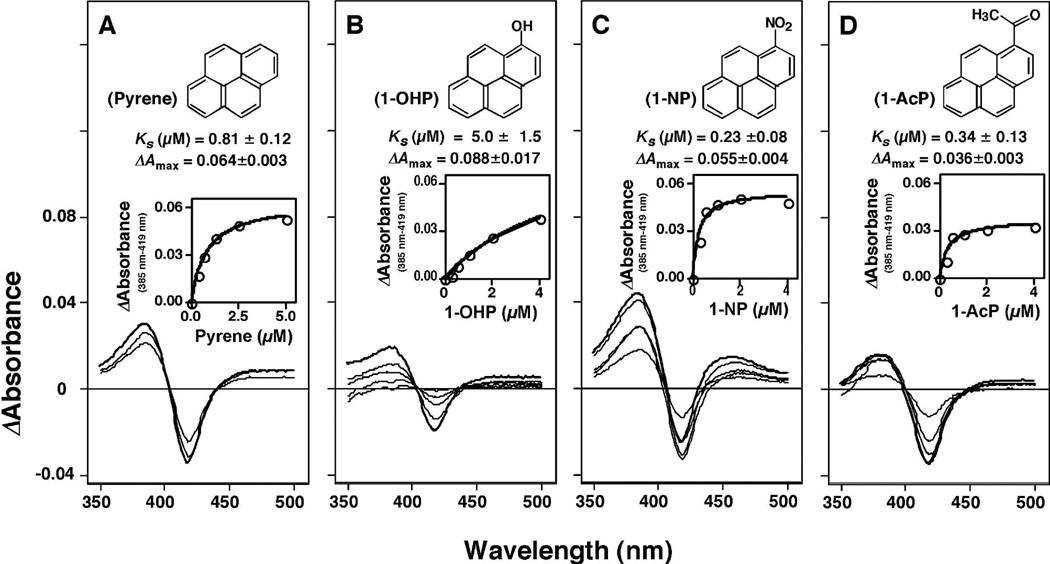
Figure 1.
Type I binding difference spectra of pyrene, 1-hydroxypyrene (1-OHP), 1-nitropyrene (1-NP), and, 1-acetylpyrene (1-AcP) with P450 2A13. Concentrations of chemicals used were 0.31, 0.63, 1.25, 2.5, and 5 µM for pyrene and 0.25, 0.5, 1.0, 2.0, and 4.0 µM for 1-OHP, 1-NP, and 1-AcP.
Enzymes
The expression and purification of P450s 2A6 and 2A13 have been described previously (Shimada et al., 2011; 2013a). Escherichia coli bicistronic P450 2A13, 2A6, 1B1, 1A2, 2C9, and 3A4 membranes (with human NADPH-P450 reductase co-expressed) were suspended in 10 mM Tris-HCl buffer (pH 7.4) containing 1.0 mM EDTA and 20% glycerol (v/v) as described. P450s 2A13, 2A6, 1B1, 1A2, 2C9, 3A4, NADPH-P450 reductase, and cytochrome b5 were purified from membranes of recombinant E. coli as described elsewhere (Shimada et al., 1998; Shimada et al., 2013a; 2013b; Sandhu et al., 1993; 1994; Gillam et al., 1993). Recombinant human P450 1A1 expressed in microsomes of Trichoplusia ni cells infected with a baculovirus containing human P450 1A1 and NADPH-P450 reductase cDNA inserts was obtained from GENTEST/BD Biosciences (Woburn, MA); the P450 content in this system was specified in the data sheet provided by the manufacturer.
Rat liver epoxide hydrolase was prepared as described previously (Guengerich et al., 1979); the enzyme has been shown to be catalytically active in forming trans -7,8-dihydroxy-7,8-dihydrobenzo[a]pyrne from benzo[a]pyrene when incubated with human P450 1B1 (Shimada et al., 1999) and also catalyzed the hydroysis of pehanthrene-9,10-oxide (Lacourciere et al., 1993). Liver microsomes prepared from β-naphthoflavone-treated rats were obtained from XenoTech (Lenexa, KS).
Spectral binding titrations
Purified P450 enzymes were diluted to 1.0 µM in 0.10 M potassium phosphate buffer (pH 7.4) containing 20% glycerol (v/v), and binding spectra were recorded with subsequent additions of chemicals in a JASCO V-550 or an OLIS-Aminco DW2a spectrophotometer (On-Line Instrument Systems, Bogart, GA) as described previously (Shimada et al., 2011: 2012a). (The chemicals in this study were dissolved in (CH3)2SO as 10 mM stock solutions and diluted, with the final solvent concentration ≤ 0.5%, v/v). Briefly, the chemicals were added to the buffer with or without the P450 and the spectra were recorded between 350 nm and 500 (or 700) nm. The substrate binding spectra were obtained by subtracting the blank spectra (in the absence of the P450) from the P450 spectra (in the presence of the P450). Spectral dissociation constants (Ks) were estimated using Prism software (GraphPad, La Jolla, CA), either using hyperbolic plots or quadratic fitting (for tight binding).
Oxidation of pyrene, 1-OHP, 1-NP, and 1-AcP
Oxidative metabolism of pyrene, 1-OHP, 1-NP, and 1-AcP by P450 enzymes was determined in a standard incubation mixture (0.50 mL) containing P450 (50 pmol) in bicistronic membranes, 50 µM chemical, and an NADPH-generating system incubating at 37°C for 20 min (Shimada et al., 2013a; 2015). In reconstitution experiments, P450 membranes were replaced by purified P450 (50 pmol), NADPH-P450 reductase (100 pmol), cytochrome b5 (100 pmol) (when required), and L-α-1,2-dilauroyl-sn-glycero-3-phosphocholine (50 µg), as described previously (Yamazaki et al., 1999). Reactions were terminated by adding 500 µL of CH3OH, and the residual substrate and products were extracted twice with a mixture of CHCl3 and ethyl acetate (1:1, v/v). The combined organic layers were evaporated to dryness under a nitrogen stream, and the extracts were dissolved in 100 µL of CH3OH for analysis by HPLC and LC-MS.
Most HPLC separations were done with a JASCO system (Tokyo, Japan) equipped with a Wakopack Navi C18-5 octadecylsilane column (2.0 mm × 150 mm) (Wako Pure Chem.) with UV detection at 254 nm and fluorescence detection (excitation wavelength 242 nm and emission wavelength 380 nm). Elution of PAHs and their metabolites utilized a linear gradient increasing from 20% to 100% CH3OH (v/v) over 25 min and then held at 100% CH3OH for 5 min, with a flow rate of 0.2 mL/min.
LC separations of P450 2A13-mediated products of pyrene and 1-OHP for time and concentration dependence experiments (as well as LC-MS analysis) were done with a Waters Acquity UPLC system (Waters, Milford, MA) equipped with an octadecylsilane (C18) column (6.2 mm × 80 mm, 3 µm; Agilent Technologies, Santa Clara, CA) and detection with UV photodiode array and fluorescence (as above). The program for elution of pyrene and its hydroxylated products was held at 20% CH3CN (v/v) for 4 min, increased to 80% CH3CN (v/v) over 11 min and then to 100% CH3CN over 2 min, and then held at 100% for 3 min, with a flow rate of 0.3 mL/min.
Quantitation of 1-OHP was accomplished by comparing peak areas from samples to those from an external standard curve of the authentic standard. Because 1,8-di-OHP is not commercially available, quantitation was accomplished by measuring the UV absorbance (275 nm) of a collected peak and using the molar extinction coefficient (ɛ275 10,800 M−1 cm−1) to convert peak area to mol (Sohl et al., 2008). The molar extinction coefficient was previously determined by quantitation of collected material by 1H-NMR (Sohl et al., 2008).
LC-MS analysis for identification of product formation
A Finnigan LTQ mass spectrometer (ThermoFisher Scientific, Waltham, MA) was used with electrospray ionization in the negative ion mode. Electrospray ionization conditions were: source voltage of 4 kV, source current 100 µA, auxiliary gas flow rate setting 20, sweep gas flow rate setting 5, sheath gas flow setting 34, capillary voltage −26 V, capillary temperature at 350 °C, and tube lens voltage of −138 V. MS-MS conditions were: normalized collision energy 35%, activation Q setting 0.250, and activation time 30 ms. For the products of pyrene and 1-OHP oxidation, selective ion monitoring scans at m/z 217 and 233 were recorded.
The separation of the products of 1-NP and 1-AcP oxidation was done with an LCMS IT-TOF mass spectrometry system equipped with an APCI/APPI ion source (Shimadzu, Kyoto, Japan). The samples were injected onto an Inert Sustain C18 column (3 µm, 2.0 mm × 100 mm; GL Science, Tokyo, Japan) equilibrated with mobile phase A (H2O containing 5 mM ammomium acetate and 0.02% formic acid, w/v) and mobile phase B (20% CH3OH containing 5 mM ammomium acetate and 0.02% formic acid, w/v). The solvent program was 20% B for 2 min, increased to 100% B over 6 min, and then held at 100 % B for 3 min, with a flow rate of 0.2 mL/min. Both positive and negative ions were detected within the same injection, using the high-speed polarity switching function. MS/MS analysis was done in the data-dependent mode.
Other enzyme assays
Coumarin 7-hydroxylation and 7-ethoxyresorufin O-deethylation activities were determined using bicistronic bacterial membranes expressing human P450s (together with human NADPH-P450 reductase), as described previously (Shimada et al., 2013a; 2013b).
Kinetic analysis
Kinetic parameters were estimated by nonlinear regression analysis using the program Kaleida-Graph (Synergy Software, Reading, PA) or Prism (Graphpad).
Docking simulations into human P450 enzyme active sites
Crystal structures of P450s 2A6 (Sansen et al., 2007a), 2A13 (Smith et al., 2007), and 1B1 (Wang et al., 2011) have been reported and were used for the analyses. Simulations were carried out for P450 enzymes using the MMFF94x force field described in the MOE Software (ver. 2013.10, Computing Group, Montreal, Canada). P450s were superimposed during modeling of the three-dimensional structures. Lower U values (ligand-interaction energy) are an indication of higher interaction between a chemical and the enzyme. The substrate pocket size and the π-H interaction between the benzene ring in the substrates and the methyl group of Ala-301 of P450 2A13 were evaluated by internal modules in the MOE Software. In addition, we measured the interaction distance between certain carbon atoms of the chemicals and P450 heme by using the MOE Software.
RESULTS
Spectral interaction and metabolism of pyrene, 1-OHP, 1-NP, and 1-AcP with human P450 2A13
Pyrene, 1-OHP, 1-NP, and 1-AcP all induced Type I binding spectra with P450 2A13 with Ks values of 0.81, 4.3, 0.23, and 0.34 µM, respectively (Fig. 1). The ΔAmax/Ks ratios for the interaction of pyrene, 1-OHP, 1-NP, and 1-AcP with P450 2A13 were found to be 0.084, 0.006, 0.24, and 0.22 (absorbance units µM−1), respectively. Such spectral interactions were not seen in the orthologous human P450, P450 2A6, with these four PAHs even at 16 µM concentration. Of four other P450 enzymes examined (P450s 1B1, 1A2, 2C9, and 3A4), only P450 1B1 showed reverse Type I binding spectra with pyrene, 1-OHP, 1-NP, and 1-AcP, with Ks values of 1.2, 0.88, 1.4, and 0.39 µM, respectively, and respective ΔAmax/Ks ratios of 0.025, 0.070, 0.026, and 0.092 (absorbance units µM−1).
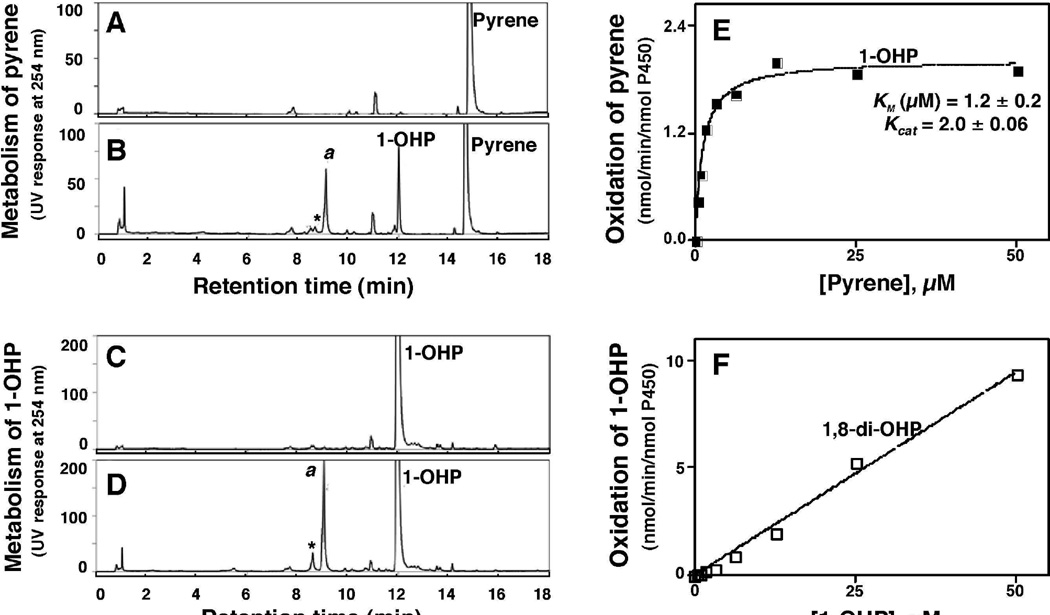
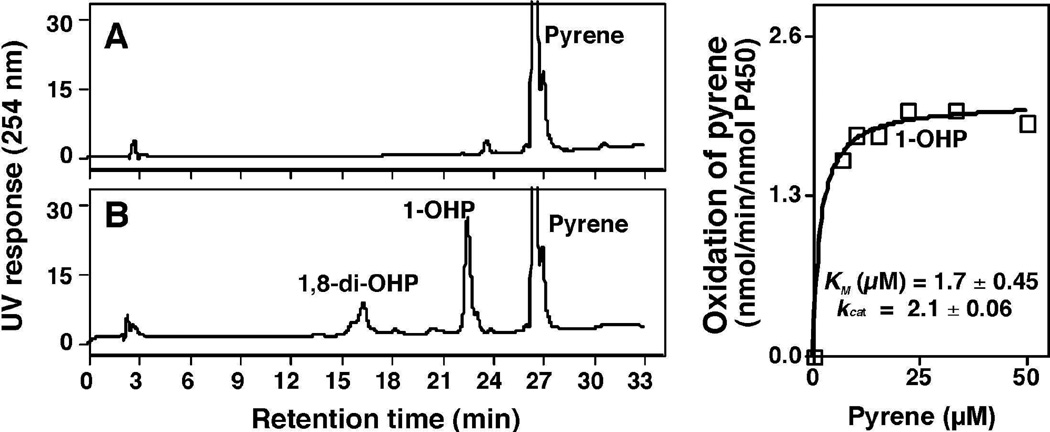
Oxidations of pyrene and 1-OHP were studied in a reconstituted monooxygenase system containing P450 2A13 and NADPH-P450 reductase in the presence or absence of an NADPH-generating system (Fig. 2). P450 2A13 converted pyrene to 1-OHP and other products only in the presence of an NADPH-generating system (Fig. 2). Peak a (tR 9.0 min) was found to be 1,8-di-OHP by comparison with a previously identified (rabbit) P450 1A2 product of 1-OHP oxidation (Sohl et al., 2008) on the basis of the HPLC tR (co-elution), LC-MS spectrum, and UV spectrum (Fig. 3). The kcat and Km values for the formation of 1-OHP from pyrene by P450 2A13 in the reconstituted system were 2.0 ± 0.1 nmol/min/nmol P450 and 1.2 ± 0.3 µM, respectively (Fig. 2E).
Figure 2.
Oxidation of pyrene (A and B) and 1-OHP (C and D) by a reconstituted system containing purified P450 2A13 and NADPH-P450 reductase in the absence (A and C) and presence (B and D) of an NADPH-generating system. Peak a was found to be 1,8-di-OHP (vide infra). Kinetic analyses of pyrene oxidation to 1-OHP (E) and of 1-OHP oxidation to 1,8-di-OHP (F) by P450 2A13 were examined.
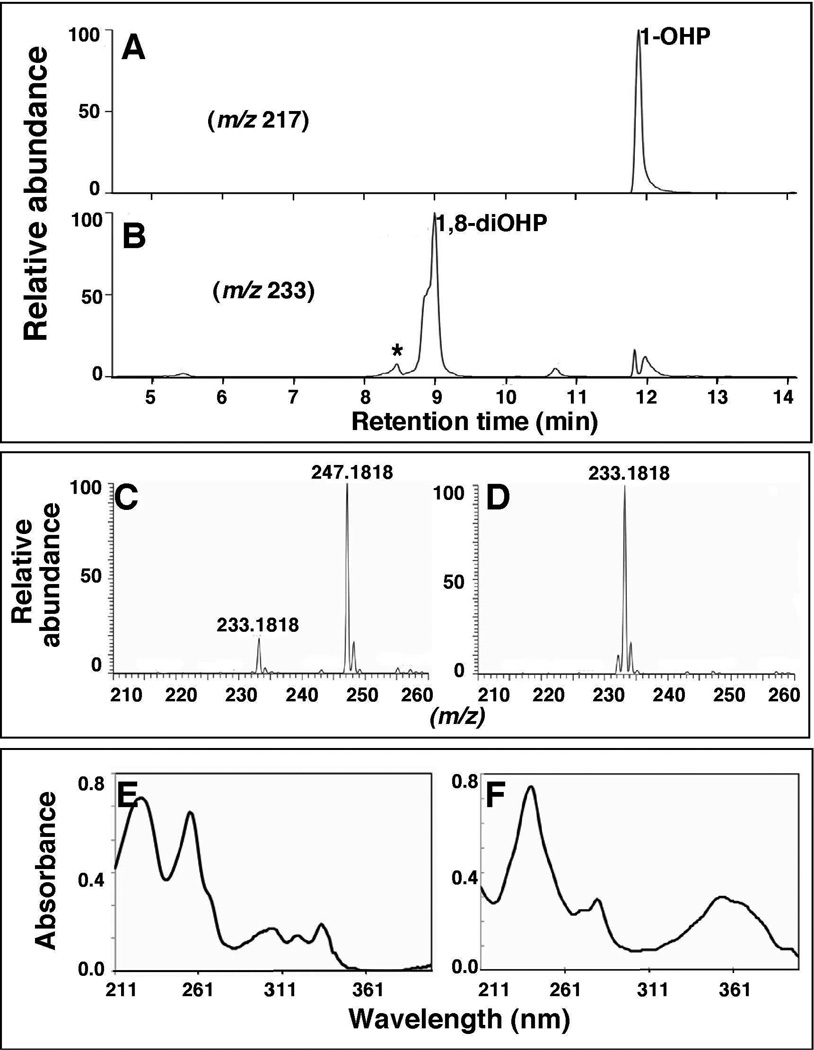
Figure 3.
LC-MS analysis of the formation of metabolites of pyrene by a reconstituted system containing purified P450 2A13 and NADPH-P450 reductase. Ions were monitored at m/z 217 (A) and m/z 233 (B) showing the formation of 1-OHP and 1,8-di-OHP, respectively. The MS and UV spectra of unidentified product “★” (Figs. 3C and 3E, respectively) and 1,8-di-OHP (Figs. 3D and 3F, respectively) in chromatogram of Fig. 3B are also shown. The peak eluting at tR 5.4 min is proposed to be 1,5-di-OHP (Sohl et al., 2008); the tR 10.8 min was not identified.
P450 2A13 was also found to oxidize 1-OHP to the major product a (1,8-di-OHP) and some minor products (Fig. 2D). In kinetic analysis to determine kcat and Km for the formation of the major product 1,8-di-OHP, we could not reach saturating conditions (Figure 2F) and thus a limit of kcat/Km of 0.2 µM−1 min−1 was estimated from the slope of the graph (compared with 1.7 µM−1 min−1 for conversion of pyrene to 1-OHP, vide supra).
The effect of rat epoxide hydrolase (50 pmol) on the metabolism of pyrene was studied in a reconstituted monooxygenase system containing P450 2A13 (50 pmol) and NADPH-P450 reductase (100 pmol) as described in Materials and methods, because Jakob et al. (1982) have reported that rat liver microsomes convert pyrene to 4,5-dihydroxy-4,5-dihydropyrene as well as 1-OHP, 1,6-di-OHP, and other metabolites, based on gas-chromatography detection. Our results showed that the HPLC profiles (in both UV and fluorescence detection) for the formation of pyrene metabolites by P450 2A13 were found to be very similar in the presence and absence of rat epoxide hydrolase in the reaction mixture and also were very similar when pyrene was incubated with liver microsomes (250 pmol of P450) of β-naphthoflavone-treated rats (results not shown).
In LC-MS analysis, P450 2A13 was found to convert pyrene to the mono-hydroxylated product 1-OHP (m/z 217, Fig. 3) and several di-hydroxy products (m/z 233, Fig. 3B, 3C, and 3D). UV scans of peaks that eluted at 8.4 min (marked with an asterisk (*)) and 8.9 min showed similar profiles of 1,6-di-OHP and 1,8-di-OHP, respectively, which have been reported previously with rabbit P450 1A2 (Sohl et al., 2008). The nature of shoulder on the early side of the peak at tR 8.9 min was not characterized in this study. An additional product peak (tR 5.4 min) was seen and is specualated to be 1,5-di-OHP, based on spectral evidence (Sohl et al., 2008), but was not further confirmed. The formation of the m/z 247.1818 product (compared to m/z 233.1818, Fig. 3C) suggested further oxidation and dehydrogenation of a di-OHP product, but we did not attempt to charcterize the product.”
Metabolism of 1-NP and 1-AcP by a reconstituted P450 2A13 system
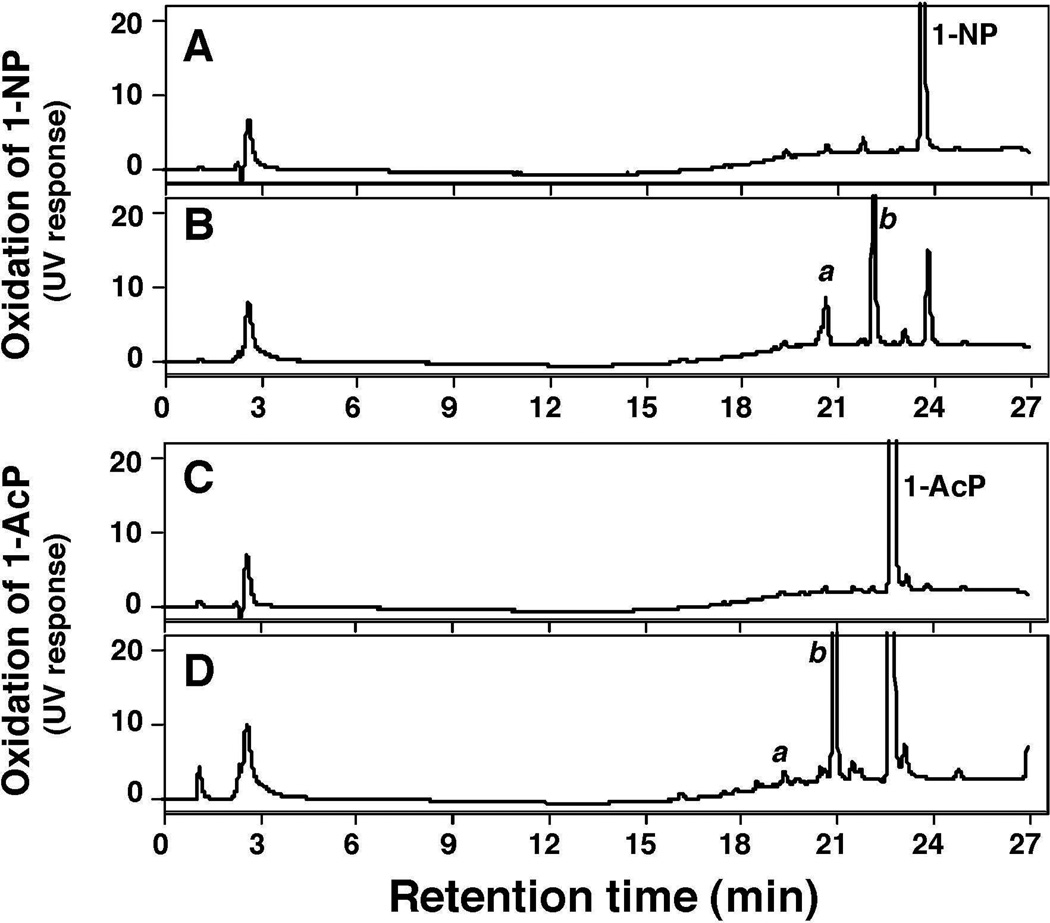
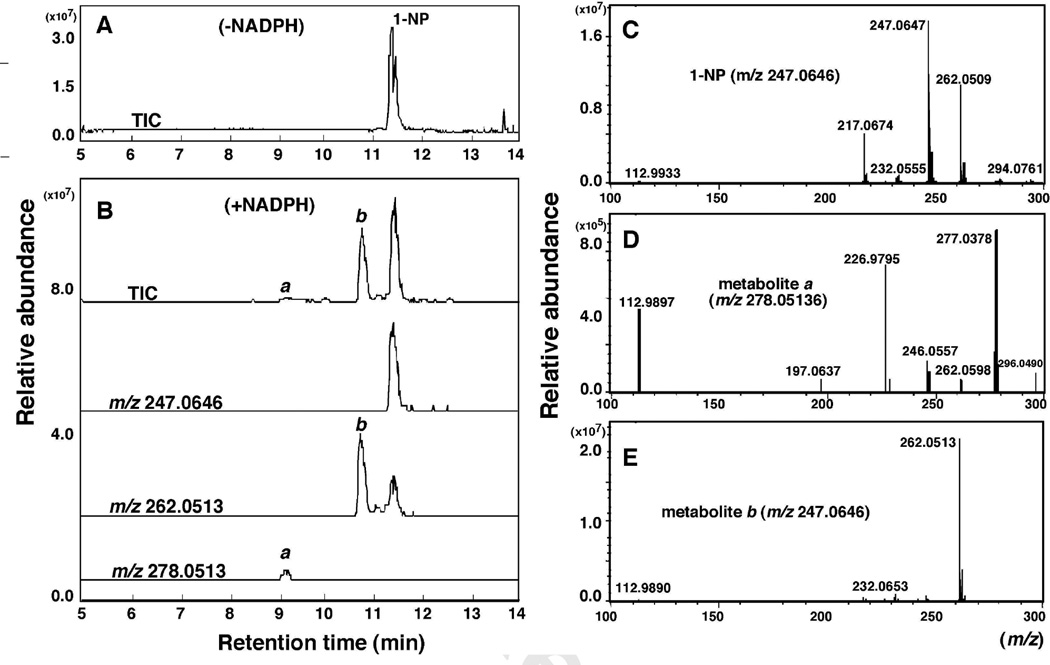
HPLC analysis showed that P450 2A13 converted 1-NP into two products, a and b, in the presence of an NADPH-generating system (Fig. 4B). The substrate 1-NP was found to contain some impurities on HPLC analysis (Fig. 5A), but the tR values of the products were different from those of the impurities. P450 2A13 also converted 1-AcP to one major and several minor products in the presence of an NADPH-generating system (Fig. 4C and 4D).
Figure 4.
Oxidation of 1-NP (A and B) and 1-AcP (C and D) by bicistronic E. coli membranes expressing both P450 2A13 and NADPH-P450 reductase in the absence (A and C) and presence (B and D) of an NADPH-generating system by incubating for 30 min (single determinations). The products a and b are unidentified.
Figure 5.
LC-MS analysis of oxidation of 1-NP by P450 2A13 in a reconstituted system. 1-NP was incubated with P450 2A13 in the absence (A) and presence (B) of an NADPH-generating system, and 1-NP and its products were extracted with ice-cold methanol and chloroform/ethyl acetate mixture (1:1, v/v). Two products, a and b, were found (B) and MS analysis indicated the formation of mono-oxygenated product a (E) and di-oxygenated product b (D) in this assay.
LC-MS analyses of the metabolism of 1-NP and 1-ACP were performed from reconstituted monooxygenase systems containing P450 2A13 and NADPH-P450 reductase in the presence and absence of an NADPH-generating system (Figs. 5 and 6, respectively). P450 2A13 converted 1-NP into a minor product (a) and a major product (b) in the presence of an NADPH-generating system (Fig. 5A and 5B). MS analysis (negative ion mode) indicated that peak a was di-oxygenated (m/z 278.0513) and peak b was mono-oxygenated product with m/z 262.0513 (Fig. 5D and 5E, respectively), although the structures of these products were not identified in this study.
Figure 6.
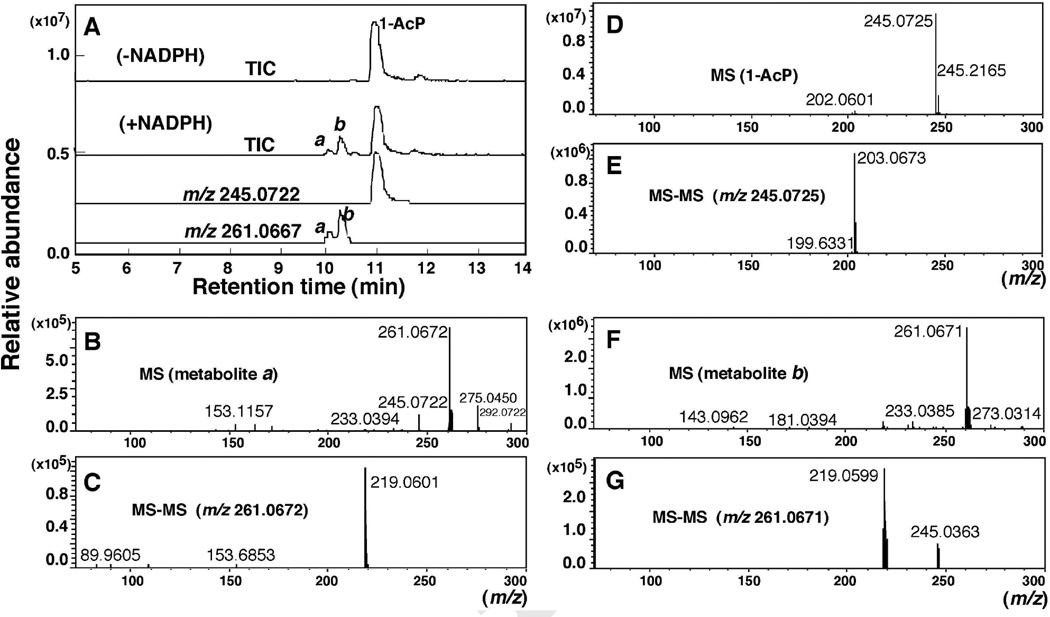
Oxidation of 1-AcP by purified P450 2A13 in a reconstituted system. LC analysis of products (A) in the presence of an NADPH-generating system showed two products a and b with m/z 261.0667, which were not detected in the absence of the NADPH system. MS/MS analysis showed a loss of 42 a.m.u. (B–G).
P450 2A13 (in the presence of an NADPH-generating system) converted 1-AcP to at least two products, a and b, on LC-MS analysis (Fig. 6A). Product b (m/z 261.0671) was found to be more prominent than product a (m/z 261.0672) (Fig. 6B and 6F). MS-MS analyses (positive ion mode) of 1-AcP and the products indicated the loss of 1-acetyl moiety during ionization (Fig. 6E, 6C, and 6G, respectively). Two fragments of m/z 219.0599 and 245.0363 (Fig. 6G) found on MS-MS analysis of the m/z 261.071 peak (Fig. 6F) suggest the formation of an epoxide or phenol metabolite during the reaction; however, we did not characterize this product in the present study.
Comparison of metabolism of pyrene, 1-NP, and 1-AcP by six human P450 enzymes
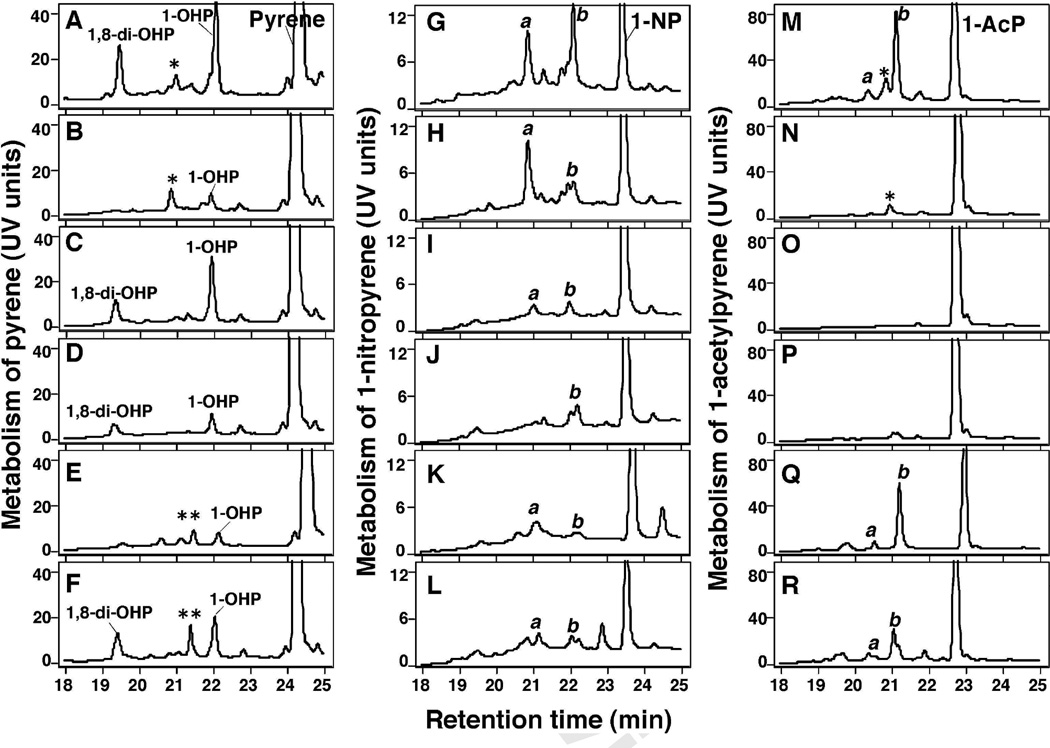
Bicistronic human P450 enzymes—P450s 2A13, 2A6, 1B1, 1A2, 2C9, and 3A4 co-expressed with NADPH-P450 reductase in E. coli membranes—were incubated with pyrene, 1-NP, and 1-AcP and the products formed were analyzed by HPLC (Fig. 7, duplicate determinations were done with these incubations and one of these results is presented in the figure). Pyrene was converted to 1-OHP at the highest rate by P450 2A13, followed by P450s 1B1 and 3A4, and at lower rates by P450s 2A6, 1A2, and 2C9 (Fig. 7A-7F). The turnover numbers (nmol/min/nmol P450 ± range of duplicate determinations) for the formation of 1-OHP from pyrene by P450s 2A13, 2A6, 1B1, 1A2, 2C9, and 3A4 were calculated to be 2.6 ± 0.2, 0.41 ± 0.11, 1.9 ± 0.3, 0.57 ± 0.08, 0.21 ± 0.03, and 1.1 ± 0.3, respectively. Formation of 1,8-di-OHP from pyrene (probably through the formation of 1-OHP) was also catalyzed by P450 2A13 at a much higher rate than by P450s 1B1, 1A2, and 3A4 and was very slow with P450s 2A6 and 2C9 (Fig. 7).
Figure 7.
Metabolism of pyrene (A–F), 1-NP (G–L), and 1-AcP (M–R) by P450s 2A13 (A, G, and M), 2A6 (B, H, and N), 1B1 (C, I, and O), 1A2 (D, J, and P), 2C9 (E, K, and Q), and 3A4 (F, L, and R) in bicistronic E. coli membranes expressing each respective P450 and NADPH-P450 reductase. The incubation time was 30 min with detection of pyrene, 1-NP, and 1-ACP and their products by UV absorbance. Products of pyrene were identified as 1-OHP and 1,8-di-OHP and other products (★ and ★★) and products of 1-NP and 1-AcP were designated a and b. Duplicate experiments produced similar results.
P450s 2A13 and 2A6 converted 1-NP to product a to similar extents, but the former enzyme was more active than the latter in forming product b (Fig. 7G and 7H). The other four P450s studied showed lower rates for oxidation of 1-NP. Oxidation of 1-AcP was also catalyzed by P450 2A13 to the highest extent, followed by P450s 2C9 and 3A4. P450s 2A6, 1B1, and 1A2 were not very active in oxidizing 1-AcP.
Oxidation of pyrene by recombinant human P450 1A1 in microsomes of T. ni cells
Kim et al. (2004) reported that human P450 1A1 showed high activity in catalyzing pyrene oxidation to 1-OHP in microsomes of Trichoplusia ni cells infected with baculovirus containing P450 and NADPH-P450 reductase cDNA inserts. Here, we re-examined how P450 1A1 (producted in a T. ni-baculovirus) system catalyzes the oxidation of pyrene in our assay system (P450 1A1 enzyme in E. coli membranes was not available). Our results showed that P450 1A1 (from the baculovirus system) formed 1-OHP, with Km and kcat values of 1.7 µM and 2.1 nmol/min/nmol P450, respectively, similar to P450 2A13 (Fig 2) and also formed a small amount of 1,8-di-OHP not observed by Kim et al. (2004) (identified by comparison with P450 2A13 reactions, Fig. 3) (Fig. 8).
Figure 8.
Oxidation of pyrene by microsomes of T. ni cells expressing P450 1A1 and NADPH-P450 reductase in the absence (A) and presence (B) of an NADPH-generating system. Kinetic analysis of formation of 1-OHP on incubation of pyrene with P450 1A1 enzyme system is also shown.
Docking simulations of interaction of pyrene, 1-OHP, 1-NP, and 1-AcP with P450s 2A13 and 2A6
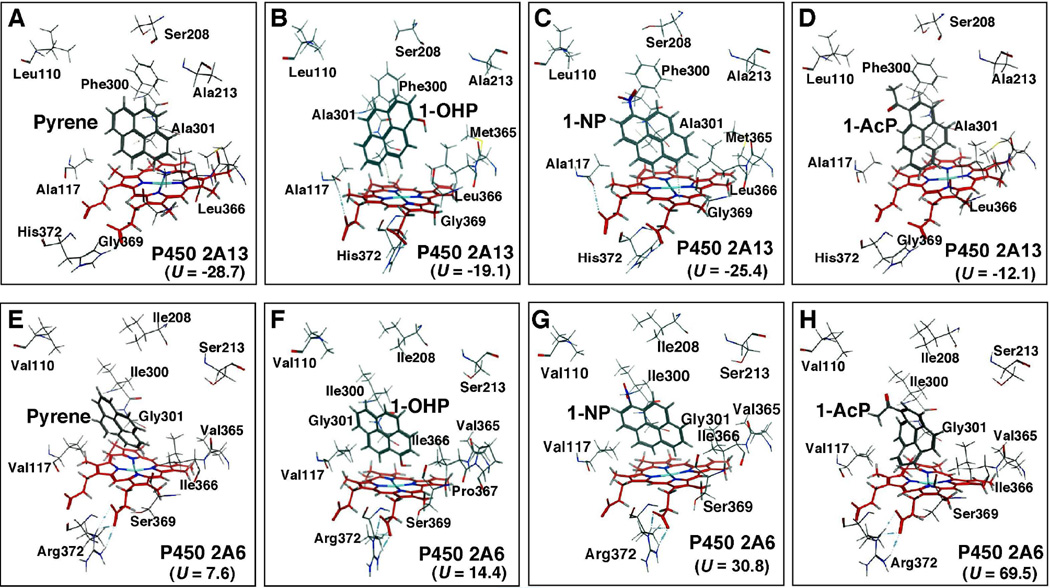
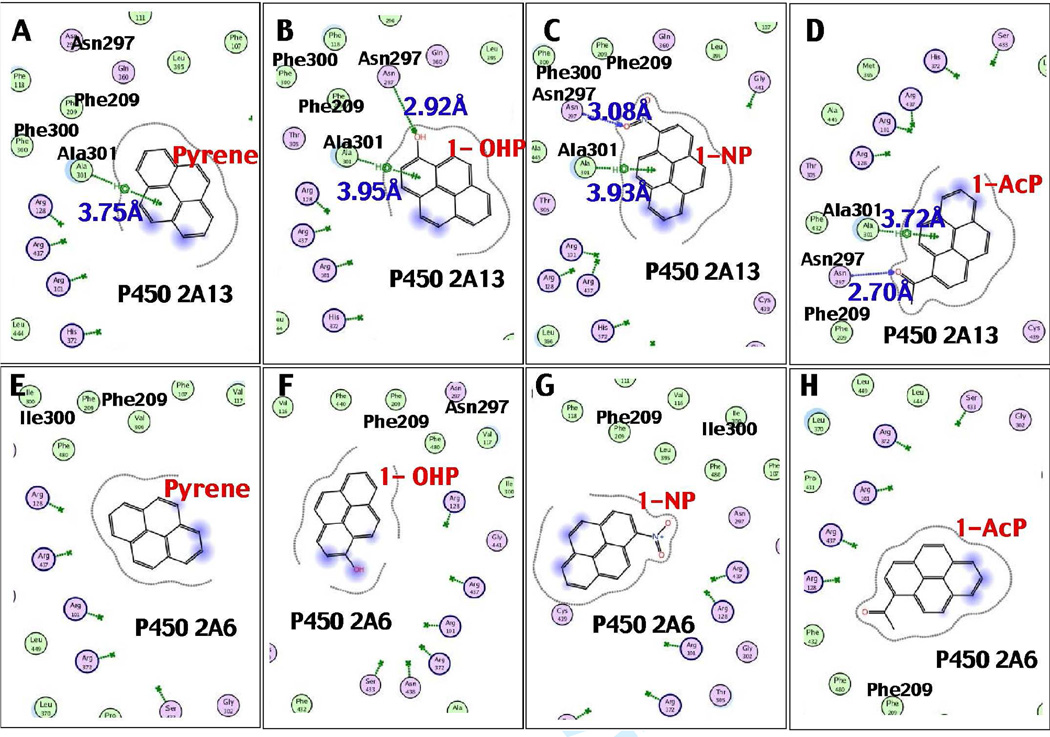
Because our results suggest that there are differences in how P450s 2A13 and 2A6 induce spectral changes with and catalyze oxidation of pyrene, 1-OHP, 1-NP, and 1-AcP, we compared the interactions of these PAHs in the active sites of these two P450s through molecular docking analyses (Fig. 9). Several different amino acid residues in P450s 2A13 and 2A6 sequences that have been reported previously (Smith et al., 2007; DeVore et al., 2008) are indicated in the figure. Pyrene was docked well into the P450 2A13 active site, with a low interaction energy of U = −28.7 (Figure 9A). The higher interaction energy (U = 7.6) of pyrene with P450 2A6 suggested poor orientation of the molecule in the active site (Figure 9E). The distance between C-1 of pyrene and the P450 2A13 heme iron was 4.05 Å; it was shortest among other possible interactions with C-2, C-10, and C-9 (Table 1). In addition, a π-H interaction (3.75 Å) between a benzene ring of pyrene and the CH3 moiety of Ala-301 was found in P450 2A13 (Figure 10); this interaction was not seen for P450 2A6. No interactions were detected between pyrene and Asn-297 in P450 2A13 or 2A6, while the importance of Asn-297 interaction with several other ligands has been previously suggested (Sansen et al., 2007a; Smith et al., 2007; DeVore et al., 2008; 2009; 2012; DeVore and Scott, 2012).
Figure 9.
Docking simulations of pyrene, 1-OHP, 1-NP, and 1-AcP with P450s 2A13 (A–D) and 2A6 (E–H). Total interaction energy values (U-values) are shown in the figures. Oxygen, nitrogen, and iron atoms are colored with red, blue, and light blue, respectively.
Table 1.
Distance between C-positions of the chemicals studied and the P450 2A13 heme.
| Carbon position |
Distance between C-position of chemicals and P450 2A13 heme (Å) |
||||
|---|---|---|---|---|---|
| (Clockwise) | (Anticlockwise) | Pyrene | 1-OHP | 1-NP | 1-AcP |
| C-1 | C-1 | 4.05 | |||
| C-2 | C-10 | 4.69 | |||
| C-3 | C-9 | ||||
| C-4 | C-8 | 4.45 | |||
| C-5 | C-7 | 4.23 | 5.39 | 5.24 | |
| C-6 | C-6 | 5.25 | 4.33 | 4.74 | |
| C-7 | C-5 | 6.30 | 4.59 | 5.05 | |
| C-8 | C-4 | 5.14 | 5.32 | ||
| C-9 | C-3 | 5.56 | |||
| C-10 | C-2 | 4.58 | |||
Figure 10.
Interaction of pyrene (A and E), 1-OHP (B and F), 1-NP (C and G), and 1-AcP (D and H) with active sites of P450 2A13 (A–D) and P450 2A6 (E–H). Interaction distances between amino acid residues Ala-301 and Asn-297 in P450 2A13 sequence and chemical portions are shown in the figure. No direct interactions between Gly-301 and Asn-297 in P450 2A6 sequence with these PAHs were observed.
1-OHP was also found to be well docked into the P450 2A13 active site, having an interaction energy of U = −27.4 with P450 2A13 as compared with U = 14.4 with P450 2A6 (Figure 9). There was a hydrogen bond interaction (2.92 Å) between Asn-297 and the -OH and a π-H interaction (3.95 Å) between Ala-301 and the aromatic ring of 1-OHP in the active site of P450 2A13 (Figure 10). The aromatic C-5 (C-7) of 1-OHP was the closest (4.23 Å) to the heme center of P450 2A13 followed by C-4 (C-8) (4.45 Å) and C-6 (5.25 Å) as reported in Table 1.
Docking studies also showed that 1-NP and 1-AcP were well oriented into the P450 2A13 active site having lower U values of –27.4 and –12.1, respectively, as compared with those in P450 2A6 active site with U values of 30.8 and 69.5, respectively (Fig. 9). 1-NP formed a hydrogen bond interaction (3.08 Å) between the NO2 moiety and Asn-297 and a π-H interaction (3.93 Å) between a benzene ring and Ala-301 of P450 2A13; such interactions were not seen for P450 2A6 (Fig. 9). The C-6 atom of 1-NP was close (4.33 Å) to the P450 2A13 heme center, as compared with C-5 and C-7 (Table 1). In addition, C-6 of 1-AcP was found to be close (4.74 Å) to P450 2A13 heme, as compared with C-7 and C-5 (Table 1). 1-AcP formed a hydrogen bond (2.70 Å) between the 1-acetyl moeity and Asn-297, as well as a π-H interaction (3.72 Å) between a benzene ring and Ala-301 in P450 2A13.
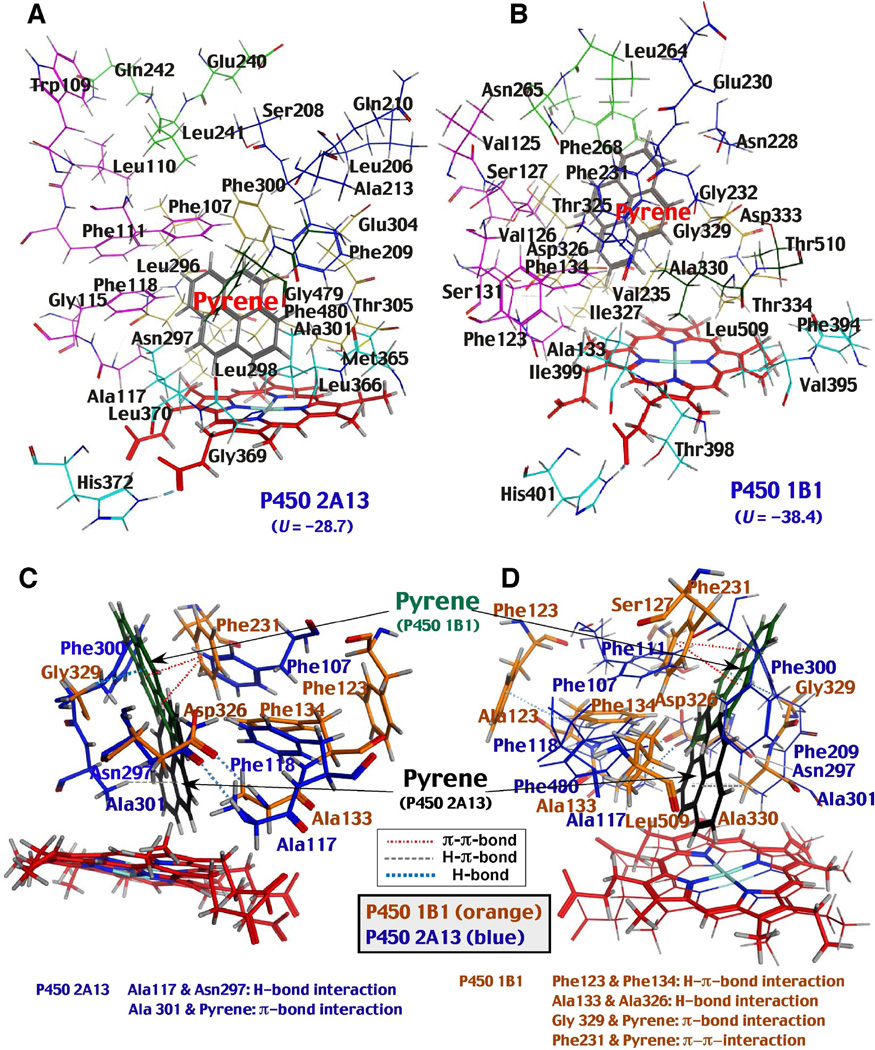
Interaction of key amino acids of P450s 2A13 and 1B1 with pyrene
Because our previous studies indicated that there are similarities in the active sites of the P450s 2A13 and 1B1 sequences in molecular docking experiments (Shimada et al., 2013b), we compared the distribution of key amino acid residues at Substrate Recognition Sites (SRS) (Gotoh, 1992) of these P450 enzymes in the presence of pyrene. First, we observed that pyrene was positioned closer to the P450 2A13 heme than to the P450 1B1 heme (Fig. 11A and 11B). In addition, Phe-107, Phe-111, Phe-118, Phe-209, Phe-300, and Phe-480 in P450 2A13 were located above the pyrene molecule; these phenylalanine residues have been reported to play important roles in holding several ligand molecules in the active sites of P450 2A enzymes (Smith et al., 2007; DeVore et al., 2008; 2009; 2012; DeVore and Scott, 2012). Second, the hydrogen-π-bond interaction was detected between Phe-123 and Phe-134 in the P450 1B1 sequence, while such interaction in the respective Phe-107 and Phe-118 residues in P450 2A13 was not found (Fig. 11C and 11D). Another interesting observation was that P450 1B1 had a wider space between Phe-123 and Ser-127 in SRS1 as compared with the respective Phe-107 and Phe-111 residues in P450 2A13, although the former enzyme had a narrower space near at the active site surrounded by Phe-134, Asp-326, Ala-330, and Leu-509 (Fig. 11D). Two π-π interations between Phe-231 and two benzene rings of pyrene were found in P450 1B1; the importance of these interactions has been reported for directing the orientation of α-naphthoflavone in the P450 1B1 active site (Wang et al., 2011). There were π-bond interactions between benzene ring of pyrene with Ala-301 in P450 2A13 and with Gly-329 in P450 1B1; such interactions may contribute to the different orientations of pyrene in the active sites of these enzymes (Fig. 11). As mentioned above, there was hydrogen-bond interaction between Ala-117 and Asn-297 in P450 2A13 (Fig. 11C and 11D); this interaction may help to hold the pyrene molecule in the active site of P450 2A13.
Figure 11.
Comparison of molecular interaction of pyrene with active sites of P450 2A13 (A) and 1B1 (B). Key amino acid residues in each P450 enzyme are shown in the figure and the substrate recognition sites (SRS1-6) are indicated with different colors; SRS1 with pink, SRS2 with blue, SRS3 with light green, SRS4 with dark yellow, SRS5 with light blue, and SRS6 with green. Two views of differences in interactions of pyrene with P450s 2A13 and 1B1. The positions of pyrene, amino acids and heme of P450s 2A13 and 1B1 were superimposed in the figure to observe the differences in the interactions of these P450 enzymes with pyrene (11C and 11D). Hydrogen-bond, hydrogen-π-bond, and π- π-bond interactions are shown by dotted lines with different colors.
Discussion
Recently we reported that P450 2A13 is one of the key enzymes that interacts with and metabolizes a number of environmental chemicals, including a variety of PAH compounds, and plays more significant roles than the orthologous P450 2A6 in activating PAH-diols and aryl- and heterocyclic amines to reactive metabolites that induce umu gene expression in the S. typhimurium 2009 tester strain (Shimada et al., 2011; 2013a, 2013b). In the current study, we examined the oxidative metabolism of pyrene, 1-OHP, 1-NP, and 1-AcP as model PAHs and found that P450 2A13 oxidized these compounds at much higher rates than did P450 2A6 and the other four human P450s examined. P450 2A13 has 94% similarity in amino acid sequence with P450 2A6 (Sansen et al., 2007a; Smith et al., 2007), but the volume of the active site cavity of P450 2A13 (307 Å3) is larger than that of P450 2A6 (260 Å3) and slightly smaller than those of P450s 1B1 (398 Å3) and 1A2 (375 Å3) (Smith et al., 2007; Wang et al., 2011; Sansen et al., 2007b). Thus, P450 2A13 may have more active site space to better fit pyrene (MW 202), 1-OHP (MW 218), 1-NP (MW 247), and 1-AcP (MW 244) than P450 2A6. The preferred catalytic activities of P450 2A13 compared to P450 2A6 have already been reported in the metabolism of nicotine, NNK, N´-nitrosonornicotine, naphthalene, styrene, toluene, 4-aminobiphenyl, and phenacetin (DeVore and Scott, 2012; Wong et al., 2005a; Su et al., 2000; Fukami et al., 2008; Nakajima et al., 2006; DeVore et al., 2008).
Molecular docking studies suggeted that pyrene, 1-OHP, 1-NP, and 1-AcP were all well oriented in the active site of P450 2A13 as compared with that of P450 2A6; the interaction energies (U values) for these four PAH compounds in the active sites were always lower in P450 2A13 than in P450 2A6 (Figure 9). There were π-H interactions of benzene rings of pyrene, 1-OHP, 1-NP, and 1-AcP with the CH3 moiety of Ala-301 in SRS4 of P450 2A13; such interactions were not seen in P450 2A6 where the residue 301 is glycine (Smith et al., 2007). Mutation of Ala-301 of P450 2A13 to Gly-301 has been shown to cause losses in catalytic activities of substrate oxidation and induces spectral changes with substrates (DeVore et al., 2008; 2009). The importance of a residue N297 in SRS4 of P450s 2A13 and 2A6 has also been reported in the oxidation of xenobiotics (Nakamura et al., 2001; Kim et al., 2005; Sansen et al., 2007a; Smith et al., 2007; DeVore et al., 2008; 2009; 2012). Our present studies showed that there were hydrogen bond interactions between Asn-297 of P450 2A13 and –OH of 1-OHP, and -NO2 of 1-NP, and -NH2 of 1-AcP; such interaction was not seen for the unsubstituted pyrene molecule. However, there was hydrogen bond interaction between Ala-117 and Asn-297 in P450 2A13 in the presence of pyrene, and it has been reported that both amino acid residues play important roles in interacting with substrates and ligands (Smith et. al., 2007; DeVore et al., 2008; 2009; 2012). Our preliminary results also showed that the pyrene molecule was vertically positioned in relation to the plane of P450 2A13 heme, and such positioning was not seen in P450 2A6, probably due to the different orientation of Phe-209 in the two P450 enzymes.
Pyrene was oxidized by P450 2A13 to form 1-OHP as a major product, which was further oxidized by the enzyme to form 1,8- and 1,6-di-OHP (and possibly also 1,5-di-OHP); these products have been reported previously in rabbit P450 1A2 studies (Sohl et al., 2008). Rat liver microsomes have been shown to oxidize pyrene to form K-region epoxides (by detecting formation of 4,5-dihydroxy-4,5-dihydropyrene) as well as 1-OHP, 1,6-diOHP, and other products on gas-chromatography analysis (Jacob et al., 1982). In this study we examined the effects of rat liver epoxide hydrolase on the metabolism of pyrene by reconstituted monooxygenase system containing P450 2A13 and NADPH-P450 reductase and compared metabolic profiles with those catalyzed by liver microsomes of β-naphthoflavone-treated rats. Our HPLC results did not show any significant formation of 4,5-dihydroxy-4,5-dihydropyrene during pyrene metabolism in either P450 2A13 and rat liver microsomes, and further work is required to address to detect K-region epoxide(s) of pyrene.
Of the six human P450s studied using enzymes expressed in E. coli membranes, P450 2A13 showed the highest activity in oxidizing pyrene to form 1-OHP and 1,8-di-OHP. Since Kim et al. (2004) reported that recombinant P450 1A1 in a baculovirus-based system showed high activity for oxidizing pyrene to 1-OHP (they did not report 1,8-di-OHP and other products on HPLC analysis), we re-examined P450 1A1 in the baculovirus system and found a Km value of 1.7 ± 0.5 µM and kcat value of 2.1 ± 0.1 nmol/min/nmol P450 for the formation of 1-OHP from pyrene. These kinetic values are similar to those found for our purified P450 2A13 (Km 1.2 ± 0.2 µM, kcat 2.0 ± 0.1 nmol/min/nmol P450 for the 1-OHP formation) (Fig. 2), indicating that both P450 2A13 and P450 1A1 can play important roles in oxidizing pyrene in humans, with tissue-selective expression playing an importatn role. It should also be mentioned that recombinant P450 enzyme systems expressed in baculovirus often show higher catalytic activities in metabolizing xenobiotic chemicals than those purified from E. coli, probably due to overexpression of the reductase (Shimada et al., 2001).
P450 1B1 induced Reverse Type I binding spectra with pyrene, 1-OHP, 1-NP, and 1-AcP with Ks values of 1.2, 0.88, 1.4, and 0.39 µM, respectively, and catalyzed the oxidation of pyrene to 1-OHP at a similar level to P450 2A13; however it did not oxidize 1-NP or 1-AcP to significant extents (Fig. 7). Docking analysis suggested that pyrene was docked at a more distant position relative to the P450 1B1 heme than to the P450 2A13 heme, and several amino acid residues may be involved in causing this. First, a π-bond interaction between pyrene and Gly-329 was observed in P450 1B1, keeping the compound away from the heme in comparison to the π-bond interaction between pyrene and Ala-301 in P450 2A13 (Fig. 11C and 11D). Second, P450 1B1 has a narrow space at the substrate binding pocket comprised of Phe-134 in SRS1, Asp-326 and Ala-330 in SRS4, and Leu-509 in SRS6 that may direct the orientation of pyrene in the active site. In addition, π-π interactions were observed between Phe231 of P450 1B1 with two benzene rings of pyrene, as has been reported in the interaction of α-naphthpflavone with P450 1B1 (Wang et al., 2011).
1-NP, a major contaminant in urban air and diesel engine exhausts, is known to cause respiratory diseases, e.g. trachea and lung cancer, in experimental animal models (El-Bayoumy et al., 1984; Hirose et al., 1984). Howard et al. (1990) reported that human P450 3A enzymes (among the 12 P450s tested) oxidized 1-NP to form 1-NP-3-ol as a major metabolite and 1-NP-6-ol and -8-ol as minor ones (using recombinant human P450s in HepG2 cells and a vaccina virus expression system). 1-NP has been reported to be oxidized by P450s 3A4 and 1A2 (Chae et al., 1999) and P450s 1A1 and 1B1 (Sun et al., 2004) to give 1-, 3-, 6-, and 8-mono-OH and several di-OH products. Our current studies showed that P450s 2A13 and 2A6 were more active in oxidizing 1-NP to form mono- and di-oxygenated products than P450s 1B1, 1A2, 2C9, and 3A4. Both 1-NP and 1-AcP induced Type I binding spectra with P450 2A13, having Ks values of 0.23 and 0.34 µM, respectively; the values were lower than those with pyrene and 1-OHP. P450 2A13 oxidized 1-AcP as well as 1-NP to form two mono-oxygenated products, a and b in each case. Little is known about the metabolism and biological significance of products of 1-AcP in humans, as well as experimental animals. This study is the first to report that 1-AcP is metabolized by P450s 2A13 and 2C9 to much higher exents than P450s 2A6, 1B1, 1A2, and 3A4. Further studies are required to identify these products and assess their biological significance in humans.
In conclusion, our current studies show that P450 2A13 is one of the important enzymes in catalyzing the oxidation of pyrene, 1-OHP, 1-NP, and 1-AcP to several mono- and di-hydroxy products. Pyrene was oxidized by P450 2A13 to 1-OHP, which was further oxidized by the enzyme to di-OHP compounds, i.e. 1,8- and 1,6-OHP (and probably 1,5-OHP). 1-NP and 1-AcP, which induced Type I binding spectra with P450 2A13, were oxidized by this enzyme to mono- and di-oxygenated products. In comparison with other recombinant human P450 enzymes co-expressed with NADPH-P450 reductase in E. coli, namely P450s 2A6, 1B1, 1A2, 2C9, and 3A4, P450 2A13 was the most efficient catalyst in oxidizing these four chemicals, and was found to have similar activity to recombinant P450 1A1 in oxidizing pyrene to 1-OHP. Molecular docking studies support good orientation of these chemicals in the active site of P450 2A13, and the importance of Ala-301 in binding pyrene and Asn-297 and Ala-301 in binding 1-OHP, 1-NP, and 1-AcP. The corresponding Gly-301 in P450 2A6 may be one of the reasons for the lower catalytic activities and poorer interactions observed for this enzyme with pyrene, 1-OHP, 1-NP, and 1-AcP compared to P450 2A13. Similar, but different, orientations seen for the interactions of the pyrene molecule with the active sites of P450s 2A13 and 1B1 may be the cause for the unique catalytic specificities of these enzymes. All of the results obtained in this study suggest that P450 2A13 plays important roles in the detoxication and activation of pyrene and its derivatives and may contribute to those of other environmental and carcinogenic PAH compounds.
Acknowledgments
This work was supported in part by Grants from the Ministry of Education, Science, and Culture of Japan, the Ministry of Health and Welfare of Japan (T.S., S.T., N.M., H.Y., M.K.), NIH grant S06 GM08008 and DOD grant W81XWH-11-1-547 0105 (M.K.F.), and NIH grants R37 CA090426, T32 ES007028, and P30 ES000267 (F.P.G.).
Abbreviations used
- P450
cytochrome P450
- 1-AcP
1-acetylpyrene
- di-OH
di-hydroxy-
- 1-OHP
1-hydroxypyrene
- 1-NP
1-nitropyrene
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- PAH
polycyclic aromatic hydrocarbons
- SRS
substrate recognition site
Footnotes
Declaration of interest Statement
-The authors declare no conflict of interest associated with this manuscript.
References
- Anttila S, Raunio H, Hakkola J. Cytochrome P450-mediated pulmonary metabolism of carcinogens: regulation and cross-talk in lung carcinogenesis. Am. J. Respir. Cell Mol. Biol. 2011;44:583–590. doi: 10.1165/rcmb.2010-0189RT. [DOI] [PubMed] [Google Scholar]
- Chae YH, Thomas T, Guengerich FP, Fu PP, El-Bayoumy K. Comparative metabolism of 1-, 2-, and 4-nitropyrene by human hepatic and pulmonary microsomes. Cancer Res. 1999;59:1473–1480. [PubMed] [Google Scholar]
- Chiang HC, Wang CY, Lee HL, Tsou TC. Metabolic effects of CYP2A6 and CYP2A13 on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) -induced gene mutation—A mammalian cell-based mutagenesis approach. Toxicol Appl Pharmacol. 2011;253:145–152. doi: 10.1016/j.taap.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Chiang H-C, Wang C-K, Tsou T-C. Differential distribution of CYP2A6 and CYP2A13 in the human respiratory tract. Respiration. 2012;84:319–326. doi: 10.1159/000339591. [DOI] [PubMed] [Google Scholar]
- Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- DeVore NM, Smith BD, Urban MJ, Scott EE. Key residues controlling phenacetin metabolism by human cytochrome P450 2A enzymes. Drug Metab Dispos. 2008;36:582–5890. doi: 10.1124/dmd.108.023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVore NM, Smith BD, Wang JL, Lushington GH, Scott EE. Key residues controlling binding of diverse ligands to human cytochrome P450 2A enzymes. Drug Metab Dispos. 2009;37:1319–1327. doi: 10.1124/dmd.109.026765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVore NM, Meneely KM, Bart AG, Stephens ES, Battaile KP, Scott EE. Structural comparison of cytochromes P450 2A6, 2A13, and 2E1 with pilocarpine. FEBS J. 2012;279:1621–1631. doi: 10.1111/j.1742-4658.2011.08412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVore NM, Scott EE. Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone binding and access channel in human cytochrome P450 2A6 and 2A13 enzymes. J Biol Chem. 2012;287:26576–26585. doi: 10.1074/jbc.M112.372813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bayoumy K, Hecht SS, Sackl T, Stoner GD. Tumorigenicity and metabolism of 1-nitropyrene in A/J mice. Carcinogenesis. 1984;11:1449–1452. doi: 10.1093/carcin/5.11.1449. [DOI] [PubMed] [Google Scholar]
- Fukami T, Katoh M, Yamazaki H, Yokoi T, Nakajima M. Human cytochrome P450 2A13 efficiently metabolizes chemicals in air pollutants: naphthalene, styrene, and toluene. Chem Res Toxicol. 2008;21:720–725. doi: 10.1021/tx700325f. [DOI] [PubMed] [Google Scholar]
- Gillam EM, Baba T, Kim BR, Ohmori S, Guengerich FP. Expression of modified human cytochrome P450 3A4 in Escherichia coli and purification and reconstitution of the enzyme. Arch Biochem Biophys. 1993;305:123–131. doi: 10.1006/abbi.1993.1401. [DOI] [PubMed] [Google Scholar]
- Gotoh O. Substrate recognition sites in cytochrome P450 family 2 (CYP2) proteins inferred from comparative analyses of amino acid and coding nucleotide sequences. J Biol Chem. 1992;267:83–90. [PubMed] [Google Scholar]
- Guengerich FP, Wang P, Mitchell MB, Mason PS. Rat and human liver microsomal epoxide hydratase. Purification and evidence for the existence of multiple forms. J Biol Chem. 1979;254:12248–12254. [PubMed] [Google Scholar]
- Hirose M, Lee MS, Wang CY, King CM. Induction of rat mammary gland tumors by 1-nitropyrene, a recently recognized environmental mutagen. Cancer Res. 1984;44:1158–1162. [PubMed] [Google Scholar]
- Howard PC, Aoyama T, Bauer SL, Gelboin HV, Gonzalez FJ. The metabolism of 1-nitropyrene by human cytochromes P450. Carcinogenesis. 1990;11:1539–1542. doi: 10.1093/carcin/11.9.1539. [DOI] [PubMed] [Google Scholar]
- Jacob J, Grimmer G, Raab G. The Metabolism of pyrene by rat liver microsomes and the influence of various mono-oxygenase inducers. Xenobiotica. 1982;12:45–53. doi: 10.3109/00498258209052453. [DOI] [PubMed] [Google Scholar]
- Kim YD, Todoroki H, Oyama T, Isse T, Matsumoto A, Yamaguchi T, Kim H, Uchiyama I, Kawamoto T. Identification of cytochrome P450 isoforms involved in 1-hydroxylation of pyrene. Environ Res. 2004;94:262–266. doi: 10.1016/S0013-9351(03)00134-8. [DOI] [PubMed] [Google Scholar]
- Kim D, Wu ZL, Guengerich FP. Analysis of coumarin 7-hydroxylation activity of cytochrome P450 2A6 using random mutagenesis. J Biol Chem. 2005;280:40319–40327. doi: 10.1074/jbc.M508171200. [DOI] [PubMed] [Google Scholar]
- Lacourciere GM, Vakharia VN, Tan CP, Morris DI, Edwards GH, Moos M, Armstrong RN. Interaction of hepatic microsomal epoxide hydrolase derived from a recombinant baculovirus expression system with an azarene oxide and an aziridine substrate analogue. Biochemistry. 1993;32:2610–2616. doi: 10.1021/bi00061a019. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Itoh M, Sakai H, Fukami T, Katoh M, Yamazaki H, Kadlubar FF, Imaoka S, Funae Y, Yokoi T. CYP2A13 expressed in human bladder metabolically activates 4-aminobiphenyl. Int J Cancer. 2006;119:2520–2526. doi: 10.1002/ijc.22136. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Martin MV, Guengerich FP. Random mutagenesis of human cytochrome P450 2A6 and screening with indole oxidation products. Arch Biochem Biophys. 2001;395:25–31. doi: 10.1006/abbi.2001.2569. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- Rendic S, Guengerich FP. Contributions of human enzymes in carcinogen metabolism. Chem Res Toxicol. 2012;25:1316–1383. doi: 10.1021/tx300132k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu P, Baba T, Guengerich FP. Expression of modified cytochrome P450 2C10 (2C9) in Escherichia coli, purification, and reconstitution of catalytic activity. Arch Biochem Biophys. 1993;306:443–450. doi: 10.1006/abbi.1993.1536. [DOI] [PubMed] [Google Scholar]
- Sandhu P, Guo Z, Baba T, Martin MV, Tukey RH, Guengerich FP. Expression of modified human cytochrome P450 1A2 in Escherichia coli: stabilization, purification, spectral characterization, and catalytic activities of the enzyme. Arch Biochem Biophys. 1994;309:168–177. doi: 10.1006/abbi.1994.1099. [DOI] [PubMed] [Google Scholar]
- Sansen S, Hsu MH, Stout CD, Johnson EF. Structural insight into the altered substrate specificity of human cytochrome P450 2A6 mutants. Arch Biochem Biophys. 2007a;464:197–206. doi: 10.1016/j.abb.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansen S, Yano JK, Reynald RL, Schoch GA, Griffin KJ, Stout CD, Johnson EF. Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P450 1A2. J Biol Chem. 2007b;282:14348–14355. doi: 10.1074/jbc.M611692200. [DOI] [PubMed] [Google Scholar]
- Shimada T. Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab Pharmacokinet. 2006;21:257–276. doi: 10.2133/dmpk.21.257. [DOI] [PubMed] [Google Scholar]
- Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, Sutter TR. Activation of chemically diverse procarcinogens by human cytochrome P450 1B1. Cancer Res. 1996;56:2979–2984. [PubMed] [Google Scholar]
- Shimada T, Wunsch RM, Hanna IH, Sutter TR, Guengerich FP, Gillam EM. Recombinant human cytochrome P450 1B1 expression in Escherichia coli . Arch Biochem Biophys. 1998;357:111–120. doi: 10.1006/abbi.1998.0808. [DOI] [PubMed] [Google Scholar]
- Shimada T, Gillam EM, Oda Y, Tsumura F, Sutter TR, Guengerich FP, Inoue K. Metabolism of benzo[a]pyrene to trans-7,8-dihydroxy-7, 8-dihydrobenzo[a]pyrene by recombinant human cytochrome P450 1B1 and purified liver epoxide hydrolase. Chem Res Toxicol. 1999;12:623–629. doi: 10.1021/tx990028s. [DOI] [PubMed] [Google Scholar]
- Shimada T, Oda Y, Gillam EMJ, Guengerich FP, Inoue K. Metabolic activation of polycyclic aromatic hydrocarbons and their dihydrodiol derivatives and other procarcinogens by cytochrome P450 1A1 and 1B1 allelic variants and other human cytochrome P450 enzymes in Salmonella typhimurium NM2009. Drug Metab. Dispos. 2001;29:1176–1182. [PubMed] [Google Scholar]
- Shimada T, Murayama N, Tanaka K, Takenaka S, Guengerich FP, Yamazaki H, Komori M. Spectral modification and catalytic inhibition of human cytochromes P450 1A1, 1A2, 1B1, 2A6, and 2A13 by four chemopreventive organoselenium compounds. Chem Res Toxicol. 2011;24:1327–1337. doi: 10.1021/tx200218u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Kim D, Murayama N, Tanaka K, Takenaka S, Nagy LD, Folkman LM, Foroozesh MK, Komori M, Yamazaki H, Guengerich FP. Binding of diverse environmental chemicals with human cytochromes P450 2A13, 2A6, and 1B1 and enzyme inhibition. Chem Res Toxicol. 2013a;26:517–528. doi: 10.1021/tx300492j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Murayama N, Yamazaki H, Tanaka K, Takenaka S, Komori M, Kim D, Guengerich FP. Metabolic activation of polycyclic aromatic hydrocarbons and aryl and heterocyclic amines by human cytochromes P450 2A13 and 2A6. Chem Res Toxicol. 2013b;26:529–537. doi: 10.1021/tx3004906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Takenaka S, Murayama N, Yamazaki H, Kim J-H, Kim D, Yoshimoto FK, Guengerich FP, Komori M. Oxidation of acenaphthene and acenaphthylene by human cytochrome P450 enzymes. Chem Res Toxicol. 2015;28:268–278. doi: 10.1021/tx500505y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BD, Sanders JL, Porubsky PR, Lushington GH, Stout CD, Scott EE. Structure of the human lung cytochrome P450 2A13. J Biol Chem. 2007;282:17306–17313. doi: 10.1074/jbc.M702361200. [DOI] [PubMed] [Google Scholar]
- Sohl CD, Isin EM, Eoff RL, Marsch GA, Stec DF, Guengerich FP. Cooperativity in oxidation reactions catalyzed by cytochrome P450 1A2: Highly cooperative pyrene hydroxylation and multiphasic kinetics of ligand binding. J Biol Chem. 2008;283:7293–7308. doi: 10.1074/jbc.M709783200. [DOI] [PubMed] [Google Scholar]
- Su T, Bao Z, Zhang Q-Y, Smith TJ, Hong J-Y, Ding X. Human cytochrome P450 CYP2A13: Predominant exression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 2000;60:5074–5079. [PubMed] [Google Scholar]
- Sun YW, Guengerich FP, Sharma AK, Boyiri T, Amin S, El-Bayoumy K. Human cytochromes P450 1A1 and 1B1 catalyze ring oxidation but not nitroreduction of environmental pollutant mono-nitropyrene isomers in primary cultures of human breast cells and cultured MCF-10A and MCF-7 cell lines. Chem Res Toxicol. 2004;17:1077–1085. doi: 10.1021/tx049889d. [DOI] [PubMed] [Google Scholar]
- Wang A, Savas U, Stout CD, Johnson EF. Structural characterization of the complex between α-naphthoflavone and human cytochrome P450 1B1. J Biol Chem. 2011;286:5736–5743. doi: 10.1074/jbc.M110.204420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HL, Murphy SE, Hecht SS. Cytochrome P450 2A–catalyzed metabolic activation of structurally similar carcinogenic nitrosamines: N´-nitrosonornicotine enantiomers, N-nitrosopiperidine, and N-nitrosopyrrolidine. Chem Res Toxicol. 2005a;18:61–69. doi: 10.1021/tx0497696. [DOI] [PubMed] [Google Scholar]
- Wong HL, Zhang X, Zhang QY, Gu J, Ding X, Hecht SS, Murphy SE. Metabolic activation of the tobacco carcinogen 4-(methylnitrosamino)-(3-pyridyl)-1-butanone by cytochrome P450 2A13 in human fetal nasal microsomes. Chem Res Toxicol. 2005b;18:913–918. doi: 10.1021/tx0500777. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Nakajima M, Nakamura M, Asahi S, Shimada N, Gillam EM, Guengerich FP, Shimada T, Yokoi T. Enhancement of cytochrome P-450 3A4 catalytic activities by cytochrome b 5 in bacterial membranes. Drug Metab Dispos. 1999;27:999–1004. [PubMed] [Google Scholar]
- Zhu LR, Thomas PE, Lu G, Reuhl KR, Yang GY, Wang LD, Wang SL, Yang CS, He XY, Hong JY. CYP2A13 in human respiratory tissues and lung cancers: an immunohistochemical study with a new peptide-specific antibody. Drug Metab Dispos. 2006;34:1672–1676. doi: 10.1124/dmd.106.011049. [DOI] [PubMed] [Google Scholar]