Abstract
Endothelial dysfunction, characterized by decreased production or availability of nitric oxide (NO), is widely believed to be the hallmark of early-stage atherosclerosis. In addition, hypercholesterolemia is considered a major risk factor for development of atherosclerosis and is associated with impaired flow-induced dilation. However, the mechanism by which elevated cholesterol levels leads to decreased production of NO is unclear. NO is released in response to shear stress and agonist-evoked changes in intracellular calcium. Although calcium signaling is complex, we have previously shown that NO production by endothelial nitric oxide synthase (eNOS) is preferentially activated by calcium influx via store-operated channels. We hypothesized that cholesterol enrichment altered this signaling pathway (known as capacitive calcium entry; CCE) ultimately leading to decreased NO. Our results show that cholesterol enrichment abolished ATP-induced eNOS phosphorylation and attenuated the calcium response by the preferential inhibition of CCE. Furthermore, cholesterol enrichment also inhibited shear stress-induced NO production and eNOS phosporylation, consistent with our previous results showing a significant role for ATP autocrine stimulation and subsequent activation of CCE in the endothelial flow response.
Keywords: Endothelial cells, Nitric oxide, Shear stress, Atherosclerosis, Cholesterol, Capacitative calcium entry
Introduction
Endothelial dysfunction, defined as decreased production or availability of nitric oxide (NO), is widely believed to be the hallmark of early-stage atherosclerosis and has been linked to hypercholesterolemia and hypertension.16,36 However, controversy exists on the role of cholesterol in endothelial dysfunction. It is known that cholesterol is important for maintaining cell membranes and affects membrane properties such as permeability, transport functions, membrane enzyme activities, substrate availability, membrane protein organization, structure and exposure of lipid and protein head groups to water (see review Ref. 4). Cholesterol levels appear to have a direct effect on endothelial dysfunction, apart from the contribution of LDL accumulation in the intima to lesion formation and eventual encroachment on the vessel lumen, which occurs only in the later stages of disease progression.20 Reduction of plasma cholesterol levels leads to recovery of vasodilatation and vasoconstriction responses, implicating high cholesterol levels, per se, as a major causal factor in endothelial dysfunction.40 Studies on high cholesterol fed rabbits have shown increased cell membrane cholesterol content in smooth muscle cells42,44 and endothelial cells,42 likely due to the transfer of cholesterol to endothelial cells from LDL.48
NO is a major vasodilator produced in response to shear stress (flow) and agonist-evoked intracellular calcium release.8,9,12,35 Numerous studies have shown that long-term high cholesterol diets impair agonist (acetylcholine, serotonin, thrombin and ATP) stimulated dilation (see review Ref. 18) and that flow-mediated endothelial dysfunction can occur on short time scales up to 4 h after ingesting a single high-fat meal.39,45 However, in vivo studies on the effect of high cholesterol diets result in changes that cannot be independently attributed to cholesterol levels. In vitro studies offer the potential to examine the effect of cholesterol on the endothelium independently of these additional factors. In fact, the effect of membrane cholesterol depletion of the endothelium is very well studied and has been shown to result in the loss of caveolae and the associated intracellular signaling.21,33,47 However, the effect of cholesterol enrichment has received less attention, and results on NO production are conflicting. Studies of ionophore-stimulated NO production in cholesterol-enriched static cultures have reported enhanced 38 or impaired15 NO in response to A23187 and no effect on ionomycin stimulated NO.49 Aside from agonist-evoked NO production, few researchers have studied the effect of cholesterol enrichment on shear stress mechanotransduction and NO production and virtually none in vitro. Overall, there is a paucity of studies on the effects of cholesterol enrichment, particularly on the response to flow, and, thus, the mechanisms by which cholesterol contributes to endothelial dysfunction remain unclear.
We have recently demonstrated a strong association between capacitive calcium entry and eNOS phosphorylation in response to ATP stimulation. Furthermore, a significant component of NO response to flow appears to be mediated by ATP autocrine signaling and activation of store-operated channels (SOCs).2 In the present study, we investigate the effect of cholesterol enrichment on ATP—induced calcium signaling and eNOS phosphorylation. Our results demonstrate that cholesterol preferentially impairs CCE, specifically by inhibiting SOCs, which in turn abolishes eNOS phosphorylation. In addition, we show evidence that cholesterol enrichment impairs shear stress-induced NO production and eNOS phosphorylation.
Materials and Methods
Cell Culture
Bovine aortic endothelial cells (BAECs) were obtained from Dr. Peter Davies’ Laboratory (University of Pennsylvania). The cells were isolated from tissue obtained from a USDA-inspected slaughterhouse, and thus were exempt from IACUC review and approval. BAECs were cultured in Dulbeccos modified Eagle’s medium (Mediatech Cellgro), supplemented with 10% fetal bovine serum (Sigma), 2 mmol/l l-glutamine (Mediatech Cellgro), and penicillin–streptomycin (Mediatech Cellgro) as described previously.24
Chemicals
Water-soluble cholesterol, SKF-96365 (SKF), Thapsigargin (Tg), l-α-Lysophosphatidylcholine (LPC) were all purchased from Sigma.
Cholesterol Enrichment and Quantification
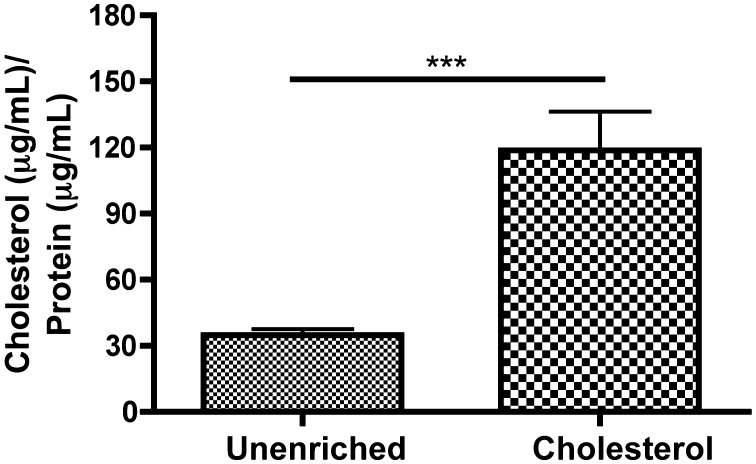
ECs were enriched using an established technique of rapid enrichment50 using methyl-β-cyclodextrin-cholesterol (37 °C, 3.5 mM for 30 min). Cholesterol content was determined by the amplex red cholesterol assay following the manufacturers’ instructions (Molecular Probes). Standards and cell samples were placed in a microplate in triplicates, Amplex Red reagent/HRP/cholesterol oxidase/cholesterol esterase solution was added to each well, and the plate and incubated for 30 min at 37 °C. Fluorescence was then read in a microplate reader (Tecan Infinite M200 Series) with excitation range 530–560 and emission detection at 590. Background was subtracted using the no-cholesterol control. A cholesterol standard curve (0–8 mg/mL) was used to quantify the total change in cholesterol (ratio of cholesterol (μg/mL)/protein (μg/mL) (Fig. 1).
Figure 1.
Quantification of cholesterol enrichment using cholesterol-cyclodextrin complexes. Cells were incubated with either PBS with calcium/magnesium or 3.5 mM cholesterol for 30 min at 37 °C. Cells were lysed, and protein and cholesterol content were assayed. Cholesterol enriched cells showed a 3.5× increase in cholesterol as compared to unenriched cells. (Means and SEM were plotted, two-tailed t test ***p < 0.0001 n = 6 unenriched, n = 6 cholesterol).
Protein Assay
Total protein content was determined using bicinchoninic acid for colorimetric detection and quantification of total protein (BCA Protein Assay Kit). Standards were created using Albumin (BSA) ranging from 2000 to 0 μg/ml. Each sample was measured in triplicate. BCA working reagent was mixed in a 1:50 ratio of BCA Reagent A to BCA Reagent B. Microplates were incubated at 37 °C for 30 min and read at 562 nm in a Tecan Infinite M200 Series. Blank absorbances were subtracted and a best-fit curve was used to calculate total protein content of experimental samples.
Nitric Oxide Measurements Under Flow
All of our NO measurements under flow were conducted in a device as described previously.2,3 The device consists of a parallel plate flow chamber in which a NO sensitive electrode is located in a stagnant upper compartment separated from the ECs and the flow field by a 10 μm thick porous membrane. Cells were grown to confluency on the underside of Transwell® membranes, which fit seamlessly into our flow chamber device. Prior to insertion, membranes were incubated in PBS with cholesterol, following the enrichment protocol described above. Unenriched controls were treated identically except that they were incubated in PBS without cholesterol. Membranes were then washed and then inserted in the flow chamber. All washes and circulating fluid was performed in PBS with calcium and magnesium supplemented with 70 μM l-arginine. Cells were exposed to 4 step changes of 0.1–10 dyn/cm2 with 3-min intervals between step changes. The procedure was repeated four times and responses for multiple membranes were then averaged and compared between treatment conditions.
eNOS Phosphorylation and Shear Stress
Cells were prepared for flow experiments as described above. The chamber was placed in a water bath for 10 min prior to exposure to 10 dyn/cm2 for 0, 1 or 3 min. Following shear stress exposure, the membrane was removed, and cells were lysed following the protocol described below.
Western Blot
Samples were lysed (20 mM Tris, 1% Deoxycholate, 150 mM NaCl, 1% Triton X-100, 2 mM EDTA, 2 mM PMSF, 0.1% SDS, 1 μg/mL leupeptin, 50 mM mM NaF, 10% glycerol, Pierce complete protease inhibitor, pH 7.4) at 4 °C for 20 min, then centrifuged for 10 min at 10,000 g, 4 °C to remove insoluble material. Cell lysates were normalized for protein content, separated by SDS-PAGE on a 4–20% Bis–Tris gel (Lonza), and transferred to a nitrocellulose membrane (Invitrogen, Iblot). Membranes were blocked with 5% Blotto (Bioexpress) and incubated overnight with primary p-eNOS (1179) antibody (Invitrogen) or eNOS antibody (BD biosciences) at 4 °C. Secondary horseradish peroxidase-conjugated antibodies were detected with an enhanced chemiluminescence kit (Western Lightning, PerkinElmer) and visualized with a Fluorchem digital imager (Alpha Innotech). Band intensity was quantified using AlphaEase FC software and expressed as a ratio of p-eNOS/eNOS. For studies on phosphorylation after shear stress, results were normalized by the no flow condition for both cholesterol-enriched and unenriched cells.
ATP Stimulation and eNOS Phosphorylation
Cells were grown on 25 mm2 round glass coverslips for 3 days prior to experiments. Cells were enriched with cholesterol as described above. Cells were washed 3× with PBS with Ca+2, stimulated with 100 μM ATP or 300 nM LPC and then harvested at time intervals 0, 1, 3, 5, 10 min.
Calcium Fluorescence Imaging
Cells were grown on glass coverslips for 2 days prior to experiments then enriched with cholesterol. Cells were incubated with 1.5 μmol/L fluo-3AM (Molecular Probes) for 40 min at room temperature. SKF treated cells (unenriched) were incubated with 50 μM 10 min prior to incubation with fluorescent dye. Cells were stimulated with 500 μL of 100 μM ATP, 500 μL of 300 nM LPC, or 1 μM Tg. For experiments with agonist stimulation in the absence of extracellular calcium: cells were washed 3× with Ca+2 free PBS prior to stimulation. Following the calcium response, the agonist was removed and replaced with 500 μL of PBS with Ca+2. Cells were viewed with an inverted light microscope 20× (Nikon TE300 Eclipse microscope). All fluorescence measurements were expressed as a ratio (R) of fluorescence intensity (F) to the basal fluorescence intensity (F 0): R = F/F 0 as described previously.24
Results
Cholesterol Attenuates eNOS Phosphorylation Through Inhibition of CCE
Cholesterol enrichment increased cholesterol content by ~3.5× as determined with the amplex red cholesterol assay (Fig. 1).
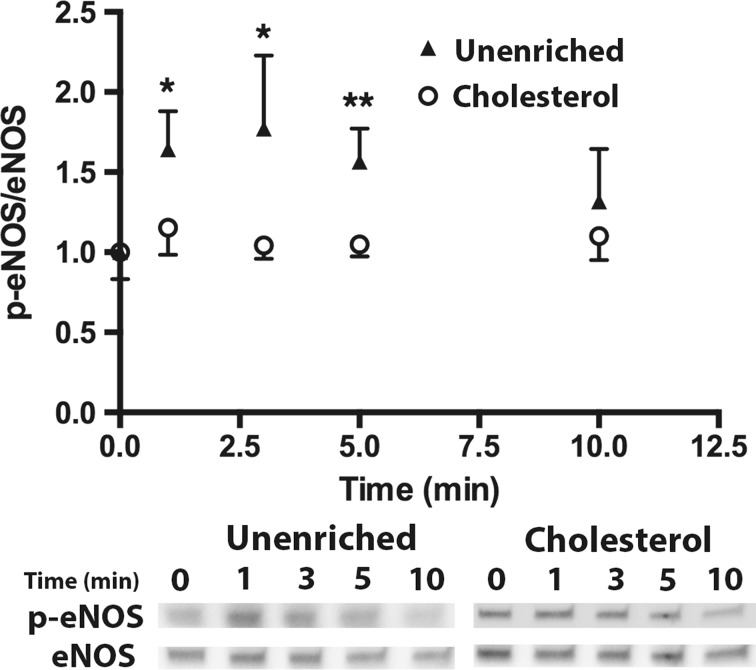
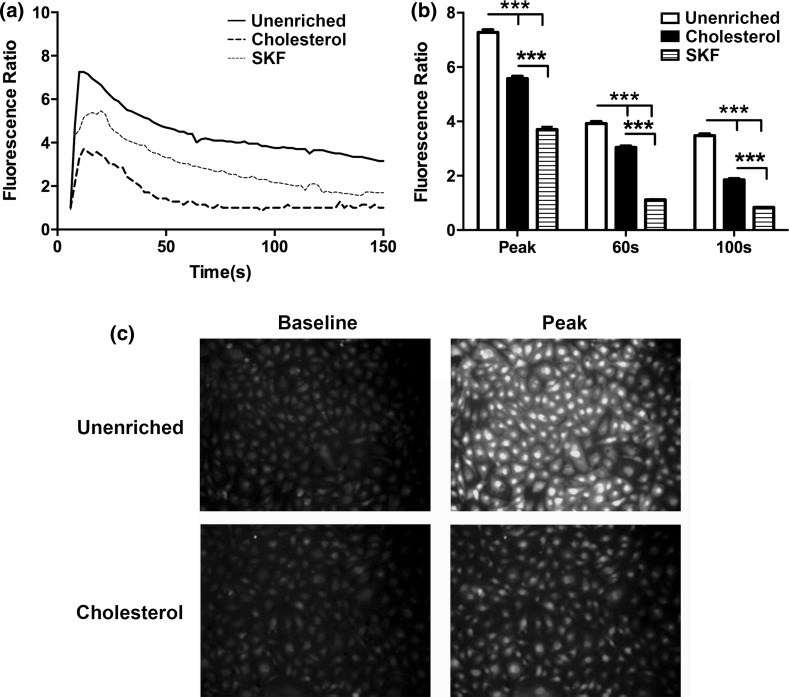
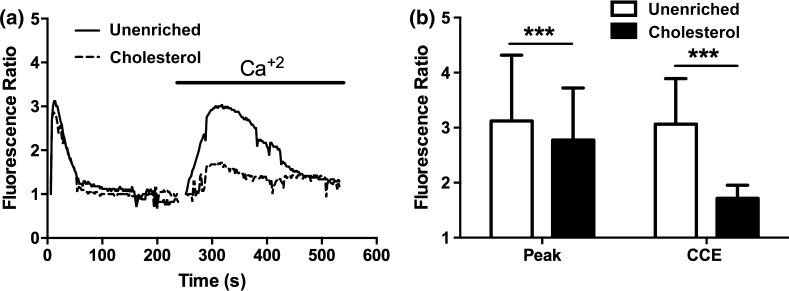
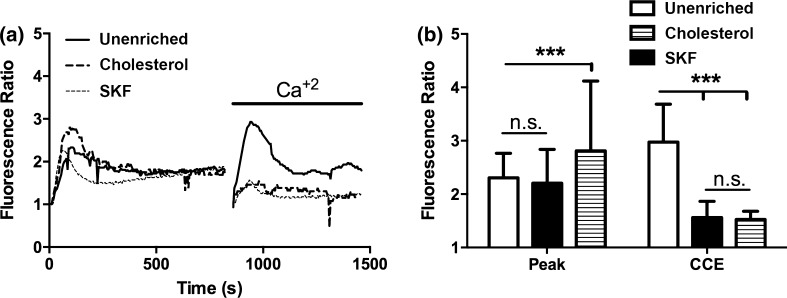
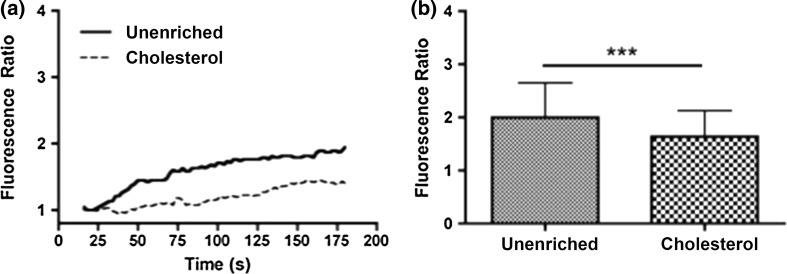
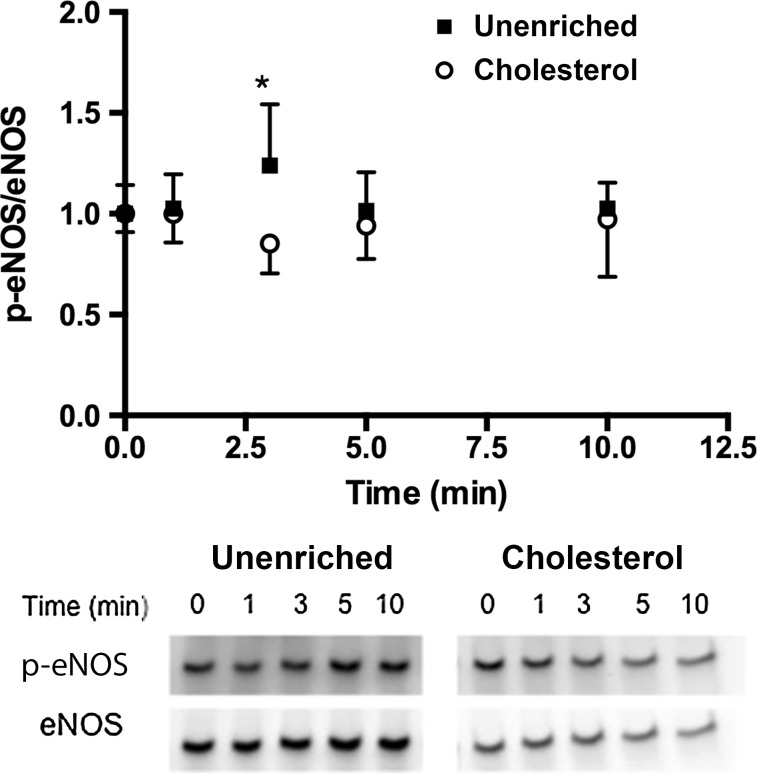
We examined the effect of cholesterol enrichment on ATP induced eNOS phosphorylation in static cultures. When stimulated with ATP, unenriched cells have a rapid increase in phosphorylation that peaks around 3 min and then returns to baseline around 10 min (Fig. 2). Enrichment with cholesterol completely abolished the increase in eNOS phosphorylation in response to stimulation with ATP. Cholesterol has been shown to affect a number of cell and membrane properties, and one of the most studied effects is the impairment or enhancement of membrane ion channels.7,11,14,15,30 We have previously shown that calcium and specifically the activation of CCE plays an important role in ATP-induced eNOS phosphorylation. ATP stimulation in unenriched cells elicited a rapid transient peak followed by a sustained plateau (Fig. 3). Inhibition of SOCs with SKF reduced both the transient peak and plateau levels of calcium. Cholesterol-enrichment inhibited the calcium response to a greater extent than SKF. It significantly reduced the transient peak and abolished the plateau phase of the response, with the calcium level returning to baseline within 50 s (Fig. 3). To further evaluate the contribution of SOCs to the calcium response in cholesterol-enriched cells, we separated the ER store release and concomitant CCE. Stimulation with ATP in the absence of extracellular calcium elicited a rapid, but brief, calcium transient that was only slightly inhibited by cholesterol enrichment. In contrast, subsequent addition of extracellular calcium produced a large sustained calcium influx that was strongly inhibited in cholesterol-enriched cells (Fig. 4). These results suggest that the effect of cholesterol enrichment on the calcium response to ATP is primarily through an inhibition of CCE, rather than a consequence of impaired upstream signaling. To confirm this, we used an alternative method to stimulate CCE. Thapsigargin (Tg) causes ER store depletion (through the inhibition of calcium pumps) and subsequent activation of CCE.26 In this experiment we also separated the ER store release from CCE by stimulating with Tg in the absence of extracellular calcium followed by the addition of calcium. Initial ER store release in response to Tg occurred over 10 min and was slower than stimulation with ATP (Fig. 5). The intracellular calcium increase in response to Tg was slightly higher in cholesterol-enriched cells. However, the subsequent influx via CCE was significantly inhibited in cholesterol-enriched cells to an extent similar to SOC blocker, SKF (Fig. 5).
Figure 2.
Cholesterol inhibits ATP-induced eNOS phosphorylation. Cells were simulated with 100 µM ATP with Ca+2. Cells were treated with either PBS with Ca+2 or 3.5 mM cholesterol at 37 °C for 30 min prior to stimulation. Cells were harvested before stimulation (t = 0) and at time points 1, 3, 5, and 10 min after stimulation. All p-eNOS/eNOS ratios were normalized by t = 0. eNOS phosphorylation in cholesterol enriched cells (Cholesterol) is abolished in response to ATP. (Mean and SEM were plotted p < 0.05*, #; p < 0.01** two-tailed t test; Unenriched n = 4, Cholesterol n = 4).
Figure 3.
Cholesterol enrichment attenuates the ATP agonist calcium response. Prior to the experiment, cells were incubated with PBS with Ca+2 for 30 min (solid line), 3.5 mM water-soluble cholesterol for 30 min (dashed line) or 50 μM of SOC inhibitor SKF-96365 for 10 min (dotted line). (a) Ca+2 response of each condition to 500 µL of 100 µM ATP dissolved in PBS with Ca+2. (b) Bar graph representing average responses for each condition at the peak response, 60 and 100 s. Cholesterol treated cells show a reduced transient and sustained calcium response, which was statically significant from unenriched and SKF treated cells. Average responses represent multiple coverslips (cs) and total cell count among coverslips (n) Unenriched: #cs = 8, n = 512; SKF: #cs = 5, n = 298; Cholesterol: #cs = 7, n = 441. Mean and SEM were plotted two-tailed t test p < 0.0001.
Figure 4.
Cholesterol inhibits ATP stimulated capacitative calcium entry (CCE). Prior to the experiment, cells were incubated with PBS with Ca+2 (solid line) or 3.5 mM water soluble cholesterol (dashed line) for 30 min. (a) Representative traces of the Ca+2 response to 500 µL of 100 µM exogenous ATP in Ca+2 free PBS. After the initial response, the solution was replaced with 500 µL of PBS with Ca+2. (b) Bar graph representing the average responses for each condition at the initial response peak and the max after re-addition Ca+2, which represents capacitative calcium entry (CCE). Cholesterol enriched cells showed a slight reduction in the initial ER calcium response and a strong attenuation of the CCE calcium response as compared to unenriched cells. Average responses represent multiple coverslips (cs) and total cell count among coverslips (n) Unenriched: cs # = 5, n = 210; Cholesterol: cs# = 6, n = 321. Mean and SEM were plotted Two-tailed t test p < 0.0001.
Figure 5.
Cholesterol attenuated the thapsigargin-activated capacitative calcium entry (CCE) similarly to SOC inhibition. Prior to experiment, cells were incubated with PBS with Ca+2 for 30 min (solid line), 3.5 mM water-soluble cholesterol for 30 min (dashed line) or 50 µM of SOC inhibitor SKF-96365 for 10 min (dotted line). (a) Shows representative traces of the calcium response to 1 μM Tg was added to cells in Ca+2 free PBS. After the initial response, the solution is replaced with 500 µL of PBS with Ca+2. (b) Bar graph representing average responses for each condition at initial response peak and after re-addition Ca+2, which represents capacitative calcium entry (CCE). Cholesterol enriched cells showed an increase in the ER calcium response and an attenuated CCE response, which was significantly different from unenriched cells. In addition, the CCE response in cholesterol-treated cells was not statistically different from cells treated with the SOC inhibitor SKF. (c) Representative images of the fluorescence signal at baseline and peak stimulation for unenriched and cholesterol treated coverslips. Average responses represent multiple coverslips (cs) and total cell count among coverslips (n) Unenriched: cs = 4, n = 225, Cholesterol: cs = 3, n = 169, SKF: cs = 3, n = 135. Mean and SEM were plotted Two-tailed t test ***p < 0.0001.
Cholesterol Impairs the l-α-lysophosphatidylcholine (LPC) Induced Calcium Response and eNOS Phosphorylation
To further investigate the effect of cholesterol on calcium-dependent eNOS phosphorylation, we examined calcium signaling and eNOS phosphorylation in response to stimulation with LPC. LPC is an iPLA2 metabolite, which has previously been suggested to be involved in activation of store operated calcium channels responsible for CCE.6 We therefore sought to use LPC as an alternative to activate CCE. In unenriched cells, LPC elicited a small gradual increase in calcium, which approached a stable plateau (Fig. 6). This calcium response was attenuated in cholesterol-enriched cells (Fig. 6). The calcium response elicited by LPC was notably different from the ATP-stimulated response in that it lacked the initial rapid transient. LPC treatment nonetheless stimulated a small increase in eNOS phosphorylation at 3 min, which was inhibited by cholesterol enrichment (Fig. 7).
Figure 6.
Cholesterol attenuates the LPC stimulated calcium response. Cells were stimulation with 300 nM of LPC. Prior to stimulation, cells were treated with either PBS with Ca+2 (solid line) or 3.5 mM cholesterol (dotted line) at 37 °C for 30 min. (a) Representative traces to LPC stimulation in unenriched and cholesterol treated cells. LPC stimulates a gradual increase in calcium that was attenuated by cholesterol enrichment. (b) Bar graph showing that cholesterol statistically attenuates the peak calcium response in 180 s to LPC. Average responses represent multiple coverslips (cs) and total cell count among coverslips (n) Unenriched: #cs = 9, n = 359, Cholesterol: #cs = 8, n = 320, Mean and SEM were plotted two-tailed t test ***p < 0.0001.
Figure 7.
Cholesterol attenuates the LPC stimulated increase in eNOS phosphorylation. Prior to stimulation, cells were treated with either PBS with Ca+2 or 3.5 mM cholesterol at 37 °C for 30 min. Cells were stimulation with 300 nM of LPC. Cells were harvested before stimulation (t = 0) and at time points 1, 3, 5, and 10 min after stimulation. All p-eNOS/eNOS ratios were normalized by t = 0. LPC stimulates a small increase in eNOS phosphorylation that was attenuated by cholesterol enrichment. (*p < 0.05 Mean and SEM were plotted two tailed t test Unenriched n = 5, Cholesterol n = 6).
Cholesterol Enrichment Attenuates the Shear Stress-Induced NO Response
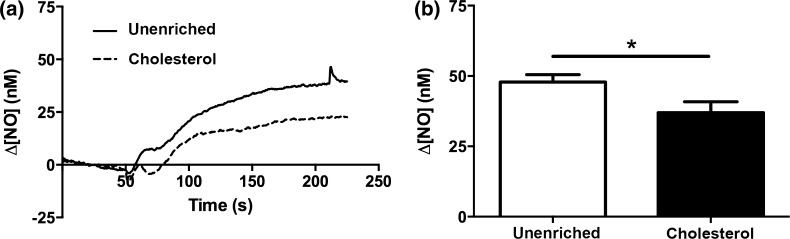
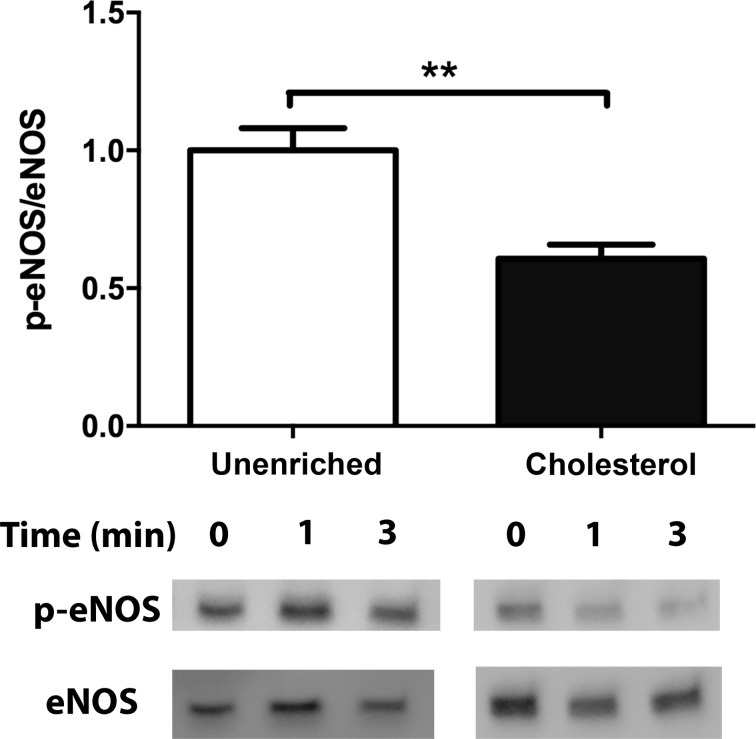
We next investigated the effect of cholesterol enrichment on the shear stress-induced NO response. We compared the steady-state changes in NO concentration (∆[NO]) in response to a step change of 10 dyn/cm2and found that cholesterol enrichment attenuated the ∆[NO] response by 24% (Fig. 8). To further evaluate the effect of cholesterol enrichment on NO production, we examined the effect of cholesterol on shear stress-induced eNOS phosphorylation. eNOS phosphorylation on Serine1179 has been reported to increase in response to shear stress within 5 min 17 and is generally accepted as an indicator of increased production of NO. Unenriched cells have increased phosphorylation following 3 min of flow,2 which is attenuated by 40% in cholesterol treated cells (Fig. 9).
Figure 8.
Cholesterol enrichment attenuates the steady state NO produced in response to flow. Cells were treated with either PBS with Ca+2 or 3.5 mM Cholesterol for 30 min at 37 °C prior to insertion into the chamber. Each membrane was exposed to a series of 4 step changes from 0.1 to 10 dyn/cm2, which represented the response of a single membrane. Response from multiple membranes were then averaged and compared. (a) Sample traces of the response for unenriched (solid line) and cholesterol treated (dotted line) cells. The steady-state concentration was offset to zero in order to show the individual NO response due to the step change occurring at 50 s. B. Bar graph showing the ∆[NO] response in unenriched and cholesterol enriched cells. Cholesterol enriched responses were reduced by 24% and were statistically significant (Mean and SEM were plotted, One-tailed t test. Unenriched n = 4, Cholesterol n = 3, *p < 0.05).
Figure 9.
Cholesterol impairs the shear stress-induced increase in eNOS phosphorylation. Bar graph representation of the ratio of phosphorylated eNOS to total eNOS for unenriched and cholesterol enriched cells following exposure to 10 dyn/cm2 for 3 min. The response is normalized by the unenriched control (Mean and SEM were plotted, ***p < 0.001 one-tailed t test n = 5 unenriched, n = 6 cholesterol).
Discussion
Our results demonstrate that cholesterol enrichment of ECs impairs the ATP-CCE pathway leading to downstream attenuation of eNOS phosphorylation and NO production. In addition, we show that cholesterol enrichment also impairs flow-induced NO production and eNOS phosphorylation.2 These results have important implications for our understanding of agonist and shear stress-induced NO signaling during pathological conditions involving elevated blood cholesterol levels.
A number of studies have shown that hypercholesterolemia leads to impaired agonist-stimulated 10,19,23 and flow-dependent vasodilation.19,45 Thus, we sought to understand the role of cholesterol enrichment in endothelial dysfunction. In our experiments, cells were enriched to ~3.5× the level of unenriched cells (Fig. 1). Few studies have measured the level of enrichment in the endothelium specifically in vivo. Aortic endothelial cells isolated from hypercholesterolemic pigs showed enrichment ranging from 1.5 to 4× of control animals.14 Additionally, cardiac myocytes isolated from hypercholesterolemic rabbits show 3× enrichment with cholesterol.43 While this level of enrichment occurs over long time scales, rapid enrichment of isolated vessels with cholesterol-loaded liposomes showed an enrichment measured as μg/ml cholesterol/μg/ml protein of the whole vessel (endothelium + smooth muscle) to be 1.7–2× higher after 4 h of enrichment.5 Overall, our level of enrichment is high but within the pathophysiological range for hypercholesterolemic conditions.
Examination of both calcium signaling and eNOS phosphorylation in cholesterol enriched ECs showed complete abolishment of eNOS phosphorylation at all time points and significant attenuation of calcium signaling, especially the sustained phase due to CCE (Figs. 2 and 3). The calcium response in cholesterol-enriched cells was lower than inhibition of SOCs alone with SKF. However, examination of ER calcium release alone following ATP or Tg showed only small differences with the primary effect of cholesterol enrichment was on CCE (Figs. 4 and 5). There was a slight increase in ER calcium release in cholesterol-enriched cells when stimulated with Tg. This might be explained by studies by Li et al. who found that cholesterol enrichment of the endoplasmic reticulum inhibits SERCA activity31 and would lead to faster store depletion.
The importance of CCE in agonist-induced NO production has been reported experimentally by us and a number of researchers,1,13,25,32 along with our recent work highlighting its importance in shear-stress induced NO production.2 In addition, mathematical modeling by our group has suggested that co-localization of SOCs with eNOS creates high local calcium gradients able to maximally activate production of NO.25 Therefore, alterations in the calcium signaling through SOCs can have a significant effect on the resulting NO release. Our data strongly support that the major effect of cholesterol enrichment is an inhibition of SOCs; however, we must consider other possible mechanisms. One potential cause for impaired calcium signaling is changes in the release of ATP. Cholesterol enrichment has been reported to reduce ATP release in arteries.22 In contrast to this, Wang et al. showed an increase in ATP release in endothelial cells with cholesterol enrichment.46 In our experiments, we did not measure ATP to determine if cholesterol enrichment caused changes in ATP release. However, our experiments showing the response of cholesterol-enriched cells to ATP (Figs. 3 and 4) indicate that if ATP levels were unchanged, the calcium and NO response would still be inhibited. In addition to the reported effects on ATP, cholesterol could inhibit upstream signaling leading to SOC activation. Chun et al. have shown that cholesterol enrichment decreases the number of active MIC channels in human embryonic kidney cells (HEK293), via downregulation of phosphatidylinositol 4,5-bisphosphate (PIP2) and thus preventing downstream activation.11 We did not measure PIP2 in our experiments, but activation with Tg, which does not involve PIP2, still resulted in CCE that was inhibited by cholesterol enrichment suggesting that upstream signaling is not the limiting factor (Fig. 5). In addition, cholesterol had minimal effects on the transient calcium response (Figs. 3 and 4), which is a result of ER calcium release and suggests that upstream signaling in unaffected. Although Chun et al. identified PIP2 as the cause of inhibition of voltage-dependent K+ (Kv) currents rather than direct channel inhibition, others have reported direct inhibition.14,30 These discrepancies may be due to differences in the level of cholesterol enrichment and cell type. Zidovetzki and Levitan et al., have reviewed the use of cyclodextrins, and detailed that the level of enrichment can vary depending on the cell type and length of incubation.50 Numerous studies have shown that cholesterol can inhibit other calcium and ion channels,7,14,29–31 and it is therefore plausible that cholesterol enrichment also inhibits SOCs. Thus, we believe that the main mechanism of impaired NO production by cholesterol enrichment is through direct inhibition of SOCs.
In order to support the idea that cholesterol enrichment directly impairs SOCs, we sought to directly activate SOCs using L-α-Lysophosphatidylcholine, which has previously been suggested to activate store operated calcium channels.6 LPC elicited a small calcium response (Fig. 6) suggesting it is not a particularly robust activator of SOCs. The magnitude and time course of the calcium response was significantly different from influx due to store depletion (Figs. 4 and 5). Store depletion due to ATP stimulation or Tg treatment caused a CCE-mediated calcium fluorescence ratio increase to 3 at 1 min as compared to LPC treatment which increased the fluorescence ratio to 2 over 3 min. Perhaps owing to the sluggish calcium response to LPC, the resulting eNOS phosphorylation showed only a small increase at 3 min. (Figure 7). Although not definitive, the inhibition of both LPC-stimulated calcium influx and eNOS phosphorylation by cholesterol enrichment is consistent with the hypothesis that cholesterol directly affects SOCs.
With our results indicating that cholesterol enrichment impairs the ATP-CCE pathway, we also examined the effect of on shear stress-induced NO production.2 Shear stress induces the production of NO through calcium dependent and independent mechanisms, which occur on different timescales (short vs. long respectively).2,28,35 Our recent work has highlighted the importance of ATP autocine activation of CCE in the shear stress-induced NO response.2 We have proposed that shear stress elicits the release of ATP, which activates purinergic receptors and the IP3 pathway. IP3 binds to the IP3 receptor on the ER and causes the release of calcium into the cytosol. This activates SOCs, which are PKC dependent, to allow the influx of extracellular calcium (termed capacitative calcium entry). The increase in calcium leads to eNOS phosphorylation and NO production. Our understanding of this pathway led us to hypothesize that the cholesterol enrichment would reduce shear stress-induced NO by inhibition of the ATP-CCE autocrine signaling pathway. We therefore utilized our novel device, which is capable of measuring NO directly and in real-time from ECs exposed to flow. Our results showed inhibition of both shear stress-induced NO production (Fig. 8) and eNOS phosphorylation (Fig. 9) which were of a similar magnitude as interference with ATP autocrine signaling shown previously by our group.2 Although we have only quantified the effect of cholesterol enrichment on the steady state NO concentration, the real-time measurements of the kinetics of NO production can potentially be used to study and validate mathematical models of NO production during physiological and disease conditions. Our results therefore offer the first report of how cholesterol enrichment affects the shear stress-induced production of NO, by direct measurement, from ECs in vitro. One previous report measuring the effect of a cholesterol derivative on NO indirectly by cGMP levels at a single time-point also supports our results. That study by Knudesen et al. found that enrichment with cholesteryl hemisuccinate decreased shear stress-induced cGMP levels. However, the extent of inhibition cannot be compared to our results due to the differences in NO quantification (cGMP vs. direct NO measurements). In addition, sterol specificity has been reported to be important for the structure and function of caveolae,27 which can impact eNOS function,41 making it unclear if cholesteryl hemisuccinate affects cells differently than cholesterol. Our results showed that cholesterol enrichment attenuated the shear stress-induced NO response by ~25% (Fig. 2). Although the inhibition we report may seem modest, little is known on how small changes in the ∆[NO] response or the time-dependent release of NO affects vasodilation and long term gene expression.
An interesting aspect of this study is the effect of cholesterol enrichment on SOCs, which are thought to reside or translocate to cholesterol rich areas such as lipid rafts or caveolae.34,37 We had speculated that cholesterol might affect channels differently depending on their location in the plasma membrane given that proteins and ion channels preferentially reside in either cholesterol rich or deficient domains due to the difference in the surrounding lipid environment and membrane fluidity. However, it appears that higher levels of cholesterol affect even channels that prefer cholesterol rich areas. Our studies demonstrate that SOCs are among the wide range of ion channels reportedly inhibited by cholesterol enrichment. Our results indicate that cholesterol enrichment impairs both ATP agonist- and shear stress-induced NO production through inhibition of CCE. In addition, these findings have provided a mechanism by which cholesterol enrichment can lead to decreased NO production and endothelial dysfunction.
Acknowledgments
We would like to acknowledge the following funding sources for this project: NIH/HL068164 (DJ, KAB), NSF/BES0301446 (DJ, KAB), NSF/CBET0730547 (DJ, KAB), NIH U01HL116256 (DJ, KAB, DGB).
Conflict of interest
Ms. Muzorewa, Ms. Zaccheo, and Dr. Buerk have nothing to disclose. Dr. Andrews, Dr. Jaron and Dr. Barbee have a patent 8,828,711 issued.
Ethical standards
No human studies were carried out by the authors for this article. No animal studies were carried out by the authors for this article.
References
- 1.Ambudkar IS, Bandyopadhyay BC, Liu XB, Lockwich TP, Paria B, Ong HL. Functional organization of TRPC-Ca2+ channels and regulation of calcium microdomains. Cell Calcium. 2006;40:495–504. doi: 10.1016/j.ceca.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Andrews AM, Jaron D, Buerk DG, Barbee KA. Shear stress-induced NO production is dependent on ATP autocrine signaling and capacitative calcium entry. Cell Mol. Bioeng. 2014;7:510–520. doi: 10.1007/s12195-014-0351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews AM, Jaron D, Buerk DG, Kirby PL, Barbee KA. Direct, real-time measurement of shear stress-induced nitric oxide produced from endothelial cells in vitro. Nitric Oxide. 2010;23:335–342. doi: 10.1016/j.niox.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastiaanse EML, Hold KM, VanderLaarse A. The effect of membrane cholesterol content on ion transport processes in plasma membranes. Cardiovasc. Res. 1997;33:272–283. doi: 10.1016/S0008-6363(96)00193-9. [DOI] [PubMed] [Google Scholar]
- 5.Bialecki RA, Tulenko TN. Excess membrane cholesterol alters calcium channels in arterial smooth muscle. Am. J. Physiol. 1989;257:C306–C314. doi: 10.1152/ajpcell.1989.257.2.C306. [DOI] [PubMed] [Google Scholar]
- 6.Boittin FX, Gribi F, Serir K, Beny JL. Ca2+-independent PLA2 controls endothelial store-operated Ca2+ entry and vascular tone in intact aorta. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2466–H2474. doi: 10.1152/ajpheart.00639.2008. [DOI] [PubMed] [Google Scholar]
- 7.Bowles DK, Heaps CL, Turk JR, Maddali KK, Price EM. Hypercholesterolemia inhibits L-type calcium current in coronary macro-, not microcirculation. J. Appl. Physiol. 2004;96:2240–2248. doi: 10.1152/japplphysiol.01229.2003. [DOI] [PubMed] [Google Scholar]
- 8.Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear-stress induced release of nitricoxide from endothelial-cells grown on beads. Hypertension. 1991;17:187–193. doi: 10.1161/01.HYP.17.2.187. [DOI] [PubMed] [Google Scholar]
- 9.Cabral PD, Hong NJ, Garvin JL. ATP mediates flow-induced NO production in thick ascending limbs. Am. J. Physiol. Ren. Physiol. 2012;303:F194–F200. doi: 10.1152/ajprenal.00504.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casino PR, Kilcoyne CM, Quyyumi AA, Hoeg JM, Panza JA. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation. 1993;88:2541–2547. doi: 10.1161/01.CIR.88.6.2541. [DOI] [PubMed] [Google Scholar]
- 11.Chun YS, Shin S, Kim Y, Cho H, Park MK, Kim TW, Voronov SV, Di Paolo G, Suh BC, Chung S. Cholesterol modulates ion channels via down-regulation of phosphatidylinositol 4,5-bisphosphate. J. Neurochem. 2010;112:1286–1294. doi: 10.1111/j.1471-4159.2009.06545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen RA, Plane F, Najibi S, Huk I, Malinski T, Garland CJ. Nitric oxide is the mediator of both endothelium-dependent relaxation and hyperpolarization of the rabbit carotid artery. Proc. Natl. Acad. Sci. USA. 1997;94:4193–4198. doi: 10.1073/pnas.94.8.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dedkova EN, Blatter LA. Nitric oxide inhibits capacitative Ca2+ entry and enhances endoplasmic reticulum Ca2+ uptake in bovine vascular endothelial cells. J. Physiol. Lond. 2002;539:77–91. doi: 10.1113/jphysiol.2001.013258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang Y, Emile RM, Hsieh E, Osman H, Hashemi SM, Davies PF, Rothblat GH, Wilensky RL, Levitan I. Hypercholesterolemia suppresses inwardly rectifying K+ channels in aortic endothelium in vitro and in vivo. CircRes. 2006;98:1064–1071. doi: 10.1161/01.RES.0000218776.87842.43. [DOI] [PubMed] [Google Scholar]
- 15.Feron O, Dessy C, Moniotte S, Desager JP, Balligand JL. Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J. Clin. Investig. 1999;103:897–905. doi: 10.1172/JCI4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferroni P, Basili S, Paoletti V, Davi G. Endothelial dysfunction and oxidative stress in arterial hypertension. Nutr. Metab. Carbiovasc. Dis. 2006;16:222–233. doi: 10.1016/j.numecd.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Fisslthaler B, Dimmeler S, Hermann C, Busse R, Fleming I. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol. Scand. 2000;168:81–88. doi: 10.1046/j.1365-201x.2000.00627.x. [DOI] [PubMed] [Google Scholar]
- 18.Flavahan NA. Atherosclerosis or lipoprotein-induced endothelial dysfunction—potential mechanisms underlying reduction in EDRF/nitric oxide activity. Circulation. 1992;85:1927–1938. doi: 10.1161/01.CIR.85.5.1927. [DOI] [PubMed] [Google Scholar]
- 19.Giannattasio C, Mangoni AA, Failla M, Carugo S, Stella ML, Stefanoni P, Grassi G, Vergani C, Mancia G. Impaired radial artery compliance in normotensive subjects with familial hypercholesterolemia. Atherosclerosis. 1996;124:249–260. doi: 10.1016/0021-9150(96)05834-0. [DOI] [PubMed] [Google Scholar]
- 20.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 21.Graziani A, Bricko V, Carmignani M, Graier WF, Groschner K. Cholesterol- and caveolin-rich membrane domains are essential for phospholipase A2-dependent EDHF formation. Cardiovasc. Res. 2004;64:234–242. doi: 10.1016/j.cardiores.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto M, Shinozuka K, Tanabe Y, Shahdat HM, Gamoh S, Kwon YM, Tanaka Y, Kunitomo M, Masumura S. Long-term supplementation with a high cholesterol diet decreases the release of ATP from the caudal artery in aged rats. Life Sci. 1998;63:1879–1885. doi: 10.1016/S0024-3205(98)00464-0. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi T, Naito M, Ishikawa T, Kuzuya M, Funaki C, Tateishi T, Asai K, Hidaka H, Kuzuya F. Beta-migrating very low density lipoprotein attenuates endothelium-dependent relaxation in rabbit atherosclerotic aortas. Blood Vessel. 1989;26:290–299. doi: 10.1159/000158778. [DOI] [PubMed] [Google Scholar]
- 24.Hong D, Jaron D, Buerk DG, Barbee KA. Heterogeneous response of microvascular endothelial cells to shear stress. Am. J. Physiol. Heart Circul. Physiol. 2006;290:H2498–H2508. doi: 10.1152/ajpheart.00828.2005. [DOI] [PubMed] [Google Scholar]
- 25.Hong D, Jaron D, Buerk DG, Barbee KA. Transport-dependent calcium signaling in spatially segregated cellular caveolar domains. Am. J. Physiol. Cell Physiol. 2008;294:C856–C866. doi: 10.1152/ajpcell.00278.2007. [DOI] [PubMed] [Google Scholar]
- 26.Jan CR, Ho CM, Wu SN, Tseng CJ. Mechanism of rise and decay of thapsigargin-evoked calcium signals in MDCK cells. Life Sci. 1999;64:259–267. doi: 10.1016/S0024-3205(98)00561-X. [DOI] [PubMed] [Google Scholar]
- 27.Jansen M, Pietiarinen VM, Polonen H, Rasilainen L, Koivusalo M, Ruotsalainen U, Jokitalo E, Ikonen E. Cholesterol substitution increases the structural heterogeneity of caveolae. J. Biol. Chem. 2008;283:14610–14618. doi: 10.1074/jbc.M710355200. [DOI] [PubMed] [Google Scholar]
- 28.Kuchan MJ, Jo H, Frangos JA. Role of G proteins in shear stress-mediated nitric oxide production by endothelial cells. Am. J. Physiol. 1994;267:C753–C758. doi: 10.1152/ajpcell.1994.267.3.C753. [DOI] [PubMed] [Google Scholar]
- 29.Lee AK, Yeung-Yam-Wah V, Tse FW, Tse A. Cholesterol elevation impairs glucose-stimulated Ca(2+) signaling in mouse pancreatic beta-cells. Endocrinology. 2011;152:3351–3361. doi: 10.1210/en.2011-0124. [DOI] [PubMed] [Google Scholar]
- 30.Levitan I, Christian AE, Tulenko TN, Rothblat GH. Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells. J. Gen. Physiol. 2000;115:405–416. doi: 10.1085/jgp.115.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li YK, Ge MT, Ciani L, Kuriakose G, Westover EJ, Dura M, Covey DF, Freed JH, Maxfield FR, Lytton J, Tabas I. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids—Implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J. Biol. Chem. 2004;279:37030–37039. doi: 10.1074/jbc.M405195200. [DOI] [PubMed] [Google Scholar]
- 32.Lin S, Fagan KA, Li KX, Shaul PW, Cooper DMF, Rodman DM. Sustained endothelial nitric-oxide synthase activation requires capacitative Ca2+ entry. J. Biol. Chem. 2000;275:17979–17985. doi: 10.1074/jbc.275.24.17979. [DOI] [PubMed] [Google Scholar]
- 33.Linder AE, McCluskey LP, Cole KR, 3rd, Lanning KM, Webb RC. Dynamic association of nitric oxide downstream signaling molecules with endothelial caveolin-1 in rat aorta. J. Pharmacol. Exp. Ther. 2005;314:9–15. doi: 10.1124/jpet.105.083634. [DOI] [PubMed] [Google Scholar]
- 34.Lockwich TP, Liu XB, Singh BB, Jadlowiec J, Weiland S, Ambudkar IS. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J. Biol. Chem. 2000;275:11934–11942. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- 35.Mochizuki S, Goto M, Chiba Y, Ogasawara Y, Kajiya F. Flow dependence and time constant of the change in nitric oxide concentration measured in the vascular media. Med. Biol. Eng. Comput. 1999;37:497–503. doi: 10.1007/BF02513336. [DOI] [PubMed] [Google Scholar]
- 36.Neishi Y, Mochizuki S, Miyasaka T, Kawamoto T, Kume T, Sukmawan R, Tsukiji M, Ogasawara Y, Kajiya F, Akasaka T, Yoshida K, Goto M. Evaluation of bioavailability of nitric oxide in coronary circulation by direct measurement of plasma nitric oxide concentration. Proc. Natl. Acad. Sci. USA. 2005;102:11456–11461. doi: 10.1073/pnas.0501392102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pani B, Singh BB. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium. 2009;45:625–633. doi: 10.1016/j.ceca.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson TE, Poppa V, Ueba H, Wu A, Yan C, Berk BC. Opposing effects of reactive oxygen species and cholesterol on endothelial nitric oxide synthase and endothelial cell caveolae. CircRes. 1999;85:29–37. doi: 10.1161/01.res.85.1.29. [DOI] [PubMed] [Google Scholar]
- 39.Plotnick GD, Corretti MC, Vogel RA, Hesslink R, Wise JA. Effect of supplemental phytonutrients on impairment of the flow-mediated brachial artery vasoactivity after a single high-fat meal. J. Am. Coll. Cardiol. 2003;41:1744–1749. doi: 10.1016/S0735-1097(03)00302-4. [DOI] [PubMed] [Google Scholar]
- 40.Saini HK, Arneja AS, Dhalla NS. Role of cholesterol in cardiovascular dysfunction. Can. J. Cardiol. 2004;20:333–346. [PubMed] [Google Scholar]
- 41.Shaul PW. Endothelial nitric oxide synthase, caveolae and the development of atherosclerosis. J. Physiol. 2003;547:21–33. doi: 10.1113/jphysiol.2002.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Troup GM, Xie Y, Boesze-Battaglia K, Huang Y, Kirk T, Hanley F, Tulenko TN. Membrane Cholesterol and the Formation of Cholesterol Domains in the Pathogenesis of Cardiovascular Disease; V C H Verlag Gmbh: Wiley; 2004. p. 25. [Google Scholar]
- 43.Troup GM, Xie Y, Boesze-Battaglia K, Huang Y, Kirk T, Hanley F, Tulenko TN. Membrane cholesterol and the formation of cholesterol domains in the pathogenesis of cardiovascular disease. Macromol. Symp. 2005;219:25–38. doi: 10.1002/masy.200550104. [DOI] [Google Scholar]
- 44.Tulenko TN, Chen M, Mason PE, Mason RP. Physical effects of cholesterol on arterial smooth muscle membranes: evidence of immiscible cholesterol domains and alterations in bilayer width during atherogenesis. J. Lipid Res. 1998;39:947–956. [PubMed] [Google Scholar]
- 45.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am. J. Cardiol. 1997;79:350–354. doi: 10.1016/S0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 46.Wang TK, Chen Z, Wang X, Shyy JYJ, Zhu Y. Cholesterol loading increases the translocation of ATP synthase beta chain into membrane caveolae in vascular endothelial cells. BBA Mol. Cell Biol. Lipids. 2006;1761:1182–1190. doi: 10.1016/j.bbalip.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Xu Y, Henning RH, van der Want JJ, van Buiten A, van Gilst WH, Buikema H. Disruption of endothelial caveolae is associated with impairment of both NO- as well as EDHF in acetylcholine-induced relaxation depending on their relative contribution in different vascular beds. Life Sci. 2007;80:1678–1685. doi: 10.1016/j.lfs.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 48.Yuan Y, Verna LK, Wang NP, Liao HL, Ma KS, Wang Y, Zhu Y, Stemerman MB. Cholesterol enrichment upregulates intercellular adhesion molecule-1 in human vascular endothelial cells. BBA Mol. Cell Biol. Lipids. 2001;1534:139–148. doi: 10.1016/S1388-1981(01)00188-3. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Q, Church JE, Jagnandan D, Catravas JD, Sessa WC, Fulton D. Functional relevance of Golgi- and plasma membrane-localized endothelial NO synthase in reconstituted endothelial cells. Arterioscler Thromb. Vasc. Biol. 2006;26:1015–1021. doi: 10.1161/01.ATV.0000216044.49494.c4. [DOI] [PubMed] [Google Scholar]
- 50.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim. Biophys. Acta Biomembr. 2007;1768:1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]