Abstract
The mechanisms contributing to the development of autoimmune insulin-dependent diabetes mellitus have been analyzed in allophenic mouse chimeras of the NOD in equilibrium with C57BL/6 strain combination (where NOD is nonobese diabetic). Occurrence of lymphoid cell infiltration (insulitis) in pancreatic islets was observed in the majority of such chimeras. The development of insulitis was found to correlate with major histocompatibility complex chimerism in lymphoid cells and in thymus cortical regions. Chimeras with more than 50% of C57BL/6 lymphoid cells rarely developed insulitis. Our data suggest that the correlation with the thymic cortical region is absolute. Thus, all individuals displaying NOD or NOD/C57BL/6 thymic cortical regions developed insulitis, whereas we have not observed insulitis in chimeras with only C57BL/6 thymic cortical regions. Thus the positive selection of T cells appears to play a crucial role in the development of insulin-dependent diabetes mellitus.
Full text
PDF
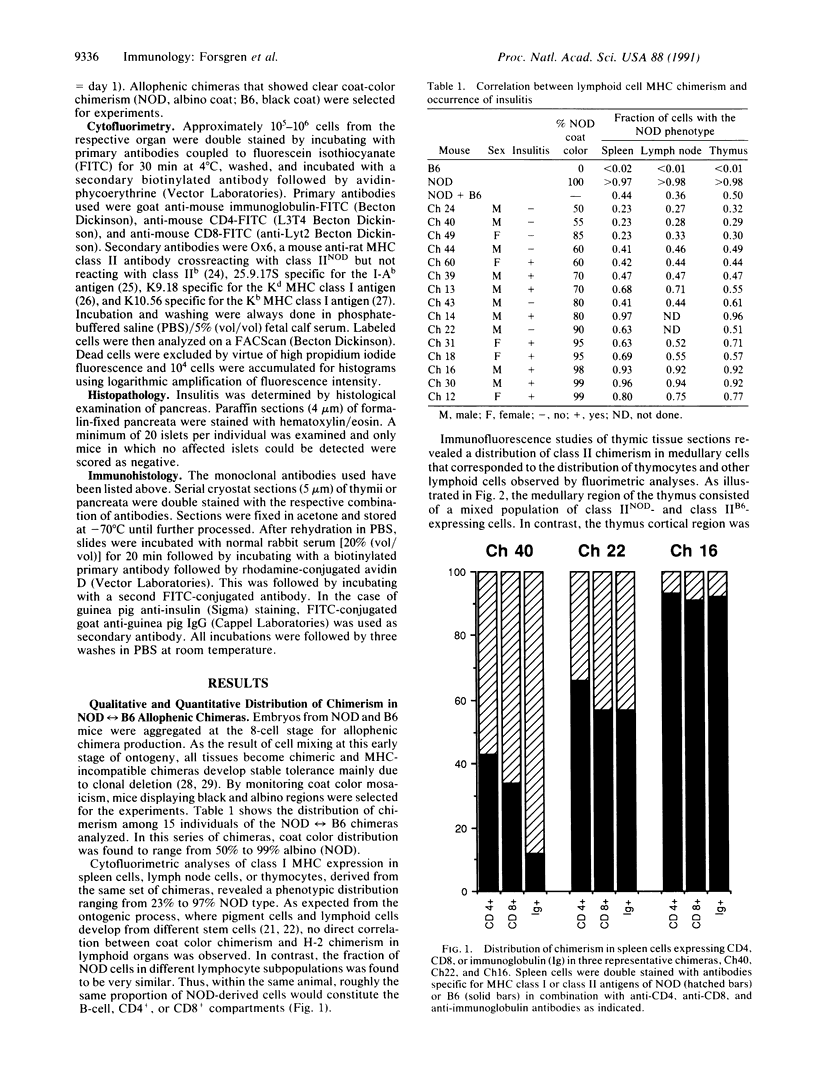

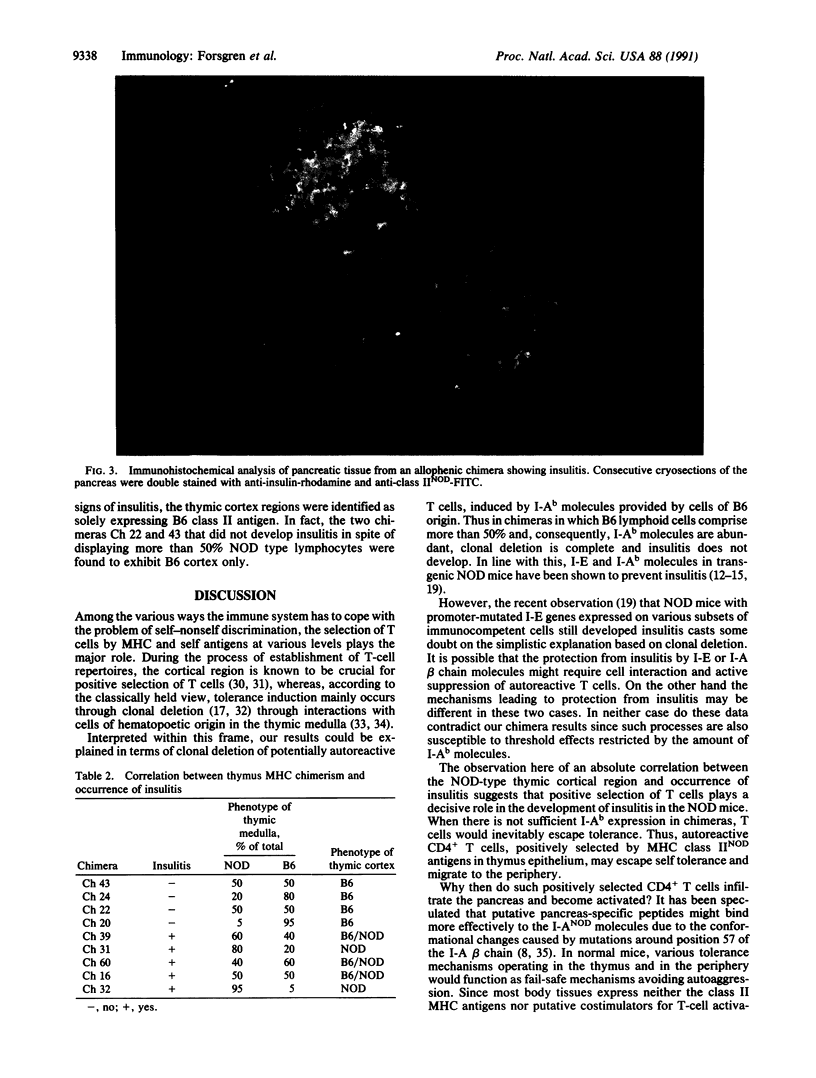

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold B., Horstmann U., Kuon W., Burgert H. G., Hämmerling G. J., Kvist S. Alloreactive cytolytic T-cell clones preferentially recognize conformational determinants on histocompatibility antigens: analysis with genetically engineered hybrid antigens. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7030–7034. doi: 10.1073/pnas.82.20.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay A. N. Different reticular elements in rat lymphoid tissue identified by localization of Ia, Thy-1 and MRC OX 2 antigens. Immunology. 1981 Dec;44(4):727–736. [PMC free article] [PubMed] [Google Scholar]
- Bendelac A., Carnaud C., Boitard C., Bach J. F. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987 Oct 1;166(4):823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottazzo G. F., Pujol-Borrell R., Hanafusa T., Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983 Nov 12;2(8359):1115–1119. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Böhme J., Haskins K., Stecha P., van Ewijk W., LeMeur M., Gerlinger P., Benoist C., Mathis D. Transgenic mice with I-A on islet cells are normoglycemic but immunologically intolerant. Science. 1989 Jun 9;244(4909):1179–1183. doi: 10.1126/science.2499048. [DOI] [PubMed] [Google Scholar]
- Böhme J., Schuhbaur B., Kanagawa O., Benoist C., Mathis D. MHC-linked protection from diabetes dissociated from clonal deletion of T cells. Science. 1990 Jul 20;249(4966):293–295. doi: 10.1126/science.2115690. [DOI] [PubMed] [Google Scholar]
- Campbell I. L., Wong G. H., Schrader J. W., Harrison L. C. Interferon-gamma enhances the expression of the major histocompatibility class I antigens on mouse pancreatic beta cells. Diabetes. 1985 Nov;34(11):1205–1209. doi: 10.2337/diab.34.11.1205. [DOI] [PubMed] [Google Scholar]
- Charlton B., Bacelj A., Mandel T. E. Administration of silica particles or anti-Lyt2 antibody prevents beta-cell destruction in NOD mice given cyclophosphamide. Diabetes. 1988 Jul;37(7):930–935. doi: 10.2337/diab.37.7.930. [DOI] [PubMed] [Google Scholar]
- Fink P. J., Bevan M. J. H-2 antigens of the thymus determine lymphocyte specificity. J Exp Med. 1978 Sep 1;148(3):766–775. doi: 10.1084/jem.148.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Buse J. B., Jackson R. A., Glimcher L., Dorf M. E., Minami M., Makino S., Moriwaki K., Kuzuya H., Imura H. The NOD mouse: recessive diabetogenic gene in the major histocompatibility complex. Science. 1986 Feb 14;231(4739):733–735. doi: 10.1126/science.3003909. [DOI] [PubMed] [Google Scholar]
- Hutchings P., Rosen H., O'Reilly L., Simpson E., Gordon S., Cooke A. Transfer of diabetes in mice prevented by blockade of adhesion-promoting receptor on macrophages. Nature. 1990 Dec 13;348(6302):639–642. doi: 10.1038/348639a0. [DOI] [PubMed] [Google Scholar]
- Hämmerling G. J., Rüsch E., Tada N., Kimura S., Hämmerling U. Localization of allodeterminants on H-2Kb antigens determined with monoclonal antibodies and H-2 mutant mice. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4737–4741. doi: 10.1073/pnas.79.15.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Wade T., White J., Kushnir E., Blackman M., Bill J., Roehm N., Marrack P. A T cell receptor V beta segment that imparts reactivity to a class II major histocompatibility complex product. Cell. 1987 Apr 24;49(2):263–271. doi: 10.1016/0092-8674(87)90567-8. [DOI] [PubMed] [Google Scholar]
- Lund T., O'Reilly L., Hutchings P., Kanagawa O., Simpson E., Gravely R., Chandler P., Dyson J., Picard J. K., Edwards A. Prevention of insulin-dependent diabetes mellitus in non-obese diabetic mice by transgenes encoding modified I-A beta-chain or normal I-E alpha-chain. Nature. 1990 Jun 21;345(6277):727–729. doi: 10.1038/345727a0. [DOI] [PubMed] [Google Scholar]
- MINTZ B. GENETIC MOSAICISM IN ADULT MICE OF QUADRIPARENTAL LINEAGE. Science. 1965 May 28;148(3674):1232–1233. doi: 10.1126/science.148.3674.1232. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Hengartner H., Pedrazzini T. Intrathymic deletion of self-reactive cells prevented by neonatal anti-CD4 antibody treatment. Nature. 1988 Sep 8;335(6186):174–176. doi: 10.1038/335174a0. [DOI] [PubMed] [Google Scholar]
- Makino S., Harada M., Kishimoto Y., Hayashi Y. Absence of insulitis and overt diabetes in athymic nude mice with NOD genetic background. Jikken Dobutsu. 1986 Oct;35(4):495–498. doi: 10.1538/expanim1978.35.4_495. [DOI] [PubMed] [Google Scholar]
- Makino S., Kunimoto K., Muraoka Y., Mizushima Y., Katagiri K., Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980 Jan;29(1):1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- Marquetant R., Desai N. M., Sabina R. L., Holmes E. W. Evidence for sequential expression of multiple AMP deaminase isoforms during skeletal muscle development. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2345–2349. doi: 10.1073/pnas.84.8.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga T., Simpson E., Meo T. Allogeneic tolerance in embryo aggregation mouse chimeras studied by mixed lymphocyte culture and cell-mediated lympholysis. Transplantation. 1980 Jul;30(1):34–39. doi: 10.1097/00007890-198007000-00007. [DOI] [PubMed] [Google Scholar]
- Matzinger P., Guerder S. Does T-cell tolerance require a dedicated antigen-presenting cell? Nature. 1989 Mar 2;338(6210):74–76. doi: 10.1038/338074a0. [DOI] [PubMed] [Google Scholar]
- Miller B. J., Appel M. C., O'Neil J. J., Wicker L. S. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988 Jan 1;140(1):52–58. [PubMed] [Google Scholar]
- Mintz B. Gene control of mammalian differentiation. Annu Rev Genet. 1974;8:411–470. doi: 10.1146/annurev.ge.08.120174.002211. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Uno M., Uehira M., Kikutani H., Kishimoto T., Kimoto M., Nishimoto H., Miyazaki J., Yamamura K. Direct evidence for the contribution of the unique I-ANOD to the development of insulitis in non-obese diabetic mice. Nature. 1990 Jun 21;345(6277):722–724. doi: 10.1038/345722a0. [DOI] [PubMed] [Google Scholar]
- Morel P. A., Dorman J. S., Todd J. A., McDevitt H. O., Trucco M. Aspartic acid at position 57 of the HLA-DQ beta chain protects against type I diabetes: a family study. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8111–8115. doi: 10.1073/pnas.85.21.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata M., Yokono K., Hayakawa M., Kawase Y., Hatamori N., Ogawa W., Yonezawa K., Shii K., Baba S. Destruction of pancreatic islet cells by cytotoxic T lymphocytes in nonobese diabetic mice. J Immunol. 1989 Aug 15;143(4):1155–1162. [PubMed] [Google Scholar]
- Nishimoto H., Kikutani H., Yamamura K., Kishimoto T. Prevention of autoimmune insulitis by expression of I-E molecules in NOD mice. 1987 Jul 30-Aug 5Nature. 328(6129):432–434. doi: 10.1038/328432a0. [DOI] [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Prochazka M., Leiter E. H., Serreze D. V., Coleman D. L. Three recessive loci required for insulin-dependent diabetes in nonobese diabetic mice. Science. 1987 Jul 17;237(4812):286–289. doi: 10.1126/science.2885918. [DOI] [PubMed] [Google Scholar]
- Reich E. P., Sherwin R. S., Kanagawa O., Janeway C. A., Jr An explanation for the protective effect of the MHC class II I-E molecule in murine diabetes. Nature. 1989 Sep 28;341(6240):326–328. doi: 10.1038/341326a0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. Acquisition of immunologic self-tolerance. Cell. 1989 Jun 30;57(7):1073–1081. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Slattery R. M., Kjer-Nielsen L., Allison J., Charlton B., Mandel T. E., Miller J. F. Prevention of diabetes in non-obese diabetic I-Ak transgenic mice. Nature. 1990 Jun 21;345(6277):724–726. doi: 10.1038/345724a0. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Acha-Orbea H., Bell J. I., Chao N., Fronek Z., Jacob C. O., McDermott M., Sinha A. A., Timmerman L., Steinman L. A molecular basis for MHC class II--associated autoimmunity. Science. 1988 May 20;240(4855):1003–1009. doi: 10.1126/science.3368786. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Bell J. I., McDevitt H. O. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987 Oct 15;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990 Dec 21;63(6):1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- Wicker L. S., Miller B. J., Chai A., Terada M., Mullen Y. Expression of genetically determined diabetes and insulitis in the nonobese diabetic (NOD) mouse at the level of bone marrow-derived cells. Transfer of diabetes and insulitis to nondiabetic (NOD X B10) F1 mice with bone marrow cells from NOD mice. J Exp Med. 1988 Jun 1;167(6):1801–1810. doi: 10.1084/jem.167.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker L. S., Miller B. J., Coker L. Z., McNally S. E., Scott S., Mullen Y., Appel M. C. Genetic control of diabetes and insulitis in the nonobese diabetic (NOD) mouse. J Exp Med. 1987 Jun 1;165(6):1639–1654. doi: 10.1084/jem.165.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker L. S., Miller B. J., Mullen Y. Transfer of autoimmune diabetes mellitus with splenocytes from nonobese diabetic (NOD) mice. Diabetes. 1986 Aug;35(8):855–860. doi: 10.2337/diab.35.8.855. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Callahan G. N., Klein J., Dennert G. Cytotoxic T cells learn specificity for self H-2 during differentiation in the thymus. Nature. 1978 Jan 19;271(5642):251–253. doi: 10.1038/271251a0. [DOI] [PubMed] [Google Scholar]