Abstract
Brain derived neurotrophic factor (BDNF) is a member of the neurotrophin family that plays a critical role in numerous neuronal activities. Recent studies report that some functions or action mechanisms of BDNF vary in a sex-dependent manner. In particular, BDNF content in some brain parts and the tendency of developing BDNF-deficient-related diseases like depression is higher in female animals. With the support of other relevant studies, it is suggested that sex hormones or steroids can modulate the activities of BDNF, which may account for its functional discrepancy in different sexes. Indeed, the cross-talk between BDNF and sex steroids has been detected for decades and some sex steroids like estrogen have a positive regulatory effect to BDNF expression and signaling. Thus, the sex of animal models used is critical when studying the functions of BDNF in vivo. In this review, we will summarize our current findings on the difference in expression, signaling, and functions of BDNF between sexes. We will also discuss the potential mechanisms in mediating these differential responses with a specific emphasis on sex steroids. By presenting and discussing these findings, we encourage taking sex influences into consideration when designing experiments, interpreting results and drawing conclusions.
Keywords: Brain, BDNF, sex, gene expression, signal transduction
INTRODUCTION
The central nervous system (CNS) is different in structure, activity, development and chemistry between female and male mammals (Forger et al. 2016; Raznahan et al. 2010; Ruigrok et al. 2014). Although these phenomena have long been recognized, the mechanism that causes the differences is still not clear (Cahill 2006). Some of the sex variations, nevertheless, could be explained by the difference in gonadal steroid (androgens in male and estrogens in female) concentration, which are mainly produced in the testicles in male and the ovaries in female mammals. There are three natural estrogens: estrone (E1), 17β-estradiol (E2) and estriol (E3) in female and a major androgen, testosterone, in male mammals. These hormones act on the brain via multiple mechanisms to shape the differences and part of their actions rely on the interaction with other factors/hormones like neurotrophins. Neurotrophins are a group of growth factors that regulates numerous activities of the nervous system. They consist of 4 members: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3) and neurotrophin-4/5 (NT-4/5). BDNF is the second neurotrophin identified, which has the most abundant and widespread expression in both developing and mature mammalian brain (Cunha et al. 2010). To date it has been shown that BDNF is involved in every aspect of neuronal activity including neuronal survival, neurogenesis, dendritic branching, neurotransmitter production and release, synapse formation and maturation, and synaptic plasticity, which indicate that BDNF is crucial for the development and maintenance of the brain’s activities (Park and Poo 2013). In addition to its contribution in normal brain functions, BDNF has been implicated in various neurological disorders. Lower concentration of BDNF in the blood stream or cerebral fluid has been correlated with the prognosis of diseases like Alzheimer’s disease, Parkinson disease, Huntington’s disease, Rett Syndrome, and amyotrophic lateral sclerosis (ALS) (Zuccato and Cattaneo 2009). In fact, administration of BDNF is an effective way to improve the neuronal deficits in a number of animal disease models, suggesting BDNF can be a potential therapeutic agent against various traumas like stroke, spinal cord injury, and aging-related neuronal loss (Nagahara and Tuszynski 2011). However, the therapeutic effect of BDNF can be affected by a lot of factors including time and place of administration, age, and even sex of the recipients. Surprisingly, the sex-specific effect on BDNF expression, signaling or activities has not raised much concern in most of the BDNF-related research, which may affect our analysis on BDNF’s functions or activities. For instance, Li et al. has demonstrated that BDNF injection can alleviate the acetic acid-induced pain response in male rats. In contrast, the pain-killing effect of BDNF was abolished when female rats were used in the examination (Li et al. 2010). Thus, the conclusion drawn from an identical study may be completely different, if a different sex of research model is used. Due to the limited amount of studies performed, the mechanism of how sex differences affect the physiological or therapeutic activity of BDNF is still unclear. This mini-review aims to present the findings on the sex effect on the expression, signaling and functions of BDNF. The possible role of sex hormones in these differential activities will also be discussed, and we will close with a brief discussion on some problems that need to be resolved in the future.
SEX DIFFERENCES IN CIRCULATING BDNF AND BDNF EXPRESSION
BDNF is mainly produced in the CNS, and can be detected in almost every brain part (Conner et al. 1997; Katoh-Semba et al. 1997). When comparing the absolute amount of BDNF in various brain regions between male and female, it is reported that female rats have higher BDNF content in the hippocampus, ventromedial hypothalamus, cortex and amygdala than male rats (Bakos et al. 2009; Bland et al. 2005; Liu et al. 2014; Snigdha et al. 2011). Franklin and Perrot-Sinal also demonstrated a lower level of BDNF in male hippocampus CA3 region (Franklin and Perrot-Sinal 2006). However, the authors also found that the dorsal dentate gyrus of male rats hippocampus has more BDNF than that in the same region of the female brain. (Franklin and Perrot-Sinal 2006). In contrast to rats, male mice have higher BDNF in the hippocampus, cortex and brain stem (Szapacs et al. 2004). In human, there is no significant difference in hippocampal BDNF content between men and women but female subjects have higher BDNF in the prefrontal context (Hayley et al. 2015). Interestingly, sex-dependent expression difference can also be detected in non-mammalian species. For example, male song birds have a higher BDNF level in the high vocal center, which is believed as an important factor to their singing behavior during mating (Rasika et al. 1999). These reports suggest that sex differences on the CNS distribution of BDNF are consistent among various species. Nevertheless, these observations were generated from fragmented and scattered studies, which were not designed primarily to examine the tissue distribution of BDNF in different sexes. Thus, a systematic and comprehensive comparison on the sex- and species-specific BDNF expression in corresponding brain regions should be performed to make a clear picture on this issue.
Besides basal transcription, stress or stimulus-induced BDNF expression also shows significant sex-dependent variations. Compelling evidence on such disparity comes from investigations on the effect of environment enrichment on brain functions, which has been shown to increase neuronal BDNF expression probably by epigenetic modifications (Ickes et al. 2000; Kuzumaki et al. 2011; Vazquez-Sanroman et al. 2013). Specifically, enriched environment induces more BDNF expression in the prefrontal cortex, hippocampus and hypothalamus of female mice (Bakos et al. 2009; Zhu et al. 2006). Chen et al. further demonstrate that environment enrichment only promotes BDNF increase in the female brain after traumatic injury (Chen et al. 2005). Stress-induced responses are also different between male and female. An early stressful event such as isolation, early weaning or mother deprivation decreases BDNF transcription solely in the male hippocampus and hypothalamus (Kikusui et al. 2009; Viveros et al. 2010; Weintraub et al. 2010). In contrast, these maltreatments cause a female-specific increase of BDNF expression in the amygdala (Hill et al. 2014). In a recent study, Liu et al. demonstrate a downregulation of BDNF expression in the male ventromedial hypothalamus (VMH) during fasting (Liu et al. 2014). This observation concurs with the function of BDNF as an anorexic factor to suppress appetite in the hypothalamus (Pelleymounter et al. 1995), which may be associated with the food intake behavioral change in response to food availability as male animals have a higher food-anticipatory activity and food intake after refeeding (Li et al. 2015). There is also a sex-specific response to pharmacological-stimulated BDNF expression in the brain. For example, NMDA receptor antagonist Dizocilpine increases BDNF expression in the cortex of female mice, whereas another NMDA receptor antagonist, Phencyclidine, decreases BDNF expression in a variety of brain regions only in female rats (Matsuki et al. 2001; Snigdha et al. 2011). In contrast, male brains are more responsive to GABA receptor agonist Diazepam and canabionoid agonist CP55940 in reducing hippocampal and hypothalamic BDNF expression (Kellogg et al. 2000; Lopez-Gallardo et al. 2012). It remains to be explored if the sex-specific BDNF expression is responsible for efficacy of these receptor (ant)agonists, which display obvious sex-specific activities (Feinstein and Kritzer 2013; Tseng and Craft 2001)
Although it has been suggested that most BDNF in the blood is released from the CNS (Rasmussen et al. 2009), the differential BDNF expression in male and female brain does not cause divergence in circulating BDNF. Several studies have reported comparable BDNF levels in circulation between men and women at various ages (Bus et al. 2012; Katoh-Semba et al. 2007; Lang et al. 2009; Ziegenhorn et al. 2007). Presumably, the differences in neuronal BDNF expression only affect the autocrine or paracrine activity of BDNF. This autocrine theory is supported by another study performed in skeletal muscle. After electroporating BDNF-overexpressing vector into the tibialis of mouse, Mathews et al. found no increase of serum BDNF concentration in the tested mice, although the BDNF content increased 20-fold in their skeletal muscles (Matthews et al. 2009). Nevertheless, the possibility that different platelet activities, the major reservoir of circulating BDNF (Fujimura et al. 2002), may contribute significantly in normalizing the blood BDNF level in different sexes could not be excluded because the hormone uptake and aggregation activities of platelets are different between male and female (Halbreich et al. 1991; Haque et al. 2001).
SEX-SPECIFIC FUNCTIONS OF BDNF
Because of the sex-specific expression in various conditions, it is expected that BDNF may induce different functions or different degrees of action in particular sexes. Comparative studies using male and female animals with ablated BDNF gene provides some evidence to support this hypothesis. In one of these studies, it is shown that conditional deletion of BDNF gene in the forebrain (cKO) causes hyperactivity in the male mice only. Female BDNF cKO mice, in contrast, display anxiety and depression behaviors, which cannot be detected in male animals (Monteggia et al. 2007). This observation of lower BDNF in female mouse brain causes depressive behaviors is an attractive explanation to the high preponderance of depression in women across cultures (Kuehner 2003). In support of this conclusion, a recent examination performed in postmortem samples from depressed women who died by suicide clearly demonstrate reduced BDNF levels within the frontopolar prefrontal cortex compared to their non-psychiatric controls (Hayley et al. 2015). However, the linkage of BDNF level in the brain and depression has not been strongly established as another study, which analyzes BDNF and its receptor Tropomyosin kinase receptor B (TrkB) expression in postmortem subjects with major depressive disorder does not show any sex-dependent effect in the subgenual anterior cingulate cortex (Tripp et al. 2012).
In contrast to the depression behavior, heterozygous depletion of BDNF gene (BDNF+/−) alleviates the anxiety behavior only in female serotonin transporter knockout (SERT−/−) mice (Ren-Patterson et al. 2006). This study reveals that female SERT−/−BDNF+/− mice have higher serotonin (5-HT) and its metabolites 5-hydroxyindoleacetic acid (5-HIAA) in the hippocampus and hypothalamus, elevated catecholamines [dopamine, dihydroxyphenylacetic acid (DOPAC) and homovanillic acid] in striatum, and augmented hypothalamic TrkB expression than SERT−/−BDNF+/+ mice. Due to an increase in the concentration of 5-HT in the hypothalamus of estradiol implanted male mice, the authors suggest that the availability of estrogen protects female SERT−/−BDNF+/− mice from monoamine depletion and thus reduces the anxiety behavior. In another study performed in neuron-specific BDNF knockout mice, Camerino et al. show that ablation of BDNF in the CNS causes more body adiposity and higher bone mass in female animals (Camerino et al. 2012). Interestingly, these phenotypes show close similarities to that of the neuronal-specific estrogen receptor knockout (ER−/−) mice (Ohlsson et al. 2012). Thus, the authors postulate that BDNF and estrogen pathways may act synergistically in female mice to modulate the skeletal and metabolic phenotypes.
In a series of studies to explore the role of BDNF signaling in various disorders using our newly identified BDNF mimetic 7,8-dihydroxyflavone (7,8-DHF) (Jang et al. 2010), it was found that this small molecule also displays differential pharmacological activities in different sexes. In the first study performed in mice with hypoxia ischemia (HI)-induced brain injury, 7,8-DHF-treatment has a more pronounced protective effect against HI-induced hippocampal neurodegeneration and astrogliosis in female animals (Uluc et al. 2013). A later study confirmed these findings that 7,8-DHF treatment has a higher potency against HI-induced hippocampal apoptosis in female mice (Cikla et al. 2016). This study also found that the protective effect of 7,8-DHF is ablated in estrogen receptor (ER)−/− animals. Together, with other signaling analysis, the authors proposed that post-HI induced ER expression in female hippocampus couples the c-Src kinase to augment the TrkB phosphorylation, thereby reduces the cellular apoptosis and neuronal injury. In our recent study, we found that 7,8-DHF initiates the AMPK/CREB/uncoupling protein 1 (UCP1) pathway to increase the lipid oxidation and energy expenditure in muscle cells (Chan et al. 2015). Concurred with these in vitro findings, administration of 7,8-DHF increased the energy expenditure of mice under high-fat diet (HFD) feeding, thus protecting these animals from gaining excess body weight. It is intriguing to note that the anti-obesity function of 7,8-DHF could only be detected in female mice as HFD feeding induced similar body mass increase in both control and 7,8-DHF administered male mice. While the TrkB-ER cross-talk proposed by Cikla et al. (Cikla et al. 2016) provides an attractive model to explain the sex-dimorphic activities of 7,8-DHF, we should also consider the fact that 7,8-DHF is an aromatase inhibitor (Chen et al. 1997; Kao et al. 1998). Thus, further studies on the role of 7,8-DHF in ER signaling or estrogen production are needed to delineate its sex-dependent activity in both neurological system and peripheral tissues.
SEX-DEPENDENT SIGNALINGS INDUCED BY BDNF
BDNF exerts its biological actions via interaction with its cognate high affinity receptor TrkB. After binding to the transmembrane receptor, BDNF induces TrkB dimerization and autophosphorylation. The phosphorylated residues in TrkB serve as the docking sites for adaptor proteins like Src homology domain 2 (SH2) or polypyrimidine tract-binding protein (PTB), which lead to the activation of phosphoinositide 3-kinase (PI3K)-Akt, mitogen-activated protein kinase (MEK)-extracellular signal regulated kinase (ERK), and phospholipase Cγ1 (PLCγ1)-CRE-responsive element binding protein (CREB) signaling (Longo and Massa 2013). Surprisingly, there is no clear evidence showing a sexual difference in BDNF-induced signaling in cells under physiological conditions, but several indirect studies suggest that female mice have a differential TrkB activation mechanism. Hill and van den Buuse demonstrated a sex-dependent variation in TrkB signaling in BDNF+/− mice. These authors reported that male BDNF+/− mice exhibited higher TrkB phosphorylation than female animals in the frontal cortex and striatum (Hill and van den Buuse 2011). Consequently, the downstream ERK signaling was enhanced in the male BDNF+/− brain. The mechanism that contributes to this differential TrkB signaling between male and female is unknown, but the authors suspect that the sex steroids may a play significant role. In another study that investigates the distribution of phosphorylated (activated)-TrkB in the hippocampus, Spencer-Segal et al. showed that the proesterous female hippocampus CA1 region has more phosphorylated TrkB proteins than the age-matched male (Spencer-Segal et al. 2011). The same research group found in an earlier study that TrkB and the downstream Akt phosphorylations fluctuated during the estrous cycle (Spencer et al. 2008), further confirming estrogen may increase TrkB signaling. In another elegant study to explain the sex-specific pattern of mouse mammary gland development, it is reported that mammary mesenchymal cells express a high level of the truncated TrkB isoform (TrkB-T1) in E13 male embryo in an androgen-dependent manner (Liu et al. 2012). Thus, the BDNF generated by mesenchymal cells in male embryo is sequestered; leading to an inhibition of sensory neuron innervation to the mammary gland, which eventually dampens the development of mammary gland in male mice.
INTERACTION OF SEX HORMONES WITH BDNF EXPRESSION AND SIGNALING
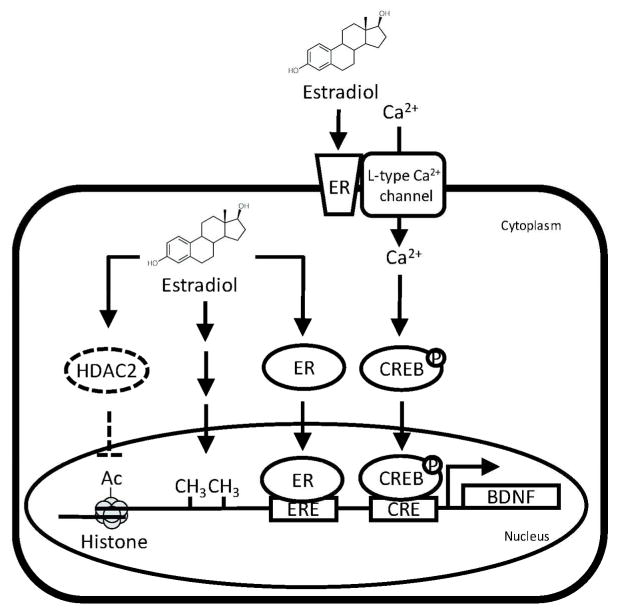
The first linkage between BDNF and sex steroids was reported from a study that showed a co-localization of BDNF and TrkB in the ER mRNA-containing neurons during forebrain development (Toran-Allerand et al. 1992). Subsequent studies performed in animals with ovariectomy reveal that estrogen replacement increases BDNF expression in hippocampus, cortex and olfactory bulb, which confirm the BDNF-inducer activity of estrogen (Solum and Handa 2002). It is now generally accepted that estrogen modulates BDNF expressions through at least four different mechanisms. First of all, estrogen can directly induce BDNF expression by activating ER. This conclusion is supported by the findings that the BDNF gene contains a canonical estrogen response element (Sohrabji et al. 1995), and gel shift and DNA foot-printing assays clearly show that estrogen-ER complexes bind to this sequence in the BDNF gene (Sohrabji et al. 1995). Second, estrogen modifies the activity of BDNF promoter epigenetically as ovariectomy-induced estrogen depletion causes an increase of methylation on BDNF promoters IV and V in the hippocampus (Moreno-Piovano et al. 2014). The mechanism of how estrogen epigenetically controls BDNF expression is obscure, but a recent study reports that estradiol infusion into the hippocampus specifically increases histone H3 acetylation at BDNF promoters II and IV, possibly through decreasing the expression of histone deacetylase 2 (HDAC2) (Fortress et al. 2014). Third, the ER regulates the activity of CREB through non-genomic activity. In cultured hippocampal neurons, estradiol induces an influx of extracellular Ca2+ influx, leading to activation of CREB (Zhao et al. 2005; Zhou et al. 2005). Given that CREB is a major transcription factor that controls BDNF expression in neurons in response to Ca2+ concentration change (Shieh et al. 1998; Tao et al. 1998), it is reasonable to induce that the ER/CREB axis may modulate the expression of BDNF (Fig 1). Lastly, ER controls BDNF expression indirectly via interneuronal activity. Based on a series of immunofluorescent staining results, it is proposed that estrogen modulates BDNF expression in a cortical neuronal circuit that contains the ERβ-bearing neurons, non-ER-β-bearing GABA(γ-aminobutyric acid)ergic interneurons, and the target BDNF producing neurons (Blurton-Jones and Tuszynski 2006). In this model, estrogen increases GABA activity or production within the ERβ-bearing neurons, which inhibits the activity of non-ER-β-bearing GABAergic interneurons. Since GABA is an inhibitory neurotransmitter of BDNF expression (Heese et al. 2000), the reduced GABA production in the interneurons results in an elevation of BDNF transcription in the target cells. This model also provides a possible explanation to the observation that prenatal (gestation days 14–20) exposure to GABA receptor agonist diazepam results in a male-specific reduction of BDNF expression in the hypothalamus (Kellogg et al. 2000), because female fetuses of the same gestational age have slightly higher estrogen levels in the hypothalamus (Konkle and McCarthy 2011).
Fig 1. Regulation of BDNF expression by estrogen.
Estrogen may induce BDNF transcription by initiating the ER/DNA tethering, enhancing CREB activity, increasing histone acetylation, and elevating methylation of BDNF promoter (CRE: cAMP responsive element; CREB: cAMP responsive element binding protein; ER: estrogen receptor; ERE: ER responsive element; HDAC2: histone deacetylase 2).
When compared with estrogen, the effect of androgen on BDNF expression is less studied. However, several studies have reported that testosterone, the major component of androgens, is a positive regulator of BDNF expression or production in the motor neurons of the spinal nucleus of the bulbocavernosus (SNB), hippocampus CA1 and brain homogenate after middle cerebral artery occlusion (Fanaei et al. 2014; Li et al. 2012; Osborne et al. 2007; Ottem et al. 2007). Progesterone, another important sex steroid produced mainly in the ovary, can also increase the production of BDNF in the cortex explant and glial cells (Kaur et al. 2007; Su et al. 2012). Most studies reporting the positive role of progesterone on BDNF expression were performed in injured neurons such as spinal cord injury, traumatic brain injury and ischemic stroke (Cekic et al. 2012; Gonzalez et al. 2005; Jiang et al. 2016). Based on the result of these studies, it has been suggested that progesterone-mediated neuroprotection is BDNF-dependent. Indeed, inhibition of TrkB activity by the kinase inhibitor K252a abolishes the protective effects of progesterone against glutamate-induced neurotoxicity (Kaur et al. 2007). Interestingly, it is also showed that progesterone attenuated the estrogen-induced BDNF and TrkB expression, although both hormones are BDNF expression enhancers (Aguirre and Baudry 2009; Bimonte-Nelson et al. 2004). The physiological significance of the contradictory effect of progesterone is unknown but it suggests that there is a complex interaction network between sex-steroids in regulating BDNF expression.
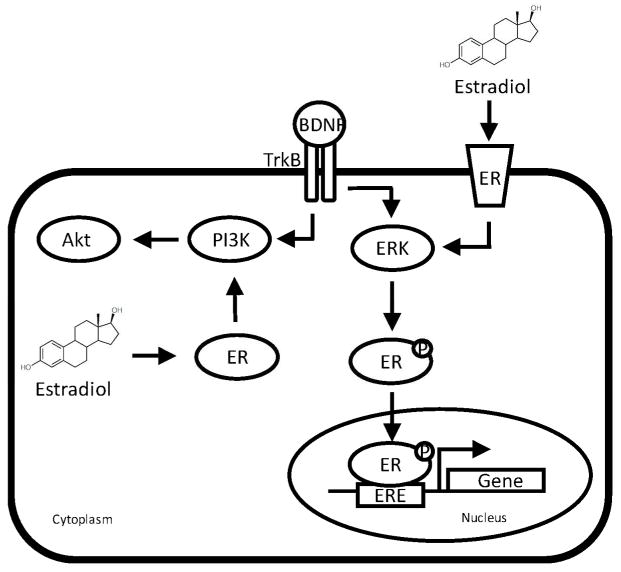
While the relationship between sex steroids and BDNF expression is evident, the interaction between steroid signaling and BDNF/TrkB pathway is largely unexplored. A recent study reported that overexpression of TrkB in Chinese Hamster Ovary (CHO) cells induced transcription of estrogen responsive elements (ERE)-containing reporter by increasing ERα phosphorylation through ERK (Wong et al. 2011), which suggested that the BDNF/TrkB and ER signaling may cross-talk with each other somehow. However, this study does not provide any evidence on the endogenous protein association, which raises a concern of non-specific interaction due to protein over-expression. Nevertheless, estrogen and BDNF trigger several common pathways such as Akt, ERK and CREB individually in neurons (Belcher et al. 2005; Spencer-Segal et al. 2012), which provides indirect evidence to support the TrkB-ER signaling cross-talk (Fig 2). While there is no study performed to investigate if these two factors provoke an additive or synergistic effect in neurons, it has been suggested that estrogen-mediated synaptogenesis and neuroprotection is through BDNF release (Krizsan-Agbas et al. 2003; Sato et al. 2007). This hypothesis is supported by a study using co-cultured uteri from wild-type or BDNF knockout mice with the rat sympathetic ganglia. In this study, the neuritogenesis induced by wild-type uteri was diminished in the presence of estrogen. However, neurite formation in the presence of BDNF−/− uteri was not affected by estrogen, indicating that estrogen alters neuritogenic properties of the rodent uterus by regulating BDNF synthesis (Krizsan-Agbas et al. 2003). In another study using BDNF heterozygous (+/−) mice, it is reported that estradiol replacement improved the performance on Y-maze and novel-object recognition test in ovariectomized wild-type but not in ovariectomized BDNF+/− mice (Wu et al. 2015). Thus, the integrity of BDNF/TrkB signaling is essential to some estrogen-mediated neurological action but how these two pathways interact with each other is still mysterious.
Fig 2.
Common signaling pathways of BDNF and estrogen.
CONCLUDING REMARK
It is now clear that sex difference is more widespread than one may assume in different physiological and pathological conditions. The diversity in body weight and composition, hormonal status, and enzyme activities between male and female interact and contribute to these differences. Since most neuroscience research has been performed using single sex animals, our understanding on the sexual differences in neurological signaling, especially those induced by BDNF, is at the early stage and most information that we have is fragmented and inconclusive. There are still many areas that require further elucidation. An example is to compare if BDNF initiates differential signaling cascades in male and females under physiological or pathological settings. Because a majority of studies were conducted to measure the functional outcome of BDNF administration in animals without elucidating the molecular mechanism or examining the cellular details, we do not have a clear picture on how BDNF causes the differential activities in different sexes. These data are critical for us to better assess the efficacy of employing BDNF therapy in human patients. Another unresolved question lies on sex steroids/TrkB signaling cross-talk. It is also necessary to determine if different sex steroids alter the signaling cascades induced by BDNF, in addition to their role in manipulating BDNF expression. Examining the functional effect of BDNF administration in animals lacking the sex steroid receptors would provide some insights. Without a full understanding on the sex-specific effect, special attention should be made when interpreting the data from studies performed in either male or female animals to conclude the overall activity of BDNF. This information is particularly important when translating the findings into BDNF-based therapeutic strategies in various neurodegenerative, psychiatric and metabolic disorders. Thus, determining and delineating the sex-specific activities of BDNF in both physiological and pathological conditions is highly warranted.
SIGNIFICANCE.
Sex influence on brain function is now being spotlighted in the field of neuroscience. As an important factor that plays a critical role in numerous neuronal activities, the effect of sex difference on the activity of brain-derived neurotrophic factor (BDNF) has not been systemically studied or reviewed. The current article aims to integrate the scattered findings on sex differences in BDNF research and discuss their potential effects on our understanding on BDNF’s functions.
Acknowledgments
This work is supported by grants from National Institute of Health (R01DK097092, P20GM104934), Presbyterian Health Foundation (Seed Grant) and Oklahoma Center for the Advancement of Science and Technology (OCAST HR14-117) to C.B. Chan, and NIH R21 AG050793; RO1 AG051538 to K. Ye. We are also thankful to Mr. Daniel Brobst for his critical reading of the manuscript.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHORS’ CONTRIBUTIONS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drafting of the manuscript: CBC and KY. Critical revision of the manuscript for important intellectual content: CBC and KY. Obtained funding: CBC and KY.
References
- Aguirre CC, Baudry M. Progesterone reverses 17beta-estradiol-mediated neuroprotection and BDNF induction in cultured hippocampal slices. Eur J Neurosci. 2009;29(3):447–454. doi: 10.1111/j.1460-9568.2008.06591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakos J, Hlavacova N, Rajman M, Ondicova K, Koros C, Kitraki E, Steinbusch HW, Jezova D. Enriched environment influences hormonal status and hippocampal brain derived neurotrophic factor in a sex dependent manner. Neuroscience. 2009;164(2):788–797. doi: 10.1016/j.neuroscience.2009.08.054. [DOI] [PubMed] [Google Scholar]
- Belcher SM, Le HH, Spurling L, Wong JK. Rapid estrogenic regulation of extracellular signal- regulated kinase 1/2 signaling in cerebellar granule cells involves a G protein- and protein kinase A-dependent mechanism and intracellular activation of protein phosphatase 2A. Endocrinology. 2005;146(12):5397–5406. doi: 10.1210/en.2005-0564. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Nelson ME, Granholm AC. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004;15(17):2659–2663. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- Bland ST, Schmid MJ, Der-Avakian A, Watkins LR, Spencer RL, Maier SF. Expression of c-fos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain Res. 2005;1051(1–2):90–99. doi: 10.1016/j.brainres.2005.05.065. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Tuszynski MH. Estradiol-induced modulation of estrogen receptor-beta and GABA within the adult neocortex: a potential transsynaptic mechanism for estrogen modulation of BDNF. J Comp Neurol. 2006;499(4):603–612. doi: 10.1002/cne.21122. [DOI] [PubMed] [Google Scholar]
- Bus BA, Arias-Vasquez A, Franke B, Prickaerts J, de Graaf J, Voshaar RC. Increase in serum brain-derived neurotrophic factor in met allele carriers of the BDNF Val66Met polymorphism is specific to males. Neuropsychobiology. 2012;65(4):183–187. doi: 10.1159/000336997. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7(6):477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Camerino C, Zayzafoon M, Rymaszewski M, Heiny J, Rios M, Hauschka PV. Central depletion of brain-derived neurotrophic factor in mice results in high bone mass and metabolic phenotype. Endocrinology. 2012;153(11):5394–5405. doi: 10.1210/en.2012-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekic M, Johnson SJ, Bhatt VH, Stein DG. Progesterone treatment alters neurotrophin/proneurotrophin balance and receptor expression in rats with traumatic brain injury. Restor Neurol Neurosci. 2012;30(2):115–126. doi: 10.3233/RNN-2011-0628. [DOI] [PubMed] [Google Scholar]
- Chan CB, Tse MC, Liu X, Zhang S, Schmidt R, Otten R, Liu L, Ye K. Activation of muscular TrkB by its small molecular agonist 7,8-dihydroxyflavone sex-dependently regulates energy metabolism in diet-induced obese mice. Chem Biol. 2015;22(3):355–368. doi: 10.1016/j.chembiol.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Kao YC, Laughton CA. Binding characteristics of aromatase inhibitors and phytoestrogens to human aromatase. J Steroid Biochem Mol Biol. 1997;61(3–6):107–115. [PubMed] [Google Scholar]
- Chen X, Li Y, Kline AE, Dixon CE, Zafonte RD, Wagner AK. Gender and environmental effects on regional brain-derived neurotrophic factor expression after experimental traumatic brain injury. Neuroscience. 2005;135(1):11–17. doi: 10.1016/j.neuroscience.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Cikla U, Chanana V, Kintner DB, Udho E, Eickhoff J, Sun W, Marquez S, Covert L, Otles A, Shapiro RA, Ferrazzano P, Vemuganti R, Levine JE, Cengiz P. ERalpha Signaling Is Required for TrkB-Mediated Hippocampal Neuroprotection in Female Neonatal Mice after Hypoxic Ischemic Encephalopathy(1,2,3) eNeuro. 2016;3(1) doi: 10.1523/ENEURO.0025-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17(7):2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front Mol Neurosci. 2010;3:1. doi: 10.3389/neuro.02.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanaei H, Karimian SM, Sadeghipour HR, Hassanzade G, Kasaeian A, Attari F, Khayat S, Ramezani V, Javadimehr M. Testosterone enhances functional recovery after stroke through promotion of antioxidant defenses, BDNF levels and neurogenesis in male rats. Brain Res. 2014;1558:74–83. doi: 10.1016/j.brainres.2014.02.028. [DOI] [PubMed] [Google Scholar]
- Feinstein I, Kritzer MF. Acute N-methyl-D-aspartate receptor hypofunction induced by MK801 evokes sex-specific changes in behaviors observed in open-field testing in adult male and proestrus female rats. Neuroscience. 2013;228:200–214. doi: 10.1016/j.neuroscience.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Strahan JA, Castillo-Ruiz A. Cellular and molecular mechanisms of sexual differentiation in the mammalian nervous system. Front Neuroendocrinol. 2016 doi: 10.1016/j.yfrne.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Kim J, Poole RL, Gould TJ, Frick KM. 17beta-Estradiol regulates histone alterations associated with memory consolidation and increases Bdnf promoter acetylation in middle-aged female mice. Learn Mem. 2014;21(9):457–467. doi: 10.1101/lm.034033.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Perrot-Sinal TS. Sex and ovarian steroids modulate brain-derived neurotrophic factor (BDNF) protein levels in rat hippocampus under stressful and non-stressful conditions. Psychoneuroendocrinology. 2006;31(1):38–48. doi: 10.1016/j.psyneuen.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, Sun B, Tandon NN. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87(4):728–734. [PubMed] [Google Scholar]
- Gonzalez SL, Labombarda F, Gonzalez Deniselle MC, Mougel A, Guennoun R, Schumacher M, De Nicola AF. Progesterone neuroprotection in spinal cord trauma involves up-regulation of brain-derived neurotrophic factor in motoneurons. J Steroid Biochem Mol Biol. 2005;94(1–3):143–149. doi: 10.1016/j.jsbmb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Rojansky N, Zander KJ, Barkai A. Influence of age, sex and diurnal variability on imipramine receptor binding and serotonin uptake in platelets of normal subjects. J Psychiatr Res. 1991;25(1–2):7–18. doi: 10.1016/0022-3956(91)90012-y. [DOI] [PubMed] [Google Scholar]
- Haque SF, Matsubayashi H, Izumi S, Sugi T, Arai T, Kondo A, Makino T. Sex difference in platelet aggregation detected by new aggregometry using light scattering. Endocr J. 2001;48(1):33–41. doi: 10.1507/endocrj.48.33. [DOI] [PubMed] [Google Scholar]
- Hayley S, Du L, Litteljohn D, Palkovits M, Faludi G, Merali Z, Poulter MO, Anisman H. Gender and brain regions specific differences in brain derived neurotrophic factor protein levels of depressed individuals who died through suicide. Neurosci Lett. 2015;600:12–16. doi: 10.1016/j.neulet.2015.05.052. [DOI] [PubMed] [Google Scholar]
- Heese K, Otten U, Mathivet P, Raiteri M, Marescaux C, Bernasconi R. GABA(B) receptor antagonists elevate both mRNA and protein levels of the neurotrophins nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) but not neurotrophin-3 (NT-3) in brain and spinal cord of rats. Neuropharmacology. 2000;39(3):449–462. doi: 10.1016/s0028-3908(99)00166-5. [DOI] [PubMed] [Google Scholar]
- Hill KT, Warren M, Roth TL. The influence of infant-caregiver experiences on amygdala Bdnf, OXTr, and NPY expression in developing and adult male and female rats. Behav Brain Res. 2014;272:175–180. doi: 10.1016/j.bbr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, van den Buuse M. Sex-dependent and region-specific changes in TrkB signaling in BDNF heterozygous mice. Brain Res. 2011;1384:51–60. doi: 10.1016/j.brainres.2011.01.060. [DOI] [PubMed] [Google Scholar]
- Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol. 2000;164(1):45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107(6):2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Zuo F, Wang Y, Lu H, Yang Q, Wang J. Progesterone Changes VEGF and BDNF Expression and Promotes Neurogenesis After Ischemic Stroke. Mol Neurobiol. 2016 doi: 10.1007/s12035-015-9651-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao YC, Zhou C, Sherman M, Laughton CA, Chen S. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: A site-directed mutagenesis study. Environ Health Perspect. 1998;106(2):85–92. doi: 10.1289/ehp.9810685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh-Semba R, Takeuchi IK, Semba R, Kato K. Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. J Neurochem. 1997;69(1):34–42. doi: 10.1046/j.1471-4159.1997.69010034.x. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Wakako R, Komori T, Shigemi H, Miyazaki N, Ito H, Kumagai T, Tsuzuki M, Shigemi K, Yoshida F, Nakayama A. Age-related changes in BDNF protein levels in human serum: differences between autism cases and normal controls. Int J Dev Neurosci. 2007;25(6):367–372. doi: 10.1016/j.ijdevneu.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Kaur P, Jodhka PK, Underwood WA, Bowles CA, de Fiebre NC, de Fiebre CM, Singh M. Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res. 2007;85(11):2441–2449. doi: 10.1002/jnr.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg CK, Yao J, Pleger GL. Sex-specific effects of in utero manipulation of GABA(A) receptors on pre- and postnatal expression of BDNF in rats. Brain Res Dev Brain Res. 2000;121(2):157–167. doi: 10.1016/s0165-3806(00)00039-0. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Ichikawa S, Mori Y. Maternal deprivation by early weaning increases corticosterone and decreases hippocampal BDNF and neurogenesis in mice. Psychoneuroendocrinology. 2009;34(5):762–772. doi: 10.1016/j.psyneuen.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Konkle AT, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 2011;152(1):223–235. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizsan-Agbas D, Pedchenko T, Hasan W, Smith PG. Oestrogen regulates sympathetic neurite outgrowth by modulating brain derived neurotrophic factor synthesis and release by the rodent uterus. Eur J Neurosci. 2003;18(10):2760–2768. doi: 10.1111/j.1460-9568.2003.03029.x. [DOI] [PubMed] [Google Scholar]
- Kuehner C. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr Scand. 2003;108(3):163–174. doi: 10.1034/j.1600-0447.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- Kuzumaki N, Ikegami D, Tamura R, Hareyama N, Imai S, Narita M, Torigoe K, Niikura K, Takeshima H, Ando T, Igarashi K, Kanno J, Ushijima T, Suzuki T. Hippocampal epigenetic modification at the brain-derived neurotrophic factor gene induced by an enriched environment. Hippocampus. 2011;21(2):127–132. doi: 10.1002/hipo.20775. [DOI] [PubMed] [Google Scholar]
- Lang UE, Hellweg R, Sander T, Gallinat J. The Met allele of the BDNF Val66Met polymorphism is associated with increased BDNF serum concentrations. Mol Psychiatry. 2009;14(2):120–122. doi: 10.1038/mp.2008.80. [DOI] [PubMed] [Google Scholar]
- Li F, Zhang JW, Wei R, Luo XG, Zhang JY, Zhou XF, Li CQ, Dai RP. Sex-differential modulation of visceral pain by brain derived neurotrophic factor (BDNF) in rats. Neurosci Lett. 2010;478(3):184–187. doi: 10.1016/j.neulet.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Li M, Masugi-Tokita M, Takanami K, Yamada S, Kawata M. Testosterone has sublayer-specific effects on dendritic spine maturation mediated by BDNF and PSD-95 in pyramidal neurons in the hippocampus CA1 area. Brain Res. 2012;1484:76–84. doi: 10.1016/j.brainres.2012.09.028. [DOI] [PubMed] [Google Scholar]
- Li Z, Wang Y, Sun KK, Wang K, Sun ZS, Zhao M, Wang J. Sex-related difference in food-anticipatory activity of mice. Horm Behav. 2015;70:38–46. doi: 10.1016/j.yhbeh.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhu Z, Kalyani M, Janik JM, Shi H. Effects of energy status and diet on Bdnf expression in the ventromedial hypothalamus of male and female rats. Physiol Behav. 2014;130:99–107. doi: 10.1016/j.physbeh.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Rutlin M, Huang S, Barrick CA, Wang F, Jones KR, Tessarollo L, Ginty DD. Sexually dimorphic BDNF signaling directs sensory innervation of the mammary gland. Science. 2012;338(6112):1357–1360. doi: 10.1126/science.1228258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo FM, Massa SM. Small-molecule modulation of neurotrophin receptors: a strategy for the treatment of neurological disease. Nat Rev Drug Discov. 2013;12(7):507–525. doi: 10.1038/nrd4024. [DOI] [PubMed] [Google Scholar]
- Lopez-Gallardo M, Lopez-Rodriguez AB, Llorente-Berzal A, Rotllant D, Mackie K, Armario A, Nadal R, Viveros MP. Maternal deprivation and adolescent cannabinoid exposure impact hippocampal astrocytes, CB1 receptors and brain-derived neurotrophic factor in a sexually dimorphic fashion. Neuroscience. 2012;204:90–103. doi: 10.1016/j.neuroscience.2011.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki H, Shirayama Y, Hashimoto K, Tanaka A, Minabe Y. Effects of age and gender on the expression of brain-derived neurotrophic factor mRNA in rat retrosplenial cortex following administration of dizocilpine. Neuropsychopharmacology. 2001;25(2):258–266. doi: 10.1016/S0893-133X(00)00246-3. [DOI] [PubMed] [Google Scholar]
- Matthews VB, Astrom MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O, Akerstrom T, Yfanti C, Broholm C, Mortensen OH, Penkowa M, Hojman P, Zankari A, Watt MJ, Bruunsgaard H, Pedersen BK, Febbraio MA. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52(7):1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61(2):187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Moreno-Piovano GS, Varayoud J, Luque EH, Ramos JG. Long-term ovariectomy increases BDNF gene methylation status in mouse hippocampus. J Steroid Biochem Mol Biol. 2014;144(Pt B):243–252. doi: 10.1016/j.jsbmb.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10(3):209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Engdahl C, Borjesson AE, Windahl SH, Studer E, Westberg L, Eriksson E, Koskela A, Tuukkanen J, Krust A, Chambon P, Carlsten H, Lagerquist MK. Estrogen receptor-alpha expression in neuronal cells affects bone mass. Proc Natl Acad Sci U S A. 2012;109(3):983–988. doi: 10.1073/pnas.1111436109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne MC, Verhovshek T, Sengelaub DR. Androgen regulates trkB immunolabeling in spinal motoneurons. J Neurosci Res. 2007;85(2):303–309. doi: 10.1002/jnr.21122. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Beck LA, Jordan CL, Breedlove SM. Androgen-dependent regulation of brain-derived neurotrophic factor and tyrosine kinase B in the sexually dimorphic spinal nucleus of the bulbocavernosus. Endocrinology. 2007;148(8):3655–3665. doi: 10.1210/en.2007-0308. [DOI] [PubMed] [Google Scholar]
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14(1):7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp Neurol. 1995;131(2):229–238. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22(1):53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH, Pedersen BK, Pilegaard H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94(10):1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, Addington A, Gogtay N, Rapoport JL, Giedd JN. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc Natl Acad Sci U S A. 2010;107(39):16988–16993. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren-Patterson RF, Cochran LW, Holmes A, Lesch KP, Lu B, Murphy DL. Gender-dependent modulation of brain monoamines and anxiety-like behaviors in mice with genetic serotonin transporter and BDNF deficiencies. Cell Mol Neurobiol. 2006;26(4–6):755–780. doi: 10.1007/s10571-006-9048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K. beta-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res. 2007;1150:108–120. doi: 10.1016/j.brainres.2007.02.093. [DOI] [PubMed] [Google Scholar]
- Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20(4):727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Snigdha S, Neill JC, McLean SL, Shemar GK, Cruise L, Shahid M, Henry B. Phencyclidine (PCP)-induced disruption in cognitive performance is gender-specific and associated with a reduction in brain-derived neurotrophic factor (BDNF) in specific regions of the female rat brain. J Mol Neurosci. 2011;43(3):337–345. doi: 10.1007/s12031-010-9447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92(24):11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22(7):2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Segal JL, Tsuda MC, Mattei L, Waters EM, Romeo RD, Milner TA, McEwen BS, Ogawa S. Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience. 2012;202:131–146. doi: 10.1016/j.neuroscience.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Segal JL, Waters EM, Bath KG, Chao MV, McEwen BS, Milner TA. Distribution of phosphorylated TrkB receptor in the mouse hippocampal formation depends on sex and estrous cycle stage. J Neurosci. 2011;31(18):6780–6790. doi: 10.1523/JNEUROSCI.0910-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, McEwen BS. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience. 2008;155(4):1106–1119. doi: 10.1016/j.neuroscience.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C, Cunningham RL, Rybalchenko N, Singh M. Progesterone increases the release of brain-derived neurotrophic factor from glia via progesterone receptor membrane component 1 (Pgrmc1)-dependent ERK5 signaling. Endocrinology. 2012;153(9):4389–4400. doi: 10.1210/en.2011-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szapacs ME, Mathews TA, Tessarollo L, Ernest Lyons W, Mamounas LA, Andrews AM. Exploring the relationship between serotonin and brain-derived neurotrophic factor: analysis of BDNF protein and extraneuronal 5-HT in mice with reduced serotonin transporter or BDNF expression. J Neurosci Methods. 2004;140(1–2):81–92. doi: 10.1016/j.jneumeth.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20(4):709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Miranda RC, Bentham WD, Sohrabji F, Brown TJ, Hochberg RB, MacLusky NJ. Estrogen receptors colocalize with low-affinity nerve growth factor receptors in cholinergic neurons of the basal forebrain. Proc Natl Acad Sci U S A. 1992;89(10):4668–4672. doi: 10.1073/pnas.89.10.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry. 2012;169(11):1194–1202. doi: 10.1176/appi.ajp.2012.12020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AH, Craft RM. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol. 2001;430(1):41–47. doi: 10.1016/s0014-2999(01)01267-5. [DOI] [PubMed] [Google Scholar]
- Uluc K, Kendigelen P, Fidan E, Zhang L, Chanana V, Kintner D, Akture E, Song C, Ye K, Sun D, Ferrazzano P, Cengiz P. TrkB receptor agonist 7, 8 dihydroxyflavone triggers profound gender- dependent neuroprotection in mice after perinatal hypoxia and ischemia. CNS Neurol Disord Drug Targets. 2013;12(3):360–370. doi: 10.2174/18715273113129990061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Sanroman D, Sanchis-Segura C, Toledo R, Hernandez ME, Manzo J, Miquel M. The effects of enriched environment on BDNF expression in the mouse cerebellum depending on the length of exposure. Behav Brain Res. 2013;243:118–128. doi: 10.1016/j.bbr.2012.12.047. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Diaz F, Mateos B, Rodriguez N, Chowen JA. Maternal deprivation induces a rapid decline in circulating leptin levels and sexually dimorphic modifications in hypothalamic trophic factors and cell turnover. Horm Behav. 2010;57(4–5):405–414. doi: 10.1016/j.yhbeh.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- Wong J, Woon HG, Weickert CS. Full length TrkB potentiates estrogen receptor alpha mediated transcription suggesting convergence of susceptibility pathways in schizophrenia. Mol Cell Neurosci. 2011;46(1):67–78. doi: 10.1016/j.mcn.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Wu YW, Du X, van den Buuse M, Hill RA. Analyzing the influence of BDNF heterozygosity on spatial memory response to 17beta-estradiol. Transl Psychiatry. 2015;5:e498. doi: 10.1038/tp.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Chen S, Ming Wang J, Brinton RD. 17beta-estradiol induces Ca2+ influx, dendritic and nuclear Ca2+ rise and subsequent cyclic AMP response element-binding protein activation in hippocampal neurons: a potential initiation mechanism for estrogen neurotrophism. Neuroscience. 2005;132(2):299–311. doi: 10.1016/j.neuroscience.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhang H, Cohen RS, Pandey SC. Effects of estrogen treatment on expression of brain-derived neurotrophic factor and cAMP response element-binding protein expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendocrinology. 2005;81(5):294–310. doi: 10.1159/000088448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SW, Yee BK, Nyffeler M, Winblad B, Feldon J, Mohammed AH. Influence of differential housing on emotional behaviour and neurotrophin levels in mice. Behav Brain Res. 2006;169(1):10–20. doi: 10.1016/j.bbr.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Ziegenhorn AA, Schulte-Herbruggen O, Danker-Hopfe H, Malbranc M, Hartung HD, Anders D, Lang UE, Steinhagen-Thiessen E, Schaub RT, Hellweg R. Serum neurotrophins--a study on the time course and influencing factors in a large old age sample. Neurobiol Aging. 2007;28(9):1436–1445. doi: 10.1016/j.neurobiolaging.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5(6):311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]