Abstract
In this randomized blinded study, we investigated caffeine 5 mg/kg treatment given directly after neonatal brain hypoxia ischemia. Brain morphology, behavior and key brain infiltrating immune populations were examined. Caffeine treatment significantly improves outcome when compared to phosphate buffered saline. Flow cytometric analysis of immune responses revealed no persistent immunological alterations. Given its safety caffeine emerges as a candidate for neuroprotective intervention after neonatal brain injury.
Introduction
Neuroprotective strategies are needed for neonatal hypoxia ischemia brain injury (HI). Mild hypothermia is currently the only available treatment in widespread clinical use [1], though difficult to achieve in low income countries. Thus, an affordable, technically uncomplicated alternative is highly desirable. When caffeine was given to treat apneas cerebral palsy significantly diminished [2], suggesting that caffeine might be a candidate for the treatment of brain HI.
Much is published concerning the risk of adverse effects of caffeine use. Dosage is of major importance since caffeine is a competitive inhibitor of adenosine A1, A2A and A2B receptors at lower doses whereas higher concentrations inhibit phosphodiesterase, responsible for many of the adverse effects reported [3]. Available prospective randomized studies of perinatal use have been unable to show adverse effects of clinically relevant doses of caffeine [3].
We investigated morphological, behavioral and immunological outcomes after HI in neonatal mice randomized to caffeine or phosphate buffered saline (PBS) treatment. Since the nature of caffeine's immunomodulatory effects has been questioned [4], a broad spectrum of brain and spleen immune cell populations were investigated.
Material and Methods
All experiments were approved by the regional ethics committee, Stockholms norra djurförsöksetiska nämnd, in accordance with local institutional guidelines and the Directive 2010/63/EU.
Hypoxic ischemic brain injury
The modified Vannucci model was used and evaluated with morphology and behavioral tests. There was no mortality, severe illness or need for early euthanasia. WT C57/bl6 specific pathogen free (SPF) mice (Charles River Laboratories) of either sex were used, with free access to pelleted food and housed in open cages with standard enrichment with daily monitoring of the animals in accordance with local institutional guidelines. Pups were kept with their mother at all times except during surgery and hypoxia. Randomly allocated (https://www.random.org), single-dose caffeine 5 mg/kg or PBS was administered i.p. directly after HI (n = 29 and n = 13 respectively). Evaluations were performed by investigators blinded to treatment. Animals were sacrificed by injection of 240 mg/kg sodium pentobarbiturate i.p. and cardiac perfusion with 12 mL phosphate buffered saline (PBS) to remove intravascular blood cells.
HI was performed at postnatal day 10. Unilateral electrocoagulation at 8 Watt of the right carotid artery was carried out via midline neck incision under isoflurane sedation and local bupivacaine infiltration to minimize pain and distress. Pups rested one hour with the dam after surgery, prior to one hour of 10% O2 in 90% N2 at 36˚C skin temperature. During sham operation the carotid artery was visualized as described above but not electrocoagulated.
Immunohistochemistry
Brains were collected after behavioral evaluation in the Caffeine or PBS treatment groups (n = 29 and n = 13 respectively) at postnatal day 24, two weeks after HI. In accordance with previous studies [5], three levels of each brain were collected, corresponding to bregma 1.3 mm, -1.64 mm and -2.92 mm in the adult mouse brain using cryostat 10–12 μm sections. Tissue sections were fixed for 10 min in 4% paraformaldehyde. Endogenous peroxidase was blocked by 0.3% H2O2 in 3% Normal Horse Serum for 10 min. Microtubule associated protein-2 (MAP-2) staining VECTOR® M.O.M.™ Immunodetection Kit and VECTASTAIN® Elite ABC-Peroxidase Kit were used according to the manufacturer’s specifications. The enzymatic coloration of immunoreactivity was performed by simultaneous full immersion in 3,3´-diaminobenzidine, DAB for all slides. Imaging was performed with a Nikon eclipse E800 microscope with an Olympus DP70 camera with DP controller, and version 3.1.1.267 acquisition software. Percent MAP-2 stained area and infarction size were manually delineated using Adobe Photoshop version 12.0.4 in brain slices with investigators blinded to genotype and treatment. Atrophy was calculated as the remaining tissue loss not described by the infarction area delineated by loss of MAP-2 compared to the uninjured hemisphere using the formula 1-(Damaged hemisphere area including infarction area)/(Undamaged hemisphere area) [6].
Behavioral tests
Behavioral assessments were done at 24 days age, two weeks after HI in caffeine or PBS treated animals (n = 29 and n = 13 respectively). Open Field test (Kungsbacka Mät- och Reglerteknik AB, Fjärås, Sweden) was used to investigate explorative behavior during 30 min. The animals were taken directly from their home litters and put in the open field box without further handling. In addition, the beam walking test, where mice walk three times back and forth on a beam (10 mm wide, 600 mm long), was performed directly after the open field test. Mice were handled with care to assure a calm explorative state of mind in the animal, and the number of slips with the hind limbs recorded by investigator blinded to treatment group.
2.6 FACS analysis
Randomization to caffeine or PBS treatment and sham or HI operation was performed at postnatal day 10 and samples were analyzed at 24h, 72h and 2 weeks after HI (total n = 52, treatment allocation is discernible in Fig 2).
Damaged and undamaged brain hemispheres were gently homogenized using a loose fit glass homogenizer, and cell suspension passed through a 100um cell strainer prior to staining with fluorochrome conjugated antibodies. Splenocyte cell suspensions were prepared by gentle homogenization through a 100um cell strainer followed by red blood cell lysis with ACK buffer for 5 min at room temperature. For antibodies used see S2 Table. Flow cytometric data was collected on a FACS Aria (BD Biosciences). 2.5x106 events were collected from each brain hemisphere.
A data driven gating strategy was used with the FlowCore package for R [7] as previously described in detail [8]. Debris was manually removed using a polygon gate in forward and side scatter. A curvfilter was used to filter out immune cell populations. Dead cells were excluded using DAPI stain. Negative population was selected as reference and cells with higher expression than that were regarded as positive cells. Median fluorescent intensities of the selected populations were used to discriminate between positive and negative populations. The same filter settings were used for each marker in all brain samples. After automated batching, all gate settings were visually confirmed and no manual adjustments were required.
Statistical analysis
Mann-Whitney U test was used to analyze data on morphology and behavior data. Statistical calculations were made in StatSoft, Inc. (2011) STATISTICA, version 10. Heatmaps were made in R version 2.15.
Results
A single dose caffeine 5 mg/kg administered directly after neonatal HI significantly decreased atrophy by 44% (p<0.05) defined by MAP-2 staining compared to PBS treatment (Fig 1A), paralleled by a borderline significant (p = 0.05) increase in time spent on the Rotarod (Fig 1B), indicating better balance and coordination. In addition, open field test activity significantly decreases after caffeine treatment compared to controls (p<0.01), signifying improved habituation (Fig 1C). Thus, caffeine given directly after HI is neuroprotective since improved neurological outcome was demonstrated in treated neonatal mice.
Fig 1. Improved outcome in caffeine treated animals compared to controls (n = 29 and n = 13 respectively).
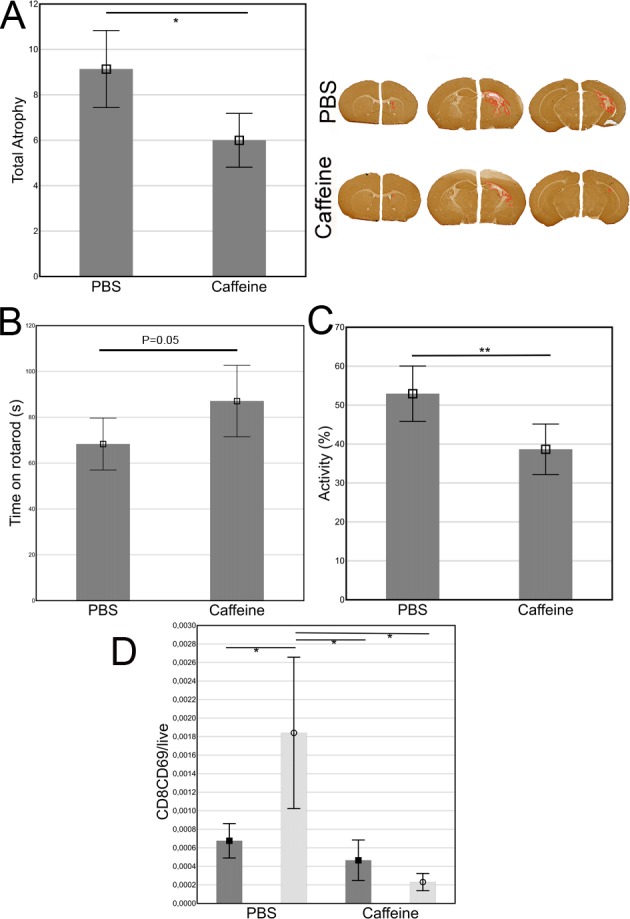
A) Brain infarction was delineated by MAP-2 and atrophy calculated as 1-(Damaged hemisphere area including infarction area) / (Undamaged hemisphere area). The depicted brains are from the animals with injury size close to mean in each group respectively. B) Time spent on the Rotarod. C) Activity and velocity in the open field test was significantly decreased in the caffeine treated group. D) The only significantly altered population after caffeine compared to PBS treatment. Dark bars and squares represent sham operation and light bars with circles HI.
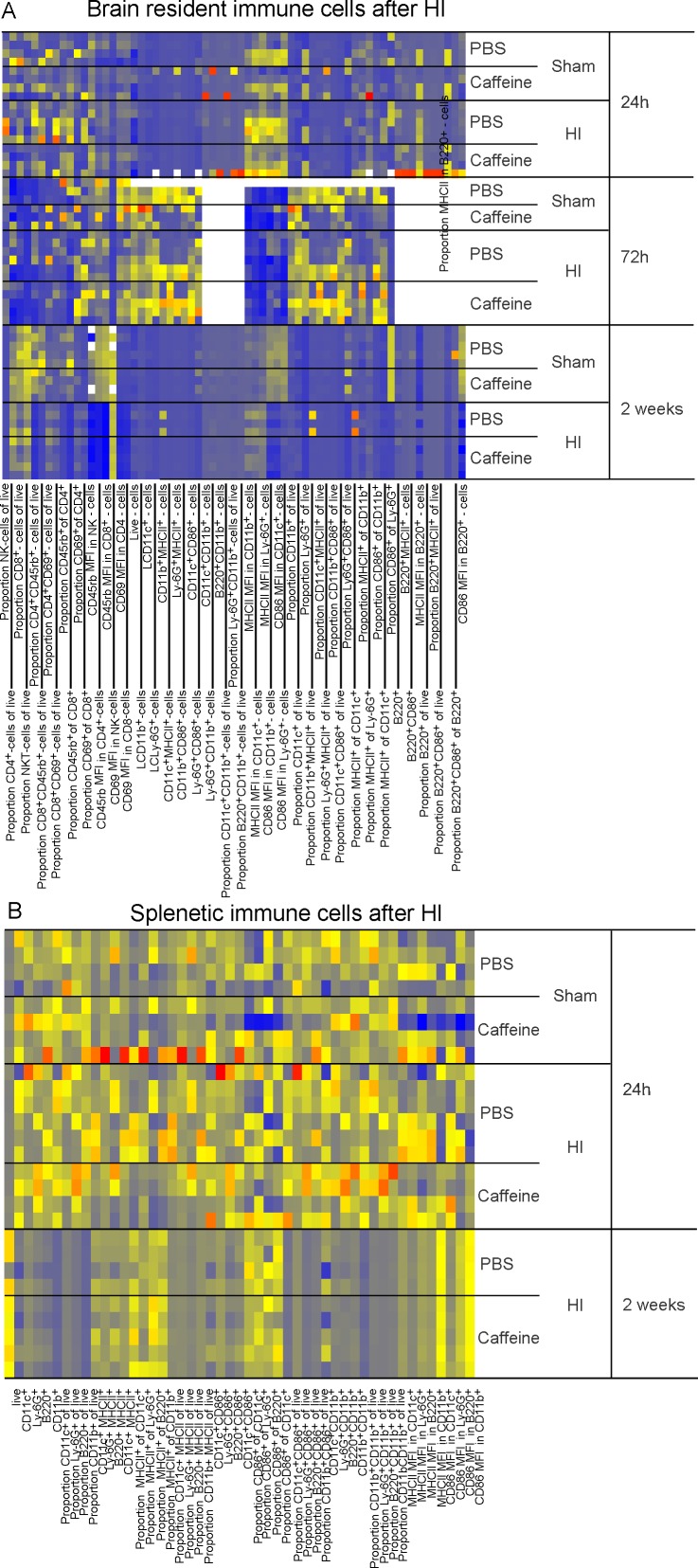
Local and systemic immune populations investigated 24h, 72h and two weeks after HI, revealed surprisingly few alterations considering the decrease in atrophy and improved outcome (Fig 2). Only the activated CD69+CD8+ T-lymphocyte population was significantly reduced in the brain 24h after caffeine treatment compared to PBS (Fig 1D). No differences in immune population frequencies or activation status were found in the brain 72h or two weeks after HI.
Fig 2. Heat map of all investigated immune populations after sham operation or HI 24h, 72h and two weeks after randomization to caffeine or PBS (n = 3–5 in each group, total n = 52).
The data was normalized to zero for the lowest value and one for the highest value in each variable with zeros in blue through yellow to ones values in red. Whites are missing values. MFI = Median florescence intensity.
Systemic effects were investigated simultaneously in spleen. No significant differences were seen between PBS and caffeine treatment animals at any time.
Discussion
Caffeine protects the neonatal mouse brain after HI, as observed by decreased brain atrophy and behavioral correlates in altered motor function and activation in the open field test. This confirms the neuroprotective properties of caffeine reported in humans [2] [9]. Of note, caffeine could potentially be administered trough breast feeding since prenatal continuous maternal caffeine intake reduces brain damage after neonatal HI in rats [10].
Of all immune populations investigated, only activated brain CD8+CD69+ T-lymphocytes 24h after HI differed significantly between caffeine and PBS. This indicates an immunomodulatory effect while caffeine is present with no apparent long term consequences. Since caffeine breakdown is slower in infants than adults, a sufficient caffeine concentration could be expected for as long as 24h but hardly for 72h or two weeks [3]. Admittedly, immune effects able to influence outcome are possible earlier than 24h after brain HI. However, our primary concern in this study was long term alterations, since the fear of persistent adverse effects have hampered the proceeding of caffeine as a neuroprotective agent.
With reference to mild or no persistent adverse effects, simplicity of administration and favorable pharmacokinetics we argue that studies addressing the therapeutic use of caffeine for neuroprotection after neonatal HI in larger animals prior to controlled prospective randomized clinical trials are urgently warranted.
Supporting Information
(TIF)
(CSV)
(DOCX)
Data Availability
Data is available at: 10.6084/m9.figshare.4249646.
Funding Statement
This work was supported by Medicinska Forskningsrådet (SE) regional agreement on medical training and clinical research, Marianne and Marcus Wallenberg foundation, Swedish Brain Foundation and Swedish Order of Freemasons in Stockholm. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jacobs SE, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cochrane Review: Cooling for newborns with hypoxic ischaemic encephalopathy. Evidence-Based Child Health: A Cochrane Review Journal. 2008;3(4):1049–115. [Google Scholar]
- 2.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357(19):1893–902. Epub 2007/11/09. 10.1056/NEJMoa073679 [DOI] [PubMed] [Google Scholar]
- 3.Aden U. Methylxanthines during pregnancy and early postnatal life. Handb Exp Pharmacol. 2011;200:373–89. Epub 2010/09/23. [DOI] [PubMed] [Google Scholar]
- 4.Horrigan LA, Kelly JP, Connor TJ. Immunomodulatory effects of caffeine: friend or foe? Pharmacol Ther. 2006;111(3):877–92. Epub 2006/03/17. 10.1016/j.pharmthera.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 5.Hedtjarn M, Mallard C, Arvidsson P, Hagberg H. White matter injury in the immature brain: role of interleukin-18. Neurosci Lett. 2005;373(1):16–20. Epub 2004/11/24. 10.1016/j.neulet.2004.09.062 [DOI] [PubMed] [Google Scholar]
- 6.Albertsson A- M, Bi D, Duan L, Zhang X, Leavenworth JW, Qiao L, et al. The immune response after hypoxia-ischemia in a mouse model of preterm brain injury. Journal of Neuroinflammation. 2014;11:153 10.1186/s12974-014-0153-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahne F, LeMeur N, Brinkman RR, Ellis B, Haaland P, Sarkar D, et al. flowCore: a Bioconductor package for high throughput flow cytometry. BMC Bioinformatics. 2009;10:106 Epub 2009/04/11. PubMed Central PMCID: PMC2684747. 10.1186/1471-2105-10-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winerdal M, Winerdal ME, Wang YQ, Fredholm BB, Winqvist O, Aden U. Adenosine A1 receptors contribute to immune regulation after neonatal hypoxic ischemic brain injury. Purinergic Signal. 2016;12(1):89–101. Epub 2015/11/27. 10.1007/s11302-015-9482-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle LW, Cheong J, Hunt RW, Lee KJ, Thompson DK, Davis PG, et al. Caffeine and brain development in very preterm infants. Annals of Neurology. 2010;68(5):734–42. 10.1002/ana.22098 [DOI] [PubMed] [Google Scholar]
- 10.Bona E, Aden U, Fredholm BB, Hagberg H. The effect of long term caffeine treatment on hypoxic-ischemic brain damage in the neonate. Pediatr Res. 1995;38(3):312–8. Epub 1995/09/01. 10.1203/00006450-199509000-00007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(CSV)
(DOCX)
Data Availability Statement
Data is available at: 10.6084/m9.figshare.4249646.