Abstract
Songbird species (order Passeriformes, suborder Oscines) are important models in various experimental fields spanning behavioural genomics to neurobiology. Although the genomes of some songbird species were sequenced recently, the chromosomal organization of these species is mostly unknown. Here we focused on the two most studied songbird species in neuroscience, the zebra finch (Taeniopygia guttata) and the canary (Serinus canaria). In order to clarify these issues and also to integrate chromosome data with their assembled genomes, we used classical and molecular cytogenetics in both zebra finch and canary to define their chromosomal homology, localization of heterochromatic blocks and distribution of rDNA clusters. We confirmed the same diploid number (2n = 80) in both species, as previously reported. FISH experiments confirmed the occurrence of multiple paracentric and pericentric inversions previously found in other species of Passeriformes, providing a cytogenetic signature for this order, and corroborating data from in silico analyses. Additionally, compared to other Passeriformes, we detected differences in the zebra finch karyotype concerning the morphology of some chromosomes, in the distribution of 5S rDNA clusters, and an inversion in chromosome 1.
Introduction
Species belonging to the suborder Oscines (Aves, order Passeriformes), also known as songbirds, have been employed as models in studies concerning neuroscience, vocal communication, development, behavioural genomics, ecology and evolution, among others [1–7]. Among songbirds, the zebra finch (Taeniopygia guttata, TGU) and the canary (Serinus canaria, SCA) belong to different families (Estrildidae and Fringillidae, respectively), are frequently used and have recently been the subjects of genomic analyses [8–11]. They are originally from different zoogeographical regions–the zebra finch is an Australian species, while the canary originates from the Canary Islands, located just off the southern coast of Africa [12].
After the chicken (Gallus gallus, GGA) the zebra finch was the second bird species to have its genome sequenced [8] which generated knowledge crucial in understanding some aspects of genome evolution in birds. Comparisons with data collected from sequencing projects of turkey (Meleagris gallopavo) and chicken revealed numerous intrachromosomal rearrangements, 10% of which are recurrent, indicating the existence of evolutionary hotspots [3]. The canary genome was sequenced and analyzed in a study dealing with the evolution of the influence of sex related hormones in gene regulation, in which an in silico karyotype was proposed, based on the alignment of the canary genome with 13 other birds, including the zebra-finch [11]. Interestingly, Romanov et al. [13] found that the zebra finch and budgerigar–representing lineages with vocal learning—showed the highest intrachromosomal rearrangement rates among birds. More recently, Farré et al. [14] published a study demonstrating how chromosomal rearrangements act as a source of phenotypic variation. Considering Passeriformes, the comparison between Oscines and Suboscines showed that the number of chromosomal rearrangements is higher in birds with vocal learning [6, 13].
In cytogenetic studies, G. gallus is also used as the main model for comparisons. Indeed, the comparison of data obtained by chromosome painting of different species of birds led Griffin et al. [15] to propose a putative avian ancestral karyotype (PAK), which showed a total correspondence to syntenic groups of G. gallus, except for the pair 4, which corresponds to two elements in the PAK (pairs 4 and 10), as found in most species of birds so far. In addition, the use of white hawk (Leucopternis albicollis, LAL) whole-chromosome paints brought new information concerning chromosomal rearrangements in birds, because many of its chromosomes correspond to regions of macrochromosomes of the PAK, and hence allow identification of the occurrence of chromosomal rearrangements, such as paracentric inversions, and breakpoints [16].
Most species of songbirds, including the zebra finch and the canary, show a diploid number close to 2n = 80. Although GGA whole chromosome probes have been used in the zebra-finch to detect some specific intrachromosomal polymorphisms little is known about the karyotype of the canary apart from the first description based on conventional staining [17]. So far, 14 species of songbirds have been analyzed by chromosome painting, most of them with GGA probes [9, 18–23]. These studies confirm the conservation of most syntenic groups found in the putative avian ancestral karyotype (PAK). An apparent centric fission in PAK chromosome 1 (GGA1) may represent a synapomorphy for the Oscines.
More recently, the use of two sets of whole chromosome probes (GGA and LAL) in the analysis of the karyotype of different species of Oscines and Suboscines from South America have confirmed the centric fission of PAK 1 as a synapomorphy, and revealed a complex pattern of paracentric and pericentric inversions in the pair corresponding to PAK 1q (GGA 1q) as a chromosome signature of this order. The results obtained corroborated the findings observed in silico [3], concerning the confirmation of the occurrence of inversions, and allowed determination of the possible sequence of some events, due to some differences among the inversions in each of the species [21, 23]. However, these studies have been performed only in South American species of Passeriformes, where the species of Oscines belonged to families Turdidae and Thraupidae.
Therefore, in order to verify and corroborate the occurrence of such intrachromosomal rearrangements in other groups of Passeriformes, and because of the importance of the zebra finch and the canary as biological models, we present here the chromosomal analysis of these two species by classical and molecular cytogenetics, using not only whole-chromosome probes of chicken and the white-hawk, but also 5S and 18S rDNA probes, and telomeric sequences.
Material and Methods
Cell culture and chromosome isolation
This study was approved by the Ethics Committee on the Use of Animals (CEUA-Universidade Federal do Pará, Permission Number: 070/2013). Tissue cultures were initiated from embryos from eggs after five to six days (HH stages 27–29, approximately) of incubation at 37°C. Sex determination was performed by molecular techniques, and cultures of one male and one female of zebra finch and canary were selected. Tissue was cut into small pieces and disaggregated in 0.5% collagenase IV for 30 min. The suspension was then washed with culture medium and diluted in DMEM (Gibco) enriched with 10% fetal calf serum and antibiotics (penicillin and streptomycin). Cells were harvested with trypsin after 2 h incubation with Colcemid. Afterwards, cell suspension were incubated for 18 minutes in KCl (0,075 M), fixed in 3:1 methanol: acetic acid, then kept at -20°C.
Classical cytogenetics
Diploid number and chromosome morphology were assessed by the analysis of 20 metaphases with conventional staining (Giemsa 5% in 0.07 M phosphate buffer, pH 6.8). G- and C-banding followed standard protocols [24, 25]. After digital image acquisition, chromosomes were ordered by centromere position and chromosome size.
Fluorescent in situ hybridization
Biotin/Fluorescein labelled 18S/28S and 5S ribosomal DNA probes were used to detect the location of ribosomal RNA gene clusters. Comparative painting was performed using whole chromosome probes generated by flow cytometry at the Cambridge Resource Centre for Comparative Genomics (Cambridge, United Kingdom): chicken, first 10 chromosomes and Z chromosome, and white hawk corresponding to chromosomes homologous to region of GGA1 (LAL 3, 6, 7, 15 and 18), 2 (LAL 2, 4, and 20), 3 (LAL 9, 13, 17 and 26), 4 (LAL 1 and 16), 5 (LAL 5) and 6 (LAL 3) [16]. Hybridization, stringency washes and detection followed standard methodologies previously described [16]. Slides were analyzed using a Zeiss Axioplan2 fluorescent microscope and Axionvisio 4.8 software (Zeiss, Germany).
Results
Classical cytogenetics: Conventional staining and banding results
In order to facilitate comparisons with other species, we decided to follow nomenclature taking into account the morphology and size of chromosomes, as proposed by the International System for Standardized Avian Karyotypes [26], instead of the nomenclature based on homology with chicken chromosomes [9–11, 27]. Hence, we present the correspondence of both systems in Table 1.
Table 1. Correspondence between nomenclature following chromosome morphology (ours), and homology with GGA [9, 27].
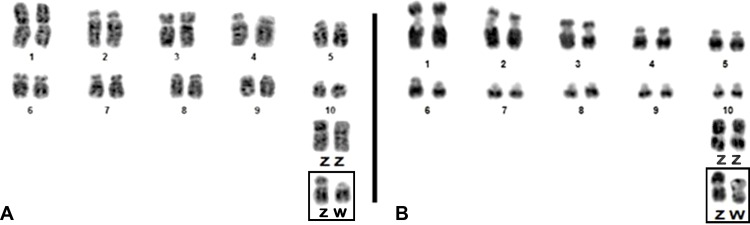
Male and female embryos of both species were studied and their diploid number was found to be 2n = 80. In the zebra finch chromosome 1 is metacentric, chromosomes 3, 4, 5 and Z submetacentric, and chromosomes 2, 6, 7 and 8 acrocentric. All other elements are telocentric. In canary, chromosomes 1–4 are submetacentric, chromosomes 5–10 are acrocentric and the remaining autosomal pairs are telocentric. The Z and the W chromosomes are metacentric. Only the largest chromosome pairs showed some distinguishable G-banding patterns (Fig 1A and 1B).
Fig 1.
G-banding patterns of the first ten pairs and sex chromosomes of zebra finch (A) and canary (B).
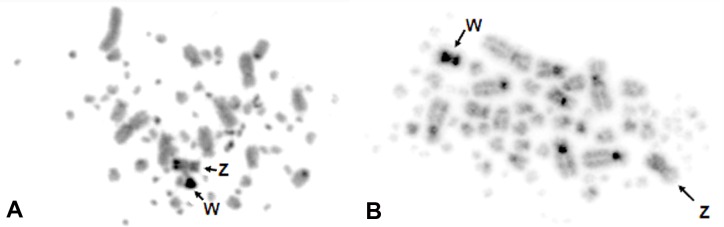
In zebra finch, C-banding revealed conspicuous heterochromatic blocks in pairs 6, Z and the W (Fig 2A), while in canary we observed C-positive segments in the centromeric region of all macrochromosomes, in the long arm of chromosome W, and in some microchromosomes (Fig 2B).
Fig 2.
C-band patternsof zebra finch (A) and canary (B). Sex chromosomes are indicated.
Telomeric sequences and 5S/18S rDNA clusters
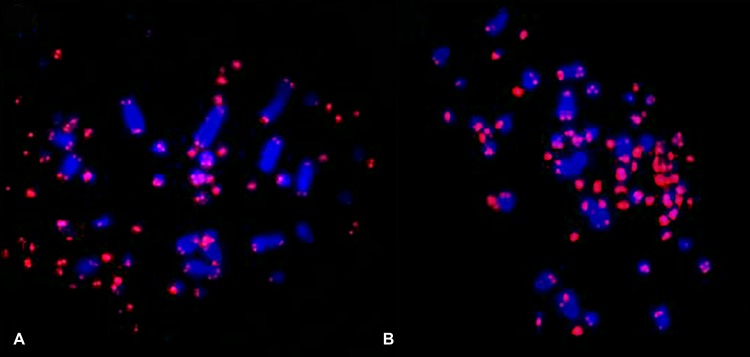
No interstitial telomeric sequences were observed in any of the two species: probes containing telomeric sequences produced signals only in the distal region of chromosome arms, and tended to be brighter in microchromosomes than in macrochromosomes (Fig 3A and 3B).
Fig 3.
Telomeric probes on chromosomes of zebra finch (A) and canary (B). No interstitial telomeric sequences were observed.
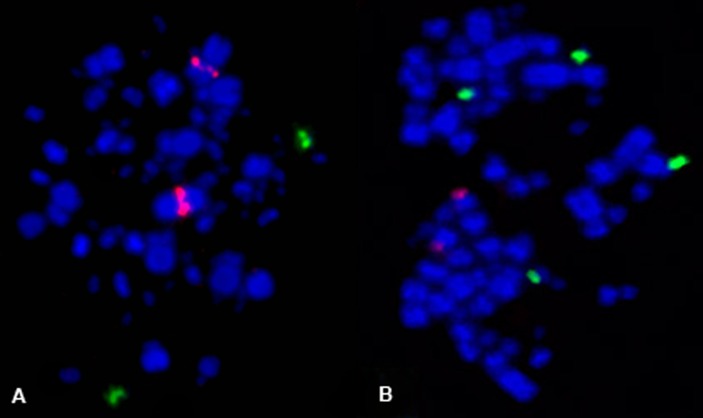
The rDNA probes revealed some karyotypical differences between the species. 5S rDNA probes hybridized to one cluster in each species. Interestingly, while this cluster was located on a pair of microchromosomes in the canary, in the zebra finch the cluster was located on the medial region of chromosome 2q. Additionally, 18/28S rDNA hybridized onto one pair of microchromosomes of zebra finch, but onto two pairs of microchromosomes in canary (Fig 4A and 4B).
Fig 4.
5S (red) and 18/28S (Green) rDNA probes on zebra finch (A) and canary (B).
Chromosome painting
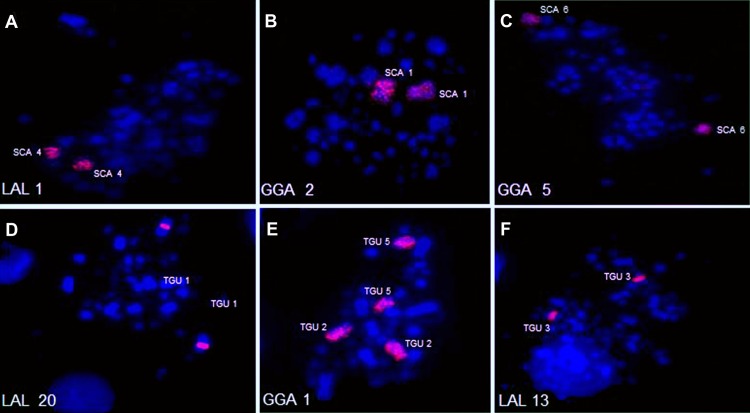
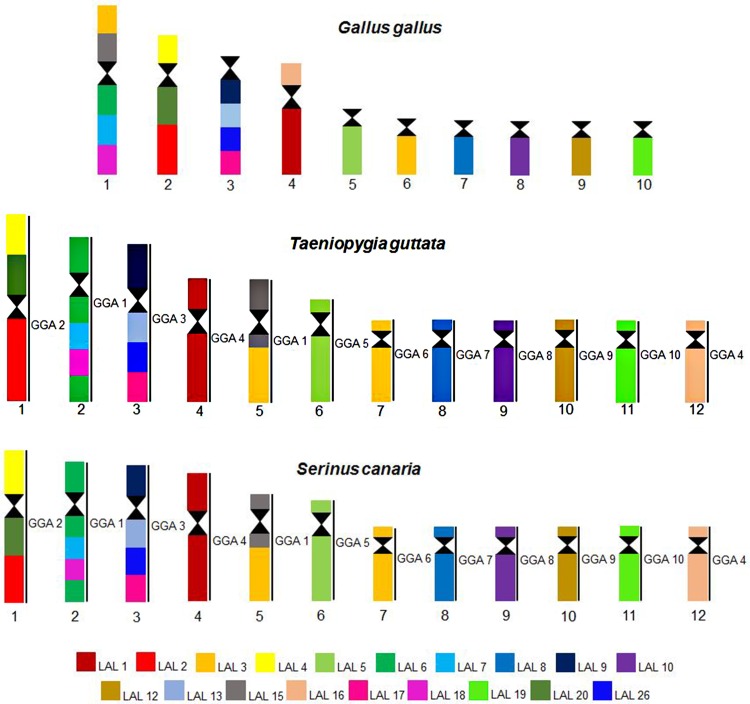
Examples of experiments using chicken and white hawk probes are shown in Fig 5, while the homology maps of zebra finch and canary with chicken and white hawk are shown in Fig 6.
Fig 5.
Representative FISH experiments using white hawk (A, D, F) and chicken (B, C, E) probes on metaphase chromosomes of canary (Serinus canaria-SCA) [A-C] and zebra finch (Taeniopygia guttata-TGU) [D-F].
Fig 6. homology maps between chicken (GGA), white hawk (LAL) and two species of Passeriformes (zebra finch and canary).
It can be noticed that the pattern of hybridization using chicken whole chromosome probes were very similar in both species; chromosomes GGA1 and 4 correspond to two different pairs each, and pairs GGA 2, 3, 5, 6, 7, 8, 9 and 10 each correspond to one pair. In canary, a gap was observed in chromosome 2 (which corresponded to GGA1q), corresponding to a block of constitutive heterochromatin.
Experiments using chromosome probes of white hawk revealed that, when compared to chicken syntenic groups, zebra finch and canary exhibited five inversions relative to chicken GGA1: four occurring in the pair corresponding to GGA1q, and one corresponding to GGA1p. In addition, zebra finch presented an inversion in pair 1 (corresponding to GGA2). The occurrence of this inversion explains the morphological differences when we compare TGU1, metacentric, to SCA1, submetacentric. No interchromosomal rearrangement was revealed by any of the probes of chicken applied to zebra finch and canary.
Discussion
Karyotype description
The zebra finch and the canary have been used as model species for many different studies. Although both passerine genomes have been fully sequenced and assembled, some aspects of their chromosome organization are still unknown; especially in the canary, published karyotypes rely on conventional staining [11, 17]. The definition of the distribution of constitutive heterochromatic segments and repetitive gene cluster (rDNA) that we report are important for a better understanding and resolution of the in silico genome assembly.
The diploid number of 2n = 80 was found both in the zebra-finch and the canary, corroborating previous reports [9–11, 17, 28]. Although the patterns of distribution of the heterochromatic blocks agreed with most reports on avian karyotypes [29], we found in the canary atypical large heterochromatic blocks in some pairs, especially in pair 2, that are not common in macrochromosomes.
rDNA and telomeric sequences
Although the number and location of repetitive ribosome gene clusters are important features for studies of chromosomal evolution, and cytotaxonomy, these features are still under-investigated in many groups, including birds [30–35]. For instance, in birds, information on the number and distribution of 18/28S is available for a limited number of species, usually using the Ag-NOR technique. In most of these 18/28S rDNA clusters are found on only one pair, usually a microchromosome, although there are already well documented variations [36–39]. For Passeriformes, most species analyzed show only one pair of microchromosomes bearing 18/28S rDNA [22, 40], such as we found in zebra finch and canary. However, there are some species which have 18/28S rDNA clusters on more than one pair [21, 41].
Even more restrictedis data concerning the multigene family 5S rDNA, found only for three species of Galliformes [34, 35], and two songbird species of genus Saltator (Passeriformes, Thraupidae) [23]. In all these species, there was only one cluster of 5S rDNA genes, located on one pair of chromosomes, similar in size to pairs 9–11 in Galliformes, and smaller in Saltator. The canary shows a similar distribution of 5S rDNA: the probes hybridized only to one pair of microchromosomes. As the identification of the chromosome bearing 5S clusters has been based solely on its size, it cannot be concluded that they are located in the same syntenic groups in both Saltator and the canary. In contrast, zebra finch shows a pattern not observed in birds so far; the cluster of 5S rDNA was found in an interstitial position on the long arm of pair 1. As chromosome painting did not detected any interchromosomal rearrangement involving this segment (corresponding to GGA1q) transposition is a possible explanation [42]. It seems that the comparative analysis of these clusters can give valuable information about karyotypical diversity during genome evolution of birds, as observed in other groups of vertebrates [43, 44]. Unfortunately, our attempt to match our findings with expectations based on genome sequences did not succeed. The blast of ribosomal genes from chicken to chicken genomes indicated that there is no clear annotation and/or overlap of the rRNAs chicken genes even using the best annotated genome and complete sequences of rRNA genes published. The most plausible reason being the excessive enrichment of repetitive sequences and artifacts of the assembly procedures of the genomes. As discussed by Dyomin et al. [45] there is no complete annotation of rRNA genes in avian genomes so far despite all efforts made.
Although many species of birds have interstitial telomeric sequences, including Passeriformes such as the Redwing (Turdus iliacus) and the chaffinch (Fringilla coelebs) [19], in canary and zebra finch telomeric sequences were restricted to the terminal regions of chromosomes. In addition, in accordance with other studies, we observed more intense telomeric probe signals in microchromosomes [19, 46–49]. According to Nanda et al. [48], the high density of (TTAGGG)n repeats in microchromosomes may contribute to the high meiotic recombination rate observed in these elements. In addition, previous studies have shown a higher number of interstitial telomeric sequences in Ratitas and Galloanserae, which led us to propose that this may represent a plesiomorphic condition, tending to diminish during avian evolution, despite the conservation of diploid number and syntenic groups.
Chromosome painting using chicken and white hawk probes
In general, zebra finch and canary show the conservation of syntenic groups. When compared to the putative ancestral karyotype, the only difference found is the fission of the first chromosome pair into two distinct elements. Furthermore, white-hawk probes confirmed most of the inversions found in other species of Passeriformes, reinforcing that these inversions, as well as the fission of pair 1, occurred very early in the history of this order.
Each chicken probe hybridized to one pair of chromosome of zebra finch and canary, except for probes GGA1 and GGA4, which hybridized to two pairs each. As GGA4 corresponds to two elements universally in different groups of birds, confirming it as the ancestral state [16], the centric fission of GGA1 seems to be apomorphic for Passeriformes. This has been described in all the species analyzed by FISH so far [18–23]. Likewise, the use of white hawk probes confirmed the results found in other species of Passeriformes, both Oscines [21, 23] and Suboscines [22], revealing the occurrence of a group of sequential chromosomal inversions in segments homologous to GGA1. However, LAL 18, which corresponds to one block in the species analyzed here, and also in Saltator, is homologous to two different segments in Turdus and Elaenia, that belong to Oscines and Suboscines, respectively [21, 23]. These findings may be explained by two different alternative scenarios. First, and maybe more parsimonious, it could be that this inversion, in which the segment homologous to LAL 18 was split into two parts, occurred some time before the split of Oscines and Suboscines. On the other hand, an alternative hypothesis based on the high level of recurrent breakpoints in birds [3] could consider a 4th inversion in Saltator species, in the canary and the zebra finch, which would have reverted the segment homologous to LAL 18 to one continuous block [23]. This proposal is supported by the fact that the genera Saltator, Serinus and Taeniopygia are included in the same phylogenetic branch, called “Core Passeroidea” [50]. The 4th inversion would have occurred in the common ancestor of this group. In any case, we are aware that only the analyses of other genera belonging to these groups may confirm one of these hypotheses.
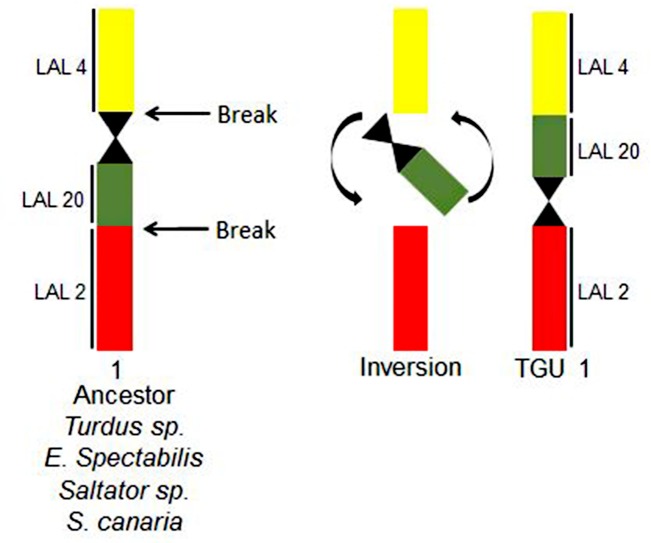
Concerning the comparison of rearrangements found in the canary and zebra finch, it is important to consider that the inversions detected by the use of white hawk probes in pairs 1 (GGA2), 2 (GGA1q) and 5 (GGA1p) corroborate the proposals of Warren et al. [8] in zebra finch, and by Frankl et al. [11] in canary, using the genomic in silico assembly data. Considering other species with data on genome sequence alignment, the occurrence of these inversions can be expanded to four other species of this group: Geospiza fortis, Zonotrichia albicollis, Pseudopodoces humilis and Ficedula albicollis [11]. In addition, the comparison of our data to previous studies indicated that TGU1 had one additional pericentric inversion when compared to Turdus, Elaenia and Saltador [21, 23] (Fig 7). Hence, while in these genera and in canary the first pair is submetacentric, in zebra finch it is metacentric. This inversion was detected by Warren et al. [8] by sequence alignment. It was proposed that some of the inversions described by Skinner & Griffin [3] in the genome study of zebra finch must be autapomorphies of this species, and not shared by others so far [11].
Fig 7. Schematic representation of the inversion found in TGU1 (GGA2).
Conclusions
The genome sequencing of an increasing number of birds, combined with cytogenomic mapping is helping to diminish the discrepancy of information concerning avian genomic organization in comparison to other Vertebrate groups. Additionally, the agreement between the data obtained by in silico assembling and FISH approaches show that these metholologies are complementary and may be used in combination to generate cytogenetic markers, or to provide information not easily obtained by sequencing and genomic assembling, such as the localization of repetitive sequences, as for example 5S and 18S rDNA clusters. Hence, a complete karyotypical characterization–including the distribution of heterochromatic blocks and rDNA blocks–may be important to complete the interpretation of data obtained by sequencing. A clear example of this is the fact that, because of the peculiar nature of the avian karyotype, the number of syntenic groups generated by bioinformatic approaches are not accurate, and usually do not match with the actual diploid number [11].
Acknowledgments
Authors would like to thank PROPESP/UFPA, Max Planck Institute for Ornithology, and Instituto Evandro Chagas, for support. We would like to thank Dr. Marcelo Cioffi (UFSC, São Carlos, SP-Brazil), for kindly providing the 5S and 18/28S rDNA probes.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors would like to thank PROPESP/UFPA, Max Planck Institute for Ornithology, and Conselho Nacional de Desenvolvimento Científico e Tecnológico, for financial support (309699/2015-0), as well as Instituto Evandro Chagas, for logistical support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Doupe AJ, Kuhl PK. Birdsong and human speech: Common Themes and Mechanisms. Annual Review of Neuroscience. 1999; 22: 567–631. 10.1146/annurev.neuro.22.1.567 [DOI] [PubMed] [Google Scholar]
- 2.Zeigler H, Marler P. Ed. Behavioral neurobiology of birdsong In: Behavioral Neurobiology of Birdsong, Dec, Hunter College, City University of New York, New York, NY, US; New York Academy of Sciences; 2002. [Google Scholar]
- 3.Skinner BM, Griffin DK. Intrachromosomal rearrangements in avian genome evolution: evidence for regions prone to breakpoints. Heredity. 2012; 108: 37–41. 10.1038/hdy.2011.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature. 2012; 491(7424):444–448. 10.1038/nature11631 [DOI] [PubMed] [Google Scholar]
- 5.Holt BG, Lessard JP, Borregaard MK, Fritz SA, Araújo MB, Dimitrov D, et al. An update of Wallace‟s zoogeographic regions of the world. Science. 2013; 339(6115):74–78. 10.1126/science.1228282 [DOI] [PubMed] [Google Scholar]
- 6.Zhang G, Li C, Li Q, Li B, Larkin DM, Lee C, et al. Comparative genomics reveals insights into avian genome evolution and adaptation. Science. 2014; 346(6215): 1311–1319. 10.1126/science.1251385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill LF, Goymann W, Maat AT, Gahr M. Patterns of call communication between group-housed zebra finches change during the breeding cycle. eLife. 2015; 4:e07770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warren WC, Clayton DF, Ellegren H, Arnold AP, Hillier LW, Künstner A, et al. The genome of a songbird. Nature. 2010; 464: 757–762. 10.1038/nature08819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itoh Y, Arnold AP. Chromosomal polymorphism and comparative painting analysis in the zebra finch. Chromosome Res. 2005; 13: 47–56. 10.1007/s10577-005-6602-x [DOI] [PubMed] [Google Scholar]
- 10.Itoh Y, Kampf K, Balakrishnan CN, Arnold AP. Karyotypic polymorphism of the zebra finch Z chromosome. Chromosoma. 2011; 120(3):255–264. 10.1007/s00412-010-0308-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frankl-Vilches C, Kuhl H, Werber M, Klages S, Kerick M, Bakker A, et al. Using the canary genome to decipher the evolution of hormone-sensitive gene regulation in seasonal singing birds. Genome Biology. 2015; 16:19 10.1186/s13059-014-0578-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrascal LM, Telleria JL, Valido A. Habitat distribution of canary chaffinches among islands: competitive exclusion or species-specific habitat preferences? J Biogeography. 1992; 19:383–390. [Google Scholar]
- 13.Romanov MN, Farré M, Lithgow PE, Fawler KE, Skinner BM, Connor RO, et al. Reconstruction of gross avian genome structure, organization and evolution suggests that the chicken lineage most closely resembles the dinosaur avian ancestor. BMC Genomics.2014; 15:1060 10.1186/1471-2164-15-1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farré M, Narayan J, Slavov GT, Damas J, Auvil L, Li C, et al. Novel insights into chromosome evolution in birds, archosaurs, and reptiles. Genome Biol. Evol. 2016; 8(8):2442–2451. 10.1093/gbe/evw166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin DK, Robertson LBW, Tempest HG, Skinner BM. The evolution of the avian genome as revealed by comparative molecular cytogenetic. Cytogenet Genome Res. 2007; 117: 64–77. 10.1159/000103166 [DOI] [PubMed] [Google Scholar]
- 16.de Oliveira EHC, Tagliarini MM, Rissino JD, Pieczarka JC, Nagamachi CY, O’Brien PCM, et al. Reciprocal chromosome painting between white hawk (Leucopternis albicollis) and chicken reveals extensive fusions and fissions during karyotype evolution of Accipitridae (Aves, Falconiformes). Chromosome Res. 2010; 18: 349–355. 10.1007/s10577-010-9117-z [DOI] [PubMed] [Google Scholar]
- 17.Ohno S, Stenius C, Christian LC, Beçak W, Beçak ML. Chromosomal uniformity in the Avian subclass Carinatae. Chromosoma. 1964; 15:280–288. [DOI] [PubMed] [Google Scholar]
- 18.Guttenbach M, Nanda I, Feichtinger W, Masabanda JS, Griffin DK, Schmid M. Comparative chromosome painting of chicken autosomal paints 1–9 in nine different bird species. Cytogenet Genome Res. 2003; 103: 173–184. [DOI] [PubMed] [Google Scholar]
- 19.Derjusheva S, Kurganova A, Haberman F, Gaginskaia E. High chromosome conservation detected by comparative chromosome painting in chicken, pigeon and passerine birds. Chromosome Res. 2004; 12: 715–723. 10.1023/B:CHRO.0000045779.50641.00 [DOI] [PubMed] [Google Scholar]
- 20.Nanda I, Benisch P, Fetting D, Haaf T, Schmid M. Synteny Conservation of Chicken Macrochromosomes 1–10 in Different Avian Lineages Revealed by Cross-Species Chromosome Painting. Cytogenet Genome Res. 2011; 132: 165–181. 10.1159/000322358 [DOI] [PubMed] [Google Scholar]
- 21.Kretschmer R, Gunski RJ, Garnero ADV, Furo IO, O’Brien PCM, Ferguson-Smith MA, et al. Molecular cytogenetic characterization of multiple intrachromosomal rearrangements in two representatives of the genus Turdus (Turdidae, Passeriformes). Plos One. 2014; 9(7): e103338 10.1371/journal.pone.0103338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kretschmer R, de Oliveira EHC, Santos MS, Furo IO, O’Brien PCM, Ferguson-Smith MA, et al. Chromosome Mapping of the Large Elaenia (Elaenia spectabilis): evidence for a cytogenetic signature for passeriform birds? Biological Journal of the Linnean Society. 2015; 115: 391–398. [Google Scholar]
- 23.dos Santos MS, Kretschmer R, Silva FA, Ledesma MA, O'Brien PCM, Ferguson-Smith MA et al. Intrachromosomal rearrangements in two representatives of the genus Saltator (Thraupidae, Passeriformes) and the occurrence of heteromorphic Z chromosomes. Genetica. 2015; 143(5):535–43. 10.1007/s10709-015-9851-4 [DOI] [PubMed] [Google Scholar]
- 24.Seabright M. 1971. A rapid banding technique for human chromosomes. Lancet. 1971; 2: 971–972. [DOI] [PubMed] [Google Scholar]
- 25.Sumner AT. 1972. A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res. 1972; 83:438–442. [DOI] [PubMed] [Google Scholar]
- 26.Ladjali-Mohammedi K, Bitgood JJ, Tixier-Boichard M, Ponce de Leon FA. International System for Standardized Avian Karyotypes (ISSAK): standardized banded karyotypes of the domestic fowl (Gallus domesticus). Cytogenet Cell Genet. 1999; 86:271–276. [DOI] [PubMed] [Google Scholar]
- 27.Stapley J, Birkhead TR, Burke T, Slate J. A linkage map of the zebra finchTaeniopygia guttata provides new insights into avian genome evolution. Genetics. 2008; 179(1):651–667. 10.1534/genetics.107.086264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pigozzi MI, Solari AJ. Germ cell restriction and regular transmission of an accessory chromosome that mimics a sex body in the zebra finch, Taeniopygia guttata. Chromosome Res.1998; 6:105–113. [DOI] [PubMed] [Google Scholar]
- 29.Shibusawa M, Nishibori M, Nishida-Umehara C, Tsudzuki M, Masabanda J, Griffin DK, et al. Karyotypic evolution in the Galliformes: An examination of the process of karyotypic evolution by comparison of the molecular cytogenetic findings with the molecular phylogeny. Cytogenet Genome Res. 2004; 106:111–119. 10.1159/000078570 [DOI] [PubMed] [Google Scholar]
- 30.Martins C, Wasko AP. Organization and evolution of 5S ribosomal DNA in the fish genome. Focus on genome research. 2004; 335–363. [Google Scholar]
- 31.Cioffi MB, Molina WF, Moreira-Filho O, Bertollo LAC. Chromosomal distribution of repetitive DNA sequences highlights the independent differentiation of multiple sex chromosome in two closely related fish species. Cytogenetic and genome research. 2011; 134: 295–302. 10.1159/000329481 [DOI] [PubMed] [Google Scholar]
- 32.Demin A, Koshel E, Saifitdinova A, Galkina S, Fukagawa T, Gaginskaya. Chicken rRNA gene cluster structure. 2015. Preprint. Available: CC-BY-NC-ND4.0. Accessed 09 June 2016. [DOI] [PMC free article] [PubMed]
- 33.Shaw P, Brown J. Nucleoli: composition, function, and dynamics. PlantPhysiol. 2012; 158(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniels LM, Delany ME. Molecular and cytogenetic organization of the 5S ribosomal DNA array in chicken (Gallus gallus). Chromosome Research. 2003; 11:305–317. [DOI] [PubMed] [Google Scholar]
- 35.McPherson MC, Robinson CM, Gehlen LP, Delany ME. Comparative cytogenomics of poultry: mapping of single gene and repeat loci in the Japanese quail (Coturnix japonica). Chromosome Res. 2014; 22(1):71–83. 10.1007/s10577-014-9411-2 [DOI] [PubMed] [Google Scholar]
- 36.Barbosa MO, da Silva RR, Correia VCS, dos Santos LP, Garnero AV, Gunski RJ. Nucleolar organizer regions in Sittasomus griseicapillus and Lepidocolaptesangustirostris (Aves, Dendrocolaptidae): Evidence of a chromosome inversion. Genetics and Molecular Biology. 2013; 36(1):70–73. 10.1590/S1415-47572013000100010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Oliveira EHC, Tagliarini MM, dos Santos MS, O’Brien PCM, Ferguson-Smith MA. Chromosome Painting in Three Species of Buteoninae: A Cytogenetic Signature Reinforces the Monophyly of South American Species. Plos One. 2013; 8(7):e70071 10.1371/journal.pone.0070071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Oliveira EHC, Habermann F, Lacerda O, Sbalqueiro IJ, Wienberg J, Muller ES. Chromosome reshuffling in birds of prey: the karyotypes of the world’s largest eagle (Harpy eagle, Harpia harpyja) compared to that of the chicken (Gallus gallus). Chromosoma. 2005; 114:338–343. 10.1007/s00412-005-0009-5 [DOI] [PubMed] [Google Scholar]
- 39.Islam FB, Uno Y, Nunome M, Nishimura O, Tarui H, Agata K, et al. Comparison of the Chromosome Structures between the Chicken and Three Anserid Species, the Domestic Duck (Anas platyrhynchos), Muscovy Duck (Cairina moschata), and Chinese Goose (Anser cygnoides), and the Delineation of their Karyotype Evolution by Comparative Chromosome Mapping. J Poultry Science. 2014; 51 (1): 1–13. [Google Scholar]
- 40.Kretschmer R, de Lima VLC, da Ponte LN, Degrandi TM, Vinadé L do C, Schünemann AL, et al. NOR- bearing as a plesiomorphic characteristic in Mimus saturninus (Passeriformes Mimidae). Journal of Biotechnology and Biodiversity. 2014; 5(2): 140–147. [Google Scholar]
- 41.Ledesma MA, Martínez PA, Calderón PS, Boeris JM, Meriles JM. Descrição do cariótipo e padrões de bandas C e NOR em Pheucticus aureoventris (Emberizidae, Cardinalinae). Revista Brasileira de Ornitologia. 2006; 14 (1) 59–62. [Google Scholar]
- 42.Merlo MA, Cross I, Manchado M, Cárdenas S, Rebordinos L. The 5S rDNA high dynamism in Diplodus sargus is a transposon-mediated mechanism. Comparison with other multigene families and Sparidae species. J Mol Evol. 2013; 76(3):83–97. 10.1007/s00239-013-9541-8 [DOI] [PubMed] [Google Scholar]
- 43.Ferreira IA, Bertollo LAC, Martins C. Comparative chromosome mapping of 5S rDNA and 5SHindIII repetitive sequences in Erythrinidae fishes (Characiformes) with emphasis on the Hopliasmalabaricus ‘species complex’. Cytogenetic Genome Research. 2007;118:78–83. 10.1159/000106445 [DOI] [PubMed] [Google Scholar]
- 44.Pinhal D, Yoshimura TS, Araki CS, Martins C. The 5S rDNA family evolves through concerted and birth-and-death evolution in fish genomes: an example from freshwater stingrays. BMC Evolutionary Biology. 2011; 11: 151 10.1186/1471-2148-11-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dyomin AG, Koshel EI, Kiselev AM, Saifitdinova AF, Galkina SA, Fukagawa T, et al. Chicken rRNA Gene Cluster Structure. Plos One. 2016; 11(6):e0157464 10.1371/journal.pone.0157464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nanda I, Schmid M. Localization of the telomeric (TTAGGG)n sequence in chicken (Gallus domesticus) chromosomes. Cytogenetic and Genome Research. 1994; 65 (3)190–193. [DOI] [PubMed] [Google Scholar]
- 47.Delany ME, Krupkin AB, Miller MM. Organization of telomere sequences in birds: evidence for arrays of extreme length and for in vivo shortening. Cytogenet Cell Genet. 2000; 90:139–145. [DOI] [PubMed] [Google Scholar]
- 48.Nanda I, Schrama D, Feichtinger W, Haaf T, Schartl M, Schmid M. Distribution of telomeric (TTAGGG)n sequences in avian chromosomes. Chromosoma. 2002; 111(4):215–227. 10.1007/s00412-002-0206-4 [DOI] [PubMed] [Google Scholar]
- 49.Raudsepp T, Houck ML, O’Brien PC, Fergunson-Smith MA, Ryder OA, Chowdhary BP. Cytogenetic analysis of California Condor (Gymnogyps californianus) chromosomes: comparison with chicken (Gallus gallus) macrochromosomes. Cytogenet Genome Res. 2002; 98:54–60. [DOI] [PubMed] [Google Scholar]
- 50.Barker FK. Mitogenomic data resolve basal relationships among passeriform and passeridan birds. Molecular Phylogenetics and Evolution. 2014; 79:313–324. 10.1016/j.ympev.2014.06.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.