Abstract
Background/aim
The role of pro-inflammatory cytokines in the immunopathogenesis of idiopathic nephrotic syndrome had been widely postulated. Reports on the release of cytokines, during idiopathic nephrotic syndrome (INS) activation, were conflicting in defining a specific interleukin pattern during relapse and remission of the disease. The aim of this study was to explore the role of IL-1β, IL-6 and IL-8 in the pathophysiology of INS during relapse and remission.
Patients and methods
A total of 37 INS patients were included. Their demographic and biochemical data were reviewed. Levels of IL-1β, IL-6 and IL-8 were measured in the urine of patients during relapse and remission of the disease. Urine samples from 30 age- and sex-matched controls were checked for the same 3 cytokines.
Results
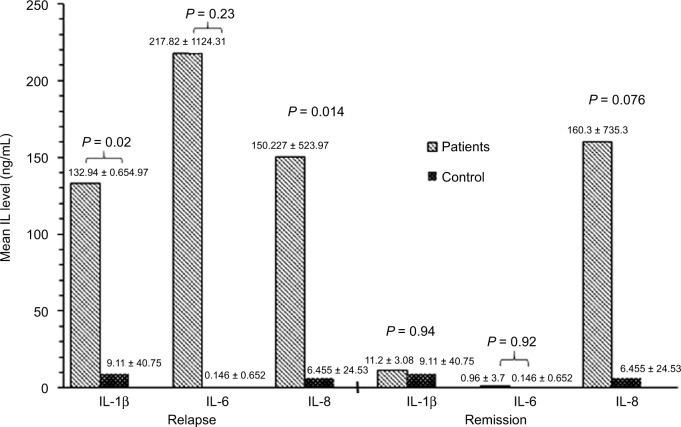
Mean age of patients at study was 6.4 ± 3.2 years (range: 14 months–12 years). Male:female ratio was 24:13. Mean serum creatinine was 47 ± 13 μmol/L, and mean serum albumin was 21 ± 7 g/L. Mean urinary IL-1β, IL6 and IL8 levels, corrected to urinary creatinine, in patients during relapse were 132.94 ± 654.97, 217.82 ± 1124.31 and 150.227 ± 523.97 pg/μmol compared to 9.11 ± 40.75, 0.146 ± 0.652, and 6.455 ± 24.53 pg/μmol in controls, respectively (P = 0.02, 0.03 and 0.014, respectively). No significant difference was reported in the mean level of the 3 cytokines compared to controls during remission (P = 0.94, 0.092 and 0.076).
Conclusion
Our results support the role of T-cell activation and the subsequent release of IL-1β, IL6 and IL8 in the pathogenesis of relapses in INS. The use of steroid-sparing cytokine blockers in managing relapses of INS remains a tempting challenge.
Keywords: cytokine, IL-1β, IL6, IL8, idiopathic nephrotic syndrome, relapse
Introduction
Idiopathic nephrotic syndrome (INS) is a common glomerular disease in children. The exact pathophysiology of this disease is not well understood. However, many studies have suggested that it is immune-mediated.1–6 These suggestions are based on the fact that most children with INS respond well to steroids and cyclophosphamide, both work on cell-mediated immunity. The fact that most attacks are triggered by viral illnesses and there is an increased incidence of allergic diseases among INS patients also supports the immune-mediated pathology.1–4
The role of T-cell lymphocytes in pathogenesis of INS was the core of many studies over the last 2 decades. Initially, imbalance between Th1 and Th2 helper cells with the increase in Th2 cytokines was postulated to be the key abnormality in INS.7–9 Nevertheless, recently, a decrease in the number and/or function of T-regulatory cells (Tregs), which are important modulators of the functions of the effector T-cells, was reported to be a prerequisite for the development of INS.1,3,10–12
Pro-inflammatory cytokines, IL-1β and IL-6, were specifically reported to be involved in the pathophysiology of INS.1,3 The chemokine IL-8 was only recently documented to act as a soluble factor that increases glomerular basement membrane permeability.1,4,13
Most previous studies reported the serum levels of IL-1β, IL-6 and IL-8 in INS patients. We believe that such cytokines are more likely produced locally in the kidneys and subsequently directly excreted in urine in order to initiate an effect on the glomerular basement membrane and subsequently provoke proteinuria. Therefore, the aim of this study was to explore the presence of IL-1β, IL6 and IL8 locally excreted in the urine of INS patients during both phases of the disease, i.e., relapse and remission, in order to speculate their role in the pathogenesis of the disease.
Patients and methods
A total of 37 Caucasian children below the age of 12 years with INS were included in the study. All patients were evaluated and managed in the renal outpatient clinics at the Department of Pediatrics at Mubarak University Teaching Hospital during the period from January to December 2013.
All patients had a confirmed diagnosis of steroid-responsive INS. They were either newly diagnosed nephrotic patients at their first presentation or already known INS patients during a relapse. All patients were not on steroid treatment at the time of recruitment in the study nor during the 2 months prior to inclusion. All patients received only prednisolone during the study period with a dose of 60 mg/m2/day at relapse. No other immunosuppressive medication was added during the study period.
Diagnosis of nephrotic syndrome was based on the presence of edema, nephrotic range proteinuria and hypoalbuminemia.14,15 Nephrotic range proteinuria was defined as the presence of ≥40 mg/m2 body surface area/hour or a protein:creatinine ratio on spot early morning urine of ≥ 2.0. Hypoalbuminemia was defined as a serum albumin level of < 25 g/L. Relapse of nephrotic syndrome was defined as the appearance of nephrotic range proteinuria or urine albumin dipstick of ≥ 2+ on 3 successive days. Remission was defined as disappearance or trace of proteinuria on albumin dipsticks for 3 successive days. Thirty healthy age-and sex-matched children of the same ethnic background were included as controls. All controls were evaluated by an experienced clinician to confirm the absence of previous illnesses or current proteinuria. All had a serum creatinine (within 15–80 μmol/L) and normal serum albumin level (range: 30–40 g/L).
Immunoassays of cytokines
Freshly voided urine samples were collected and centrifuged at 1500 ×g at 4°C for 20 minutes.
The supernatant was then collected, aliquoted and immediately frozen at −80°C till the time of assessment. Urine samples, collected from patients and controls, were analyzed to determine concentrations of secreted cytokines, IL-1β, IL6 and IL8, using FlowCytomix kits (Bender Medsystems GmbH, Vienna, Austria), according to manufacturer’s instructions. These kits allow the simultaneous quantification of 10 cytokines at the same time. In brief, FlowCytomix technology is based on spectrally discrete microspheres that are used as solid phase in an immunoassay. The beads are internally dyed with Starfire Red, a far red (685–690 nm)-emitting fluorochrome, which is excited by UV, argon or HeNe lasers. The test samples were analyzed by flow cytometry using Coulter EPICS FC500 (Beckman Coulter Inc., Indianapolis, IN, USA). For each analysis, up to 10,000 events were acquired. The mean concentration of each cytokine was expressed as picogram per milliliter. Using the abovementioned kits, the minimum detectable concentrations of cytokines were IL-1β (4.2 pg/mL), IL-6 (1.2 pg/mL) and IL-8 (0.5 pg/mL).
The study was approved by the institutional ethics committee for the protection of human subjects in research at Health Sciences Center – Kuwait University. Written informed consent was obtained from caregivers of all patients and controls. Statistical analysis and graphical presentation were performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corporation, Armonk, NY, USA). Mann–Whitney nonparametric test was used to compare the significant difference in the values between the groups. χ2 or Fisher’s exact test was applied to evaluate differences in proportions. All P-values are two-sided, and P < 0.05 indicates the statistical significance.
Results
A total of 37 patients with INS were included (24 males and 13 females). The mean age of patients at study was 6.4 ± 3.2 years (range: 14 months–12 years). All INS patients were steroid-responsive to minimal change nephrotic syndrome (MCNS). Thirteen patients were newly diagnosed with INS at presentation while the other 24 patients were frequent relapsers who relapsed while off steroids for > 2 months. Eleven patients had renal biopsy during the course of the disease and all proved to have minimal change disease (MCNS). From those who were frequent relapsers, 14 had cyclophosphamide and 6 had cyclosporine but were off such medication for > 6 months prior to recruiting them in the study. All patients had normal serum creatinine with a mean of 47 ± 13 μmol/L. Mean serum albumin level during relapse was 21 ± 7 g/L. Table 1 summarizes the clinical and biochemical data of the patients and controls. Mean urinary IL-1β, IL-6 and IL-8, corrected to urinary creatinine, in patients during relapse were 132.94 ± 654.97, 217.82 ± 1124.31 and 150.227 ± 523.97 pg/μmol, respectively. Compared to the same cytokines in healthy controls (9.11 ± 40.75, 0.146 ± 0.652 and 6.455 ± 24.53 pg/μmol, respectively), the difference was statistically significant (P = 0.02, 0.03 and 0.014, respectively). During remission, no significant difference was reported in the mean level of the 3 cytokines (11.2 ± 30.8, 0.96 ± 3.7 and 160.3 ± 735.3, respectively) compared to controls (P = 0.94, 0.092 and 0.076). Figure 1 summarizes the results of IL-1β, IL-6 and IL-8 levels in INS patients and controls during relapse and remission.
Table 1.
Clinical and laboratory data of patients with INS and controls
| Patients’ parameters | INS patients (n = 37) |
Controls (n = 30) |
Normal range |
|---|---|---|---|
| Mean age (years) | 6.4 ± 3.2 | 7.1 ± 2.3 | |
| Sex | |||
| Male | 24 | 19 | |
| Female | 13 | 11 | |
| Mean serum creatinine (μmol/L) | 47 ± 13 | 51 ± 11 | 15–88 |
| Mean serum protein (g/L) | 52 + 6 | 72 + 2 | 6–80 |
| Mean serum albumin (g/L) | 21 ± 7 | 36 ± 3 | 30–40 |
| Mean serum cholesterol (mmol/L) | 6.3 ± 0.7 | 3.1 ± 1.5 | 3.1–5.2 |
| Mean UP:Cr ratio (mg/mg) | 2.6 ± 0.4 | 0.02 ± 0.07 | ≤ 0.2 |
Abbreviations: INS, idiopathic nephrotic syndrome; UP, urine protein; Cr, creatinine
Figure 1.
IL-6, IL-8 and IL-1β mean levels during relapse and remission of idiopathic nephrotic syndrome patients compared to controls.
Discussion
The role of the immune factors in triggering a relapse of idiopathic nephrotic syndrome has been a matter of debate during the past decade. The activation of T-cells leads to release of local cytokines that work as soluble circulating factors provoking increased glomerular permeability and podocytes’ barrier dysfunction with subsequent proteinuria.16 IL-1β and IL-6 are acute-phase proteins involved in T-cell, macrophage and B-cell activation. IL8 (CXCL8) is a chemokine produced by many cells, including macrophages, fibroblasts, renal mesangial, tubular cells and epithelial cells.1,3 It is involved in chemotaxis and angiogenesis of leukocytes.4 The close association of suppressed immune responsiveness with the occurrence of MCNS has suggested that the immunological and renal abnormalities in this condition have a common pathogenesis.17 Many previous studies reported that urine and serum from certain nephrotic patients contain soluble immune response suppressor (SIRS).18,19 Others showed an antigen-nonspecific suppressor lymphokine that inhibits antibody production in vitro20 as well as antibody production and delayed-type hypersensitivity response in vivo.21
In our study, we detected increase in IL-1β, IL-6 and IL-8 in the urine of INS patients during relapse which disappeared, except IL-8, during remission of the disease. These findings support the assumption of the important role of these cytokines in the immune process during a relapse.
Many previous studies had documented high serum levels of these 3 cytokines in INS patients,3,4,12–17 nevertheless the literature on the level of these cytokines in the urine of INS patients in particular and in patients with kidney disease, in general, is scarce. It is more likely that most cytokines exert their effects in situ at their site of production; therefore, their level in urine is more reflective, than serum levels, of the immune changes taking place during relapse and remission. Moreover, cytokines in the circulation might bind to their soluble receptors or other antagonistic molecules in the serum, which leads to some confusion in interpreting their exact levels and their significance.
Schnaper and Aune18 in their study on the SIRS lymphokine in the urine of nephrotic children had reported the increased levels of SIRS in the urine of the majority of INS patients studied (11 patients) while SIRS was increased in the serum of only some of these patients (4 patients). This supports the superiority of urine cytokine levels over serum levels in reflecting the immune process in the kidneys in INS.
Previous reports had suggested that some factors may increase the permeability of the glomerular basement membrane (GBM) in the kidneys.22,23 Moreover, these pro-inflammatory cytokines had distinctive roles not only in disease pathology but also played a critical role as progressive factors in INS.24,25 Zhang et al26 have shown that IL-6 plays an important role in angiotensin II-induced hypertension, proteinuria and renal fibrosis in chronic kidney disease (CKD). IL-6 was increased in the renal biopsies of CKD patients compared to normal control, and its levels were further elevated in CKD patients with hypertension. Zhang’s work on IL-6 deficient mice has provided direct evidence of the effect of IL-6 on GBM in the kidneys.26
IL-8 (also named CXCL-8) was shown to induce changes in the permeability of the GBM via decreasing the synthesis of heparan sulfate proteoglycans, which eventually induced proteinuria in rats.7 Podocytes express CXCR chemokine receptors and usually has the potential to respond to various soluble products of the immune system.27
The decrease in IL-1β and IL-6 urinary levels, but not IL-8, in INS patients during remission is expected and probably a response to treatment with steroids. Similarly, Schnaper and Aune18 could elicit that suppressive activity disappeared from the urine of INS patients after the initiation of treatment but before remission of NS.
Our finding of high levels of IL-8 during remission may suggest a prolonged exertion of such cytokine or probably a decreased urinary clearance rates or slow degradation of the cytokine.
While conducting this study we faced some limitations that had limited the number of patients recruited in this study. Many patients were recruited at the beginning, and a relapse urine sample was taken but unfortunately no relapse samples were collected as these patients did not show up for follow-up and subsequently had to be excluded.
Conclusion
The 3 pro-inflammatory cytokines, IL-1β, IL-6 and IL-8, play a major role during relapse in INS. Identifying such role might be a crucial step in paving the way to adopt novel therapeutic steroid-sparing options such as interleukin antibodies specific to these cytokines.
Acknowledgments
We thank the study subjects and their families for participation in this study. The assistance of Mrs Amani Al-Fadhli in statistical analysis is thankfully acknowledged.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pereira Wde F, Brito-Melo GE, Guimarães FT, Carvalho TG, Mateo EC, Simões e Silva AC. The role of the immune system in idiopathic nephrotic syndrome: a review of clinical and experimental studies. Inflamm Res. 2014;63(1):1–12. doi: 10.1007/s00011-013-0672-6. [DOI] [PubMed] [Google Scholar]
- 2.Shalaby SA, Al-Edressi HM, El-Tarhouny SA, Fath El-Bab M, Zolaly MA. Type 1/type 2 cytokine serum levels and role of interleukin-18 in children with steroid-sensitive nephrotic syndrome. Arab J Nephrol Transplant. 2013;6(2):83–88. [PubMed] [Google Scholar]
- 3.Shao XS, Yang XQ, Zhao XD, et al. The prevalence of Th17 cells and FOXP3 regulate T cells (Treg) in children with primary nephrotic syndrome. Pediatr Nephrol. 2009;24(9):1683–1690. doi: 10.1007/s00467-009-1194-x. [DOI] [PubMed] [Google Scholar]
- 4.Neuhaus TJ, Wadhwa M, Callard R, Barratt TM. Increased IL-2, IL-4 and interferon-gamma (IFN-gamma) in steroid-sensitive nephrotic syndrome. Clin Exp Immunol. 1995;100(3):475–479. doi: 10.1111/j.1365-2249.1995.tb03725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlotti AP, Franco PB, Elias LL, et al. Glucocorticoid receptors, in vitro steroid sensitivity, and cytokine secretion in idiopathic nephrotic syndrome. Kidney Int. 2004;65(2):403–408. doi: 10.1111/j.1523-1755.2004.00392.x. [DOI] [PubMed] [Google Scholar]
- 6.Yildiz B, Cetin N, Kural N, Colak O. CD19 + CD23+ B cells, CD4 + CD25+ T cells, E-selectin and interleukin-12 levels in children with steroid sensitive nephrotic syndrome. Ital J Pediatr. 2013;39:42. doi: 10.1186/1824-7288-39-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Q, Wu J, Tao J, et al. Role of basophils in the pathogenesis of minimal change nephrotic syndrome: a literature review. Exp Ther Med. 2014;8(4):1027–1031. doi: 10.3892/etm.2014.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanai T, Shiraishi H, Yamagata T, et al. Th2 cells predominates in idiopathic steroid sensitive nephrotic syndrome. Clin Exp Nephrol. 2010;14(6):578–583. doi: 10.1007/s10157-010-0330-z. [DOI] [PubMed] [Google Scholar]
- 9.Youn YS, Lim HH, Lee JH. The clinical characteristics of steroid responsive nephrotic syndrome of children according to the serum immunoglobulin E levels and cytokines. Yonsei Med J. 2012;53(4):715–722. doi: 10.3349/ymj.2012.53.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu LL, Qin Y, Cai JF, et al. Th17/Treg imbalance in adult patients with minimal change nephrotic syndrome. Clin Immunol. 2011;139(3):314–320. doi: 10.1016/j.clim.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Prasad N, Jaiswal AK, Agarwal V, et al. Differential alteration in peripheral T-regulatory and T-effector cells with change in P-glycoprotein expression in Childhood Nephrotic Syndrome: a longitudinal study. Cytokine. 2015;72(2):190–196. doi: 10.1016/j.cyto.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 12.Jaiswal A, Prasad N, Agarwal V, et al. Regulatory and effector T cells changes in remission and resistant state of childhood nephrotic syndrome. Indian J Nephrol. 2014;24(6):349–355. doi: 10.4103/0971-4065.132992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanai T, Yamagata T, Momoi MY. Macrophage inflammatory protein-1beta and interleukin-8 associated with idiopathic steroid-sensitive nephrotic syndrome. Pediatr Int. 2009;51(4):443–447. doi: 10.1111/j.1442-200X.2008.02759.x. [DOI] [PubMed] [Google Scholar]
- 14.International Study on Kidney Diseases in Children Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Diseases in Children. Kidney Int. 1978;13(2):159–165. doi: 10.1038/ki.1978.23. [DOI] [PubMed] [Google Scholar]
- 15.International Study on Kidney Diseases in Children The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Diseases in Children. J Pediatr. 1981;98(4):561–564. doi: 10.1016/s0022-3476(81)80760-3. [DOI] [PubMed] [Google Scholar]
- 16.Shimada M, Araya C, Rivard C, Ishimoto T, Johnson RJ, Garin EH. Minimal change disease: a “two-hit” podocyte immune disorder? Pediatr Nephrol. 2011;26(4):645–649. doi: 10.1007/s00467-010-1676-x. [DOI] [PubMed] [Google Scholar]
- 17.Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2(7880):556–560. doi: 10.1016/s0140-6736(74)91880-7. [DOI] [PubMed] [Google Scholar]
- 18.Schnaper HW, Aune TM. Identification of the lymphokine soluble immune response suppressor in urine of nephrotic children. J Clin Invest. 1985;76(1):341–349. doi: 10.1172/JCI111967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnaper HW. A regulatory system for soluble immune response suppressor production in steroid–responsive nephrotic syndrome. Kidney Int. 1990;38(1):151–159. doi: 10.1038/ki.1990.180. [DOI] [PubMed] [Google Scholar]
- 20.Schnaper HW, Pierce CW, Aune TM. Identification and initial characterization of concanavalin A- and interferon-induced human suppressor factors: evidence for a human equivalent of murine soluble immune response suppressor (SIRS) J Immunol. 1984;132(5):2429–2435. [PubMed] [Google Scholar]
- 21.Schnaper HW, Aune TM. Suppression of immune response to sheep erythrocytes by the lymphokine soluble immune response suppressor (SIRS) in vivo. J Immunol. 1986;137(3):863–868. [PubMed] [Google Scholar]
- 22.Zhang SY, Audard V, Fan Q, Pawlak A, Lang P, Sahali D. Immunopathogenesis of idiopathic nephrotic syndrome. Contrib Nephrol. 2011;169:94–106. doi: 10.1159/000313947. [DOI] [PubMed] [Google Scholar]
- 23.Davin JC. The glomerular permeability factors in idiopathic nephrotic syndrome. Pediatr Nephrol. 2016;31(2):207–215. doi: 10.1007/s00467-015-3082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho BS, Yoon SR, Jang JY, et al. Upregulation of interleukin-4 and CD23/Fc epsilon RII in minimal change nephrotic syndrome. Pediatr Nephrol. 1999;13:199–204. doi: 10.1007/s004670050592. [DOI] [PubMed] [Google Scholar]
- 25.Noronha IL, Niemir Z, Stein H, et al. Cytokines and growth factors in renal disease. Nephrol Dial Transplant. 1995;10(6):775–786. [PubMed] [Google Scholar]
- 26.Zhang W, Wang W, Yu H, et al. Interleukin 6 underlies angiotensin II induced hypertension and chronic renal damage. Hypertension. 2012;59(1):136–144. doi: 10.1161/HYPERTENSIONAHA.111.173328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber TB, Reinhart HC, Exner MT, et al. Expression of functional CCR and CXCR chemokine receptors in podocytes. J Immunol. 2002;168(12):6244–6252. doi: 10.4049/jimmunol.168.12.6244. [DOI] [PubMed] [Google Scholar]