Abstract
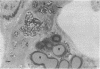
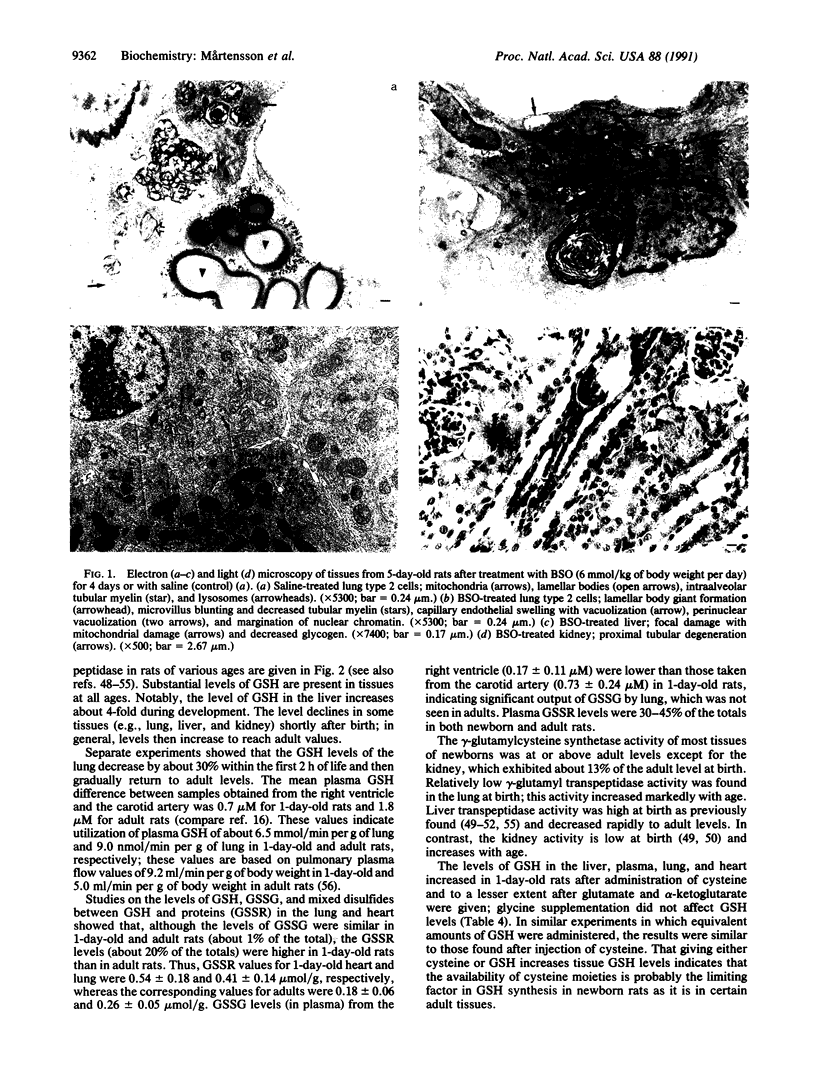
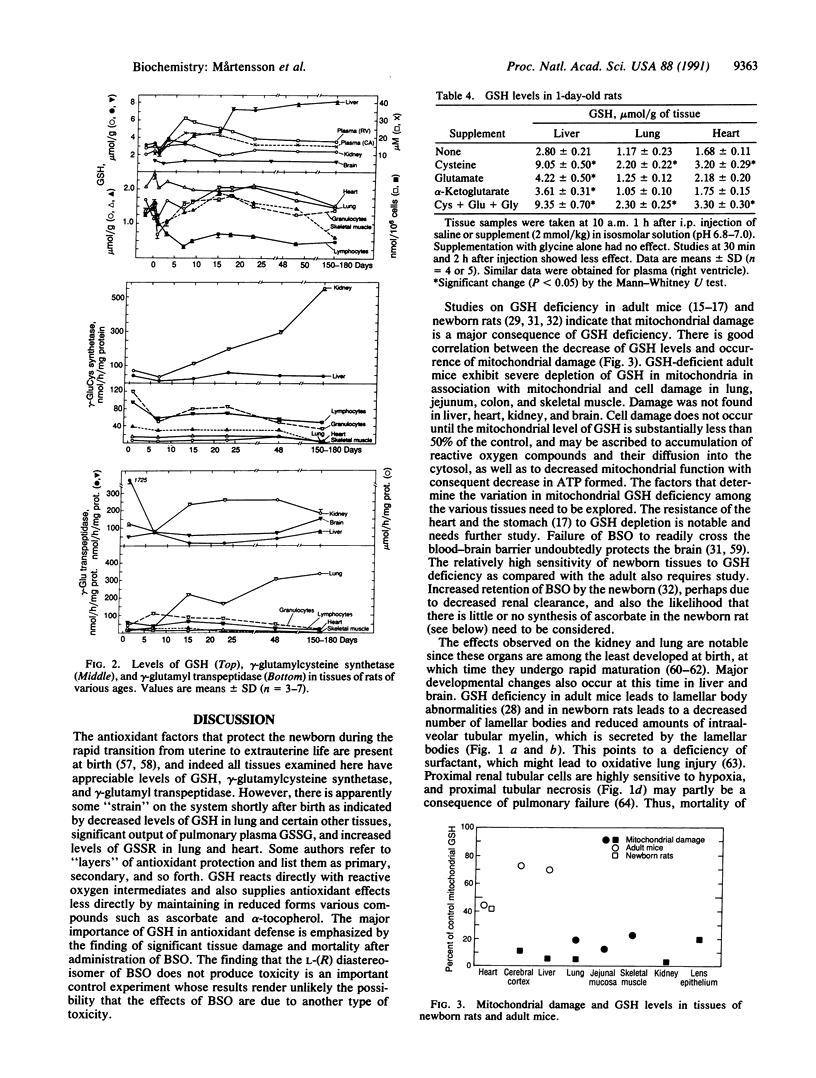
A model for oxidative stress is described in which glutathione (GSH) synthesis is selectively blocked in newborn rats by administration of L-buthionine-(S,R)-sulfoximine (BSO). In this model, the normal endogenous physiological formation of reactive oxygen species is largely unopposed, and therefore oxidative tissue damage occurs; because GSH is used for reduction of dehydroascorbate, tissue ascorbate levels decrease. In lung there are decreased numbers of lamellar bodies and decrease of intraalveolar surfactant. Proximal renal tubular, hepatic, and brain damage also occur. A diastereoisomer of BSO that does not inhibit GSH synthesis, L-buthionine-R-sulfoximine, does not produce toxicity; this control experiment renders it unlikely that the observed effects of BSO are produced by the sulfoximine moiety itself. There is correlation between the decrease of mitochondrial GSH levels and mitochondrial and cell damage. Oxidative stress as evaluated by mitochondrial damage and mortality can be prevented by treatment with GSH esters or ascorbate. There is apparent linkage between the antioxidant actions of GSH and ascorbate. This model, which may readily be applied to evaluation of the efficacy of other compounds in preventing oxidative stress, offers an approach to study of other effects of GSH deficiency (e.g., on lipid metabolism, hematopoiesis), and closely resembles oxidative stress that occurs in certain human newborns and in other clinical states.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Meister A. Glutathione monoesters. Anal Biochem. 1989 Nov 15;183(1):16–20. doi: 10.1016/0003-2697(89)90164-4. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Meister A. Transport and direct utilization of gamma-glutamylcyst(e)ine for glutathione synthesis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):707–711. doi: 10.1073/pnas.80.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. E., Powrie F., Puri R. N., Meister A. Glutathione monoethyl ester: preparation, uptake by tissues, and conversion to glutathione. Arch Biochem Biophys. 1985 Jun;239(2):538–548. doi: 10.1016/0003-9861(85)90723-4. [DOI] [PubMed] [Google Scholar]
- Aperia A., Herin P. Development of glomerular perfusion rate and nephron filtration rate in rats 17-60 days old. Am J Physiol. 1975 May;228(5):1319–1325. doi: 10.1152/ajplegacy.1975.228.5.1319. [DOI] [PubMed] [Google Scholar]
- Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973 Jul;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972 Jul;128(3):617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Calvin H. I., Medvedovsky C., Worgul B. V. Near-total glutathione depletion and age-specific cataracts induced by buthionine sulfoximine in mice. Science. 1986 Aug 1;233(4763):553–555. doi: 10.1126/science.3726547. [DOI] [PubMed] [Google Scholar]
- Campbell E. B., Griffith O. W. Glutathione monoethyl ester: high-performance liquid chromatographic analysis and direct preparation of the free base form. Anal Biochem. 1989 Nov 15;183(1):21–25. doi: 10.1016/0003-2697(89)90165-6. [DOI] [PubMed] [Google Scholar]
- Dauber I. M., Krauss A. N., Symchych P. S., Auld P. A. Renal failure following perinatal anoxia. J Pediatr. 1976 May;88(5):851–855. doi: 10.1016/s0022-3476(76)81130-4. [DOI] [PubMed] [Google Scholar]
- Frank L., Bucher J. R., Roberts R. J. Oxygen toxicity in neonatal and adult animals of various species. J Appl Physiol Respir Environ Exerc Physiol. 1978 Nov;45(5):699–704. doi: 10.1152/jappl.1978.45.5.699. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981 Nov 10;256(21):10986–10992. [PubMed] [Google Scholar]
- Gleason C. A. Prostaglandins and the developing kidney. Semin Perinatol. 1987 Jan;11(1):12–21. [PubMed] [Google Scholar]
- Griffith O. W., Anderson M. E., Meister A. Inhibition of glutathione biosynthesis by prothionine sulfoximine (S-n-propyl homocysteine sulfoximine), a selective inhibitor of gamma-glutamylcysteine synthetase. J Biol Chem. 1979 Feb 25;254(4):1205–1210. [PubMed] [Google Scholar]
- Griffith O. W., Bridges R. J., Meister A. Transport of gamma-glutamyl amino acids: role of glutathione and gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6319–6322. doi: 10.1073/pnas.76.12.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem. 1982 Nov 25;257(22):13704–13712. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Translocation of intracellular glutathione to membrane-bound gamma-glutamyl transpeptidase as a discrete step in the gamma-glutamyl cycle: glutathionuria after inhibition of transpeptidase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):268–272. doi: 10.1073/pnas.76.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guignard J. P. Renal function in the newborn infant. Pediatr Clin North Am. 1982 Aug;29(4):777–790. doi: 10.1016/s0031-3955(16)34211-0. [DOI] [PubMed] [Google Scholar]
- Hawgood S., Clements J. A. Pulmonary surfactant and its apoproteins. J Clin Invest. 1990 Jul;86(1):1–6. doi: 10.1172/JCI114670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A., Mårtensson J., Stole E., Auld P. A., Meister A. Glutathione deficiency leads to mitochondrial damage in brain. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1913–1917. doi: 10.1073/pnas.88.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen G. L., Meister A. Radioprotection of human lymphoid cells by exogenously supplied glutathione is mediated by gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4714–4717. doi: 10.1073/pnas.80.15.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J. C., Clark J. B. Preparation of synaptic and nonsynaptic mitochondria from mammalian brain. Methods Enzymol. 1979;55:51–60. doi: 10.1016/0076-6879(79)55008-3. [DOI] [PubMed] [Google Scholar]
- Lambert G. H., Thorgeirsson S. S. Glutathione in the developing mouse liver--I. Developmental curve and depletion after acetaminophen treatment. Biochem Pharmacol. 1976 Aug 1;25(15):1777–1781. doi: 10.1016/0006-2952(76)90415-9. [DOI] [PubMed] [Google Scholar]
- Loschen G., Flohé L., Chance B. Respiratory chain linked H(2)O(2) production in pigeon heart mitochondria. FEBS Lett. 1971 Nov 1;18(2):261–264. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- Marklund S. A simple specific method for the determination of the hemoglobin content of tissue homogenates. Clin Chim Acta. 1979 Mar 1;92(2):229–234. doi: 10.1016/0009-8981(79)90117-7. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Mãrtensson J., Meister A., Mrtensson J. Glutathione deficiency decreases tissue ascorbate levels in newborn rats: ascorbate spares glutathione and protects. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4656–4660. doi: 10.1073/pnas.88.11.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Frayer W., Meister A. Glutathione metabolism in the lung: inhibition of its synthesis leads to lamellar body and mitochondrial defects. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5296–5300. doi: 10.1073/pnas.86.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Meister A. Glutathione is required for intestinal function. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1715–1719. doi: 10.1073/pnas.87.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Lai J. C., Meister A. High-affinity transport of glutathione is part of a multicomponent system essential for mitochondrial function. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7185–7189. doi: 10.1073/pnas.87.18.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Meister A. Mitochondrial damage in muscle occurs after marked depletion of glutathione and is prevented by giving glutathione monoester. Proc Natl Acad Sci U S A. 1989 Jan;86(2):471–475. doi: 10.1073/pnas.86.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J. Method for determination of free and total glutathione and gamma-glutamylcysteine concentrations in human leukocytes and plasma. J Chromatogr. 1987 Sep 4;420(1):152–157. doi: 10.1016/0378-4347(87)80166-4. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Steinherz R., Jain A., Meister A. Glutathione ester prevents buthionine sulfoximine-induced cataracts and lens epithelial cell damage. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8727–8731. doi: 10.1073/pnas.86.22.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J., Cannon B. Overview--preparation and properties of mitochondria from different sources. Methods Enzymol. 1979;55:3–28. doi: 10.1016/0076-6879(79)55003-4. [DOI] [PubMed] [Google Scholar]
- Redinbaugh M. G., Turley R. B. Adaptation of the bicinchoninic acid protein assay for use with microtiter plates and sucrose gradient fractions. Anal Biochem. 1986 Mar;153(2):267–271. doi: 10.1016/0003-2697(86)90091-6. [DOI] [PubMed] [Google Scholar]
- Rollins D., Larsson A., Steen B., Krishnaswamy K., Hagenfeldt L., Moldéus P., Rane A. Glutathione and gamma-glutamyl cycle enzymes in human fetal liver. J Pharmacol Exp Ther. 1981 Jun;217(3):697–700. [PubMed] [Google Scholar]
- Slivka A., Spina M. B., Calvin H. I., Cohen G. Depletion of brain glutathione in preweanling mice by L-buthionine sulfoximine. J Neurochem. 1988 May;50(5):1391–1393. doi: 10.1111/j.1471-4159.1988.tb03021.x. [DOI] [PubMed] [Google Scholar]
- States B., Foreman J. W., Segal S. Cysteine and glutathione levels in developing rat kidney and liver. Pediatr Res. 1987 Nov;22(5):605–608. doi: 10.1203/00006450-198711000-00025. [DOI] [PubMed] [Google Scholar]
- Steinherz R., Mårtensson J., Wellner D., Meister A. Transport into brain of buthionine sulfoximine, an inhibitor of glutathione synthesis, is facilitated by esterification and administration of dimethylsulfoxide. Brain Res. 1990 Jun 4;518(1-2):115–119. doi: 10.1016/0006-8993(90)90961-a. [DOI] [PubMed] [Google Scholar]
- Suthanthiran M., Anderson M. E., Sharma V. K., Meister A. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc Natl Acad Sci U S A. 1990 May;87(9):3343–3347. doi: 10.1073/pnas.87.9.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M., Inoue M. Ontogenic changes in metabolism and transport of glutathione in the rat. J Biochem. 1986 Dec;100(6):1457–1463. doi: 10.1093/oxfordjournals.jbchem.a121851. [DOI] [PubMed] [Google Scholar]
- Tanswell A. K., Freeman B. A. Pulmonary antioxidant enzyme maturation in the fetal and neonatal rat. I. Developmental profiles. Pediatr Res. 1984 Jul;18(7):584–587. doi: 10.1203/00006450-198407000-00003. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Meister A. Identity of maleate-stimulated glutaminase with gamma-glutamyl transpeptidase in rat kidney. J Biol Chem. 1975 Jun 25;250(12):4619–4627. [PubMed] [Google Scholar]
- Tate S. S., Meister A. gamma-Glutamyl transpeptidase from kidney. Methods Enzymol. 1985;113:400–419. doi: 10.1016/s0076-6879(85)13053-3. [DOI] [PubMed] [Google Scholar]
- Tateishi N., Higashi T., Nakashima K., Sakamoto Y. Nutritional significance of increase in gamma-glutamyltransferase in mouse liver before birth. J Nutr. 1980 Mar;110(3):409–415. doi: 10.1093/jn/110.3.409. [DOI] [PubMed] [Google Scholar]
- Tsui E., Yeung D. Development of gamma-glutamylcysteine synthetase and oxoprolinase in rat kidney. Experientia. 1979 Oct 15;35(10):1293–1294. doi: 10.1007/BF01963965. [DOI] [PubMed] [Google Scholar]
- Wellner V. P., Anderson M. E., Puri R. N., Jensen G. L., Meister A. Radioprotection by glutathione ester: transport of glutathione ester into human lymphoid cells and fibroblasts. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4732–4735. doi: 10.1073/pnas.81.15.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellner V. P., Sekura R., Meister A., Larsson A. Glutathione synthetase deficiency, an inborn error of metabolism involving the gamma-glutamyl cycle in patients with 5-oxoprolinuria (pyroglutamic aciduria). Proc Natl Acad Sci U S A. 1974 Jun;71(6):2505–2509. doi: 10.1073/pnas.71.6.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells W. W., Xu D. P., Yang Y. F., Rocque P. A. Mammalian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase activity. J Biol Chem. 1990 Sep 15;265(26):15361–15364. [PubMed] [Google Scholar]