More than 30 years ago it was observed that the storage proteins of maize (Zea mays) seeds accumulate as “bulges or localized dilatations along the endoplasmic reticulum cisternae” of developing endosperm cells (Khoo and Wolf, 1970). It later became evident that, whereas the widespread seed storage proteins of the 7S and 11S classes travel from the endoplasmic reticulum (ER) to the Golgi complex and are then deposited in vacuoles, a number of cereal storage proteins instead form electron-dense, round-shaped structures with diameters of 0.5 to 2.0 μm, termed protein bodies, within the ER lumen (Herman and Larkins, 1999). These large aggregates are then either permanently stored in the ER or delivered to storage vacuoles by unconventional protein traffic pathways. Today, the mechanisms by which some of the most important proteins for human nutrition form protein bodies within the ER remain a fascinating but still puzzling issue in cell biology. This developmentally programmed use of the ER to store vast amounts of specific proteins in highly condensed forms has been found only in plants. On the other hand, aggregation of newly synthesized proteins in the ER, due to stress or genetic defects, is usually treated by the cell as a pathology that must be avoided by disposing of the misfolded proteins (Sitia and Braakman, 2003). Many ER resident proteins indeed have the role of preventing protein aggregation, maintaining nascent and newly synthesized polypeptides in a state that is compatible with further structural maturation or, for defective proteins, with degradation. Thus, proteins destined for storage in the ER must have evolved to condense in a controlled fashion and thus to avoid both export from the ER and degradation. How this might be achieved is the topic of this update.
The ER is part of the endomembrane system, which also includes the Golgi complex, vacuoles, and the plasma membrane as major components. The system hosts the secretory pathway, which synthesizes and delivers to the correct location most of the proteins of the above-mentioned compartments and of the cell wall. These proteins are collectively termed secretory proteins. The ER plays the role of a protein nursery, assisting in the folding and assembly of newly synthesized secretory proteins before they traffic to the Golgi complex and then the vacuoles or the cell surface (Vitale and Denecke, 1999). Many residents of the ER have signals that promote their localization in this compartment (Vitale and Denecke, 1999), but storage proteins that accumulate within the ER do not carry any of these known signals. How do they form stable structures in the ER? To try answering, we need to take into consideration many aspects of ER functions and their regulation.
PROTEIN QUALITY CONTROL
Sequencing of plant genomes shows that thousands of genes encode proteins with signals for insertion into the secretory pathway; consistently, the ER is very well equipped to assist in the folding and assembly of newly synthesized secretory polypeptides (Vitale and Denecke, 1999). Indeed, it seems that, directly or indirectly, most ER residents are helpers of protein folding. Proteins that fail to undergo correct conformational maturation are dislocated from the ER into the cytosol or delivered to vacuoles and then degraded. The promotion of protein folding and the disposal of defective proteins are strictly related and are collectively termed quality control (Vitale and Denecke, 1999; Sitia and Braakman, 2003).
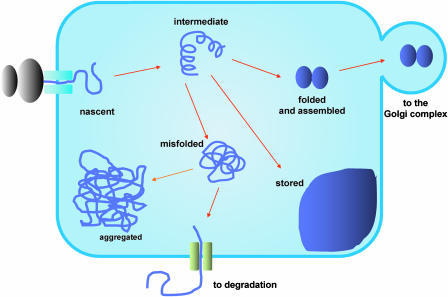
The difference between a polypeptide in the process of folding and a defective one that cannot fold or assemble may, however, be very subtle; thus, the ER is subject to an apparent paradox: it must degrade defective proteins but is continuously loaded with proteins that are in the process of folding and assembly and therefore could be considered transiently defective (Fig. 1). How does the ER quality control machinery discriminate between the two? A timing mechanism within the ER seems to be the answer in the case of glycoprotein disposal (Sitia and Braakman, 2003). In addition, the interactions of structurally defective proteins with chaperones and other helpers are more persistent than those of wild-type proteins, and this may be crucial in maintaining unfolded proteins in a state that is compatible with degradation.
Figure 1.
A schematic view of quality control. Secretory proteins emerge cotranslationally into the ER lumen. Nascent chains undergo intermediate steps of structural maturation. During this process they are retained in the ER. Once a protein has folded and, in the case of multimeric proteins, assembled, it is available for traffic to its final destination. Proteins with permanent defects are retained in the ER for prolonged periods of time (or rapidly retrieved from the Golgi complex) and often eventually dislocated into the cytosol for degradation; alternatively, they accumulate as aggregates in the ER. In plant cells, a number of proteins are naturally retained and stored permanently or transiently in the ER as large assembled/aggregated structures.
However, some native or artificial storage proteins accumulated as protein bodies in the ER also associate for a long time after synthesis with the plant homologue of the immunoglobulin heavy chain binding protein (BiP), which is the ER member of the heat shock 70 chaperone family (Li et al., 1993; and in this issue Mainieri et al., 2004). Persistent interactions with the ER helpers are therefore not sufficient per se for degradation, and this seems to constitute a second paradox, at least in the cells that accumulate storage proteins in the ER.
Storage proteins accumulating in the ER must therefore be able to take advantage of the folding machinery of this compartment to reach a conformational state that is not compatible with export and that, at the same time, does not direct the protein to the degradative phase of quality control. To understand how this can be achieved we first need to examine the mechanisms of protein export from the ER.
LEAVING THE ER
Export from the ER requires the recruitment of a specific coat protein complex, termed COPII, on the cytosolic face of the ER membrane, and it is thought to occur at the level of a subcompartment (transitional ER) defined by the presence of COPII components (Barlowe, 2003). This forward movement is balanced by backwards recycling traffic that retrieves membrane lipids and specific proteins and uses a different coat, termed COPI. Two nonexclusive models have been proposed for the export of soluble proteins (Vitale and Denecke, 1999; Barlowe, 2003). According to the first, the bulk-flow model, proteins are not concentrated at ER exit sites and diffuse in a passive fashion into COPII vesicles. Concentration of secretory cargo would not occur at the exit of the ER, but rather by exclusion from the COPI vesicles originating from the Golgi complex that recycle ER proteins. According to the second model, receptor-mediated mechanisms allow cargo concentration into COPII vesicles, and evidence has been collected indicating that membrane proteins ERGIC53, Emp24p, and Erv29p are implicated in the recognition of certain soluble proteins and in their export from the ER (Barlowe, 2003). These receptors must be able to bind sorting signals present in soluble secretory proteins and to actively recruit COPII. These signals may be concealed in misfolded proteins, and this could contribute to their retention in the ER.
Retention of misfolded proteins may also be due to their interaction with ER folding factors. ER chaperones and folding helpers often contain signals (such as the KDEL/HDEL motif in soluble ER residents and the dilysine/diarginine motif in membrane residents; Vitale and Denecke, 1999) that are recognized in the Golgi complex and promote retrieval into the ER by COPI vesicles. Certain misfolded proteins can thus be retrieved from the Golgi complex by virtue of their association with a recycling chaperone. Chaperones may also be retained because they form an insoluble protein matrix that effectively reduces their inclusion in budding COPII vesicles. It would seem reasonable to assume that binding to such a resident protein matrix could effectively hamper the export of unfolded proteins from the ER (Sitia and Braakman, 2003).
In conclusion, depending on the protein and on the mechanism of export, the lack of (exposed) export signals in misfolded proteins, together with the binding to molecular chaperones in the ER and to retrieval systems, can contribute to quality control.
PROTEIN AGGREGATES
One additional mechanism of protein retention in the ER is mediated by the formation of protein aggregates. In this case, the intrinsic biophysical properties of the protein lead to the formation of complexes that are unable to be inserted in the lumen of COPII vesicles. The accumulation of protein aggregates in the ER is normally induced by stress conditions or by the expression of defective proteins. In these situations, the degradative capacity of the ER may be insufficient to take care of the bulk of misfolded proteins, which may then be segregated into subregions of the ER (Sitia and Braakman, 2003).
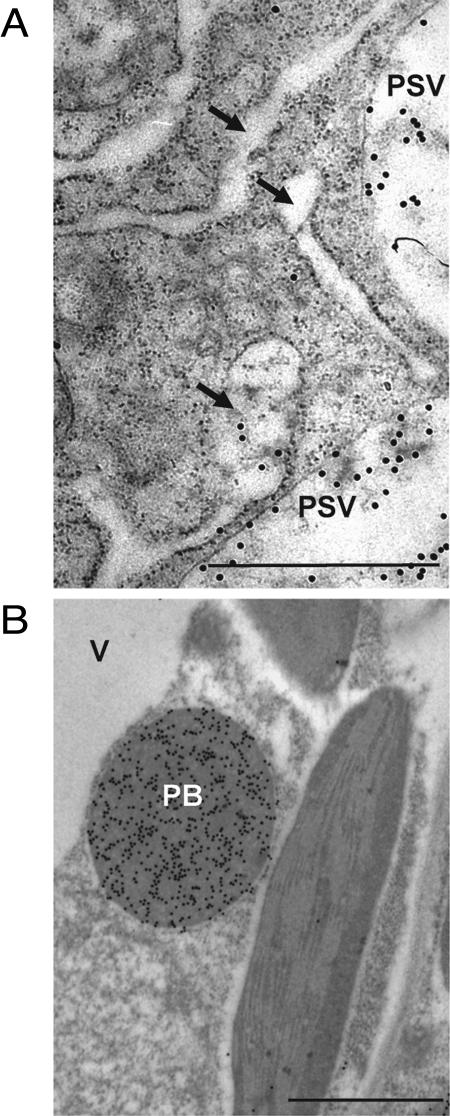
It should be stressed that the accumulation of aggregated proteins in the ER is clearly not sufficient to drive the formation of protein body-like structures, as illustrated in the following examples. Tunicamycin is a bacterial antibiotic that inhibits N-glycosylation, a process that occurs cotranslationally in the ER lumen and is required for proper folding of many glycoproteins. In developing lima bean (Phaseolus lunatus) cotyledons, tunicamycin treatment leads to gross misfolding of a set of storage glycoproteins, and BiP is found in association with the aggregated proteins (Sparvoli et al., 2000). The aggregates are detected by centrifugation in velocity gradients, but they do not form specific protein body-like structures within the ER, which enlarges in an apparently uniform process (Fig. 2A). Similarly, in Phaseolus vulgaris developing cotyledons, heat shock blocks traffic of the vacuolar lectin phytohemagglutinin and causes uniform dilatation of ER cisternae (Chrispeels and Greenwood, 1987). These results imply that specific features of the aggregating proteins are required for the formation of discrete protein body-like accretions within the ER lumen.
Figure 2.
Artificial overaccumulation of protein in the ER does not always result in the same alteration of ER morphology. When developing lima bean cotyledons are treated with tunicamycin (A), the storage proteins destined for storage vacuoles aggregate and the ER cisternae (arrows) enlarge, maintaining an electron-transparent aspect. When zeolin, a chimeric protein composed of phaseolin and γ-zein domains, is expressed in tobacco leaves (B), it forms round-shaped, electron-dense protein bodies within the ER. Immuno-gold labeling in A, performed with anti-7S storage protein antiserum, decorates the storage protein already accumulated in storage vacuoles before the tunicamycin treatment, as well as some aggregates within the ER. Immuno-gold labeling in B, performed with antiphaseolin antiserum, decorates the protein body. PB, Protein body; PSV, protein storage vacuole; V, vegetative vacuole. Bars = 1 μm. For more details on these experiments see Sparvoli et al. (2000) and Mainieri et al. (2004).
Depending on the protein, individual chaperones can be included or excluded from the aggregates, but sequestration of excessive amounts of folding helpers in these aggregates is likely to be detrimental to the cell. Consistent with this view, it has been proposed that BiP sequestration into aggregates can lead to a reduction of free BiP to a level that cannot be tolerated by a yeast (Saccharomyces cerevisiae) strain unable to respond to ER stress (Umebayashi et al., 1999).
PROTEIN BODIES
Storage proteins accumulating in the ER may take advantage of any of the above-mentioned retention or retrieval mechanisms and use controlled aggregation as a way to escape the final degradative phase of quality control and accumulate in the ER lumen without exposing this compartment to an excessive level of stress.
Protein body formation has been extensively studied in maize. The protein bodies that form in the ER of maize endosperm cells contain a set of polypeptides that are classified as α-, β-, γ-, and δ-zeins. Individual β- or γ- zeins can form stable protein body-like accretions in the ER of transgenic plants in the absence of other subunits (for review, see Herman and Larkins, 1999), but it is clear that formation of protein bodies in maize normally involves both homotypic and heterotypic interactions (Kim et al., 2002). The latter have been shown to be important for the stability of α- and δ-zeins in transgenic tobacco (Nicotiana tabacum) plants. The accumulation of these two proteins is highly increased when they are coexpressed with γ-zein or β-zein, respectively (Herman and Larkins, 1999). Interestingly, γ- and β-zeins are synthesized before the α- and δ- polypeptides during maize seed development, supporting the hypothesis of their fundamental role in the formation of a stable protein body.
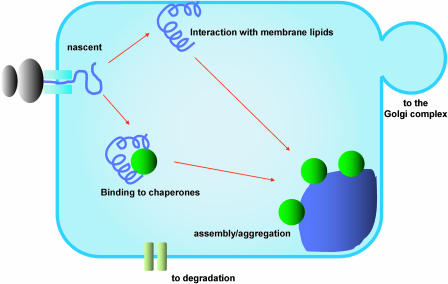
The mechanisms that determine the retention of zein polypeptides in the ER are still unclear. According to the models of protein export outlined above, either binding to ER chaperones or lack of export signals could raise the concentration of all these proteins into the ER to a level compatible with the formation of protein aggregates. Alternatively, aggregation of these proteins could be so fast as to avoid altogether their diffusion to ER export sites. Direct interactions with membrane lipids may also occur, due to the particular secondary structure of certain domains of γ-zein (Kogan et al., 2004). Which of these models is correct is at present unknown (Fig. 3).
Figure 3.
Possible mechanisms of protein body biogenesis. Newly synthesized polypeptides destined for storage in the ER interact with chaperones and folding helpers, similarly to the other secretory proteins. These interactions are, however, persistent. Specific domains may also interact directly with the lipids of the ER membrane. The possible relationships between the two events are unknown, and both may contribute to retention in the ER until protein bodies are formed. The involvement of each of these processes is likely to vary between different protein body proteins. The mature protein bodies are too large to enter COPII vesicles and are permanently retained in the ER, or transported to the vacuole by alternative pathways. Chaperones like BiP may remain present on the surface of the protein body, to assist the late steps of assembly and avoid uncontrolled exposure of hydrophobic domains that could negatively affect ER functions.
A region necessary for retention within the ER has been identified in a γ-zein polypeptide by mutagenesis and expression in transgenic Arabidopsis (Arabidopsis thaliana) plants. This polypeptide consists of a Pro-rich tandem repeat domain, followed by a linker region and by a C-terminal Cys-rich domain. While the wild-type protein is retained by Arabidopsis leaf protoplasts and localizes to protein bodies from which BiP is largely excluded, a deletion mutant lacking the repeat and linker domains is secreted (Geli et al., 1994). These results strongly suggest that retention in the ER is not simply due to the absence of an active export signal and implicate the repeat domain in retention. Interestingly, a truncated version of the protein consisting of the repeat and linker domains accumulates in enlarged regions of the ER of Arabidopsis cells that can also be stained with an anti-BiP antiserum (Geli et al., 1994). Whether these sequences contain a BiP-binding site remains to be determined, but these data would be compatible with the view that the N-terminal region of the γ-zein protein may mediate retention within the ER by first binding to BiP and then driving protein body formation. Consistently, when the repeat and linker regions of γ-zein are appended to the trimeric vacuolar protein phaseolin, the fusion protein, termed zeolin, is retained in the ER and stably deposited in protein body-like structures (Fig. 2B; see Mainieri et al., 2004). These protein bodies sequester a proportion of BiP, which can be released by ATP in vitro treatment, indicating that BiP is not trapped but is acting as a chaperone (heat shock-70 chaperones are ATPases and use ATP hydrolysis to perform their function). Zeolin protein bodies are insoluble and can be solubilized and disassembled by reducing agents (Mainieri et al., 2004). These results indicate that disulfide bonds are a key factor in zeolin protein body assembly and that the process is extensively assisted by BiP. Further work directly addressing the question whether BiP or other chaperones bind the N-terminal domain of γ-zeins within the context of the native protein will be necessary to clarify the mechanism by which the repetitive domain drives retention in the ER. As mentioned above, a role can also be played by direct interactions with lipids because a synthetic version of the repetitive domain of γ-zein strongly interacts with liposomes in vitro (Kogan et al., 2004).
γ-Zeins belong to the prolamin superfamily of storage proteins, which has representatives in a range of cereals such as wheat (Triticum aestivum), oats (Avena sativa), and rice (Oryza sativa). The rice prolamins do not contain a repeated sequence, but their deposition has been shown to be assisted by the chaperone BiP (Li et al., 1993). BiP is associated to the surface of rice prolamin protein bodies (Muench et al., 1997) and has been proposed to be necessary to maintain the prolamins in a competent state for subsequent assembly in the ER. In wheat endosperm, BiP is found within the prolamine protein bodies rather than bound to their surface, possibly suggesting that this chaperone plays different functions in the biogenesis of protein bodies in different species (Levanony et al., 1992).
It is also worth noting that proteins with similar primary structure may be differently recognized by the ER quality control system and that the level of export competency of a given protein may be dependent on the environment encountered within the ER lumen. In wheat, the sulfur-rich prolamins include both low Mr (LMW) glutenin subunits and γ-gliadins. The two classes of proteins share the same domain architecture, with an N-terminal repetitive domain rich in Gln and Pro, and a C-terminal domain stabilized by a set of conserved disulfide bonds. However, while gliadins are mainly monomeric, LMW glutenin subunits assemble into disulfide-linked polymers. Individual members of these two classes of proteins have different intracellular fates when expressed in Xenopus oocytes. While a γ-gliadin polypeptide is in large part export competent, an LMW glutenin subunit is instead fully retained, possibly at the level of the ER (Altschuler and Galili, 1994). The mechanism(s) that mediate the complete retention of the LMW glutenin subunit remain unclear, but structural features within the C-terminal nonrepetitive domain may be involved (Altschuler and Galili, 1994).
It is interesting to note that the γ-gliadin polypeptide was found to be transport competent also when expressed in transgenic tobacco plants (Napier et al., 1997), but it was found to form protein body-like structures in the yeast ER (Rosenberg et al., 1993). The cell type-specific destiny of a γ-gliadin when expressed in heterologous systems may reflect differences in the ER machinery or in the concentration reached by the newly synthesized protein in the ER lumen. Indeed, protein assembly is a concentration-dependent process and can be dramatically affected by changes in the rate of protein synthesis (Ceriotti et al., 1991). In the native tissue, high biosynthetic levels, interactions with other polypeptides, or other seed-specific factors might reduce the ability of γ-gliadins to leave the ER, as reflected by the scarce proliferation of Golgi complex in wheat endosperm cells during the onset of storage protein synthesis and deposition (Levanony et al., 1992).
The role of tissue-specific modifications or interactions with partner prolamins in determining the final destiny of a polypeptide after translocation into the ER is also highlighted by the observation that a modified γ-zein is correctly incorporated into native protein bodies when expressed in maize endosperm but, unlike its wild-type counterpart (see above), in part secreted in transgenic Arabidopsis plants (Alvarez et al., 1998).
THE UNFOLDED PROTEIN RESPONSE
The abundance and workload of the ER are certainly not uniform in different tissues, as for example testified by the high ER proliferation during seed development, and are also influenced by stress situations or pathogen attacks (most pathogen defense proteins are secretory proteins). An interesting issue of cell biology is how the ER regulates its functions to accommodate increased production of secretory proteins or to alleviate the stress imposed by sudden perturbation of protein folding. This process has been termed unfolded protein response (UPR) and has been more deeply studied in yeast and in mammalian cells (Rutkowski and Kaufman, 2004).
UPR has obvious relationships with quality control (Sitia and Braakman, 2003; Rutkowski and Kaufman, 2004). The major link seems to be constituted by BiP. Besides its interactions with newly synthesized secretory proteins, in mammalian cells BiP also binds more permanently three transmembrane proteins that reside in the ER: PERK, IRE1, and ATF6 (Rutkowski and Kaufman, 2004). When, for any reason, the amount of BiP ligands in the ER increases, the chaperone is displaced from these three proteins to become involved as a folding helper. As a result, PERK and IRE1 dimerize, and ATF6 is transported to the Golgi complex. These three events start a cascade of reactions resulting in the specific transcription of genes that encode folding helpers of the ER, coupled to a general inhibition of protein synthesis. If these UPR reactions do not succeed in alleviating the stress, apoptosis can eventually be induced. Therefore, the cell senses the amount of BiP that is not working as a chaperone. Structurally defective proteins introduced into the ER undergo abnormally persistent interactions with BiP before being targeted for degradation, and therefore they displace a high proportion of BiP from the three sensors (Rutkowski and Kaufman, 2004). A rapid increase of workload of the ER, as a result of programmed or stress-induced increase in the expression of secretory protein genes, can also have the same effect.
WHO COMES FIRST?
Because many secretory proteins are N-glycosylated and because glycosylation is often important for protein folding and solubility, tunicamycin is a potent and widely used inducer of UPR but, obviously, tunicamycin is not a frequent inducer of UPR during the life of a plant. It is therefore interesting to analyze other situations in which increased levels of ER folding helpers have been detected. BiP and protein disulfide isomerase (an ER-located oxidoreductase) are up-regulated in storage cells of developing seeds, compared to vegetative green tissues (Kalinski et al., 1995; Shimoni et al., 1995; Muench et al., 1997). In developing seeds, UPR induction could be due to displacement of BiP from the transmembrane ER effectors because of its action as a chaperone on the newly synthesized storage proteins. However, in wheat endosperm the levels of protein disulfide isomerase are already up-regulated days before storage protein accumulation can be detected by protein blot (Shimoni et al., 1995). This suggests that, at least initially, induction is independent of the known UPR signaling. Similarly, during pathogen response, up-regulation of the mRNA of BiP occurs before that of pathogen-induced proteins (Jelitto-Van Dooren et al., 1999). A recent proteomic study on the changes occurring when mammalian B cells differentiate into immunoglobulin-secreting cells confirms the plant biology results: induction of the ER machinery precedes immunoglobulin production (van Anken et al., 2003). It is reasonable to hypothesize that once the high synthesis of storage or pathogen-induced proteins has started, ER loading induces further UPR, but the first stage of ER preparation must be triggered by something else than the entry of these new proteins into the ER.
In this respect, the observation that UPR is induced by a number of opaque endosperm maize mutations is important (Herman and Larkins, 1999; Hunter et al., 2002). The mutations resulting in stronger induction directly affect zein structural genes. The fl2 and De*B-30 mutants have been extensively characterized: a single amino acid change inhibits removal of the signal peptide in the two affected zein proteins (Herman and Larkins, 1999; Kim et al., 2004). Therefore, in spite of the high level of zein synthesis, wild-type maize still has spare inducibility of UPR, indicating that protein body formation in not an unspecific aggregation process and that zein storage proteins are not treated as defective proteins. This situation recalls the one observed in cells expressing proteins engineered to form conditional aggregates in the ER (Rivera et al., 2000).
Abnormal protein body formation is observed in the above-described fl2 and De*B-30 maize mutants (Zhang and Boston, 1992). Although the altered morphology of protein bodies could be directly ascribed to the presence of the mutated zein polypeptides, an alternative and intriguing possibility is that chaperone overexpression due to UPR interferes with the process of protein body formation. The influence of altered chaperone levels in a protein assembly process is testified by the observation that the artificial overexpression of BiP transiently inhibits protein assembly in the plant ER (Foresti et al., 2003).
CONCLUSIONS
The synthesis of large amounts of secretory proteins requires substantial remodeling at the subcellular level and a coordinated effort between various cellular pathways. Work performed during the last decades has started shedding light on the way these processes are integrated and elucidating the mechanisms that allow the cell to cope with the heavy biosynthetic load encountered during the period of storage protein deposition.
In some instances, plant cells have exploited the flexibility of the ER to directly accumulate the synthesized polypeptides without the need for further transport along the secretory pathway. Although this process could be seen as the mere aggregation of essentially insoluble polypeptides, it is clear that specific characteristics of the accumulated proteins and specific interactions with cellular factors must be important to allow the successful deposition of the storage proteins without subjecting the ER to an intolerable level of stress. The details of these processes have started being investigated in some species, but many questions still remain unanswered. The role of the UPR in the developmental process leading to ER proliferation during seed development needs to be analyzed in more detail. The requirement of high rates of storage protein synthesis for the formation of protein bodies is an issue that should be investigated. The widespread occurrence of interchain disulfide bonds in cereal storage proteins calls for a more detailed analysis of their role in the aggregation/assembly process. The search for more mutants of protein body formation can also lead to the discovery of novel protein maturation helpers. The question whether there are specialized regions of the ER where protein body formation can begin remains open. Addressing these issues should help us understand not only the mechanism of storage protein deposition but also how eukaryotic cells in general manage to deal with aggregation-prone proteins translocated into the ER.
Acknowledgments
We thank Franco Faoro and Michele Bellucci for the pictures in Figure 2, A and B, respectively. We thank the many colleagues with whom we have discussed during the last years several of the issues detailed in this update, and we apologize to those whose work could not be cited due to space constraints.
This work was supported by the Ministero dell'Istruzione, dell'Università e della Ricerca – Fondo per gli Investimenti della Ricerca di Base (project nos. RBNE018BHE and RBNE01TYZF) and by the European Union Research Training Networks Contract HPRN–CT–2002–00262 (BioInteractions).
References
- Altschuler Y, Galili G (1994) Role of conserved cysteines of a wheat gliadin in its transport and assembly into protein bodies in Xenopus oocytes. J Biol Chem 269: 6677–6682 [PubMed] [Google Scholar]
- Alvarez I, Geli MI, Pimentel E, Ludevid D, Torrent M (1998) Lysine-rich γ-zeins are secreted in transgenic Arabidopsis plants. Planta 205: 420–427 [DOI] [PubMed] [Google Scholar]
- Barlowe C (2003) Signals for COPII-dependent export from the ER: what's the ticket out? Trends Cell Biol 13: 295–300 [DOI] [PubMed] [Google Scholar]
- Ceriotti A, Pedrazzini E, Fabbrini MS, Zoppé M, Bollini R, Vitale A (1991) Expression of the wild-type and mutated vacuolar storage protein phaseolin in Xenopus oocytes reveals relationships between assembly and intracellular transport. Eur J Biochem 202: 959–968 [DOI] [PubMed] [Google Scholar]
- Chrispeels MJ, Greenwood JS (1987) Heat stress enhances phytohemagglutinin synthesis but inhibits its transport out of the endoplasmic reticulum. Plant Physiol 83: 778–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti O, Frigerio L, Holkeri H, de Virgilio M, Vavassori S, Vitale A (2003) A phaseolin domain involved directly in trimer assembly is a determinant for binding by the chaperone BiP. Plant Cell 15: 2464–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geli MI, Torrent M, Ludevid D (1994) Two structural domains mediate two sequential events in γ-zein targeting: protein endoplasmic reticulum retention and protein body formation. Plant Cell 6: 1911–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman EM, Larkins BA (1999) Protein storage bodies and vacuoles. Plant Cell 11: 601–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter BG, Beatty MK, Singletary GW, Hamaker BR, Dilkes BP, Larkins BA, Jung R (2002) Maize opaque endosperm mutations create extensive changes in patterns of gene expression. Plant Cell 14: 2591–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelitto-Van Dooren EP, Vidal S, Denecke J (1999) Anticipating endoplasmic reticulum stress: a novel early response before pathogenesis-related gene induction. Plant Cell 11: 1935–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinski A, Rowley DL, Loer DS, Foley C, Buta G, Herman EM (1995) Binding-protein expression is subject to temporal, developmental and stress-induced regulation in terminally differentiated soybean organs. Planta 195: 611–621 [DOI] [PubMed] [Google Scholar]
- Khoo U, Wolf MJ (1970) Origin and development of protein granules in maize endosperm. Am J Bot 57: 1042–1050 [Google Scholar]
- Kim CS, Hunter BG, Kraft J, Boston RS, Yans S, Jung R, Larkins BA (2004) A defective signal peptide in a 19-kD α-zein protein causes the unfolded protein response and an opaque endosperm phenotype in the maize De*-B30 mutant. Plant Physiol 134: 380–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Woo Ym YM, Clore AM, Burnett RJ, Carneiro NP, Larkins BA (2002) Zein protein interactions, rather than the asymmetric distribution of zein mRNAs on endoplasmic reticulum membranes, influence protein body formation in maize endosperm. Plant Cell 14: 655–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan MJ, Lopez O, Cocera M, Lopez-Iglesias C, De La Maza A, Giralt E (2004) Exploring the interaction of the surfactant N-terminal domain of γ-zein with soybean phosphatidylcholine liposomes. Biopolymers 73: 258–268 [DOI] [PubMed] [Google Scholar]
- Levanony H, Rubin R, Altschuler Y, Galili G (1992) Evidence for a novel route of wheat storage proteins to vacuoles. J Cell Biol 119: 1117–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wu Y, Zhang DZ, Gillikin JW, Boston RS, Franceschi VR, Okita TW (1993) Rice prolamine protein body biogenesis: a BiP-mediated process. Science 262: 1054–1056 [DOI] [PubMed] [Google Scholar]
- Mainieri D, Rossi M, Archinti M, Bellucci M, De Marchis F, Vavassori S, Pompa A, Arcioni S, Vitale A (2004) Zeolin. A new recombinant storage protein constructed using maize γ-zein and bean phaseolin. Plant Physiol 136: 3447–3456 [DOI] [PMC free article] [PubMed]
- Muench DG, Wu Y, Zhang Y, Li X, Boston RS, Okita TW (1997) Molecular cloning, expression and subcellular localization of a BiP homolog from rice endosperm tissue. Plant Cell Physiol 38: 404–412 [DOI] [PubMed] [Google Scholar]
- Napier JA, Richard G, Turner MF, Shewry PR (1997) Trafficking of wheat gluten proteins in transgenic tobacco plants: γ-gliadin does not contain an endoplasmic reticulum-retention signal. Planta 203: 488–494 [DOI] [PubMed] [Google Scholar]
- Rivera VM, Wang X, Wardwell S, Courage NL, Volchuk A, Keenan T, Holt DA, Gilman M, Orci L, Cerasoli F Jr, et al (2000) Regulation of protein secretion through controlled aggregation in the endoplasmic reticulum. Science 287: 826–830 [DOI] [PubMed] [Google Scholar]
- Rosenberg N, Shimoni Y, Altschuler Y, Levanony H, Volokita M, Galili G (1993) Wheat (Triticum aestivum L.) γ-gliadin accumulates in dense protein bodies within the endoplasmic reticulum of yeast. Plant Physiol 102: 61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ (2004) A trip to the ER: coping with stress. Trends Cell Biol 14: 20–28 [DOI] [PubMed] [Google Scholar]
- Shimoni Y, Zhu XZ, Levanony H, Segal G, Galili G (1995) Purification, characterization, and intracellular localization of glycosylated protein disulfide isomerase from wheat grains. Plant Physiol 108: 327–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R, Braakman I (2003) Quality control in the endoplasmic reticulum protein factory. Nature 426: 891–894 [DOI] [PubMed] [Google Scholar]
- Sparvoli F, Faoro F, Daminati MG, Ceriotti A, Bollini R (2000) Misfolding and aggregation of vacuolar glycoproteins in plant cells. Plant J 24: 825–836 [DOI] [PubMed] [Google Scholar]
- Umebayashi K, Hirata A, Horiuchi H, Ohta A, Takagi M (1999) Unfolded protein response-induced BiP/Kar2p production protects cell growth against accumulation of misfolded protein aggregates in the yeast endoplasmic reticulum. Eur J Cell Biol 78: 726–738 [DOI] [PubMed] [Google Scholar]
- van Anken E, Romijn EP, Maggioni C, Mezghrani A, Sitia R, Braakman I, Heck AJ (2003) Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity 18: 243–253 [DOI] [PubMed] [Google Scholar]
- Vitale A, Denecke J (1999) The endoplasmic reticulum: gateway of the secretory pathway. Plant Cell 11: 615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Boston RS (1992) Increases in binding protein (BiP) accompany changes in protein body morphology in three high-lysine mutants of maize. Protoplasma 171: 142–152 [Google Scholar]