Abstract
Background
Among the Reduviidae family, triatomines are giant blood-sucking bugs. They are well known in Central and South America where they transmit Trypanosoma cruzi to mammals, including humans, through their feces. This parasitic protozoan is the causative agent of Chagas disease, a major public health issue in endemic areas. Because of the medical and economic impact of Chagas disease, the presence of other arthropod-borne pathogens in triatomines was rarely investigated.
Methodology/Principal findings
In this study, seven triatomines species involved in the transmission of T. cruzi were molecularly screened for the presence of known pathogens generally associated with arthropods, such as Rickettsia, Bartonella, Anaplasmataceae, Borrelia species and Coxiella burnetii. Of all included triatomine species, only Eratyrus mucronatus specimens tested positive for Bartonella species for 56% of tested samples. A new genotype of Bartonella spp. was detected in 13/23 Eratyrus mucronatus specimens, an important vector of T. cruzi to humans. This bacterium was further characterized by sequencing fragments of the ftsZ, gltA and rpoB genes. Depending on the targeted gene, this agent shares 84% to 91% of identity with B. bacilliformis, the agent of Carrion’s disease, a deadly sandfly-borne infectious disease endemic in South America. It is also closely related to animal pathogens such as B. bovis and B. chomelii.
Conclusions
As E. mucronatus is an invasive species that occasionally feeds on humans, the presence of potentially pathogenic Bartonella-infected bugs could present another risk for human health, along with the T. cruzi issue.
Author Summary
Triatomines are hematophagous insects including vectors of T. cruzi, the agent of Chagas disease, a huge public health issue, especially in South America. Whether these arthropods carry other pathogenic microorganisms is currently unknown. We investigated the presence of different arthropod-borne pathogens, including Bartonella spp., by quantitative PCR. Bartonella species were identified using ftsZ, gltA and rpoB gene sequencing and a new genotype of Bartonella spp. was detected in Eratyrus mucronatus specimens, an important vector of T. cruzi to humans. This agent is closely related to several human and animal pathogens. Depending on the gene fragment used, this agent shares 84% to 91% of identity with B. bacilliformis, the agent of the deadly Carrion’s disease. The possibility of transmission of potentially pathogenic bacteria could be an additional threat to human health since E. mucronatus bugs are more and more anthropophilic.
Introduction
Triatomine bugs (order Hemiptera, family Reduviidae, subfamily Triatominae) are blood-sucking arthropods (“kissing bugs”), most of which can feed both on animals and humans. All stages from first instar to male and female adults are strictly hematophagous and responsible for a relatively large blood intake due to their large size. They are mainly sylvatic and feed on small wild mammals but can also feed on birds and bats [1]. Triatomines occupy diverse natural ecotopes, such as mammal and bird nests, hollow trees, caves and rock fissures [2], but also rural environments, as they can prosper in crevices in houses [1]. These arthropods are distributed world-wide but the vast majority of the 140 recognized species is found in the Americas [3]. They are particularly well studied in South America, where they transmit an endemic flagellate pathogen, T. cruzi, the etiological agent of Chagas disease [1]. Also known as the American trypanosomiasis, Chagas disease is a neglected tropical disease, the first human parasitic disease in the endemic areas. T. cruzi is transmitted through the feces of infected kissing bugs, causing heart failure 10 to 30 years post-infection for almost 30% of individuals [4].
Because of the public health impact of Chagas disease, studies on kissing bugs are mainly focused on this theme. As a matter of fact, the presence of other human pathogens was never described in the hundred years that it has been known that kissing bugs could transmit pathogens. Only the presence of Wolbachia and Arsenophonus species was investigated based on the fact that these obligate intracellular bacteria are known to be endosymbionts of many arthropods [5,6]. The presence of zoopathogenic arthropod-borne viruses was also investigated. To our knowledge, there is no report of pathogen detection in dejections or in triatomines themselves, except for T. cruzi, although Arsenophonus nasoniae was once reported to be detected in an eschar of a human [7]. Regarding viruses, two have been described in these bugs. Triatoma virus is reported as strictly entomopathogenic, particularly for its principal host, Triatoma infestans [8], while African swine fever virus was detected in Triatoma gerstaeckeri but not transmitted to pigs [9].
French Guiana is an 84,000 km2 overseas department and region of France bordered by Brazil and Suriname. Because of its many different ecosystems, particularly a dense rainforest, French Guiana is a biodiversity hotspot and one of the 21 areas where Chagas disease is endemic [10]. Among the 27 described species of triatomines in this area, many are invasive species. That is to say that many of them temporarily leave their sylvatic or peridomestic dwellings in order to invade houses. The main vector community of French Guiana comprises highly anthropophilic bugs belonging to the Panstrongylus, Rhodnius and Eratyrus genera [10]. They accidentally feed on humans [11] and also on potentially infected animals since they easily feed on domestic animals or wild mammals.
Aiming to add to knowledge regarding bacteria and triatomine association, we screened seven species of triatomines bugs from French Guiana by molecular biology for the presence of arthropod-borne bacteria such as Rickettsia, Bartonella, Borrelia, Anaplasma, Wolbachia, Ehrlichia species and Coxiella burnetii.
Methods
Triatomine collection, identification and selection
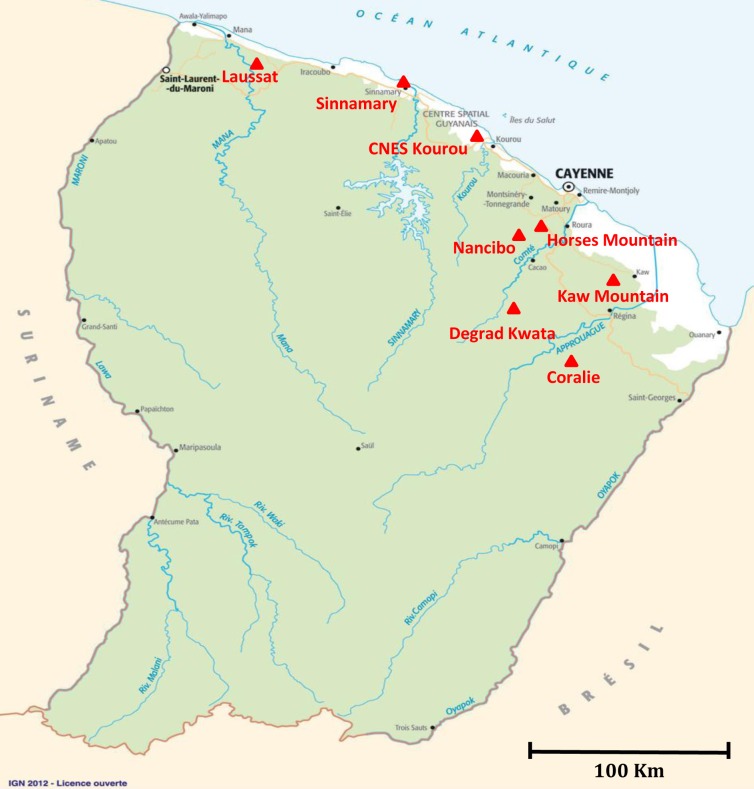
Triatomine specimens were collected in French Guiana from 1991 to 2013 using light traps or interception traps by one of the authors (JMB) and by the Société Entomologique Antilles-Guyane (SEAG) as part of an inventory of French Guiana’s insects. Triatomines were caught in forests (Horses Mountains, Kaw Mountains), peridomiciliary areas (Degrad Kwata, Kaw Mountains, Nancibo) or urban areas (Sinnamary, Kourou savannah) as displayed in Fig 1.
Fig 1. Distribution of sampling areas in French Guiana.
Exact sampling sites are indicated by a triangle.
All triatomine specimens were morphological identified with the Bérenger et al. morphological key [12] and kept dried as insect collections. Seven T.cruzi vectors were included in this study: Rhodnius prolixus (n = 10), Rh. pictipes (n = 10), Rh. robustus (n = 10), Triatoma infestans (n = 10), Panstrongylus geniculatus (n = 10), P. rufotuberculatus (n = 4), Eratyrus mucronatus (n = 23).
DNA extraction
Dried triatomines were rinsed in sterile water and air-dried on filter paper before cutting lengthwise in two equal halves, using a sterile surgical blade for each specimen. One half and the legs were stored at -20°C as a backup sample and the other legless half was selected for molecular analyses. Each half triatomine was crushed with a sterile pestle in 400 μL of a G2 buffer solution containing 40 μM of proteinase K (Qiagen) and incubated at 56°C overnight. After 1 minute of centrifugation at 7000 x g, 200 μL of the supernatant was then collected prior to DNA extraction. Triatominae genomic DNA was individually extracted using the EZ1 DNA tissue extraction kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Triatominae DNAs were then eluted in 100 μL of Tris EDTA (TE) buffer using the DNA extracting EZ1 Advanced XL Robot (Qiagen) as previously described [13]. DNAs were either immediately used or stored at -20°C until used for molecular analysis. The DNA extracting EZI Advanced XL Robot was disinfected after each batch of extraction as per the manufacturer recommendations to avoid cross-contamination.
Molecular analysis
DNA samples were individually tested by genus-specific PCR using primers and probes targeting specific sequences of Bartonella spp., but also Rickettsia spp., Coxiella burnetii, Borrelia spp., and all Anaplasmataceae species [14] as previously described [15] (Table 1). Real-time quantitative PCR (qPCR) was carried out according to the manufacturer’s protocol using a CFX Connect Real-Time PCR Detection System (Bio-rad, Hercules, CA, USA) with the Eurogentec Takyon qPCR kit (Eurogentec, Seraing, Belgium).
Table 1. Sequences of qPCR primers used to investigate the presence of pathogens’ DNA in the E. mucronatus samples. F: forward primer, R: reverse primer, P: qPCR probe.
| Target organism | Target gene | Primer’s name | Sequence (5’-3’) |
|---|---|---|---|
| Bartonella spp. | Intergenic spacer | IT2_F | GGGGCCGTAGCTCAGCTG |
| ITS2_R | TGAATATATCTTCTCTTCACAATTTC | ||
| ITS2_P | 6FAM-CGATCCCGTCCGGCTCCACCA | ||
| Rickettsia spp. | gltA | gltA_F | GTGAATGAAAGATTACACTATTTAT |
| gltA_R | GTATCTTAGCAATCATTCTAATAGC | ||
| gltA_P | 6FAM-CTATTATGCTTGCGGCTGTCGGTTC | ||
| Coxiella burnetii | IS30A | ITS30A_F | CGCTGACCTACAGAAATATGTCC |
| ITS30A_R | GGGGTAAGTAAATAATACCTTCTGG | ||
| ITS30A_P | 6FAM-CATGAAGCGATTTATCAATACGTGTATGC | ||
| Borrelia spp. | 16S | 16S_F | AGCCTTTAAAGCTTCGCTTGTAG |
| 16S_R | GCCTCCCGTAGGAGTCTGG | ||
| 16S_P | 6FAM- CCGGCCTGAGAGGGTGAACGG | ||
| Anaplasmataceae | 23S | 23S_F | TGACAGCGTACCTTTTGCAT |
| 23S_R | GTAACAGGTTCGGTCCTCCA | ||
| 23S_P | 6FAM- GGATTAGACCCGAAACCAAG |
Bartonella elizabethae, Rickettsia montanensis, Coxiella burnetii, Anaplasma phagocytophilum and Borrelia crocidurae DNAs were used as positive qPCR controls for the primers and probe targeting respectively all Bartonella, Rickettsia, Coxiella burnetii and Borrelia species.
DNAs were tested at different concentrations to avoid PCR inhibition. For each run, a PCR mix without DNA was used as negative control. Standard PCR targeting a 710 bp region of the invertebrate cytochrome oxidase I (COI) gene was performed on PCR negative triatomines to control DNA extraction.
Sequencing and GenBank accession numbers
DNA samples that were positive with Bartonella-qPCR were submitted to conventional PCR amplification using a Bio-Rad Thermocycler (Bio-Rad Laboratories, Hercules, CA) prior to sequencing. For Bartonella species identification, primers targeting Bartonella rpoB, gltA and ftsZ genes fragments were used as previously described [16]. DNA from Bartonella elizabethae served as PCR positive control and mixture without DNA as negative control. The cycling protocol consisted of 15 min at 95°C followed by 35 cycles of denaturing at 95°C for 30 s, annealing at 50°C for 30 s (58°C for rpoB gene), extension 1 min at 72°C, followed by a final cycle of 1 min at 72°C and sampling held at 4°C. Amplification products were separated by electrophoresis through a 1.5% agarose-tris-borate-EDTA gel containing ethidium bromide. PCR products were sequenced using a Big Dye Terminator kit and an ABI PRISM 3130 Genetic Analyser (Applied BioSystems, Courtabeauf, France). The sequences were analyzed using the ABI PRISM DNA Sequencing Analysis software version 3.0 (Applied BioSystems) and compared to sequences available in the GenBank database using the BLAST algorithm (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The partial sequences of ftsZ and rpoB genes of Bartonella amplified from the sample EmG01 are available in GenBank at #KX377404 and #KX377405.
Phylogenic analysis
Phylogeny of the detected Bartonella with other members of the Bartonella genus was established with TOPALi 2.5 software (Biomathematics and Statistics Scotland, Edinburgh, UK). Available sequences of ftsZ, gltA and rpoB genes of validated Bartonella species were retrieved from the National Center for Biotechnology Information (NCBI) based on the results of the BLAST program. Multiple sequence alignment was performed with the ClustalW multiple sequence alignment program, which is included in the BioEdit software.
Results
Triatominae collection
Triatomines were collected in eight different localities in French Guiana with no selection regarding species and sex (convenient sampling). Among the triatomines collected, Eratyrus mucronatus (Fig 2) accounted for 20% of catches and 29.8% of the specimens analyzed. Details related to collection, such as sampling area and triatomines’ sex, are indicated in Table 2. Of 23 E. mucronatus samples, 22 (95.6%) were male. Further details regarding other collected species have been listed elsewhere [12].
Fig 2. Pictures of alive and dead Eratyrus mucronatus in its environment and dried on paper.
Table 2. Details of Eratyrus mucronatus collect from 1991 to 2013 in French Guiana.
1,2,3,4,5,6,7: different areas of French Guiana. SEAG: Société Entomologique Antilles-Guyane (Entomological Society for French West-Indies and Guiana). JMB: Jean-Michel Bérenger. BH: Bernard Hermier. CNES Kourou: Centre National d’Etudes Spatiales (National Center for Spatial Studies).
| Eratyrus mucronatus specimens | Sex | Date of collection | Location | Area type | Sampling person | Ct values |
|---|---|---|---|---|---|---|
| EmG01 | M | 1998 | Kaw Mountains | Primary forest—peridomestic | JMB | 20.20 |
| EmG02 | M | 1997 | Kaw Montains1 | Primary forest—peridomestic | JMB | 29.64 |
| EmG03 | M | 1998 | Kaw Mountains | Primary forest—peridomestic | JMB | 28.56 |
| EmG04 | M | 1995 | Nancibo2 | Rural- peridomestic | BH | Neg |
| EmG05 | M | 2010 | Laussat 3 | SEAG | 21.38 | |
| EmG06 | M | 2013 | Horses Mountains4 | Sylvatic | SEAG | Neg |
| EmG07 | M | 2013 | Horses Mountains5 | Sylvatic | SEAG | Neg |
| EmG08 | M | 2013 | Horses Mountains5 | Sylvatic | SEAG | 19.91 |
| EmG09 | M | 2013 | Horses Mountains5 | Sylvatic | SEAG | 23.41 |
| EmG10 | M | 2013 | Horses Mountains5 | Sylvatic | SEAG | Neg |
| EmG11 | M | 2013 | Horses Mountains5 | Sylvatic | SEAG | 24.78 |
| EmG12 | F | 1993 | Sinnamary6 | Urban—peridomestic | JMB | Neg |
| EmG13 | M | 2013 | Horses Mountains5 | Sylvatic | SEAG | 13.98 |
| EmG14 | M | 2013 | Horses Mountains5 | Sylvatic | SEAG | 13.23 |
| EmG15 | M | 1995 | Degrad Kwata7 | Sylvatic–primary forest | JMB | 20.12 |
| EmG16 | M | 1998 | Kaw Mountains | Primary forest—peridomestic | JMB | 19.69 |
| EmG17 | M | 1998 | Kaw Mountains | Primary forest—peridomestic | JMB | 24.47 |
| EmG18 | M | 1996 | Kaw Montains1 | Primary forest—peridomestic | JMB | Neg |
| EmG19 | M | 1996 | Kaw Montains1 | Primary forest—peridomestic | JMB | Neg |
| EmG20 | M | 2003 | CNES Kourou | Savannah | JMB | Neg |
| EmG21 | M | 2003 | CNES Kourou | Savannah | JMB | 25.91 |
| EmG22 | M | 1991 | Coralie8 | Sylvatic–secondary forest | JMB | Neg |
| EmG23 | M | 1993 | Kaw Montains1 | Primary forest—peridomestic | JMB | Neg |
Molecular detection
DNAs extracted from all the triatomines were included to assess the presence of Bartonella species. Of 23 Eratyrus mucronatus samples, 13 (56.5%) were positive by Bartonella spp.-specific qPCR, with cycle threshold (Ct) values ranging from 13.23 to 25.91 (mean: 21.44) (Table 2). These specimens were from six distinct regions and collected between 1993 and 2003. All positive specimens were male, and a majority of them were collected in sylvatic and peridomestic areas: the Kaw Mountains (38.4%) and Horses Mountains (38.4%).
All samples tested negative for the presence of Rickettsia spp., Borrelia spp. Anaplasma spp., Ehrlichia spp., Wolbachia spp. and Coxiella burnetii. Bartonella spp. was only detected in Eratyrus mucronatus specimens.
Sequencing
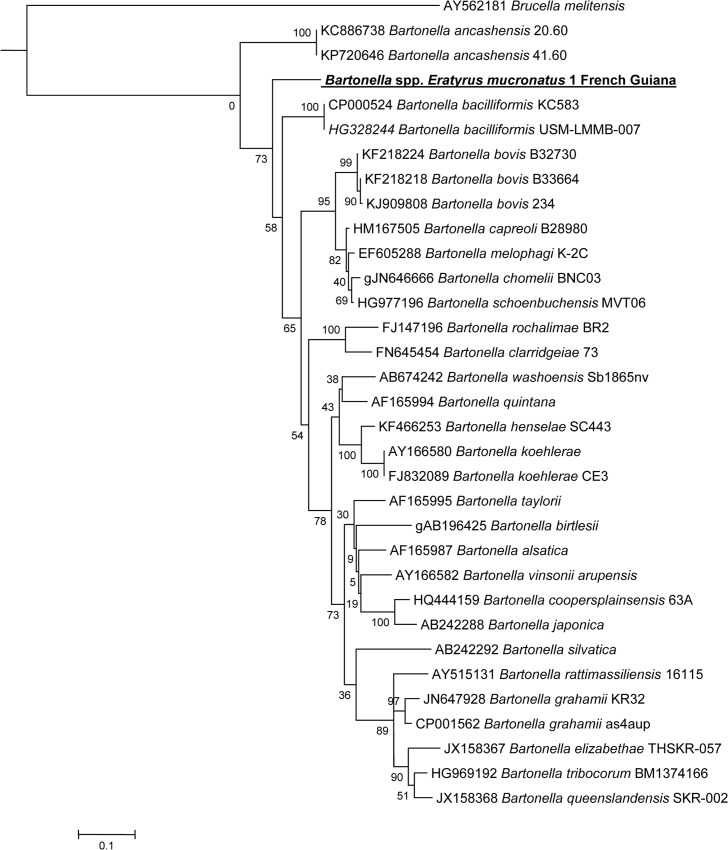
A 787 bp fragment of the Bartonella spp. rpoB gene was amplified using conventional PCR primers prior to sequencing. Only three ITS2-qPCR positive samples were also positive for rpoB by standard PCR. Sequencing failed for two of them. Comparison of the one rpoB resulting sequence against the NCBI database using the BLASTN program indicated that it possessed 90% identity with the ATCC Bartonella bacilliformis 35685D-5 strain (#CP014012.1) and with the B. bacilliformis KC583 strain (#CP000524.1). The next closest cultivated strains were a B. bovis strain [17] and a B. chomelii strain [18], both with 89% identity. Our genotype also possesses 87% identity with a Bartonella ancashensis strain [19,20]. Available sequences of Bartonella rpoB gene were retrieved from NCBI and compared to the Bartonella sequence described hereby. This Bartonella genotype formed a distinct clade, with a strain of Bartonella bacilliformis as the closest clade based on rpoB gene analysis (Fig 3).
Fig 3. A consensus phylogenetic tree showing the relationships of the studied species of Bartonella species based on a portion of rpoB gene sequence comparison.
GenBank accession numbers (or the only genome accession number) are indicated when the sequences initially originated from Genbank. The sequences were aligned using ClustalW, and phylogenetic inferences were obtained using Bayesian phylogenetic analysis with TOPALi 2.5 software (Biomathematics and Statistics Scotland, Edinburgh, UK) within the integrated Maximum Likelihood application using the TrN + I + Г model. Numbers at the nodes are percentages of bootstrap values obtained by repeating the analysis 100 times to generate a majority consensus tree. Bootstrap values below 80 were deleted from the final tree. The final set includes 756 base pairs. The new Bartonella sequence described in the present study is written in red.
All samples allowed amplification of a single 333 bp fragment of the ftsZ gene by standard PCR. Blast analysis revealed 91% identity with the aforementioned 35685D-5 and KC583 B. bacilliformis strains (Fig 4).
Fig 4. A consensus phylogenetic tree showing the relationships of the Bartonella species studied based on a portion of ftsZ gene sequence comparison.
GenBank accession numbers (or the only genome accession number) are indicated when the sequences originated from Genbank at the beginning. The sequences were aligned using ClustalW, and phylogenetic inferences were obtained using Bayesian phylogenetic analysis with TOPALi 2.5 software (Biomathematics and Statistics Scotland, Edinburgh, UK) within the integrated Maximum Likelihood application using the ML SYM+I+Г model. Numbers at the nodes are percentages of bootstrap values obtained by repeating the analysis 100 times to generate a majority consensus tree. Bootstrap values below 80 were deleted from the final tree. The final set includes 292 base pairs. The new Bartonella sequence described in this study is written in red.
A total of 12 out of 13 samples were successfully amplified by standard PCR targeting a fragment of the gltA gene. BLAST analysis showed 88% identity with uncultured Bartonella species detected in bank voles [21], deer [22] and bats from Africa [23], but also with B. bovis and B. chomelii strains (Fig 5). Based on the gltA gene, our genotype is 84% similar to B. bacilliformis strains. Only a single rpoB sequence was obtained but all ftsZ and gltA sequences obtained were identical for all E. mucronatus specimens.
Fig 5. A consensus phylogenetic tree showing the relationships of the Bartonella species studied based on a portion of gltA gene sequence comparison.
GenBank accession numbers (or the only genome accession number) are indicated when the sequences originated from Genbank at the beginning. The sequences were aligned using ClustalW, and phylogenetic inferences were obtained using Bayesian phylogenetic analysis with TOPALi 2.5 software (Biomathematics and Statistics Scotland, Edinburgh, UK) within the integrated Maximum Likelihood application using the K81uf + I + Г model. Numbers at the nodes are percentages of bootstrap values obtained by repeating the analysis 100 times to generate a majority consensus tree. Bootstrap values below 80 were deleted from the final tree. The final set includes 200 base pairs. The new Bartonella sequence described in this study is written in red.
BLAST analysis of the concatened sequence of the three genes revealed 99% of coverage and 90% similarity with the two aforementioned B. bacilliformis strains. Phylogenetic analysis based on the concatened sequences revealed clustering of our Bartonella strain with two B. bacilliformis and B. ancashensis strains (Fig 6).
Fig 6. A consensus concatened phylogenetic tree showing the relationships of the Bartonella species studied based on a concatened sequence of Bartonella rpoB, ftsZ and gltA gene fragment.
Concatenated rpoB, ftsZ and gltA sequences were aligned using CLUSTALW and phylogenetic inferences obtained using Bayesian phylogenetic analysis [Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bio-informatics 2003; 19:1572–1574] with the TOPALi 2.5 software (Biomathematics and Statistics Scotland, Edinburgh, UK) with the integrated MrBayes application [ttp://mrbayes.csit.fsu.edu] with the HKY+I+Г substitution model. GenBank accession numbers are indicated at the beginning. Numbers at the nodes are bootstrap values obtained by repeating the analysis 100 times to generate a majority consensus tree. There were a total of 1245 positions in the final dataset. The scale bar indicates a 10% nucleotide sequence divergence.
Discussion
Bartonella species are small fastidious gram-negative bacteria belonging to the Alphaproteobacteria class that are able to infect many mammals, including humans [24]. They are mostly transmitted by arthropod vectors such as sandflies (Lutzomyia verrucarum), human body lice (Pediculus humanus humanus), different fleas including cat fleas (Ctenocephalides felis), biting flies and ticks [25]. Among the several Bartonella species, some have been identified as human pathogens, causing well-known vector-borne diseases such as Carrion’s disease (B. bacilliformis), trench fever (B. quintana), cat scratch disease (B. henselae) as well as endocarditis [24].
We hereby describe a novel Bartonella genotype, phylogenetically related to several human and animal pathogens, as shown by the phylogenetic analyses. B. bacilliformis, a closely related species, is the causative agent of the first and well-described human bartonellosis called Carrion’s disease [26]. Transmitted through the bite of an infected phlebotomine sand fly, L. verrucarum, this South American endemic bacterium can induce a biphasic illness with two distinct syndromes that can be concomitant or independent. An acute phase known as Oroya fever manifests as a hemolytic fever linked to bacteremia that can range from 10 to 210 days and can be fatal in 40–88% of individuals without treatment. The second syndrome called verruga peruana manifests as blood-filled hemangiomas due to infection of the endothelium [26]. No human cases of B. bacilliformis infection have been reported in French Guiana to date [27]. Our genotype is also closely related to a strain of B. ancashensis, a recently described Bartonella species closely related to B. bacilliformis that was isolated from the blood of two patients diagnosed with a chronic stage of verruga peruana in Peru [20]. All data suggest that B. ancashensis could be a second agent. Our new agent is also closely related to B. bovis strains. Isolated from cats, which are only accidental hosts, this endocarditis [28].
E. mucronatus is a sylvatic triatominae bug involved in the transmission of T. cruzi [11]. It is recognized now as an invasive species as it has been described around and inside houses since 1959 [29] because of its attraction to artificial light sources [30]. They are known to feed on bats, but also on small mammals such as xenarthrans and opossums [11]. Bats are widely reported to be sources of many viral and bacterial pathogens [31], including Bartonella spp. worldwide, including in French Guiana [32], Nigeria [33], Guatemala [34] and Vietnam, for example [35]. Therefore, Bartonella spp. were frequently detected in hematophagous arthropods feeding on bats such as bat flies (Hippoboscidae, Streblidae, Nycteribiidae) [36] or Cimex adjunctus [37]. Triatomine vectors belonging to genera Triatoma, Rhodnius, Panstrongylus and Eratyrus can be totally domiciliated or invasive, since they occasionally visit houses as described in Bolivia [38], Brazil [39], Argentina [40] and Venezuela [11]. The presence of these bugs around houses has long been known and has justified the establishment of chemical control campaigns, which after 5 years remain a failure in Bolivia [38]. The invasive behavior of E. mucronatus has not yet been described in French Guiana but data from Bolivia suggest that eliminating it once it is settled is challenging [38]. Living in various ecotopes and not host-specific [41], these bugs can easily feed both on humans and animals [38], both of them potentially bacteriemic, parasitemic or viremic at the blood meal time point.
Triatominae species are well-studied bugs, however, this work provides the first evidence to our knowledge of infection with a bacterium that is not a priori endosymbiotic. The specimens we analyzed were dry, with no information regarding their engorgement status at the time of collection. However, as they were collected using light traps or interception traps, we can assume that they were seeking hosts and therefore probably non-engorged. Thus, we can suppose that we did not detect DNA of a bacterium present in recently ingested blood but a genuine infection. To support this hypothesis, the infection rate was considerable (56%) among triatomines collected in very distant sampling periods, geographically and over time. Ct values were also very low, increasing the possibilities that this bacterium multiplies within the bug's body. However, to evaluate the possibility of transmission of these Bartonella spp., an experimental model of infection, or at least information regarding the location of the bacteria in the bug, would be necessary. Such information could not be obtained from our samples as they were dry and old. Cultivation of any bacteria or any attempt to localize with immunofluorescence, for example, was not possible.
In continuing this work, it would be interesting to collect wild E. mucronatus specimens in order to isolate the bacterium and establish an experimental model of infection with this arthropod/pathogen pair. This would reveal whether the bug is a simple carrier or an efficient vector of this Bartonella. The possible interaction between T. cruzi and this Bartonella spp. in this insect is also unknown and could be investigated by monitoring the trypanosome’s cycle and transmission in co-infected E. mucronatus. Being phylogenetically closely related to two severe human pathogens (B. bacilliformis and B. ancashensis), it would also be important to evaluate its pathogenicity. Because of the huge public health impact of Chagas disease in South America, investigations on Triatominae were limited to the study of their interactions with T. cruzi. In fact, Triatominae bugs may host such bacteria as Bartonella species and, probably, may be its vector.
Acknowledgments
We would like to thank the SEAG (Société Entomologique Antilles Guyane) and Bernard Hermier for their contribution to the sample collection.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Lazzari CR, Pereira MH, Lorenzo MG, Lazzari CR, Pereira MH, Lorenzo MG. Behavioural biology of Chagas disease vectors. Mem Inst Oswaldo Cruz. 2013;108: 34–47. 10.1590/0074-0276130409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monte GLS, Tadei WP, Farias TM. Ecoepidemiology and biology of Eratyrus mucronatus Stål, 1859 (Hemiptera: Reduviidae: Triatominae), a sylvatic vector of Chagas disease in the Brazilian Amazon. Rev Soc Bras Med Trop. 2014;47: 723–727. 10.1590/0037-8682-0263-2014 [DOI] [PubMed] [Google Scholar]
- 3.Schofield CJ, Galvão C. Classification, evolution, and species groups within the Triatominae. Acta Trop. 2009;110: 88–100. [DOI] [PubMed] [Google Scholar]
- 4.Longo DL, Bern C. Chagas’ Disease. N Engl J Med. 2015;373: 456–466. 10.1056/NEJMra1410150 [DOI] [PubMed] [Google Scholar]
- 5.Espino CI, Gómez T, González G, do Santos MFB, Solano J, Sousa O, et al. Detection of Wolbachia bacteria in multiple organs and feces of the triatomine insect Rhodnius pallescens (Hemiptera, Reduviidae). Appl Environ Microbiol. 2009;75: 547–550. 10.1128/AEM.01665-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorfová P, Skeríková A, Hypsa V. An effect of 16S rRNA intercistronic variability on coevolutionary analysis in symbiotic bacteria: molecular phylogeny of Arsenophonus triatominarum. Syst Appl Microbiol. 2008;31: 88–100. 10.1016/j.syapm.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 7.Edouard S, Subramanian G, Lefevre B, Santos Dos A, Pouedras P, Poinsignon Y, et al. Co-infection with Arsenophonus nasoniae and Orientia tsutsugamushi in a traveler. Vector Borne Zoonotic Dis. 2013;13: 565–571. 10.1089/vbz.2012.1083 [DOI] [PubMed] [Google Scholar]
- 8.Rozas-Dennis GS, Cazzaniga NJ. Effects of Triatoma virus (TrV) on the fecundity and moulting of Triatoma infestans (Hemiptera: Reduviidae). Ann Trop Med Parasitol. 2000. September;94(6):633–41. [DOI] [PubMed] [Google Scholar]
- 9.Hess WR, Endris RG, Haslett TM, Monahan MJ, McCoy JP. Potential arthropod vectors of African swine fever virus in North America and the Caribbean basin. Vet Parasitol. 1987;26: 145–155. [DOI] [PubMed] [Google Scholar]
- 10.Péneau J, Nguyen A, Flores-Ferrer A, Blanchet D, Gourbière S. Amazonian triatomine biodiversity and the transmission of Chagas Disease in French Guiana: In Medio Stat Sanitas. PLoS Negl Trop Dis. 2016;10: e0004427 10.1371/journal.pntd.0004427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrasco HJ, Segovia M, Londoño JC, Ortegoza J, Rodríguez M, Martínez CE. Panstrongylus geniculatus and four other species of triatomine bug involved in the T. cruzi enzootic cycle: high risk factors for Chagas' disease transmission in the Metropolitan District of Caracas, Venezuela. Parasit Vectors. 2014;7: 602 10.1186/s13071-014-0602-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berenger J-M, Pluot-Sigwalt D, Pagés F, Blanchet D, Aznar C. The triatominae species of French Guiana (Heteroptera: Reduviidae). Mem Inst Oswaldo Cruz. 2009;104: 1111–1116. [DOI] [PubMed] [Google Scholar]
- 13.Yssouf A, Almeras L, Terras J, Socolovschi C, Raoult D, Parola P. Detection of Rickettsia spp in ticks by MALDI-TOF MS. PLoS Negl Trop Dis. 2015;9: e0003473 10.1371/journal.pntd.0003473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahmani M, Davoust B, Benterki MS, Fenollar F, Raoult D, Mediannikov O. Development of a new PCR-based assay to detect Anaplasmataceae and the first report of Anaplasma phagocytophilum and Anaplasma platys in cattle from Algeria. Comp Immunol Microbiol Infect Dis. 2015;39: 39–45. 10.1016/j.cimid.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 15.Bessas A, Leulmi H, Bitam I, Zaidi S, Ait-Oudhia K, Raoult D, et al. Molecular evidence of vector-borne pathogens in dogs and cats and their ectoparasites in Algiers, Algeria. Comp Immunol Microbiol Infect Dis. 2016;45: 23–28. 10.1016/j.cimid.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 16.Scola BL, Zeaiter Z, Khamis A, Raoult D. Gene-sequence-based criteria for species definition in bacteriology: the Bartonella paradigm. Trends in Microbiology. 2003;11: 318–321. [DOI] [PubMed] [Google Scholar]
- 17.Bai Y, Malania L, Alvarez Castillo D, Moran D, Boonmar S, Chanlun A, et al. Global distribution of Bartonella infections in domestic bovine and characterization of Bartonella bovis strains using multi-locus sequence typing. PLoS ONE. 2013;8: e80894 10.1371/journal.pone.0080894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antequera-Gómez ML, Lozano-Almendral L, Barandika JF, González-Martín-Niño RM, Rodríguez-Moreno I, García-Pérez AL, et al. Bartonella chomelii is the most frequent species infecting cattle grazing in communal mountain pastures in Spain. Appl Environ Microbiol. 2015;81: 623–629. 10.1128/AEM.03159-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hang J, Mullins KE, Clifford RJ, Onmus-Leone F, Yang Y, Jiang J, et al. Complete Genome Sequence of Bartonella ancashensis Strain 20.00, isolated from the blood of a patient with Verruga Peruana. Genome Announc. 2015;3: e01217–15. 10.1128/genomeA.01217-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullins KE, Hang J, Jiang J, Leguia M, Kasper MR, Ventosilla P, et al. Description of Bartonella ancashensis sp. nov., isolated from the blood of two patients with verruga peruana. International Journal of Systematic and Evolutionary Microbiology. 2015;65: 3339–3343. 10.1099/ijsem.0.000416 [DOI] [PubMed] [Google Scholar]
- 21.Buffet J-P, Marsot M, Vaumourin E, Gasqui P, Masséglia S, Marcheteau E, et al. Co-infection of Borrelia afzelii and Bartonella spp. in bank voles from a suburban forest. Comp Immunol Microbiol Infect Dis. 2012;35: 583–589. 10.1016/j.cimid.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 22.Sato S, Kabeya H, Yamazaki M, Takeno S, Suzuki K, Kobayashi S, et al. Prevalence and genetic diversity of Bartonella species in sika deer (Cervus nippon) in Japan. Comp Immunol Microbiol Infect Dis. 2012;35: 575–581. 10.1016/j.cimid.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 23.Dietrich M, Tjale MA, Weyer J, Kearney T, Seamark ECJ, Nel LH, et al. Diversity of Bartonella and Rickettsia spp. in bats and their blood-feeding ectoparasites from South Africa and Swaziland. PLoS ONE. 2016;11: e0152077 10.1371/journal.pone.0152077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regier Y, O’Rourke F, Kempf VAJ. Bartonella spp.—a chance to establish One Health concepts in veterinary and human medicine. Parasit Vectors. 2016;9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai Y-L, Chang C-C, Chuang S-T, Chomel BB. Bartonella species and their ectoparasites: selective host adaptation or strain selection between the vector and the mammalian host? Comp Immunol Microbiol Infect Dis. 2011;34: 299–314. 10.1016/j.cimid.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 26.Minnick MF, Anderson BE, Lima A, Battisti JM, Lawyer PG, Birtles RJ. Oroya fever and verruga peruana: bartonelloses unique to South America. PLoS Negl Trop Dis. 2014;8: e2919 10.1371/journal.pntd.0002919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez Clemente N, Ugarte-Gil CA, Solórzano N, Maguiña C, Pachas P, Blazes D, et al. Bartonella bacilliformis: a systematic review of the literature to guide the research agenda for elimination. PLoS Negl Trop Dis. 2012;6: e1819 10.1371/journal.pntd.0001819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maillard R, Petit E, Chomel B, Lacroux C, Schelcher F, Vayssier-Taussat M, et al. Endocarditis in cattle caused by Bartonella bovis. Emerging Infect Dis. 2007;13: 1383–1385. 10.3201/eid1309.070236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vivas AS, Barazarte H, Molina-de-Fernández D. Primer registro de Eratyrus mucronatus. Stal; 1959.
- 30.Castro MCM, Barrett TV, Santos WS, Abad-Franch F, Rafael JA. Attraction of Chagas disease vectors (Triatominae) to artificial light sources in the canopy of primary Amazon rainforest. Mem Inst Oswaldo Cruz. 2010;105: 1061–1064. [DOI] [PubMed] [Google Scholar]
- 31.Mühldorfer K. Bats and Bacterial Pathogens: A Review. Zoonoses and Public Health. Blackwell Publishing Ltd; 2013;60: 93–103. 10.1111/j.1863-2378.2012.01536.x [DOI] [PubMed] [Google Scholar]
- 32.Davoust B, Marié J-L, Dahmani M, Berenger J-M, Bompar J-M, Blanchet D, et al. Evidence of Bartonella spp. in blood and ticks (Ornithodoros hasei) of bats, in French Guiana. Vector Borne Zoonotic Dis. 2016;16: 516–519. 10.1089/vbz.2015.1918 [DOI] [PubMed] [Google Scholar]
- 33.Kamani J, Baneth G, Mitchell M, Mumcuoglu Y, Gutiérrez R, Harrus S. Bartonella species in bats (Chiroptera) and bat flies (Nycteribiidae) from Nigeria, West Africa. Vector-Borne and Zoonotic Diseases. 2014;14: 625–632. 10.1089/vbz.2013.1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai Y, Kosoy M, Recuenco S, Alvarez D, Moran D, Turmelle A, et al. Bartonella spp. in Bats, Guatemala. Emerging Infect Dis. 2011;17: 1269–1272. 10.3201/eid1707.101867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anh PH, Van Cuong N, Son NT, Tue NT, Kosoy M, Woolhouse MEJ, et al. Diversity of Bartonella spp. in Bats, Southern Vietnam. Emerging Infect Dis. 2015;21: 1266–1267. 10.3201/eid2107.141760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morse SF, Olival KJ, Kosoy M, Billeter S, Patterson BD, Dick CW, et al. Global distribution and genetic diversity of Bartonella in bat flies (Hippoboscoidea, Streblidae, Nycteribiidae). Infection, Genetics and Evolution. 2012;12: 1717–1723. 10.1016/j.meegid.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 37.Reeves WK, Loftis AD, Gore JA, Dasch GA. Molecular evidence for novel Bartonella species in Trichobius major (Diptera: Streblidae) and Cimex adjunctus (Hemiptera: Cimicidae) from two southeastern bat caves, U.S.A. J Vector Ecol. 2005;30: 339–341. [PubMed] [Google Scholar]
- 38.Depickère S, Durán P, López R, Martínez E, Chávez T. After five years of chemical control: colonies of the triatomine Eratyrus mucronatus are still present in Bolivia. Acta Trop. 2012;123: 234–238. 10.1016/j.actatropica.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro G, Gurgel-Gonçalves R, Reis RB, Santos CGSD, Amorim A, Andrade SG, et al. Frequent house invasion of T. cruzi-infected triatomines in a suburban area of Brazil. PLoS Negl Trop Dis. 2015;9: e0003678 10.1371/journal.pntd.0003678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavallo MJ, Amelotti I, Gorla DE. Invasion of rural houses by wild Triatominae in the arid Chaco. J Vector Ecol. 2016;41: 97–102. 10.1111/jvec.12199 [DOI] [PubMed] [Google Scholar]
- 41.Farfán-García AE, Angulo-Silva VM. Triatoma dimidiata populations' (Hemiptera: Reduviidae: Triatominae) feeding behaviour in an endemic zone and related epidemiological implications. Revista de Salud Pública. 2011;13: 163–172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.