Diverse organisms contain neutral lipids in subcellular particles for food reserves and other purposes. These lipid particles are present in seeds, flowers, pollen and fruit of higher plants, the vegetative and reproductive organs of primitive plants, algae, fungi, nematodes, mammalian glands and brown adipose tissue of mammals, and bacteria. Of all these lipid particles, the oil bodies (OBs) in seeds are the most prominent and best studied.
Seeds of most plant species store oils (triacylglycerols [TAGs]) as a food reserve for germination and postgerminative growth. TAGs are present in small subcellular spherical OBs of approximately 1 μm in diameter. Each OB has a matrix of TAGs surrounded by a layer of phospholipids (PLs) and structural proteins termed oleosins. The small size of OBs provides a large surface area per unit TAG, which would facilitate lipase binding and lipolysis during germination. OBs inside the cells of mature seeds or in isolated preparations are remarkably stable and do not aggregate or coalesce. This stability is in contrast to the instability of artificial liposomes made from amphipathic and neutral lipids; the liposomes gradually coalesce after formation. Seed OBs are stable because their surface is shielded by a layer of oleosins. In maturing seeds, TAGs, PLs, and oleosins are synthesized in the endoplasmic reticulum (ER), from which budding OBs are released.
Research on seed OBs and oleosins has been reviewed by Huang (1992, and an earlier Update article in 1996), Napier et al. (1996), Galili et al. (1998), Frandsen et al. (2001), and Murphy (2001). This Update provides brief reviews of earlier work and emphasizes recent major discoveries. Detailed information and references to earlier work can be found in the previous reviews.
OLEOSIN DISTRIBUTION, STRUCTURE, AND EVOLUTION
Oleosins in seeds are small proteins of about 15 to 26 kD. They completely cover the surface of the subcellular OB (Fig. 1B). They can be abundant in seeds with a high proportion of oils and small OBs (therefore more OB surface area). For example, Arabidopsis (Arabidopsis thaliana) seeds have more than 40% (wt/wt) oils and small OBs of approximately 0.5-μm diameter, and 10% of the seed proteins are oleosins. The ratio of oils to oleosins determines the size and even the shape of the OBs. This phenomenon is best illustrated in maize (Zea mays) lines that have been bred for high and low oil contents; kernels containing a high oil-to-oleosin ratio have large and spherical OBs, whereas those containing a low ratio have small and irregularly shaped OBs.
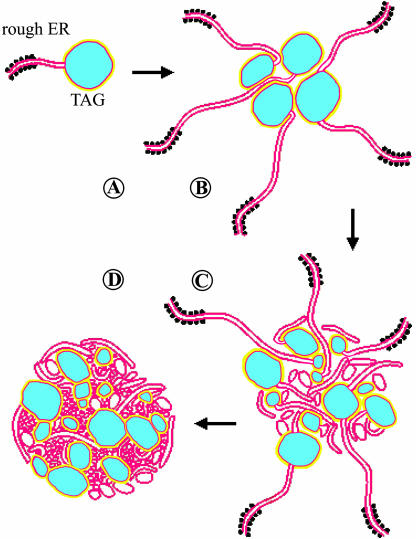
Figure 1.
Models of an oleosin molecule, a seed oil body, and the synthesis of an oil body on the endoplasmic reticulum. A, The three portions of an oleosin molecule (yellow), showing the N-terminal hydrophilic portion, the central hydrophobic hairpin (and residues at the turn, including the Pro knot of three Pro residues and one Ser residue), and the C-terminal hydrophilic portion. The number of residues and their ranges in the 3 portions in all 17 Arabidopsis oleosins are shown. B, An OB having oleosins (yellow) and PLs (red) enclosing the matrix TAGs (blue). All molecules are drawn approximately to scale, whereas the diameter of the OB has been reduced 24 times to magnify the surface structure. C, A budding OB being produced on the RER. The ER lumen, the two PL layers (red), the sequestered TAGs (blue) in a budding OB, a ribosome with an mRNA synthesizing an oleosin polypeptide (dark line, of an unknown configuration), and enzymes (irregular circles) for the synthesis of TAGs and PLs are shown.
More than 200 genes encoding oleosins have been identified. Nonplant organisms do not have oleosins. Recently, genes encoding oleosins on the storage OBs in Arabidopsis pollen (Kim et al., 2002) and tropical cacao (Theobroma cacao) seeds (Guilloteau et al., 2003) have been described. These findings have negated a proposal of having an alternative subcellular mechanism for stabilizing the OBs in pollen and another proposal explaining the short life span of tropical seeds such as cacao because of unstable OBs. The transcript of a gene encoding an oleosin in the moss Physcomitrella can be found in an expressed sequence tag database; this is the most primitive plant known to contain oleosins. Whether algae contain oleosins is not known. Arabidopsis has 17 genes encoding oleosins: 9 (8 in tandem on chromosome 5) that are active in the tapetum cells, 5 active in seeds, and 3 active in both seeds and pollen (Kim et al., 2002). Minor proteins present in isolated OBs of some seeds have been termed caoleosin and steroleosin (Frandsen et al., 2001). They do not have a long hydrophobic sequence, although they have sequences similar to but less conserved than the Pro knot sequence in oleosins (to be described). Their mode of association with the OBs and the possibility of their being contaminants of the OB fractions need to be explored.
An oleosin molecule can be divided into three portions according to its amino acid sequence (Fig. 1A). The N-terminal portion can be short or long (e.g. 6–68 residues in Arabidopsis) and is hydrophilic. The central portion is a long hydrophobic stretch of 72 residues. The C-terminal portion can be short or very long (e.g. 28–1,000 residues in Arabidopsis oleosins), and its approximately 30 residues adjacent to the central hydrophobic stretch can form an amphipathic α-helical structure that interacts horizontally with the charged phosphate and choline groups of the PL layer on the OB surface. The C-terminal portions of most Arabidopsis oleosins consist of fewer than 100 residues; a few have 100 to 150 residues, one has 403, and another has 1,000 residues. Each of the longer C-terminal portions contains many repeats of short peptides, which are not conserved among oleosins and may not have functional significance. Some of the repeated short peptides have several Gly residues; thus, these oleosins are Gly-rich. These proteins are sometimes called Gly-rich proteins, although such a term does not describe the important characteristics of the proteins.
The central hydrophobic stretch of 72 uninterrupted hydrophobic residues is the hallmark of an oleosin. No other protein in any organism has such a long hydrophobic stretch. Proteins on the surface of extracellular or intracellular lipid droplets, such as apolipoproteins, perilipin, adipophilin, and caveolin in mammals, phasin in bacteria, and the plastid lipid-associated protein (PAP), do not have a long hydrophobic stretch; their polypeptides run parallel to the surface of, rather than penetrate into, the lipid droplets. The 72-residue hydrophobic stretch of an oleosin is long enough (a trans-membrane [PL bilayer] peptide has 20–25 residues) to form a hairpin that penetrates the surface PL monolayer of an OB into the matrix (Fig. 1B).
The center of the hydrophobic stretch has three Pro residues and one Ser residue that could interact to form a “Pro knot.” This proposal (Fig. 1A) is based on the presence of the relatively less hydrophobic Pro and Ser residues among the other more hydrophobic residues and TAGs in the matrix of an OB, as well as the well-known fact that Pro residues on polypeptides are breakers or turners of α-helical and β-structures. The formation of the Pro knot could permit the creation of a hairpin structure of the whole hydrophobic stretch, with 2 arms of 30 residues each joined by a turn of 12 residues. The 72 residues of the hydrophobic stretch are conserved in terms of hydrophobicity among oleosins of diverse species, and the conservation is much higher at the Pro knot and its immediate vicinity (-PX5SPX3P-). All oleosins contain the three Pro and one Ser residues at identical locations in the center of the hydrophobic stretch.
All researchers agree with the central hydrophobic stretch forming a hairpin structure with a Pro knot at the turn but disagree on the secondary structures of the two hairpin arms. Earlier, the two arms were proposed to be an anti-parallel α-helical structure on the basis of an algorithm prediction (actually no database for predicting secondary structures of polypeptides in a hydrophobic environment exists) or an anti-parallel β-structure on the basis of high symmetry of residues between the two arms (Huang, 1996). If the two arms had an anti-parallel β-structure, they could bend at several locations where pairing of small Gly residues occurs; the bending would create more interactions among residues and thus offer higher stability. Two laboratories used circular dichromism and Fourier transform infrared spectroscopy to determine the secondary structures of the oleosin hairpin in its imitated native conditions; they came to opposite conclusions of an α-helical structure (Alexander et al., 2002) or β-structure (Li et al., 2002). The controversy underlines the difficulties in measuring the uniquely long hydrophobic polypeptide in a neutral-lipid environment. In addition, the arms or even the turn could interact with those in adjacent oleosins in the OB matrix. Such interactions could provide higher stability to the oleosin hairpin, in which the peptide bonds are relatively hydrophilic, in the hydrophobic environment. In maize, oleosins of two isoforms coexist in a 1:1 ratio, and interaction between the pair is likely. Furthermore, the N- and C-terminal portions of an oleosin, even though on the OB surface, may play a role in maintaining the hairpin in a special configuration.
It has been hypothesized that the long hydrophobic stretch was evolved from duplications of a trans-membrane peptide in a primitive plant or algae. This could explain the residue symmetry of the 2 arms of the hairpin structure and the length of the 72-residue hydrophobic stretch being 4 times that of a trans-membrane peptide (2 × 2 × approximately 20 residues; Huang, 1996). The hypothesis can be tested by comparing the amino acid sequences of oleosins (and the nucleotide sequences of the genes) with those of transmembrane segments of proteins, especially of enzymes related to TAG synthesis, in the most primitive organisms (currently, the moss Physcomitrella). Other than the hairpin hydrophobic stretch, the N- and C-terminal portions are quite variable, which indicates minimal constraints on their structures to perform functions. Analyses of the nine oleosin genes, all but one in tandem, in Arabidopsis chromosome 5 have confirmed the rapid evolution of the N and C termini of tapetum oleosins (Fiebig et al., 2004; Schein et al., 2004), as has been observed in seed oleosins. It is likely that for oleosins to perform their functions, the major structural requirement is the central hydrophobic stretch.
The N- and C-terminal portions of an oleosin on the surface of a seed OB may act as a receptor for the binding of lipase or glyoxysomes during germination. This possibility can be tested. The test can be made with use of seed lipase protein derived from a cloned lipase gene or with Arabidopsis mutants defective of glyoxysomal surface proteins.
ER AND THE SYNTHESIS OF OILS, OLEOSINS, AND OBS
OBs and their constituent TAGs, PLs, and oleosins are all synthesized on the ER. Diacylglycerol acyltransferase (DAG AT), the last enzyme and the only one unique to the synthesis of TAG, as well as enzymes for the synthesis of precursor DAG and PLs, are associated with the rough ER (RER). It is most likely that an alternative TAG-synthesizing enzyme that can transfer the acyl moiety from PLs instead of acyl-CoA to DAG is also located in the ER. The presence of these enzymes in the ER is not surprising in view of the hydrophobicity of the TAG and its metabolic precursors. TAGs synthesized in the ER are sequestered in the hydrophobic region (i.e. the acyl region of the PL bilayer). Continuation of TAG accumulation at a region of the ER forms a budding OB, which is enclosed by a single layer of PLs (Fig. 1C). This budding OB covered with a PL monolayer is stabilized by the inclusion of oleosins to its surface.
The ribosome-mRNA with a nascent oleosin peptide can be guided to the ER via the signal-recognition particle (SRP) pathway. The mRNA for the synthesis of oleosin is associated with the RER. Translation of oleosin mRNA in an in vitro synthesis system is retarded or enhanced when SRP or microsomes are added, respectively (Loer and Herman, 1993; Thoyts et al., 1995; Abell et al., 2002; Beaudoin and Napier, 2002). The findings suggest that translation of the oleosin mRNA pauses after binding of the SRP to the nascent peptide and accelerates when the newly synthesized oleosins are incorporated into the ER. In addition, stable incorporation of the in vitro-synthesized oleosin (commercial in vitro synthesis systems usually contain SRP) into microsomes is inhibited when the SRP receptor (SRP-60) on the microsomes is removed beforehand with proteolysis, and this inhibition can be restored with reconstituted SRP receptor. Yeast (Saccharomyces cerevisiae) transformed with an oleosin gene synthesizes and targets the oleosin to the yeast OBs (Ting et al., 1997). When the transformed yeast strains are mutants defective in SRP components, the oleosin is not targeted to the OBs, and the nontargeted oleosin is proteolyzed (Beaudoin et al., 2000).
The targeting of oleosin to the ER occurs with use of SRP components and microsomes from yeast, mammals, or plants, and thus the unique aspect of the targeting is the targeting signals in the oleosin molecule. Modified oleosins produced via gene recombination can be tested for their stable insertion into microsomes in vitro or the ER in vivo (Abell et al., 1997, 2002; Beaudoin and Napier, 2002). The N- and C-terminal portions of the oleosin molecule are relatively unimportant in targeting the protein to the ER. Rather, the long hydrophobic stretch of the oleosin is the predominant factor for targeting. No specific signal sequence in the hydrophobic stretch is required. Instead, any of the multiple and probably overlapping sequences along the hydrophobic stretch can target the protein to the ER. Significantly, the highly conserved Pro knot is not important, because replacement of the three Pro residues with Leu residues does not affect targeting of the modified oleosin to the microsomes. The finding that multiple peptides along the hydrophobic stretch can be the targeting signals is consistent with the knowledge that the hydrophobic pocket of an SRP can recognize a diverse array of hydrophobic ER-targeting peptides at the N termini or interior of many proteins.
The nascent oleosin polypeptide synthesized or being synthesized on the ER assumes a topology on the basis of its hydrophobic and hydrophilic interactions with the PL bilayer. The hydrophilic N- and C-terminal portions interact with the PL layers on the cytosolic side of the ER (Fig. 1C). The central hydrophobic stretch buries itself in the hydrophobic acyl portion of the PL bilayer. There is much evidence from in vivo and in vitro experiments for such a topology of the oleosin (Abell et al., 1997, 2002; Beaudoin et al., 2000). The N- and C-terminal portions but not the hydrophobic stretch of the oleosin in isolated microsomes are susceptible to proteolysis by added proteases; this observation is similar to that of the oleosins on mature OBs. The secondary structure of the 72-residue hydrophobic stretch in the hydrophobic portion of the PL bilayer is unknown but likely differs from that in a mature OB. The hydrophobic region of the ER does not provide an excess of hydrophobic volume for the hydrophobic stretch to assume its presumably most stable hairpin configuration, but the matrix of a mature OB does. The hydrophobic stretch of the oleosin within the hydrophobic region of the ER could assume a bent hairpin structure or an extended structure with or without coiling, running parallel to the PL bilayer (Fig. 1C). An additional consideration is the actual thickness of the hydrophobic region of the PL bilayer. While the ER is synthesizing oleosins, it also produces massive amounts of TAGs, which will be temporarily sequestered in and thus enlarge the hydrophobic region of the PL bilayer. Thus, the hydrophobic region of the PL bilayer may have more room for the hydrophobic stretch of an oleosin than that confined by the length of the two acyl chains.
Both the newly synthesized oleosins and the temporarily located TAGs on the ER diffuse to the budding OBs. This movement is made possible in accordance with the fluid mosaic model of membrane action and thermodynamic considerations. The TAGs and the oleosins will both be more stable in the hydrophobic environment of a budding OB.
A native oleosin stably inserted into the ER diffuses to the budding OB, but a stably inserted artificially modified oleosin may not. The latter scenario has been used to study the signals on the oleosin that allow the protein on the ER to diffuse to the OB (Abell et al., 1997, 2002, 2004; Beaudoin and Napier, 2002). The mechanism of this oleosin movement has been studied by in vivo experiments with use of modified oleosins and measurements of oleosins recovered in the ER and OB fractions. Strictly speaking, this approach measures not just targeting success per se but also the stability of the modified oleosins in OBs. Modified oleosins that can diffuse to the OBs may be unstable there and be removed by native proteolysis. The molecular requirements for an oleosin to diffuse successfully to and be incorporated stably into the OBs are similar to those for targeting the protein to the ER; however, more are required. The Pro knot in the hydrophobic stretch is also essential, presumably for the stable incorporation into the OBs. It is likely that a modified oleosin without the Pro knot (e.g. having the three Pro residues replaced with Leu) can insert into the ER and also diffuse to the OB but is unstable there and thus eliminated by native proteolysis. In addition to the need for the Pro knot, decreased length or elimination of the N- or C-terminal portions or decreased length of the hydrophobic stretch leads to a reduced recovery of the oleosin in OBs.
Oleosins must be on the cytosolic side of the ER to be able to diffuse to the budding OBs. An N-terminal ER targeting peptide from a nonoleosin protein attached to the N terminus of an oleosin, produced via gene cloning, can pull the N-terminal portion of the oleosin but not the hydrophobic stretch with or without the C-terminal portion into the ER lumen (Abell et al., 2002, 2004). Apparently, the hydrophobic interaction between the long hydrophobic stretch and the acyl moieties of the PL bilayer, with or without the added hydrophilic interaction between the C-terminal portion of the oleosin and the PL layer on the cytosolic side, is too strong for the oleosin to leave the PL bilayer into the lumen. This modified oleosin can be incorporated into the ER but cannot be inserted into the OBs. Obviously, its polypeptide spanning across the whole PL bilayer of the ER cannot diffuse to the PL monolayer of a budding OB (Fig. 1C). Even if it could, it would be unstable in the OBs.
Can a ribosome-mRNA-oleosin complex be targeted to the ER or budding OBs directly without the involvement of the SRP pathway? All the evidence from in vitro experiments shows that the SRP system can be involved. If the SRP is not involved in vivo, the ribosome-mRNA-oleosin complex with the hydrophobic stretch dangling outward in vitro could bind to the hydrophobic pocket of added SRP. Certainly, it has been shown that in vitro-synthesized oleosin cannot insert into mature OBs co- or posttranslationally (Hills et al., 1993). However, a mature OB is packed with oleosins on its surface and has no extra room for new oleosins. In vitro-synthesized oleosin can insert into artificial OBs whose surface has not been filled with oleosins (Chen and Tzen, 2001). A ribosome-mRNA-oleosin complex with the hydrophobic stretch dangling outward could theoretically bind to the hydrophobic region of the ER or a budding OB whose surface has not been filled with oleosins. Nevertheless, the strongest evidence for the requirement for the SRP pathway to guide the oleosin to the ER has come from in vivo studies with yeast mutants defective in SRP components (Beaudoin et al., 2000). This finding with yeast should be tested with plants. In addition, whether oleosin synthesis employs both the SRP system and a direct insertion mechanism has not been evaluated.
As the newly synthesized TAGs and oleosins on the ER diffuse to and converge at the budding OB, a gradient of enrichment of these two components should exist from the point of synthesis to the budding OB. This concentration gradient can explain the immunocytochemical observation that more oleosins are present in the ER near the budding OBs (Herman, 1987). Whether subdomains of ER for TAG and oleosin synthesis are present remains to be documented. In an in vitro study, sunflower seed microsomes supplied with precursors synthesized TAGs, and after this synthesis, were subfractionated by density gradient centrifugation into fractions (Lacey et al., 1999). The fraction with the lowest buoyant density contained more TAG, oleosin, and lipid synthesis activity on a per fraction basis. This fraction may represent ER subdomains for TAG and oleosin synthesis, or simply fragments of ER regions originally closest to the budding OBs and thus have more TAGs and a lower buoyant density. In an earlier experiment, when an extract of maturing maize kernel was subfractionated by density gradient centrifugation, DAG AT, the last and unique enzyme for TAG synthesis, was found with cytochrome reductase in RER fragments of diverse buoyant densities (Cao and Huang, 1986); the DAG AT was not concentrated in ER fragments with the lowest buoyant densities and therefore most TAGs (or fewer polysomes). Thus, in the maize kernel cells, TAGs are likely synthesized in diverse regions of the ER and diffuse to the budding OBs.
When will a budding OB on the ER be released (Fig. 1C)? An early release will generate a smaller OB, and vice versa. The size of an OB is determined partly or completely by the relative amount or rate of synthesis of oils and oleosins. High-oil maize kernels (having a high oil-to-oleosin ratio) generated by breeding have larger OBs, whereas low-oil kernels have smaller OBs with irregularly shaped surface. If the cells do not synthesize oleosins, as those in the fatty mesocarp of fruits, the OBs become very large (following paragraph). Is there a special mechanism for the physical release of the budding OB from the ER? The oleosins accumulated on the bud surface may interact among themselves to produce a physical force of constriction at the neck of the bud, thereby releasing the OB. Or, the physical release may require specific proteins (e.g. dynamins) or actions of the ER or cytosol. This possibility can be tested by screening for Arabidopsis mutants whose seeds have larger or smaller OBs or only budding OBs with use of light microscopy after lipid staining; some of these mutants may be defective in the action for the physical release of OBs from the ER.
In the fatty mesocarp of fruits such as avocado (Persea americana), oil palm (Elaeis guineensis), and olive (Olea europaea), each cell has only several large lipid globules, which occupy the bulk of the cell volume. There is little or no oleosins on the lipid globules. The mesocarp lipids are for attraction to animals for seed dispersion and thus are not required to be in small entities as the OBs in seeds. Mostly likely, the TAGs are synthesized in the ER, as are the seed OBs, but without the cosynthesis of oleosins (Fig. 1C). As a consequence, the budding OB enclosed only by PL becomes larger before it is released from the ER; this is equivalent to the synthesis of larger OBs in maize kernels having a high oil-to-oleosin ratio. It is possible that the mesocarp cells can be modified to synthesize small OBs instead of large lipid globules if oleosin is allowed to be cosynthesized via genetic engineering. Although conceptually this genetic engineering project can be easily achieved, in practice it is difficult because the better-known avocado, oil palm, and olive containing fatty mesocarp are tree crops.
OLEOSINS IN TAPETUM CELLS AND THE NOVEL ORGANELLE TAPETOSOME
The presence of oleosins in tapetum cells of anthers in Arabidopsis and Brassica was discovered a decade ago from unexpected gene cloning results (de Oliveira et al., 1993; Roberts et al., 1994) because tapetum cells were not known to contain OBs similar to those in seeds. These oleosins are present in a novel, neutral lipid-containing organelle, which has been termed the tapetosome because of its unique presence in tapetum cells. To date, the findings of oleosins in tapetum cells are limited to the insect/self-pollinating Brassicaceae family, especially Brassica and Arabidopsis.
In Arabidopsis, nine genes encode the tapetum oleosins, eight of which are in tandem on chromosome 5 (Kim et al., 2002; Fiebig et al., 2004; Schein et al., 2004). One of these genes is highly expressed to produce an oleosin of 53 kD, which represents about 70% of all the tapetum oleosins. Most of the other Arabidopsis tapetum oleosins are smaller (10–23 kD), but one has 115 kD. As expected, Brassica has a similar oleosin gene system (Roberts et al., 1994; Ross and Murphy, 1996; Ruiter et al., 1997), and the most active ortholog gene produces a major oleosin of 45 or 48 kD (from the Brassica rapa AA genome or Brassica oleracea CC genome, respectively). Genes encoding the tapetum oleosins have undergone rapid evolution, likely because the constraints for protein structures and thus functions are not high.
Tapetum oleosins are located in a novel organelle called the tapetosome (Wu et al., 1997). The tapetum is a one-cell layer enclosing the anther locule, in which microspores mature to become pollen. Tapetum cells are the only anther sporophytic cells that are metabolically very active and control the maturation of the microspores. At an early stage of anther development, the tapetum cells are specialized for active secretion and contain abundant RER and secretory vesicles. At a mid stage of anther development, the cells have become chiefly a temporary storehouse of ingredients to be deposited onto the maturing pollen as the pollen coat. In Brassicaceae, the tapetum cells at this mid stage of development are packed with two predominant storage organelles, the elaioplasts and the tapetosomes (Platt et al., 1998). The elaioplasts, of 3 to 4 μm in diameter, are specialized plastids largely devoid of thylakoids but filled with small spherical lipid droplets of steryl esters enclosed by the structural protein PAP. Although elaioplasts of similar morphology can be found in nontapetum cells, such as fruit and petal cells, the tapetosomes are unique to the tapetum cells. Each spherical tapetosome, of 2 to 3 μm in diameter, has droplets of TAGs, presumably enclosed and stabilized by oleosins and PLs as those in seed oil bodies, and vesicles presumably derived from the ER.
The contents of the tapetosomes and elaioplasts are selectively retained and discharged to the anther locule after the death of the tapetum cells during the final stage of anther development. The oleosins, but not the TAGs of the tapetosomes, and the steryl esters, but not the structural protein PAP of the elaioplasts, are selectively retained and transferred to the pollen surface, forming the bulk of the pollen coat (Wu et al., 1997, 1999). The rationale and mechanism for the selectivity are unclear. It is intriguing that in seed OBs, TAGs are the prime ingredient for physiological function and the oleosins are the accessories, whereas in the tapetosomes, oleosins may be the main element for physiological function (to be described) and TAGs are the accessories. The tapetum TAGs disappear after the death of the tapetum. Where do they go? They could be used as an energy source for active metabolism of the tapetum cells. Their fatty acids could be used to produce jasmonic acids as floral maturation hormones. Or, their fatty acids could be converted to alkanes as one of the two major lipid constituents (the other being the elaioplast steryl esters) for deposition onto the maturing pollen. These possibilities are testable with Arabidopsis mutants defective in tapetum TAG synthesis or degradation.
Although the steryl esters and other lipids on the pollen form a useful waterproofing layer, the function of the abundant oleosins there is not clear. In Brassica, the predominant 45- or 48-kD oleosin on the pollen has been cleaved selectively into two fragments, one containing the N-terminal portion and the central hydrophobic stretch, and the other the long hydrophilic C-terminal portion (Ross and Murphy, 1996; Ting et al., 1998). Whether other smaller oleosins on the pollen are cleaved is not known. The cleavage may not have physiological relevance, as will be described. The most abundant oleosin on the pollen has a large size (53 kD in Arabidopsis and 45 or 48 kD in Brassica) owing to its possession of numerous repeats of short peptides at its C terminus. Each of these repeats possesses several Gly residues, which makes the protein Gly-rich.
Because of its Gly-rich nature, this oleosin (and extrapolating to other oleosins) on the pollen has been speculated to be involved in interacting with the cell walls of the stigma cells. Such a speculation should be taken with caution. Oleosins have undergone rapid evolutionary changes, and both the tapetum and seed oleosins have repeats of short peptides at the C termini; some of these repeats have high Gly contents, whereas others do not. The rapidity and extensiveness of the changes at the C termini may reflect the minimal structural constraints on this part of the protein to perform functions. The high Gly contents at the C termini of oleosins may be fortuitous, and certainly the Gly-rich C termini in some seed oleosins do not have an apparent function for interaction with the cell wall. In fact, the short repeats at the C termini of the most abundant tapetum oleosins have not just high Gly content but also high Ser and Lys content, making the oleosin also Ser-rich and Lys-rich (the Arabidopsis 53-kD oleosin has 26 mol %, 16%, and 14%, and the Brassica 48-kD oleosin 21%, 16%, and 11% Gly, Ser, and Lys, respectively).
An oleosin molecule may serve dual functions on the pollen and subsequently on the stigma because of its amphipathic property. Its N- and C-terminal portions are hydrophilic, and its central portion is hydrophobic. The amphipathic oleosin can act as an emulsifying agent to uniformly coat the pollen with steryl esters and other ingredients. It can also aid water uptake for germination after the pollen grain has landed on the stigma. Brassicaceae have dry stigmas, and water must be drawn from the stigma interior to the pollen for germination and tube growth. Steryl esters and other neutral lipids are not amphipathic, and no other coat ingredient is known to be able to act as a wick. The abundant and amphipathic oleosins could be such a wick. On the basis of these two proposed functions, the addition of repeats of short peptides, which are all fairly hydrophilic, to the C termini of different oleosins and the fragmentation of the Brassica 45- or 48-kD oleosins into two halves do not affect the functioning of the oleosins. The proposed functions are in agreement with the observation that the pollen of an Arabidopsis mutant null in the major pollen coat oleosin does not hydrate efficiently on the stigma (Mayfield and Preuss, 2000). The major structural constraints on the oleosins to perform the proposed functions are a long hydrophobic stretch to interact with the TAG droplets in the tapetosomes (not a function per se but for storage in the organelles) and an amphipathic molecule to emulsify the pollen coat materials and take up water from the stigma. All the observed rapid evolutionary changes on the tapetum oleosins have not affected these constraints.
The tapetosome is a novel organelle with a unique morphology (Wu et al., 1997; Platt et al., 1998). It has a nonhomogeneous interior in situ by transmission electron microscopy, and its structures cannot be recognized. Its structures can be observed clearly after it has been isolated and subjected to osmotic swelling. A tapetosome has droplets of TAGs distributed among vesicles (Fig. 2). During development, massive ER cisternae are connected to the surface of the maturing tapetosomes, as observed by electron microscopy. A model of the synthesis of the tapetosome from the RER has been proposed. Initially, TAG droplets are produced by an ER budding mechanism identical to that in maturing seeds. Presumably these TAG droplets are covered by PLs and oleosins. Many of these droplets are produced and converge. More ER cisternae are connected to the droplet clusters and eventually break off. As a consequence, a tapetosome is produced. The model shown in Figure 2 is constructed largely on the basis of electron microscopic observations, and biochemical evidence is lacking.
Figure 2.
A model of the synthesis of a tapetosome in Brassica tapetum cells. A, Formation of an oil droplet from the RER by a mechanism similar to that in Figure 1C. Each oil droplet consists of an oil matrix (blue) enclosed by layers of PL (red) and oleosins (yellow). Presence of PLs and oleosins on the oil droplet is speculative. B, Association of several oil droplets and ER cisternae. C, A maturing tapetosome containing detached ER vesicles. D, A mature tapetosome. Modified from Platt et al. (1998).
What is the function of the putative ER-derived vesicles in the tapetosomes? The constituents of their membrane and lumen in comparison with those of the ER cisternae are not known; this information is crucial. These vesicles in the tapetosomes may aid the transfer of oleosins from the lyzed tapetum cells to the pollen surface. They may possess proteins, such as cell incompatibility factors and other signaling proteins, for the pollen surface; these proteins would subsequently exert action on the stigma. They may contain ions such as calcium and boron for the pollen surface; these ions would subsequently modulate the cell wall structures of the stigma. Or, they may contain flavonoids and other secondary metabolites for the pollen surface; these pollen-surface metabolites are well-known but of undefined functions. Subcellular fractionation and modern microscopy can be used to test the presence of these possible ingredients in the tapetosome vesicles and the putative interaction between the ER and the vesicles during biogenesis.
Future studies of the tapetum oleosins and tapetosomes should aim at expanding the existing findings to non-Brassicaceae species, exploring the structure and function relationship between the tapetosomes and the ER, pinpointing the roles of the oleosins on pollen, and examining the contents of the putative ER-derived vesicles in the tapetosomes. Working hypotheses exist and are testable. In addition, use of Arabidopsis mutants defective in individual constituents will aid these tests.
Acknowledgments
Figure 1B was prepared by Dr. Jason Tzen while in the authors' laboratory.
This work was supported by the National Science Foundation (MCB–0131358) and by the U.S. Department of Agriculture (National Research Initiative Competitive Grant no. 2000–01512).
References
- Abell BM, Hahn M, Holbrook LA, Moloney MM (2004) Membrane topology and sequence requirements for oil body targeting of oleosin. Plant J 37: 461–470 [DOI] [PubMed] [Google Scholar]
- Abell BM, High S, Moloney MM (2002) Membrane protein topology of oleosin is constrained by its long hydrophobic domain. J Biol Chem 277: 8602–8610 [DOI] [PubMed] [Google Scholar]
- Abell BM, Holbrook LA, Abenes M, Murphy DJ, Hills MJ, Moloney MM (1997) Role of the proline knot motif in oleosin endoplasmic reticulum topology and oil body targeting. Plant Cell 9: 1481–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander LG, Sessions RB, Clarke AR, Tatham AS, Shewry PR, Napier JA (2002) Characterization and modelling of the hydrophobic domain of a sunflower oleosin. Planta 214: 546–551 [DOI] [PubMed] [Google Scholar]
- Beaudoin F, Napier JA (2002) Targeting and membrane-insertion of a sunflower oleosin in vitro and in Saccharomyces cervisiae: The central hydrophobic domain contains more than one signal sequence, and directs oleosin insertion into the endoplasmic reticulum membrane using a signal anchor sequence mechanism. Planta 215: 293–303 [DOI] [PubMed] [Google Scholar]
- Beaudoin F, Wilkinson BM, Stirling C, Napier JA (2000) In vivo targeting of a sunflower oil body protein in yeast secretory (sec) mutants. Plant J 23: 159–170 [DOI] [PubMed] [Google Scholar]
- Cao YZ, Huang AHC (1986) Diacylglycerol acyltransferase in maturing oil seeds of maize and other species. Plant Physiol 82: 813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JCF, Tzen JTC (2001) An in vitro system to examine the effective phospholipids and structural domain for protein targeting to seed oil bodies. Plant Cell Physiol 42: 1245–1252 [DOI] [PubMed] [Google Scholar]
- de Oliveira DE, Franco LO, Simoens C, Seurinck J, Coppieters J, Botterman J, van Montagu M (1993) Inflorescence-specific genes from Arabidopsis thaliana encoding glycine-rich proteins. Plant J 3: 495–507 [DOI] [PubMed] [Google Scholar]
- Fiebig A, Kimport R, Preuss D (2004) Comparisons of pollen coat genes across Brassicaceae species reveal rapid evolution by repeat expansion and diversification. Proc Natl Acad Sci USA 101: 3286–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen GI, Mundy J, Tzen JT (2001) Oil bodies and their associated proteins, oleosin and caleosin. Physiol Plant 112: 301–307 [DOI] [PubMed] [Google Scholar]
- Galili G, Sengupta-Gopalan C, Ceriotti A (1998) The endoplasmic reticulum of plant cells and its role in protein maturation and biogenesis of oil bodies. Plant Mol Biol 38: 1–29 [PubMed] [Google Scholar]
- Guilloteau M, Laloi M, Blais D, Crouzillat D, Mc Carthy J (2003) Oil bodies in Theobroma cacao seeds: cloning and characterization of cDNA encoding the 15.8 and 16.9 kDa oleosins. Plant Sci 164: 597–606 [Google Scholar]
- Herman EM (1987) Immunogold-localization and synthesis of an oil-body membrane protein in developing soybean seeds. Planta 172: 336–345 [DOI] [PubMed] [Google Scholar]
- Hills MJ, Watson MD, Murphy DJ (1993) Targeting of oleosins to the oil bodies of oilseed rape (Brassica napus L.). Planta 189: 24–29 [DOI] [PubMed] [Google Scholar]
- Huang AHC (1992) Oil bodies and oleosins in seeds. Annu Rev Plant Physiol Plant Mol Biol 43: 177–200 [Google Scholar]
- Huang AHC (1996) Oleosins and oil bodies in seeds and other organs. Plant Physiol 110: 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HU, Hsieh K, Ratnayake C, Huang AHC (2002) Expression of Arabidopsis oleosin genes and characterization of their encoded oleosins. J Biol Chem 277: 22677–22684 [DOI] [PubMed] [Google Scholar]
- Lacey DJ, Beaudoin F, Dempsey CE, Shewry PR, Napier JA (1999) The accumulation of triacylglycerols within the endoplasmic reticulum of developing seeds of Helianthus annuus. Plant J 17: 397–405 [Google Scholar]
- Li M, Murphy DJ, Lee KHK, Wilson R, Smith LJ, Clark DC, Sung JY (2002) Purification and structural characterization of the central hydrophobic domain of oleosin. J Biol Chem 277: 37888–37895 [DOI] [PubMed] [Google Scholar]
- Loer DS, Herman EM (1993) Cotranslational integration of soybean (Glycine max) oil body membrane protein oleosin into microsomal membranes. Plant Physiol 101: 993–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield JA, Preuss D (2000) Rapid initiation of Arabidopsis pollination requires the oleosin-domain protein GRP17. Nat Cell Biol 2: 128–130 [DOI] [PubMed] [Google Scholar]
- Murphy DJ (2001) The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Plant J 13: 1–16 [DOI] [PubMed] [Google Scholar]
- Napier JA, Stobart AK, Shewry PR (1996) The structure and biogenesis of plant oil bodies: the role of the ER membrane and the oleosin class of proteins. Plant Mol Biol 31: 945–956 [DOI] [PubMed] [Google Scholar]
- Platt KA, Huang AHC, Thomson WW (1998) Ultrastructural study of lipid accumulation in tapetal cells of Brassica napus L. cv. Westar during microsporogenesis. Int J Plant Sci 159: 724–737 [Google Scholar]
- Roberts LS, Gerster J, Allard S, Cass L, Simmonds J (1994) Molecular characterization of two Brassica napus genes related to oleosins which are highly expressed in the tapetum. Plant J 6: 927–933 [DOI] [PubMed] [Google Scholar]
- Ross JHE, Murphy DJ (1996) Characterization of anther-expressed genes encoding a major class of extracellular oleosin-like proteins in the pollen coat of Brassicaceae. Plant J 9: 625–637 [DOI] [PubMed] [Google Scholar]
- Ruiter RK, Vaneldik GJ, Vanherpen RMA, Schrauwen JAM, Wullems GJ (1997) Characterization of oleosins in the pollen coat of Brassica oleracea. Plant Cell 9: 1621–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein M, Yang ZH, Mitchell-Olds T, Schmid KJ (2004) Rapid evolution of a pollen-specific oleosin-like gene family from Arabidopsis thaliana and closely related species. Mol Biol Evol 21: 659–669 [DOI] [PubMed] [Google Scholar]
- Thoyts PJ, Millichip MI, Stobart AK, Griffiths WT, Shewry PR, Napier JA (1995) Expression and in vitro targeting of a sunflower oleosin. Plant Mol Biol 29: 403–410 [DOI] [PubMed] [Google Scholar]
- Ting JTL, Balsamo RA, Ratnayake C, Huang AHC (1997) Oleosin of plant seed oil bodies is correctly targeted to the lipid bodies in transformed yeast. J Biol Chem 272: 3699–3706 [DOI] [PubMed] [Google Scholar]
- Ting JTL, Wu SSH, Ratnayake C, Huang AHC (1998) Constituents of the tapetosomes and elaioplasts in Brassica campestris and their degradation and retention during microsporogenesis. Plant J 16: 541–551 [DOI] [PubMed] [Google Scholar]
- Wu SSH, Moreau RA, Whitaker BD, Huang AHC (1999) Steryl esters in the elaioplasts of the tapetum in developing Brassica anthers and their recovery on the pollen surface. Lipids 34: 517–523 [DOI] [PubMed] [Google Scholar]
- Wu SSH, Platt KA, Ratnayake C, Wang TW, Ting JTL, Huang AHC (1997) Isolation and characterization of novel neutral-lipid-containing organelles and globuli-filled plastids from Brassica napus tapetum. Proc Natl Acad Sci USA 94: 12711–12716 [DOI] [PMC free article] [PubMed] [Google Scholar]