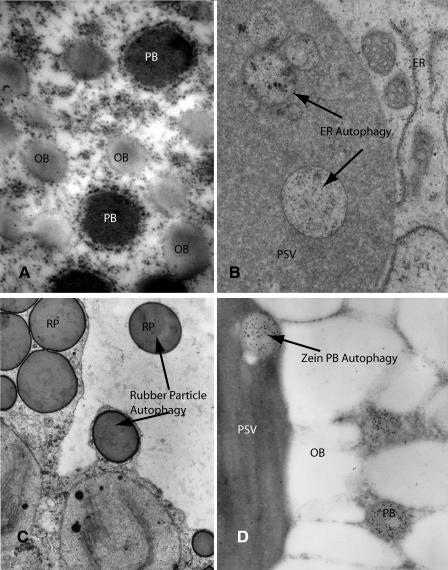
One of the emerging differences that appears to separate plants from the other eukaryotes is the plasticity of the endoplasmic reticulum (ER) to form protein, oil, or rubber containing subcellular structures best termed ER bodies, as proposed by Hara-Nishimura and colleagues (Matsushima et al., 2003), that either stably accumulate or are subsequently transported to the vacuole. ER bodies are in most instances spherical, less than 1 μm in diameter, and consist of a dense core consisting of a self-assembling or aggregating protein, oil, or rubber encased by a membrane that is ER in origin (Fig. 1A). The ER-derived limiting membrane of ER bodies is variable in composition and appearance ranging from a naked phospholipids monolayer of fruit oil bodies (OBs), to specialized single unit membranes containing unique proteins in pollen and seed OBs and rubber bodies, to membranes that appear to be identical to rough ER such as seed protein bodies (PB) and protein precursor accumulating (PAC) vesicles. The common characteristic of all of these organelles is that they appear to be physiologically inert and osmotically inactive. How plants form and use the potential of ER bodies separates them from other eukaryotes, yet in their fate plants appear to have evolved a separate convergent solution for a nonclassical Golgi-bypass trafficking of proteins to the vacuole. This process parallels the cytoplasm to vacuole trafficking (Cvt) pathway that has been described in detail in yeast (Saccharomyces cerevisiae; Klionsky and Ohsumi, 1999). In this Update, the biology of ER bodies is extended to discuss the deposition of protein precursors and other substances in the vacuole that constitutes an ER to vacuole trafficking (ERvt) pathway.
Figure 1.
A, Shown are OBs and PBs of a late maturation soybean cotyledon from a transgenic plant that suppresses conglycinin, one of the two major storage proteins. A large fraction of the other storage protein glycinin is diverted from endomembrane progression and forms ER-derived protein bodies. Both the OBs and PBs have ribosomes associated with the surface that is a remnant of the ER origin of both of these organelles. See Kinney et al. (2001) for details. B, Autophagic vesicles in the PSV of mung bean cotyledons after germination is shown. The autophagic vesicles contain fragmented remnants of the ER network that remains from seed maturation and is degraded postgermination. See Herman et al. (1981) for details. C, Rubber particles (RP) in the cytoplasm and taken in the vacuole by autophagy in Guayule (Parthenium argentatum) are shown. Although ER-derived, the rubber particles do not possess bound ribosomes like PBs and some OBs. See Backhaus and Walsh (1983) for details. D, The autophagy of zein PBs into the PSV of transgenic tobacco seed cells is shown. Zein PBs are initially assembled by the ER and then subsequently sequestered into the protein storage vacuole by selective autophagy. While autophagy of the zein PBs is occurring, other cellular structures and organelles are not subjected to autophagy. This separates the selective autophagy of PBs from the nonspecific autophagy that is central to programmed cell death and senescence. See Coleman et al. (1996) for details.
THE PLANT ER IS AN ORGANELLE WITH DIFFERENTIATED DOMAINS THAT IS REGULATED BY DEVELOPMENT AND OTHER FACTORS
The plant ER is a large network of interconnected sheets and tubes (Staehelin, 1997) contiguous with the nuclear envelope and exists as a patchwork of specialized domains that are differentiated with respect to the ability of ER to synthesize proteins and other substances (Staehelin, 1997; Choi et al., 2000). The specialization of ER domains provides the functional capacity to compartmentalize the synthesis of specific proteins and glycoproteins and other substances synthesized and processed by the ER, such as lipids, oil, waxes, rubber, sterols, and isoprenoids regulated by development, stress, and environmental conditions. While there is still a great deal to be learned about how various domains and/or components of the ER are used by plants to produce specific components, there is considerable data that supports the concept of specialized ER subdomains with specific functions.
ER AND ER BODIES MAY BE TRANSFERRED TO THE VACUOLE BY AUTOPHAGY EITHER ACCUMULATING OR FOR DISPOSAL CHANGING VACUOLE COMPOSITION AND FUNCTION
Autophagy in plants appears to be a continuous process in most cells that is illustrated by the presence of partially degraded material in the vacuoles of cells in most tissues. Although first detailed a few decades ago (e.g. Matile, 1975), much remains to be elucidated on autophagy's regulation and function. The amount of apparent autophagy is greatly enhanced during cellular remodeling events that occurs in development, in reaction to stress and recovery, and during senescence. For example, Suc starvation induces autophagy in cultured cells to recover carbon and nitrogen resources to maintain life under nutrient-challenged circumstances (Moriyasu and Ohsumi, 1996). Autophagy also appears to have a significant role in renewal and turnover of the tonoplast (Melroy and Herman, 1991). The tonoplast is variable in composition with major compositional changes occurring during development and in response to stress.
Autophagy in plants results from the engulfment of cytoplasmic materials by the tonoplast, resulting in formation of a vesicle within the vacuole sequestering the engulfed material (for review, see Herman, 1994). For membranous organelles this results in the appearance of a double unit membrane of the engulfed material with the inner membrane being that of the organelle and the outer membrane being that of the engulfing tonoplast. Once isolated within the vacuole the action of vacuolar hydrolases lyses the outer former tonoplast membrane, exposing the sequestered material to the vacuolar hydrolases that include proteases, glycosidases, phosphatases, nucleases, and phospholipases that then degrade the inner organelle membrane and its contents. How cytoplasmic material is selectively identified for autophagy remains unknown and an important subject for future research. The engulfment of cytoplasmic material by the tonoplast in plants differs from the autophagic process observed in animals and yeast. Although the processes are superficially similar, ending with deposition in the lytic compartment, the process of sequestration into the lytic compartment is different. In yeast and animals, cytoplasmic material is engulfed in membrane that is either de novo synthesized or is Golgi-derived and subsequently is recruited and encases the material targeted for autophagy. This results in a cytoplasmic structure with a double unit membrane. The outer membrane fuses with the lysosome (animals) or vacuole (yeast), releasing the sequestered organelle into the lytic compartment for degradation (for review, see Klionsky and Ohsumi, 1999).
Because the ER has specialized functions, ER membrane turnover is necessary to dispose of specialized ER inventory and/or redundant components by changing developmental or environmental circumstances. There are few studies on the disposal of ER membranes. The role of the vacuole to use autophagy to sequester and degrade the ER was established by observations on postgermination mung beans (Vigna radiate; Herman et al., 1981). The mung bean cotyledon ER undergoes massive proliferation during seed maturation to produce storage proteins, and on desiccation this ER network is fragmented but remains in the dormant seed (Gilkes et al., 1979). Immediately after germination this ER is engulfed by the protein storage vacuoles (PSVs) and degraded while a de novo synthesized ER network is formed to support the mobilization of seed storage substances (Fig. 1B). Therefore, in at least some circumstances there is selective autophagy of ER by the vacuole for disposal.
The vacuolar autophagy of ER bodies is a variation on this autophagic destruction of redundant ER. The first unambiguous demonstration of an ER body trafficking to the vacuole is sequestration of PBs containing hydrophobic prolamin proteins in wheat (Triticum aestivum; Levanony et al., 1992). Wheat prolamins are initially assembled as protein accretions in the ER that are then budded from the ER as PBs with a rough ER-limiting membrane. Electron microscopy shows that the PBs are engulfed protovacuoles by autophagy. As the small vacuoles fuse the PB is left encased in the enlarging vacuole. This is then a variation on the theme of normal vacuole ontogeny in which prevacuoles bud from the Golgi, and fuse one to another, gradually enlarging the protovacuole forming the large central vacuole. The ER-derived limiting membrane is lysed, releasing the prolamin storage protein accretions that are apparently resistant to the action of the vacuolar hydrolases. The prolamin accretions merge in parallel with the enlargement of the vacuole, resulting in a large central vacuole of the endosperm with one or more large prolamin accretions at the end of seed maturation. Wheat endosperm, like that of other monocotyledonous plants, dies at the end of maturation, leaving the prolamin accretions within the dead endosperm.
Not all prolamin PBs are targeted for vacuolar sequestration. Maize, for example, possesses zein-containing ER-PBs that at least in part remain contiguous with the ER from which they were synthesized or are free in the cytoplasm (for review, see Herman and Larkins, 1999). Curiously, replicating the process of zein-PB assembly in transgenic tobacco (Nicotiana tabacum) seeds results in the formation of PBs containing zein accretions surrounded by an ER-derived ribosome-studded membrane, but these PBs are subject to autophagy by tobacco seed PSVs (Coleman et al., 1996; Fig. 1D).
A similar process is the sequestration of rubber in the vacuole. Rubber particles are ER bodies containing a central core with an accretion of ER-synthesized high-Mr rubber (Backhaus and Walsh, 1983; Fig. 1C). Rubber particles bud from the ER, but unlike PBs with a ribosome studded membrane, rubber particles possess a membrane that primarily consists of a protein that is a member of the Cytochrome P450 family (Pan et al., 1995). After sequestration in the vacuole the rubber membrane or coat is digested, leaving intravacuolar naked rubber particles that self-aggregate, forming large rubber complexes as occurs with the wheat prolamin PB cores.
Hydrophilic storage proteins of the 2, 7, and 11S families are conserved and widely distributed in plant seeds. These proteins are all synthesized by the ER and are largely deposited in the PSVs by endomembrane progression through the Golgi. Targeting sequences have been identified for many of these proteins that are recognized by a Golgi receptor, and in some instances glycan side chains of the precursor storage proteins are modified by Golgi enzymes, further demonstrating an endomembrane progression path for deposition. Yet, Hara-Nishimura and co-workers observed that precursors of pumpkin (Cucurbita pepo) 2S storage proteins are also found in small dense vesicles surrounded by a rough ER membrane (Hara-Nishimura et al., 1998). Unlike prolamins that self-aggregate to produce PBs, these globulins are hydrophilic proteins that were previously all assumed to be deposited in vacuoles by endomembrane progression through the Golgi. There were prior hints that hydrophilic globular storage proteins might aggregate in the ER, producing PBs by expressing proteins such as 7S vicilin with an ER-retention sequence K/HDEL (Wandelt et al., 1992), but this is an artificial transgenic consequence. Storage globulins normally have a relatively short T1/2 in the ER lumen before progressing to the Golgi, but greatly increasing the ER residence time promotes the self-aggregation of the protein leading to self-assembly of PBs. There are now other examples of this phenomenon that extend to other foreign proteins expressed in transgenic plant cells. But the Hara-Nishimura et al. (1998) experiments showed that a phenomenon that had previously been known only as the consequence of expressing transgenes also occurred as a naturally occurring mechanism for storage protein accumulation. In their model the pro2S storage protein is aggregated in the ER and budded from the ER in PBs (termed by them as PAC vesicles) that are sequestered in the vacuole, where the pro2S protein is processed to its mature form (Hara-Nishimura et al., 1998). Whether the transfer into the vacuole is accomplished by vacuolar engulfment autophagy remains to be demonstrated. The pumpkin 2S protein fused to phosphoinothricin acetyltransferase induces protein body formation as a transgene expressed in Arabidopsis (Arabidopsis thaliana; Hayashi et al., 1999). The 2S storage protein family is distributed among diverse plant species. Hence, the pumpkin 2S globulin results suggest that there are likely many other examples of both endomembrane progression and ERvt paths for storage protein accumulation to be identified. What would be of particular interest is to investigate whether the same seeds use both mechanisms, and if so whether there is a process of regulating the choice of pathway either by developmental stage or environmental circumstance or other biological factors.
Other storage proteins of the 7S (vicilin-type) and 11S (legumin-type) have been shown to be synthesized by the ER, form oligomers, and are then transported to the vacuole by endomembrane progression through the Golgi. However, suppression of the soybean (Glycine max) 7S conglycinin storage protein by silencing results in seeds that accumulate 11S storage protein that has been substituted for the missing 7S protein. The pro11S protein glycinin forms accretions in the ER, forming PBs that accumulate during seed maturation (Fig. 1A; Kinney et al., 2001). The accumulated inventory of PBs is present in the fully mature soybeans. The 11S PBs are eventually engulfed by the vacuole by autophagy, but this does not occur until after seed germination.
Storage proteins are also found in vegetative cells including leaves, stems, tubers, and pollen and are different genes and gene families than the seed storage proteins. Although it is widely assumed these proteins are deposited in the vacuole by endomembrane progression through the Golgi, in most instances there is little experimental evidence to prove this. It is possible that PBs and the ERvt pathway may have some role in nonseed storage protein accumulation, and this needs to be a subject of future investigations. Vegetative cells are often subjected to wide variations of environmental conditions, and it is possible that the endomembrane progression and ER body formation occurs simultaneously or under specific circumstances as occurs in transgenic soybean seeds lacking α-conglycinin (Kinney et al., 2001).
The discovery that KDEL tailed-Cys protease precursors (Tanaka et al., 1991) form into the core of ER bodies termed protease precursor vesicles (PPV; Schmid et al., 1998; Toyooka et al., 2000; for review, see Chrispeels and Herman, 2001) is a remarkable adaptation by plants and as far is presently known plant-specific. The Cys protease papain superfamily is a large diverse family of enzymes of ancient origin found in most if not all eukaryotes. Cys proteases are initially synthesized as inactive precursors by the ER with a large prodomain that lies in the groove containing the active site precluding proteolytic activity until the enzymes are activated at their destination site. In general, the proteases exit the ER, are transported to and through the Golgi where, depending on the targeting information, they are then transported to the cell's lytic compartment, lysosomes in animals, vacuoles in plants, or are secreted into extracellular space. The plant papain superfamily is large with individual members used in developmentally specific as well as general proteolytic functions involving both intracellular and extracellular protein degradation. The PPVs are assembled in cells undergoing remodeling and senescence, with storage protein mobilization and programmed cell death as examples (Schmid et al., 1998, 2001; Toyooka et al., 2000; Hayashi et al., 2001). That PPVs have been found to be associated with these two processes suggests one possible role for Golgi-bypass of Cys protease precursors. By forming PPVs the plant cell has the potential to accumulate a large inventory of protease in a stored, stable precursor form that is only activated when autophagy of the PPVs is initiated. By this means, instead of gradual changes in protein composition as the proteases are accumulated and function, this would instead confer the ability to mediate rapid changes that could be useful in storage protein mobilization and programmed cell death.
For what are normally soluble monomeric precursors of Cys proteases to be sequestered into ER bodies requires that the protease precursors be separated from the bulk of secretory and ER-lumen proteins aggregated and marshaled into the nascent PPV ER body. This probably requires that the proteins undergo self-aggregation to provide a self-assembly mechanism to separate the aggregating protein from all of the other proteins present in the ER lumen. One approach to promote self-aggregation is to utilize the ER-retention system. ER-lumen proteins possess a carboxy-terminal retention sequence, H/KDEL, and some other variants such as KEEL that function in identifying the ER-lumen proteins for retrieval from the bulk protein flow at the cis-Golgi. Biologists working with plants, animal cells, and yeast have discovered that placing the ER-retention sequence on some secretory proteins results in these proteins being retained and/or retarded in the ER. In many, but not all, instances the normally soluble proteins aggregate as the result of engineering an ER-retention sequence onto the protein (Wandelt et al., 1992). These experiments anticipated the discovery of the aggregation of KDEL-containing Cys proteases (Schmid et al., 1998, 2001; Toyooka et al., 2000) in some plants to form precursor protease vesicles, a form of ER body. How the repeated cycles of ER-Golgi trafficking promote the self-aggregation of some proteins is unknown, but because this occurs with only some proteins it is likely an intrinsic property of those proteins.
However, the KDEL sequence may not be absolutely essential to divert enzyme precursors into ER bodies. The presence of vacuolar processing enzyme, an Asn-specific protease that functions to convert vacuolar precursor proteins to mature hydrolases and storage proteins, has been discovered in ER bodies (Rojo et al., 2003), making it possible for components of PPV to include other protease precursors that do not possess the ER-retention sequence. It is likely any secretory protein precursor could conceivably be an ER-body protein accretion if it could be induced to aggregate. Experiments with zein expression in Xenopus oocytes indicate that ER body formation is a self-assembly process (Wallace et al., 1988), suggesting that any protein that is induced to be destabilized in the ER and formed into accretions could then result in the cascade of events to form an ER body and subsequently be deposited in the vacuole by autophagy. It is likely that further examples of precursor proteins aggregating in the ER will be found.
ARE ER BODIES INERT ORGANELLES AFTER ASSEMBLY AND BEFORE SEQUESTRATION IN THE VACUOLE?
All of the present evidence indicates that the ER bodies once released from the ER are inert organelles. The presence of ribosomes on PBs, PAC vesicles, and PPVs suggests the potential that further protein modifications could be made to the organelles even after budding from the ER network has occurred. Whether this would be with mRNAs already available or by recruiting new mRNAs could then be analyzed if these organelles persisted in being translation competent. However, experiments to translate in vitro any mRNAs sequestered on any member ER body family or organelle's rough membranes have not yet been published or reported. It would be of considerable interest to see if the mRNAs encode only the prolamin storage protein core of the protein body or whether there are other not previously characterized proteins encoded that could be involved in further maturation ER body.
THE ERvt OF ER BODIES CONTAINING PRECURSOR PROTEINS IN PLANTS CONSTITUTES A CONVERGENT ANALOGUE TO THE Cvt PATHWAY OF YEAST
The primary pathway of ER bodies ending with the autophagic sequestration in the vacuole constitutes an ERvt pathway that has parallel functions to the cytoplasm-vacuole (lysosomal)-trafficking (Cvt) pathway described in yeast and that also apparently exists in animal cells. It is therefore useful to consider how this ERvt pathway resembles and differs from the Cvt process. In yeast microautophagy, where the Cvt autophagic vesicle is assembled de novo, it is necessary for the cargo protein to recruit membrane to encase the protein aggregate and subsequently to mediate its fusion with the lysosome/vacuole (for review, see Moriyasu and Ohsumi, 1996). This is accomplished by a cascade of events where the cargo protein is synthesized, aggregated, and bound to other proteins and then enclosed by membrane for the cytoplasmic assembly of the Cvt vesicle. Yeast Cvt functions to transfer cytoplasmic synthesized aminopeptidase to the vacuole (Scott et al., 1997). The product of the Atg19 gene binds to proaminopeptidase I dodecamer that in turn forms larger aggregates and higher order structure. This complex recruits Ams1 and Atg11 proteins that encase the complex producing a Cvt complex. The Atg11 interacts with Atg8 protein that induces the complex to recruit and be associated with a de novo synthesized sequestering vesicle membrane that encases the Cvt complex, resulting in the formation of a Cvt body with a double unit membrane. The outer membrane of Cvt body then fuses with the vacuole, releasing a single unit vesicle sequestering the cargo into the vacuole where the membrane is lysed by vacuolar lipase, Aut5/Cvt17p, releasing the cargo for processing into a mature enzyme.
For larger structures disposed via macro-autophagy the processes are analogous to Cvt where cytosolic organelles and large structures are encased in a membrane, which in turn fuses with the vacuole/lysosome releasing the encased structure into the vacuole where it is subject to the action of lytic hydrolases. By using one common mechanism of encasing proteins and structures in a membrane to mediate sequestration into the lytic compartment, yeast and animal cells have evolved a conserved mechanism that merges processes of altering lytic enzyme composition of the vacuole in parallel with protein turnover and organelle disposal.
There is evidence for nonconventional ER trafficking bypassing the Golgi to the lysosome in animal cells. A liver disease that results from the synthesis of mutant α1-antitrypsin Z causes chronic hepatitis and is associated with a carcinoma. The mutant protein aggregates in the ER, and the ER containing the protein is localized in autophagosomes (Teckman and Perlmutter, 2000). The ER to lysosome transfer occurs by a Cvt-like mechanism where the ER is encased in a de novo derived autophagosome membrane and the phagosome is delivered to the lytic compartment by fusion of the phagosome membrane rather than engulfment and sequestration (Teckman and Perlmutter, 2000). The function of Cvt pathway is a key aspect of human protein conformational disorder diseases such as Alzheimer's, Huntington's, and Parkinson's disease. In some instances these diseases are caused by codon reiteration that causes cytosolic proteins to be expressed with polyamino acid inserts producing misfolded and aggregated proteins. Although the accumulation of these proteins is related to pathology, a fraction of the protein may be continuously cleared from the cells by transfer to the lytic compartment by the Cvt (see Ravikumar et al., 2002 and refs. within for examples). It is particularly interesting that human protein aggregation diseases result in forming protein accretions that are targeted for sequestration in the lytic compartment. Although this is an overtly parallel process to the ERvt path of protein-containing ER bodies in plants, the underlying biology is completely different.
WHY DO PLANTS, ANIMALS, AND YEAST NEED AN ENDOMEMBRANE BYPASS PATHWAY TO DEPOSIT PROTEINS IN THE VACUOLE?
While questions of why biological processes occur are often speculative it is useful to consider what advantages the ERvt and Cvt pathways do provide beyond the capacity of avoiding endomembrane progression through the Golgi. Nonclassical protein and rubber transport by the ERvt pathway provides a means to break possible size limits on transport through the endomembrane system. For endomembrane organelles, proteins are folded in conjunction with their synthesis and transferred from organelle to organelle via vesicular transport. Receptor mediated targeting and intermediate processing occurs during trafficking, and for many of the vacuolar and secreted enzymes final processing and activation occurs after arrival at the terminal destination. The vacuoles of plants and yeast have not been shown to possess any protein deposited directly after translation by facilitated transport through a membrane pore as occurs in plastids, mitochondria, and peroxisomes. This means that all the proteins within the vacuole must be produced elsewhere and taken into the vacuole by secondary postsynthesis mechanisms. Broadly, there are two major mechanisms of trafficking proteins to the vacuole. One is endomembrane progression where newly synthesized and folded proteins are transported to and through the Golgi and then to the vacuole. Protein targeting to the vacuole requires positive targeting information that is mediated by Golgi receptor interaction with peptide sequences on the vacuole protein. Many of the proteins sequestered in the vacuole are large oligomers, particularly seed storage proteins, of 200 kD or more. There may be limits of maximum size of cargo protein oligomer/complexes for endomembrane progression from the ER, to and through the Golgi, and deposition of vacuole. The cargo size might exceed that maximum size possible for endomembrane progression, allowing larger protein complexes to be transported to the vacuole that would otherwise be impossible via conventional endomembrane progression through the Golgi.
The ERvt pathway should in theory have much broader cargo capacity than the similar Cvt pathway. Because Cvt pathway requires specific interactions with proteins that mediate formation of the Cvt complex and vesicle, it is likely that these are protein-specific. Without recruiting membrane material to form the Cvt vesicle, fusion and release into the vacuole is not possible. In contrast, for the ERvt pathway, any substance that might accrete in the ER should by self-assembly mechanisms result in the formation of an ER body that can be taken into the vacuole by autophagy. Although the only forms of ER bodies now known to be sequestered in the vacuole by ERvt are those containing either protein or rubber, this still constitutes a diverse array of hydrophobic and hydrophilic storage proteins and enzyme precursors that is much broader than that documented for the Cvt pathway.
The vast majority of plant cell biology research is conducted on a small group of plants, primarily crop plants and a few model plants, most often grown under ideal conditions and for major vegetative and reproductive organs. Detailed studies on less well-known plants, less studied portions of plants, as well as extremes of growth conditions, will likely identify other examples of ER bodies and other constituent proteins and other substances. The potential of ER bodies to sequester ER-synthesized material and deposit that material in vacuoles is only now being recognized as an important aspect of plant cell biology. A reinterpretation of electron microscopy observations acquired since the 1960s will likely result in many other examples of ER-bodies being identified and new perspectives on the growth, development and environmental responses of plants. At a time when electron microscopy skills are becoming increasingly scarce because of lack of replacement for aging investigators there are new opportunities to correlate microscopy with other forms of cell and molecular biology.
This paper is dedicated to Dr. Takao Minamikawa, Professor of Tokyo Metropolitan University, on his retirement. His discovery of the KDEL-tailed Cys proteases resulted in research that changed our perspective of ER bodies from apparently inert storage protein organelles to active participants in cellular remodeling.
References
- Backhaus RA, Walsh S (1983) Ontogeny of rubber formation in guayule, Parthenium argentatum Gray. Bot Gaz 144: 391–400 [Google Scholar]
- Chrispeels MJ, Herman EM (2001) Endoplasmic reticulum-derived compartments function in storage and as mediators of vacuolar remodeling via a new type of organelle, precursor protease vesicles. Plant Physiol 123: 1227–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SB, Wang C, Muench DG, Ozawa K, Franceschi VR, Wu Y, Okita TW (2000) Messenger RNA targeting of rice seed storage proteins to specific ER subdomains. Nature 407: 765–767 [DOI] [PubMed] [Google Scholar]
- Coleman CE, Herman EM, Takasaki K, Larkins BA (1996) The maize α-zein sequesters γ-zein and stabilizes its accumulation in protein bodies of transgenic tobacco endosperm. Plant Cell 8: 2335–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkes NR, Herman EM, Chrispeels MJ (1979) Rapid degradation and limited synthesis of phospholipids in cotyledons of mung bean seedlings. Plant Physiol 64: 38–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura I, Shimada T, Hatano K, Takeuchi Y, Nishimura M (1998) Transport of storage proteins to protein storage vacuoles is mediated by large precursor-accumulating vesicles. Plant Cell 10: 825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Toriyama K, Kondo M, Hara-Nishimura I, Nishimura M (1999) Accumulation of a fusion protein containing 2S albumin induces novel vesicles in vegetative cells of Arabidopsis. Plant Cell Physiol 40: 263–272 [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Yamada K, Shimada T, Matsushima R, Nishizawa NK, Nishimura M, Hara-Nishimura I (2001) A proteinase-storing body that prepares for cell death or stresses in the epidermal cells of Arabidopsis. Plant Cell Physiol 42: 894–899 [DOI] [PubMed] [Google Scholar]
- Herman EM (1994) Multiple origins of intravacuolar protein accumulation of plant cells. Adv Struct Biol 3: 243–283 [Google Scholar]
- Herman EM, Baumgartner B, Chrispeels MJ (1981) Uptake and apparent digestion of cytoplasmic organelles by protein bodies (protein storage vacuoles in mung bean cotyledons). Eur J Cell Biol 24: 226–235 [PubMed] [Google Scholar]
- Herman EM, Larkins BA (1999) Protein storage bodies. Plant Cell 11: 601–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney AJ, Jung R, Herman EM (2001) Cosuppression of the α-subunits of β-conglycinin in transgenic soybean seeds induces the formation of endoplasmic reticulum-derived protein bodies. Plant Cell 13: 1165–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Ohsumi Y (1999) Vacuolar import of proteins and organelles from the cytoplasm. Annu Rev Cell Dev Biol 15: 1–32 [DOI] [PubMed] [Google Scholar]
- Levanony H, Rubin R, Altshuler Y, Galili G (1992) Evidence of a novel route of wheat storage proteins to vacuoles. J Cell Biol 119: 1117–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matile P (1975) The Lytic Compartment of Plant Cells. Cell Biology Monographs, Vol 1. Springer-Verlag, Wien, Germany
- Matsushima R, Hayashi Y, Yamada K, Shimada T, Nishimura M, Hara-Nishimura I (2003) The ER body, a novel endoplasmic reticulum-derived structure in Arabidopsis. Plant Cell Physiol 44: 661–666 [DOI] [PubMed] [Google Scholar]
- Melroy DL, Herman EM (1991) TIP, an integral membrane protein of the soybean seed protein storage vacuole, undergoes developmentally regulated membrane insertion and removal. Planta 184: 113–122 [DOI] [PubMed] [Google Scholar]
- Moriyasu Y, Ohsumi Y (1996) Autophagy in tobacco suspension culture in response to sucrose starvation. Plant Physiol 111: 1233–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Durst F, Werck-Reichert D, Gardner HW, Camara B, Cornish K, Backhaus RA (1995) The major protein of guayule rubber particles is a cytochrome P450. J Biol Chem 270: 8487–8494 [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Duden R, Rubinsztein DC (2002) Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet 11: 1107–1117 [DOI] [PubMed] [Google Scholar]
- Rojo E, Zouhar J, Carter C, Kovaleva V, Raikhel N (2003) A unique mechanism for protein processing and degradation in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 7389–7394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Simpson D, Kalousek F, Gietl C (1998) A cysteine endopeptidase with a C-terminal KDEL motif isolated from castor bean endosperm is a marker enzyme for the ricinosome, a putative lytic compartment. Planta 206: 466–475 [DOI] [PubMed] [Google Scholar]
- Schmid M, Simpson DJ, Sarioglu H, Lottspeich F, Gietl C (2001) The ricinosomes of senescing plant tissue bud from the endoplasmic reticulum. Proc Natl Acad Sci USA 98: 5353–5358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SV, Baba M, Ohsumi Y, Klionsky DJ (1997) Aminopeptidase I is targeted to the vacuole by a nonclassical vesicular mechanism. J Cell Biol 138: 37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin LA (1997) The plant ER: a dynamic organelle composed of a large number of discrete functional domains. Plant J 11: 1151–1165 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yamauchi D, Minamikawa T (1991) Nucleotide sequence of cDNA for an endopeptidase (EP-C1) from pods of maturing Phaseolus vulgaris fruits. Plant Mol Biol 16: 1083–1084 [DOI] [PubMed] [Google Scholar]
- Teckman JH, Perlmutter DH (2000) Retention of mutant α1-antitrypsin Z in endoplasmic reticulum is associated with an autophagic response. Am J Physiol Gastrointest Liver Physiol 279: G961–G974 [DOI] [PubMed] [Google Scholar]
- Toyooka K, Okamoto T, Minamikawa T (2000) Mass transport of proform of a KDEL-tailed cysteine proteinase (SH-EP) to protein storage vacuoles by endoplasmic reticulum-derived vesicle is involved in protein mobilization in germinating seeds. J Cell Biol 148: 453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JC, Galili G, Kawata EE, Cuellar RE, Shotwell MA, Larkins BA (1988) Aggregation of lysine-containing zeins into protein bodies in Xenopus oocytes. Science 240: 662–664 [DOI] [PubMed] [Google Scholar]
- Wandelt CI, Khan MRI, Craig S, Schroeder HE, Spencer D, Higgins TJV (1992) Vicilin with carboxy-terminal KDEL is retained in the endoplasmic reticulum and accumulates to high levels in the leaves of transgenic plants. Plant J 2: 181–192 [DOI] [PubMed] [Google Scholar]