Abstract
The major seed storage proteins of maize (Zea mays) and bean (Phaseolus vulgaris), zein and phaseolin, accumulate in the endoplasmic reticulum (ER) and in storage vacuoles, respectively. We show here that a chimeric protein composed of phaseolin and 89 amino acids of γ-zein, including the repeated and the Pro-rich domains, maintains the main characteristics of wild-type γ-zein: It is insoluble unless its disulfide bonds are reduced and forms ER-located protein bodies. Unlike wild-type phaseolin, the protein, which we called zeolin, accumulates to very high amounts in leaves of transgenic tobacco (Nicotiana tabacum). A relevant proportion of the ER chaperone BiP is associated with zeolin protein bodies in an ATP-sensitive fashion. Pulse-chase labeling confirms the high affinity of BiP to insoluble zeolin but indicates that, unlike structurally defective proteins that also extensively interact with BiP, zeolin is highly stable. We conclude that the γ-zein portion is sufficient to induce the formation of protein bodies also when fused to another protein. Because the storage proteins of cereals and legumes nutritionally complement each other, zeolin can be used as a starting point to produce nutritionally balanced and highly stable chimeric storage proteins.
Seeds accumulate very large amounts of a few classes of storage proteins that are used during early germination as a source of reduced nitrogen. Seed storage proteins of legumes and cereals are also the major food proteins for humans, and, nutritionally, they largely complement each other: Storage proteins from legumes are poor in sulfur amino acids and those of cereal are poor in Lys and Trp. Experiments that used genes encoding wild-type or mutated storage proteins to improve by genetic engineering the nutritional value of seeds or vegetative plant tissues indicated that the subcellular location of these proteins is important for stable accumulation (for review, see Tabe and Higgins, 1998). All storage proteins are translocated cotranslationally into the lumen of the endoplasmic reticulum (ER), and then they either accumulate therein or traffic through the secretory pathway to storage vacuoles (for review, see Müntz, 1998). Storage proteins of the 7S and 11S classes, which are particularly abundant in legumes, accumulate in protein storage vacuoles, whereas cereal prolamins are mainly located in the ER (Müntz, 1998). These proteins are structurally well characterized, and the vacuolar sorting signals of two 7S proteins have been identified, but the molecular interactions that lead to their subcellular location are still largely unknown (Frigerio et al., 1998; Müntz, 1998; Nishizawa et al., 2003).
Two well-studied examples of storage proteins are γ-zein of maize (Zea mays) and phaseolin of bean (Phaseolus vulgaris). Zeins are the prolamins of maize. In the ER of maize endosperm cells, γ-zein forms large complexes termed protein bodies, together with the other classes of zein polypeptides (Lending and Larkins, 1989). Resident proteins of the ER lumen, which in all eukaryotic cells are mainly helpers of protein folding, have a tetrapeptide at the C terminus, KDEL, or variations of this motif, which is necessary and sufficient for ER localization (Pelham, 1990). ER residents leave this compartment along the secretory pathway, but are brought back into it by the KDEL receptor located in the Golgi complex (Pelham, 1990). Zeins, however, do not have this signal. The interactions that retain zeins and cereal prolamins in general in the ER and hold protein bodies together are not clear in detail, but γ-zein is able to form ER-located protein bodies also when expressed in storage (Coleman et al., 1996) or vegetative (Geli et al., 1994) tissues of transgenic plants in the absence of its partner zein subunits, indicating that no tissue-specific helping factors are required. The 27-kD γ-zein polypeptide can be divided into three major domains: an N-proximal domain constituted by eight repeats of the motif PPPVHL is followed by a Pro-rich domain and finally by a Cys-rich domain (Prat et al., 1985). The repeated domain contains sufficient information to accumulate within the ER but does not form protein body-like structures (Geli et al., 1994). Inclusion of the Pro-rich region does not change its localization. The Cys-rich domain is secreted when fused to the signal peptide in the absence of the other domains (Geli et al., 1994). Secretion is the default destination for secretory proteins that do not have additional signals apart from the transient signal peptide that determines translocation into the ER (Denecke et al., 1990; Hunt and Chrispeels, 1991). The two events of γ-zein cell biology, ER retention and protein body formation, are therefore independent; the Cys-rich domain lacks ER retention information but seems to have the role of favoring protein body assembly (Geli et al., 1994).
Like all 7S storage proteins, phaseolin is a soluble homotrimer. The subunits rapidly assemble within the ER lumen (Vitale et al., 1995) and are held together mainly by hydrophobic interactions (Lawrence et al., 1990). The trimers traffic through the Golgi complex and then to not well-characterized dense vesicles before deposition into storage vacuoles (Chrispeels, 1983). A short C-terminal propeptide, AFVY, is necessary for vacuolar sorting: A deletion construct without the propeptide forms trimers efficiently but is secreted (Frigerio et al., 1998). The receptor system involved in the recognition of this sorting signal is still unknown. Assembly defective constructs of phaseolin are unable to enter vesicle traffic that leaves the ER and are degraded after extensive interactions with the ER chaperone BiP (Pedrazzini et al., 1997; Nuttall et al., 2003).
To understand whether γ-zein domains can alter the destiny of a vacuolar storage protein, here we have fused the repeated and Pro-rich domains of γ-zein at the C terminus of phaseolin and have studied the synthesis and accumulation of this construct in plant cells.
RESULTS
Zeolin Accumulates to High Amounts in Leaves of Transgenic Tobacco
We prepared the zeolin fusion protein starting from T343F phaseolin, which accumulates similarly to wild-type phaseolin but contains only one glycosylation site instead of two (Pedrazzini et al., 1997). Zeolin contains the entire T343F sequence, including the signal peptide, followed by the unstructured 15 amino acid linker (GGGGS)3 and 89 amino acids of mature γ-zein, starting from the fifth residue after the γ-zein signal peptide (Fig. 1). The total number of amino acids is 525, including the 24 residues of the N-terminal signal peptide of phaseolin, which is removed cotranslationally. The zein sequence includes the repeated and Pro-rich domains.
Figure 1.
Sequence of zeolin. Residues 1 to 421 are the complete sequence of T343F phaseolin (Pedrazzini et al., 1997). The N-terminal signal peptide, which is removed cotranslationally, is dark-gray shaded. The synthetic linker is light-gray shaded. Residues 437 to 525 are 89 residues of γ-zein clone G1L (Bellucci et al., 1997), starting from the fifth amino acid after the γ-zein signal peptide cleavage site. The Cys residues are underlined.
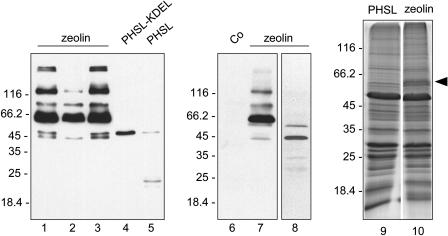
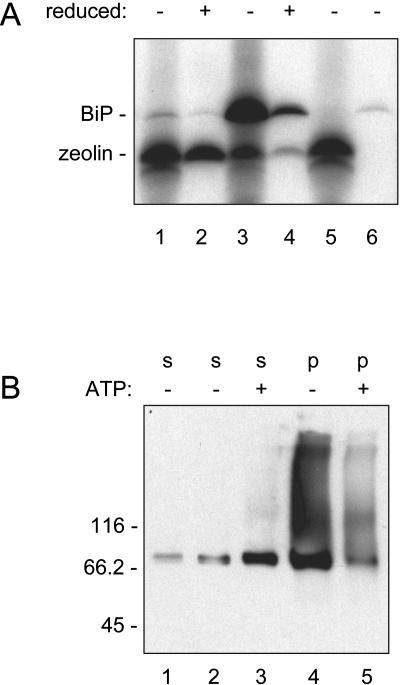
The zeolin construct was placed under the transcriptional control of the cauliflower mosaic virus 35S promoter and used to produce transgenic tobacco (Nicotiana tabacum) by Agrobacterium transformation. Young (5–7 cm long) leaves of the transgenic plants were homogenized in the presence of nonionic detergent and the reducing agent 2-mercaptoethanol, to favor protein solubilization, and analyzed by protein blotting using antiphaseolin antiserum. Leaves from plants transformed with the empty plasmid vector, used as control, gave no signal (Fig. 2, lane 6). The protein pattern obtained from three independent plants transformed with the zeolin construct was very similar (Fig. 2, lanes 1–3). The most abundant polypeptide had a molecular mass around 60 kD, expected for intact zeolin. Higher Mr forms were also present, indicating that the protein is difficult to denature. The oligomers are, however, minor components; the time of film exposure needed to allow detection of products from the other phaseolin constructs (lanes 4 and 5) caused saturation of the very strong signal from the 60-kD monomer in lanes 1 to 3 and did not allow evaluation of the quantitative difference with respect to the higher Mr forms, which is clearer in the blot in lane 7. Polypeptides of around 45 kD were also detected. This is about the molecular mass expected for phaseolin, suggesting that zeolin could be in part fragmented at the junction between the zein and phaseolin portions. This was confirmed by analysis of protein blots using anti-γ-zein antiserum. All the zeolin polypeptides shown in Figure 2 reacted, except those at 45 kD (not shown). T343F phaseolin synthesized in transgenic tobacco leaves clearly accumulated to much lower levels than zeolin (Fig, 2, lane 5). T343F phaseolin was detectable as the intact, newly synthesized polypeptide around 46 kD and as fragments around 20 to 25 kD formed upon vacuolar delivery in transgenic tobacco (Pedrazzini et al., 1997). A KDEL ER localization tetrapeptide added at the C terminus of phaseolin markedly increases protein accumulation because to a great extent it prevents vacuolar delivery. Consistently, phaseolin-KDEL was mostly intact (Fig. 2, lane 4; Frigerio et al., 2001). However, the accumulation of zeolin was also much higher than that of phaseolin-KDEL. Approximate quantitation of zeolin was made by comparing different dilutions of leaf extracts and known amounts of purified bean phaseolin (not shown). Protein blots indicated that in a typical transgenic plant zeolin accumulated to about 3.5% of total extractable protein, whereas the values were about 0.5% for phaseolin-KDEL and 0.05% for T343F, with less than 2-fold variability between independent transgenic plants for each construct. It should be taken into account that about one-fifth of intact zeolin is made of zein and therefore is not detected by anti-phaseolin antiserum; therefore, the amount of zeolin was slightly underestimated in our assay.
Figure 2.
Accumulation of zeolin in leaves of transgenic tobacco. Proteins were extracted from young leaves of plants expressing zeolin, T343F phaseolin (PHSL), phaseolin-KDEL (PHSL-KDEL), or transformed with the empty vector pGA470 (Co) and were analyzed by SDS-PAGE. Extraction was performed in the presence (lanes 1–7, 9, and 10) or absence (lane 8) of 2-mercaptoethanol. Extract containing 50 μg of protein was loaded in each lane. Lanes 1 to 8, Protein blots using antiphaseolin antiserum. Lanes 9 and 10, Gel stained with Coomassie blue. Numbers at left indicate the positions of molecular mass markers, in kD. The arrowhead at right marks the position of zeolin in lane 10.
We conclude that the γ-zein portion efficiently increased accumulation of the recombinant protein when added to phaseolin. The band corresponding to intact zeolin can be detected in Coomassie blue-stained gels (Fig. 2, lanes 9 and 10).
Zeolin Forms Protein Bodies
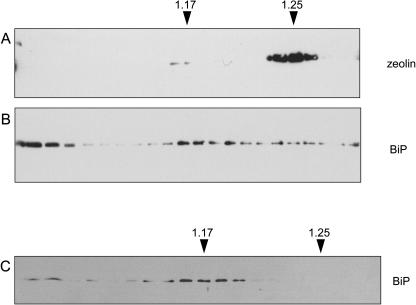
Wild-type γ-zein is insoluble unless its disulfide bridges are reduced (Vitale et al., 1982). Zeolin has similar solubility properties: When leaves from transgenic tobacco expressing zeolin were homogenized in the absence of reducing agent, intact zeolin and the larger oligomers were not solubilized (Fig. 2, lane 8; compare with lane 7). We therefore established whether zeolin also forms protein bodies. In maize endosperm, the ER-located zein protein bodies are spherical structures with a diameter between 0.3 and 1.4 μm, depending on the stage of seed development (Lending and Larkins, 1989) and with a density around 1.21 to 1.22 g/mL (Hurkman et al., 1981; Galante et al., 1983). Young leaves from transgenic tobacco were homogenized in a buffer in the absence of detergent and the presence of Suc and the subcellular compartments were fractionated by isopycnic ultracentrifugation in Suc gradients. Most zeolin was recovered in a subcellular fraction with density around 1.25 g/mL (Fig. 3A), and a very small proportion was in a fraction around 1.17 g/mL, which is the density of the ER in tobacco leaf cells (Pedrazzini et al., 1997; Frigerio et al., 2001). Consistently, BiP, a widely used ER marker, formed a major peak at density 1.17 g/mL (Fig. 3B). BiP was also present at the top of the gradient, as already observed (Gomord et al., 1997; Pedrazzini et al., 1997). This might reflect partial release of the chaperone from the ER during homogenation. BiP, however, also formed a minor but clearly detectable peak at density 1.25 g/mL (Fig. 3B), which was not detectable in extracts of plants transformed with the empty vector (Fig. 3C) or expressing phaseolin-KDEL (Frigerio et al., 2001). We conclude that zeolin mainly accumulated together with a proportion of BiP in a subcellular fraction with a density similar to that of zein protein bodies.
Figure 3.
Subcellular distribution of zeolin. Young leaves from transgenic tobacco expressing zeolin (A and B) or from tobacco transformed with empty vector (C) were homogenized in the absence of detergent and the presence of Suc. The homogenates were fractionated by centrifugation on isopycnic Suc gradient. Proteins in each gradient fraction were analyzed by SDS-PAGE and protein blot, using antiphaseolin (A) or anti-BiP (B and C) antiserum. Top of gradients is at left. Numbers at top indicate density (grams per milliliter).
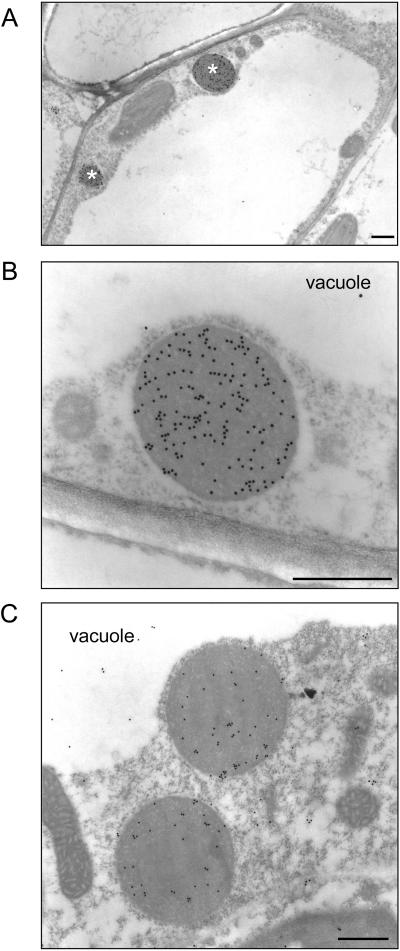
Zein protein bodies are easily detectable by electron microscopy (Lending and Larkins, 1989). Thus, we analyzed leaf cells of zeolin plants by immunoelectron microscopy. Several spherical, electron-opaque protein bodies with diameter around 0.5 to 1.0 μm were detectable (Fig. 4A). These structures were not observed in untransformed tobacco cells (not shown) and were never detected within the vacuole. Conversely, in cells of plants expressing T343F phaseolin, aggregates of the storage protein were visible in the vacuole (Frigerio et al., 1998) but were never observed within the cytoplasm. The protein bodies of zeolin plants were immunolabeled with antiphaseolin antiserum (Fig. 4A, and see Fig. 4B for an enlarged detail) and anti-γ-zein antiserum (Fig. 4C), indicating that they contained zeolin and constituted the subcellular fraction with density around 1.25 g/mL. Zeolin protein bodies were not labeled when preimmune serum was used (not shown). Despite the poor preservation of membranes due to fixation for immunocytochemistry, ribosomes were visibly clustered around the protein body periphery, indicating that zeolin protein bodies were located within the ER. The size and morphology of zeolin protein bodies were very similar to those of natural zein protein bodies in maize endosperm cells (Lending and Larkins, 1989).
Figure 4.
Zeolin protein bodies. Thin sections prepared from young leaves of transgenic tobacco expressing zeolin were treated with antiphaseolin (A and B) or anti-γ-zein (C) antiserum and the bound antibodies visualized with 1:25 goat anti-rabbit 15-nm gold complex. B is an enlargement of A. Asterisks in A mark protein bodies. Bars = 500 nm.
Zeolin Has Slow Turnover and Long-Lasting Interactions with BiP
The large accumulation of zeolin suggested the protein is very stable, and the subcellular fractionation experiments suggested a prolonged interaction with BiP. Consequently, the stability of zeolin and its interactions with BiP were investigated.
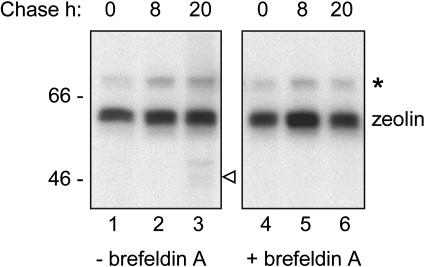
Protoplasts were prepared from young leaves of transgenic tobacco expressing zeolin and subjected to pulse-chase labeling. At each time point, an aliquot of protoplasts was homogenized and immunoselected in the presence of 2-mercaptoethanol with antiphaseolin antiserum. After a 1-h pulse, a radioactive polypeptide with the expected molecular mass of around 60 kD was immunoselected from protoplast homogenates by antiphaseolin antiserum (Fig. 5, lane 1). No marked decrease in zeolin recovery was observed after an 8- or 20-h chase (lanes 2 and 3). This indicates a half-life of more than 20 h. In protoplasts similarly prepared from leaves of transgenic tobacco expressing T343F phaseolin, the half-life of phaseolin was between 2 and 3 h (Pedrazzini et al., 1997). Fragmentation products of zeolin were detectable after the 20-h chase (Fig. 5, lane 3). Longer exposure of gels indicated that zeolin fragments start to be detectable by 8-h chase and increase in proportion after longer chase times (not shown). One radioactive fragment (arrowhead at right of lane 3) had a molecular mass corresponding to that of intact T343F detectable by protein blot in extracts of plants expressing zeolin. Pulse-chase experiments performed in the presence of the N-glycosylation inhibitor tunicamycin showed that the fragment is glycosylated, as expected for T343F phaseolin released from zeolin (not shown). The fungal metabolite brefeldin A is widely used to inhibit protein trafficking along the secretory pathway (see, for example, Pedrazzini et al., 1997). When the pulse-chase was performed in the presence of brefeldin A, the fragments were not detected (Fig. 5, lanes 4–6). We conclude that zeolin is very stable and that the release of T343F from the chimeric protein is a posttranslational process that affects a minor proportion of zeolin molecules and requires traffic along the secretory pathway.
Figure 5.
Pulse-chase analysis of zeolin. Protoplasts isolated from young leaves of transgenic tobacco expressing zeolin were pulse labeled with 35S-Met and 35S-Cys for 1 h and subjected to chase for the indicated times. The experiment was performed in the absence (lanes 1–3) or presence (lanes 4–6) of brefeldin A. Cell homogenates were then immunoselected using antiphaseolin antiserum. Analysis was by SDS-PAGE and fluorography. Numbers at left indicate the positions of molecular mass markers in kD. The positions of zeolin, putative BiP (asterisk), and putative T343F (open arrowhead) are indicated.
A polypeptide around 78 kD, which is the molecular mass of BiP, was coimmunoprecipitated with zeolin (asterisk in Fig. 5). To confirm its identity and to establish whether its association occurred with soluble or insoluble zeolin, we performed the following experiment. Protoplasts were pulse labeled for 1 h, and zeolin was immunoselected in the absence of 2-mercaptoethanol. The 78-kD polypeptide was still coselected (Fig. 6A, lane 1). Like the other members of the heat shock 70 class of chaperones, BiP has ATPase activity and can be released from its bound ligands by ATP treatment (Munro and Pelham 1986; for experiments on phaseolin, see Pedrazzini et al., 1994, 1997; Vitale et al., 1995). When the immunoprecipitate was treated with ATP, the 78-kD polypeptide was released and could be immunoselected with anti-BiP antiserum, confirming it was BiP (Fig. 6A, lanes 5 and 6). Consistently, when the protoplast homogenate was treated with anti-BiP antiserum (without adding ATP), a significant proportion of zeolin was coselected (Fig. 6A, lane 3). Therefore, BiP has affinity for soluble, newly synthesized zeolin. The immunoprecipitation protocol involved a centrifugation step before adding antiserum to discard insoluble material that could contaminate the immunoprecipitate. We collected the pellet of this centrifugation step and treated it with the homogenization buffer containing 2-mercaptoethanol. We then treated the homogenate with antiphaseolin or anti-BiP antiserum. A significant proportion (when compared to the first immunoselection) of both proteins was recovered in this second round of immunoprecipitation, each in association with detectable amounts of the other protein (Fig. 6A, lanes 2 and 4). We concluded that zeolin rapidly becomes insoluble due to the formation of disulfide bonds and that insoluble zeolin still interacts with BiP. This must not be an unspecific trapping event, because the two proteins remain largely associated even after solubilization of zeolin with reducing agents. BiP bound to insoluble zeolin can be released by ATP treatment, confirming that it is not trapped but is associated due to its chaperone-binding properties (not shown).
Figure 6.
Association of BiP with zeolin. A, protoplasts isolated from young leaves of transgenic tobacco expressing zeolin were pulse labeled with 35S-Met and 35S-Cys for 1 h and homogenated in nonreducing conditions. After preclearing by centrifugation, equal aliquots of the soluble homogenate were immunoselected with antiphaseolin (lane 1) or anti-BiP (lane 3) antiserum. Another aliquot was selected with antiphaseolin antiserum and treated with ATP (lane 5); the released material was then immunoselected with anti-BiP antiserum (lane 6). The pellet from the preclearing of the immunoprecipitations in lanes 1 and 3 was treated with homogenation buffer in the presence of 2-mercaptoethanol and immunoselected with antiphaseolin (lane 2) or anti-BiP (lane 4) antiserum. Analysis was by SDS-PAGE and fluorography. B, young leaves from transgenic tobacco expressing zeolin were homogenized in the presence of nonionic detergent in nonreducing conditions. After centrifugation at 1,500g the soluble (s) fraction was recovered (lane 1). The precipitate was treated again with homogenation buffer (lanes 2 and 4) or with buffer supplemented with ATP (lanes 3 and 5); after a new centrifugation at 1,500g the soluble fractions (s, lanes 2 and 3) and the insoluble precipitates (p, lanes 4 and 5) were recovered. Analysis was by SDS-PAGE and protein blot, using anti-BiP antiserum. Numbers at left indicate the positions of molecular mass markers in kD.
Finally, we asked whether a significant proportion of total BiP is associated with insoluble zeolin. Leaves were homogenized with nonionic detergent in the absence of reducing agent and the extract centrifuged at 1,500g. This caused the precipitation of zeolin (see Fig. 2). Protein blot revealed that a large proportion of BiP remained in the pellet. Long exposure revealed that it was in part present as higher Mr forms (Fig. 6B, lane 4 and compare with lane 1). When a similar experiment was performed on untransformed tobacco or tobacco expressing wild-type phaseolin or phaseolin-KDEL, the higher Mr forms were not detectable and BiP partitioned preferentially in the soluble fraction (not shown). When the pellet from the preparation of zeolin-expressing plants was treated with ATP and centrifuged again, a large proportion of BiP originally in the pellet was solubilized and the amount of higher Mr forms was substantially reduced (Fig. 6B, lanes 3 and 5, and compare with the control treatment in the absence of ATP in lanes 2 and 4). This indicated that a relevant proportion of total BiP was associated in an ATP-sensitive manner to insoluble zeolin and was part of the high Mr forms detectable by protein blot.
Zeolin Forms Large Oligomers Held Together by Disulfide Bonds, But Phaseolin Released in Vivo from Zeolin Is Trimeric
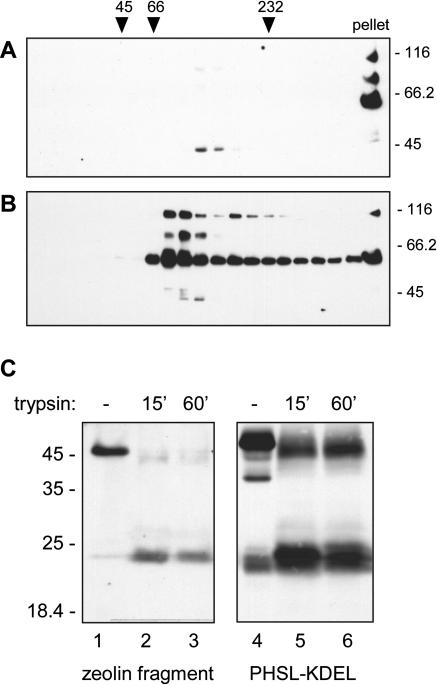
Phaseolin is a homotrimer with a determined crystal structure (Lawrence et al., 1990). The conformation of γ-zein is unknown, although a synthetic (VHLPPP)8 molecule adopts an amphipathic poly-Pro II conformation and self-assembles into cylindrical micelles in vitro (Kogan et al., 2002). We analyzed in more detail the in vivo assembly of zeolin by sedimentation in velocity Suc gradients. When protein extraction and sedimentation were performed in the absence of reducing agents, intact zeolin pelleted at the bottom of the tube, indicating high molecular-mass polymerization (Fig. 7A). The fragment around 45 kD, instead, sedimented at a position between the 66-kD and 232-kD markers, suggesting assembly into trimers (Fig. 7A). In the presence of reducing agent, intact zeolin formed a major peak migrating slightly faster than the 66-kD marker (Fig. 7B). A second, not well-separated peak migrated slightly slower than the 232-kD marker, and a minor but relevant proportion pelleted at the bottom of the tube. The 45-kD fragment did not change its behavior, as expected, since phaseolin does not contain Cys residues. These results confirmed that assembly of zeolin is due to disulfide bonds and suggested that, once these bonds are reduced, zeolin is either a monomer or a dimer. The experiment also suggested that the released phaseolin assembled into trimers, like wild-type phaseolin.
Figure 7.
Oligomeric state of zeolin. A and B, young leaves of transgenic tobacco expressing zeolin were homogenated in the presence of nonionic detergent and in the absence (A) or presence (B) of reducing agent. The homogenate were subjected to sedimentation velocity centrifugation on a continuous 5% to 25% (w/v) Suc gradient. Aliquots of gradient fractions and a proportional amount of the material precipitated at the bottom of the tubes (pellet) were analyzed by protein blot and visualized using antiphaseolin antiserum. The top of the gradients is at left. Numbers on top indicate the position along the gradient and the molecular mass, in kD, of sedimentation markers. Numbers at right indicate the positions of molecular mass markers along the SDS-PAGE gel. C, An aliquot of the fraction containing the 45-kD zeolin fragments from the gradient shown in A (zeolin fragment, lanes 1–3) or a total protein extract from leaves of transgenic tobacco expressing phaseolin-KDEL (PHSL-KDEL, lanes 4–6) were subjected to trypsin digestion in vitro for the indicated minutes, or incubated for 60 min in digestion buffer without enzyme as control (–) and then analyzed by protein blot using antiphaseolin antiserum. Numbers at left indicate the positions of molecular mass markers in kD.
Phaseolin trimers are quite resistant to trypsin digestion in vitro: The enzyme gives rise to fragments corresponding roughly to halves of the polypeptide that are not digested further and remain assembled into the original oligomeric structure (Deshpande and Damodaran, 1989). We therefore assessed the effect of trypsin on the 45-kD zeolin fragment. Products of digestion treatment around 25 kD were rapidly formed and remained stable upon longer treatment (Fig. 7C, lanes 1–3). The result was very similar to that obtained by digesting phaseolin-KDEL (Fig. 7C, lanes 4–6), which correctly forms trimers (Frigerio et al., 2001). Therefore, phaseolin released from zeolin appears to be trimeric.
DISCUSSION
A Dominant Effect of the N-Terminal Region of γ-Zein
We have shown that 89 amino acids of mature γ-zein, including the repeated and Pro-rich domains, have a dominant effect on phaseolin intracellular traffic: The zein fragment prevents zeolin from being delivered to the vacuole. Geli et al. (1994) reported that the N-terminal-repeated region of γ-zein is ER located when expressed in leaves of transgenic plants but the C-terminal region is necessary for protein body formation. We have extended the first of these two conclusions: ER retention can be conferred to another protein by γ-zein domains. The assembly/aggregation of zeolin into protein bodies is not expected based on the second conclusion of Geli et al. (1994). It is, however, possible that once the zein segment of zeolin has promoted ER retention, the characteristics of phaseolin contribute to protein body formation. Indeed, addition of the ER localization signal, KDEL, to pea (Pisum sativum) vicilin (the 7S storage protein of pea) causes, in transgenic leaves, the formation of protein bodies morphologically very similar to those composed of zein and zeolin (Wandelt et al., 1992). This suggests that 7S storage proteins have a tendency to form large complexes independently of the site of accumulation. However, zeolin is insoluble in the absence of reducing agents, whereas, for example, phaseolin-KDEL accumulated to large amounts in the ER but does not require reducing agents to be solubilized (Frigerio et al., 2001). This suggests that the protein interactions are different in the cases of zeolin and KDEL-modified 7S storage proteins. Insolubility due to disulfide bonds is a characteristic of γ-zein (Vitale et al., 1982) that was transferred to zeolin. Out of the 15 Cys residues of γ-zein, only six are present in zeolin (Fig. 1). Consequently, our results indicate that these six Cyss are sufficient to confer the solubility characteristics of γ-zein.
ER Retention and Stability of Zeolin
In tobacco leaves, zeolin accumulates to much larger amounts than phaseolin-KDEL, which is a very stable protein compared to wild-type phaseolin expressed in the same tissue (here, and see Frigerio et al., 2001). The KDEL ER localization signal is widely used to increase accumulation of foreign proteins in transgenic plants, because the ER has low hydrolytic activity. However, a proportion of phaseolin-KDEL is slowly delivered to the vacuole by a pathway that does not seem to be mediated by the Golgi complex (Frigerio et al., 2001). Other experiments also suggest that the KDEL/HDEL ER localization signal may also promote vacuolar delivery in plant cells (Gomord et al., 1997). We do not know whether this is a normal route for the disposal of natural ER residents, which are usually quite stable but eventually must be turned over. It should be noted that KDEL provides a retrieval mechanism from the Golgi complex and is not a retention mechanism (Pelham, 1990). Addition of the γ-zein sequence could instead confer ER retention, not allowing the vacuolar delivery possibly caused by the KDEL retrieval mechanism. Once assembled, protein bodies seem too large to enter the vesicles that leave the ER for the Golgi complex. Before assembly into protein bodies, γ-zein may be retained in the ER by its low solubility. Interchain disulfide bonds probably contribute to this, but the PPPVHL repeats may also play a role: A synthetic polypeptide constituted of eight PPPVHL repeats folds in vitro into an amphipathic poly-Pro II conformation and, when added to liposomes, extensively interacts with membrane lipids (Kogan et al., 2004). Therefore, the mechanism of zein and zeolin localization in the ER may be a retention system operating through low solubility and direct interactions with lipids. Extensive association with chaperones may also contribute to this ER-localization process, as we discuss below.
Zeolin Extensively Interacts with BiP But Is Very Stable
BiP is a major chaperone of the ER (Vitale and Denecke, 1999). BiP transiently binds many newly synthesized secretory proteins and most probably prevents unspecific aggregation by interacting with regions rich in hydrophobic amino acids; such regions are exposed in folding and assembly intermediates but not in structurally mature proteins, which are therefore permanently released from the chaperone. Prolamins are, however, peculiar. Interactions between wild-type zeins and BiP have not been studied, but BiP interacts with rice (Oryza sativa) prolamins during their cotranslational translocation into the ER lumen and has been detected at the periphery of rice protein bodies, from which it can be released by ATP treatment in vitro, suggesting long-lasting interactions (Li et al., 1993; Muench et al., 1997). BiP has also high affinity for severely defective proteins, which often extensively interact with BiP before being eventually degraded by ER quality control (for plant examples, see Pedrazzini et al., 1997; Brandizzi et al., 2003; Nuttall et al., 2003). Zeolin is very stable, and its interactions with BiP are extensive. BiP is even found in association with insoluble zeolin and, consistently, is present in zeolin protein bodies. This association is not the result of unspecific trapping within the protein body, because the chaperone remains associated when the originally insoluble zeolin is solubilized by treatment with 2-mercaptoethanol and can be released by ATP. Therefore, the interactions of BiP with zeolin resemble more those with rice prolamins than the ones with structurally defective proteins, and it is possible that wild-type γ-zein similarly interacts with the chaperone. Extensive interactions with BiP can contribute to the ER residence of newly synthesized zeolin monomers and of polypeptides that are assembling into the protein body. On the whole, our results suggest that BiP directly interacts with the zein portion of zeolin, but this remains to be demonstrated. The C-terminal α-helical region of phaseolin, which is involved in trimerization, is a BiP-binding determinant in phaseolin monomers (Foresti et al., 2003), but we do not know if it is still available for BiP interactions in disulfide-bonded, insoluble zeolin oligomers. Finally, it should be noted that phaseolin-KDEL, which is also efficiently retained in the ER, does not interact with BiP more extensively than wild-type phaseolin (Frigerio et al., 2001), indicating that residence in the ER, per se, is not sufficient to create a strong BiP ligand.
Our results extend the observation that a single zein polypeptide is sufficient to cause the formation of what can be considered an ER subcompartment (Geli et al., 1994; Bagga et al., 1995; Coleman et al., 1996). However, the detailed composition of maize protein bodies and the molecular interactions of zeins are not yet fully clarified. It will be interesting to characterize further the protein composition of zeolin protein bodies and compare them with the natural maize structures.
Zeolin Assembly
Velocity Suc gradient analysis showed that zeolin forms large polymers held together by disulfide bonds. Once these bonds are reduced, most of the protein migrates in a position corresponding to a monomer or a dimer. Analysis of the supramolecular properties of the synthetic polypeptide (VHLPPP)8 in aqueous solution or in the presence of phosphatidylcholine liposomes indicated the formation of cylindrical micelles (Kogan et al., 2002, 2004). These are held together by hydrophobic interactions between the monomers, which have an amphiphatic poly-Pro II conformation and align in the direction of their helical axis. In the presence of liposomes, the hyrophobic side of the helix interacts directly with lipids (Kogan et al., 2004). Possibly, the presence of phaseolin in the zeolin molecule inhibits the formation of long micelles, allowing only the hydrophobic interaction between two helical poly-Pro II structures; this would produce a dimer that further assembles into large oligomers thanks to the formation of interchain disulfide bonds.
The phaseolin portion of zeolin is released in vivo as an approximately full-length polypeptide. The process is very inefficient, but it produces a phaseolin trimer and requires vesicular trafficking that can be inhibited by brefeldin A. It seems very unlikely that the trimers originate by processing of the assembled zeolin oligomers. More likely, we suggest that a very small proportion of zeolin polypeptides fail to undergo the above-described oligomerization process mediated by the zein domain and assemble into trimers due to the interactions between the phaseolin portions. These would be similar to normal phaseolin trimers, but with an extended C-terminal tail constituted by the zein portion. In theory, the known three-dimensional structure of phaseolin would allow this, because the C-terminal tail would protrude from the corners of the trimer (Lawrence et al., 1990). These trimers, unlike the large, disulfide-linked zeolin oligomers, would remain available for intracellular trafficking and be transported to vacuoles, where proteases could cleave off the zein portion. This proteolytic event is in agreement with known phaseolin processing: Phaseolin has a short propeptide at the C terminus, which is naturally removed in storage vacuoles. In zeolin, cleavage of the propeptide obviously releases the zein portion. Altogether, our model suggests a competition between the zein-like and phaseolin-like assembly processes in zeolin, with a strong prevalence of the former. It will be interesting to investigate whether the equilibrium can be shifted by pharmacological treatments or chaperone overexpression. This would be relevant if the zein portion will be used for the large production of other and more complex proteins in transgenic plants.
A New Chimeric-Storage Protein
We have shown here that 89 amino acids of the 27-kD γ-zein polypeptide markedly stabilize phaseolin in transgenic tobacco leaves. The introduction of a 15-amino acid Met-rich peptide of β-zein into phaseolin was the first effort to improve the nutritional value of a storage protein (Hoffman et al., 1988). β-Zein is also a component of maize protein bodies (Lending and Larkins, 1989; Müntz, 1998). From a production point of view, that experiment failed because the protein was very unstable in storage vacuoles, but scientifically it represented the first important demonstration that even relatively minor sequence modifications can lead to protein instability in transgenic plants (Hoffman et al., 1988). The construct we have produced is much more stable. Zeolin has six Cys residues not present in phaseolin and 25 Lys residues not present in γ-zein. Zeolin is therefore more nutritionally balanced than the parent molecules and can be used as a starting point to produce an even better storage protein.
MATERIALS AND METHODS
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
Recombinant DNA and Tobacco Transformation
Zeolin was produced as follows. The sequence encoding 89 amino acids of mature γ-zein, starting from the fifth residue after the signal peptide, was amplified from plasmid pBSKS.G1L (Bellucci et al., 1997) using primers 5′-TGTGGGGGGATCCGGAGGGGGCGGTTCAGGCGGAGGTGGCTCAGGCGGCTGCGGCTGCCAG-3′ and 5′-TGTGGGGCTGCAGCTACTGGCACGGGCTTGGATGCG-3′. The first primer also introduced most of the sequence encoding the (GGGGS)3 linker ahead of the zein sequence. The amplified sequence was restricted with BamHI and PstI and inserted into the BamHI/PstI-restricted pDHA plasmid. T343F phaseolin inserted into the vector pDHA (Pedrazzini et al., 1997) was amplified using primers 5′-TGTGGGGGGATCCATGATGAGAGCAAGGGTTCCAC-3′ and 5′-TCTCCCCGGATCCCCCACCTCCATACACAAATGCACCCTTTCTTCC-3′. The amplified sequence was restricted with BamHI and inserted into the BamHI-restricted pDHA vector containing the zein sequence. For the production of transgenic plants, the plasmid was restricted with HindIII and introduced into the HindIII site of the binary vector pGA470. Strain LBA4404 of Agrobacterium tumefaciens was transformed by electroporation and used to produce transgenic tobacco (Nicotiana tabacum) cv Petit Havana SR1 as described (Pedrazzini et al., 1997).
Protein Analysis
For the extraction of total proteins in nonreducing conditions, young (5–7 cm long) leaves of transgenic tobacco were homogenized in an ice-cold mortar with homogenation buffer (200 mm NaCl, 1 mm EDTA, 0.2% Triton X-100, 100 mm Tris-Cl, pH 7.8, supplemented with Complete protease inhibitor cocktail [Roche, Basel]). For homogenation in reducing conditions, the buffer was supplemented with 4% 2-mercaptoethanol. The homogenate was centrifuged at 1,500g for 10 min at 4°C. Supernatant and, in some cases, pellet were then analyzed by protein blot. To disrupt BiP ligand interactions, the pellet was resuspended in homogenation buffer supplemented with 5 mm ATP and 10 mm MgCl2, incubated at 4°C for 70 min and centrifuged as described above.
For subcellular fractionation, young tobacco leaves were homogenized in an ice-cold mortar with 10 mm KCl, 100 mm Tris-Cl, pH 7.8, containing 12% (w/w) Suc and 2 mm MgCl2, using 6 mL of buffer per gram of fresh leaf tissue. Six hundred microliters were loaded on a 12-mL linear 16% to 55% (w/w) Suc gradient made in the same buffer. After centrifugation at 35,000 rpm, for 2 h at 4°C in a Beckman SW40 rotor (154,400g average; Beckman Instruments, Fullerton, CA), fractions of about 650 μL were collected. An equal aliquot of each fraction (usually 40 μL) was analyzed by SDS-PAGE and protein blot.
For velocity centrifugation on Suc gradients, young leaves were homogenized using the above described homogenation buffer with or without 4% 2-mercaptoethanol. The homogenate made in nonreducing conditions was loaded on a linear 5% to 25% (w/v) Suc gradient made in 150 mm NaCl, 1 mm EDTA, 0.1% Triton X-100, 50 mm Tris-Cl, pH 7.5. The homogenate made in reducing condition was loaded on a similar gradient supplemented with 2% dithiothreitol. After centrifugation at 39,000 rpm, for 20 h at 4°C in a Beckman SW40 rotor, fractions of about 650 μL were collected. An equal aliquot of each fraction (usually 40 μL) was analyzed by SDS-PAGE and protein blot. For treatment with trypsin, 200-μL aliquots of one gradient fraction containing the zeolin fragment with molecular mass around 45 kD were supplemented with 5 μL of trypsin (from a 10 mg/mL stock solution prepared in 1 mm HCl) or with 5 μL of 1 mm HCl as a control. The samples were then incubated at 37°C for 15 or 60 min. The reaction was terminated by adding Complete protease inhibitor cocktail and cooling to 0°C. Samples were then analyzed by protein blot. Two hundred-microliter aliquots of a total soluble protein extract from leaves of transgenic tobacco expressing phaseolin-KDEL (Frigerio et al., 2001) were treated with trypsin in the same way.
For protein blot, after SDS-PAGE, proteins were electrotransferred to Hybond-P membrane (Amersham Biosciences, Little Chalfont, UK) and revealed using antiphaseolin (Pedrazzini et al., 1997, 1:1,500 dilution) or anti-BiP (Pedrazzini et al., 1997, 1:10,000 dilution) antiserum and the SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical, Rockford, IL), following the manufacturer's protocol. In some cases, proteins were instead directly stained in the gel using Coomassie Brilliant Blue. Protein Molecular Weight Markers (Fermentas, Vilnius, Lithuania) were used as molecular mass markers.
Approximate quantitation of zeolin and phaseolin proteins in leaves was performed by loading dilutions of leaf extracts (from 20–0.2 μg of total protein) and phaseolin purified from bean (Phaseolus vulgaris) seeds (from 10–0.2 ng) on SDS-PAGE, followed by protein blot with antiphaseolin antiserum.
For pulse-chase labeling, protoplasts were prepared from transgenic plants as described by Pedrazzini et al. (1994) and subjected to pulse-chase radioactive labeling in the absence or presence of brefeldin A (Roche) as described by Pedrazzini et al. (1997).
Proteins were immunoprecipitated from protoplast homogenates using antisera raised against either phaseolin or binding protein (BiP), as described by Pedrazzini et al. (1997). For immunoprecipitation in reducing conditions, the immunoprecipitation buffer was supplemented with 4% 2-mercaptoethanol. ATP-mediated release of BiP from immunoprecipitates was performed as described by Foresti et al. (2003). Analysis of immunoprecipitated material was by SDS-PAGE followed by fluorography. Gels were usually treated with 2,5-diphenyloxazole dissolved in dimethyl sulfoxide and dried. Alternatively, gels were dried without treatment and exposed using the intensifying screen BioMax Transcreen LE (Kodak, Rochester, NY). Rainbow 14C-methylated proteins (Amersham Biosciences) were used as molecular mass markers.
Electron Microscopy
Small pieces of young leaves were fixed in 1.6% (w/v) paraformaldehyde mixed with 1.5% (v/v) glutaraldehyde in 0.1 m phosphate buffer pH 6.9 for 1 h at room temperature. After washing with 0.1 m phosphate buffer, the samples were dehydrated in ethanol and embedded overnight in LR White resin at 60°C. Ultrathin sections (70–80 nm) were cut using a Leica Microsystems Ultracut E (Leica Microsystems Nussloch GmbH, Nussloch, Germany), mounted on 300-mesh nickel grids and immunogold labeled. Grids were floated on drops of double-distilled water, phosphate-buffered saline (PBS), normal goat serum diluted 1:10 in PBS for 10 min, and 5% bovine serum albumin (BSA) in PBS for 10 min. They were then incubated with antiphaseolin antiserum (1:1,000 dilution) or anti-γ-zein antiserum (1:200 dilution) in 0.1% BSAc (Aurion, Wageningin, The Netherlands) in PBS for 1 h at room temperature. Controls were incubated in preimmune rabbit serum. After washing with 0.1% BSAc in PBS, the sections were incubated in the same buffer with goat anti-rabbit secondary antibody (1:25 dilution) conjugated with 15 nm gold particles (BBInternational, Cardiff, UK). The grids were washed in drops of 0.1% BSAc in PBS, PBS and double-distilled water, poststained in uranyl acetate, and examined under an electron microscope (EM 400 T; Philips, Eindhoven, The Netherlands).
Acknowledgments
We thank Maria Gloria Daminati for providing purified phaseolin, Emanuela Pedrazzini for the stimulating discussions, and Eva Maria Klein for critical reading of the manuscript.
This work was supported in part by the Consiglio Nazionale delle Ricerche Target Project in Biotechnology and by a Consiglio Nazionale delle Ricerche fellowship (to D.M.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.046409.
References
- Bagga S, Adams H, Kemp JD, Sengupta-Gopalan C (1995) Accumulation of 15-kilodalton zein in novel protein bodies in transgenic tobacco. Plant Physiol 107: 13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellucci M, Lazzari B, Viotti A, Arcioni S (1997) Differential expression of a γ-zein gene in Medicago sativa, Lotus corniculatus and Nicotiana tabacum. Plant Sci 127: 161–169 [Google Scholar]
- Brandizzi F, Hanton S, DaSilva LL, Boevink P, Evans D, Oparka K, Denecke J, Hawes C (2003) ER quality control can lead to retrograde transport from the ER lumen to the cytosol and the nucleoplasm in plants. Plant J 34: 269–281 [DOI] [PubMed] [Google Scholar]
- Chrispeels MJ (1983) The Golgi apparatus mediates the transport of phytohemagglutinin to the protein bodies in bean cotyledons. Planta 158: 140–151 [DOI] [PubMed] [Google Scholar]
- Coleman CE, Herman EM, Takasaki K, Larkins BA (1996) The maize γ-zein sequesters α-zein and stabilizes its accumulation in protein bodies of transgenic tobacco endosperm. Plant Cell 8: 2335–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke J, Botterman J, Deblaere R (1990) Protein secretion in plant cells can occur via a default pathway. Plant Cell 2: 51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande SS, Damodaran S (1989) Heat-induced conformational changes in phaseolin and its relation to proteolysis. Biochim Biophys Acta 998: 179–188 [Google Scholar]
- Foresti O, Frigerio L, Holkeri H, de Virgilio M, Vavassori S, Vitale A (2003) A phaseolin domain involved directly in trimer assembly is a determinant for binding by the chaperone BiP. Plant Cell 15: 2464–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio L, de Virgilio M, Prada A, Faoro F, Vitale A (1998) Sorting of phaseolin to the vacuole is saturable and requires a short C-terminal peptide. Plant Cell 10: 1031–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio L, Pastres A, Prada A, Vitale A (2001) Influence of KDEL on the fate of trimeric or assembly-defective phaseolin: selective use of an alternative route to vacuoles. Plant Cell 13: 1109–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galante E, Vitale A, Manzocchi L, Soave C, Salamini F (1983) Genetic control of a membrane component and zein deposition in maize endosperm. Mol Gen Genet 192: 316–321 [Google Scholar]
- Geli MI, Torrent M, Ludevid D (1994) Two structural domains mediate two sequential events in γ-zein targeting: protein endoplasmic reticulum retention and protein body formation. Plant Cell 6: 1911–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomord V, Denmat LA, Fitchette-Laine AC, Satiat-Jeunemaitre B, Hawes C, Faye L (1997) The C-terminal HDEL sequence is sufficient for retention of secretory proteins in the endoplasmic reticulum (ER) but promotes vacuolar targeting of proteins that escape the ER. Plant J 11: 313–325 [DOI] [PubMed] [Google Scholar]
- Hoffman LM, Donaldson DD, Herman EM (1988) A modified storage protein is synthesized, processed and degraded in the seeds of transgenic plants. Plant Mol Biol 11: 717–729 [DOI] [PubMed] [Google Scholar]
- Hunt DC, Chrispeels MJ (1991) The signal peptide of a vacuolar protein is necessary and sufficient for the efficient secretion of a cytosolic protein. Plant Physiol 96: 18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurkman WJ, Smith LD, Richter J, Larkins BA (1981) Subcellular compartmentalization of maize storage proteins in Xenopus oocytes injected with zein messenger RNAs. J Cell Biol 89: 292–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan MJ, Dalcol I, Gorostiza P, Lopez-Iglesias C, Pons R, Pons M, Sanz F, Giralt E (2002) Supramolecular properties of the proline-rich gamma-zein N-terminal domain. Biophys J 83: 1194–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan MJ, Lopez O, Cocera M, Lopez-Iglesias C, De La Maza A, Giralt E (2004) Exploring the interaction of the surfactant N-terminal domain of gamma-zein with soybean phosphatidylcholine liposomes. Biopolymers 73: 258–268 [DOI] [PubMed] [Google Scholar]
- Lawrence MC, Suzuki E, Varghese JN, Davis PC, Van Donkelaar A, Tulloch PA, Colman PM (1990) The three-dimensional structure of the seed storage protein phaseolin at 3 Å resolution. EMBO J 9: 9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lending CR, Larkins BA (1989) Changes in the zein composition of protein bodies during maize endosperm development. Plant Cell 1: 1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wu Y, Zhang DZ, Gillikin JW, Boston RS, Franceschi VR, Okita TW (1993) Rice prolamine protein body biogenesis: a BiP-mediated process. Science 262: 1054–1056 [DOI] [PubMed] [Google Scholar]
- Muench DG, Wu Y, Zhang Y, Li X, Boston RS, Okita TW (1997) Molecular cloning, expression and subcellular localization of a BiP homolog from rice endosperm tissue. Plant Cell Physiol 38: 404–412 [DOI] [PubMed] [Google Scholar]
- Munro S, Pelham HRB (1986) An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobilin heavy chain binding protein. Cell 46: 291–300 [DOI] [PubMed] [Google Scholar]
- Müntz K (1998) Deposition of storage proteins. Plant Mol Biol 38: 77–99 [PubMed] [Google Scholar]
- Nishizawa K, Maruyama N, Satoh R, Fuchikami Y, Higasa T, Utsumi S (2003) A C-terminal sequence of soybean beta-conglycinin α′ subunit acts as a vacuolar sorting determinant in seed cells. Plant J 34: 647–659 [DOI] [PubMed] [Google Scholar]
- Nuttall J, Vitale A, Frigerio L (2003) C-terminal extension of phaseolin with a short methionine-rich sequence can inhibit trimerisation and result in high instability. Plant Mol Biol 51: 885–894 [DOI] [PubMed] [Google Scholar]
- Pedrazzini E, Giovinazzo G, Bielli A, de Virgilio M, Frigerio L, Pesca M, Faoro F, Bollini R, Ceriotti A, Vitale A (1997) Protein quality control along the route to the plant vacuole. Plant Cell 9: 1869–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzini E, Giovinazzo G, Bollini R, Ceriotti A, Vitale A (1994) Binding of BiP to an assembly-defective protein in plant cells. Plant J 5: 103–110 [Google Scholar]
- Pelham HR (1990) The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem Sci 15: 483–486 [DOI] [PubMed] [Google Scholar]
- Prat S, Cortadas J, Puigdombech P, Palau J (1985) Nucleic acid (cDNA) and amino acid sequences of the maize endosperm protein glutelin-2. Nucleic Acids Res 13: 1493–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabe L, Higgins TJV (1998) Engineering plant protein composition for improved nutrition. Trends Plant Sci 3: 282–286 [Google Scholar]
- Vitale A, Bielli A, Ceriotti A (1995) The binding protein associates with monomeric phaseolin. Plant Physiol 107: 1411–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A, Denecke J (1999) The endoplasmic reticulum: gateway of the secretory pathway. Plant Cell 11: 615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A, Smaniotto E, Longhi R, Galante E (1982) Reduced soluble proteins associated with maize endosperm protein bodies. J Exp Bot 33: 439–448 [Google Scholar]
- Wandelt CI, Khan MR, Craig S, Schroeder HE, Spencer D, Higgins TJ (1992) Vicilin with carboxy-terminal KDEL is retained in the endoplasmic reticulum and accumulates to high levels in the leaves of transgenic plants. Plant J 2: 181–192 [DOI] [PubMed] [Google Scholar]