Abstract
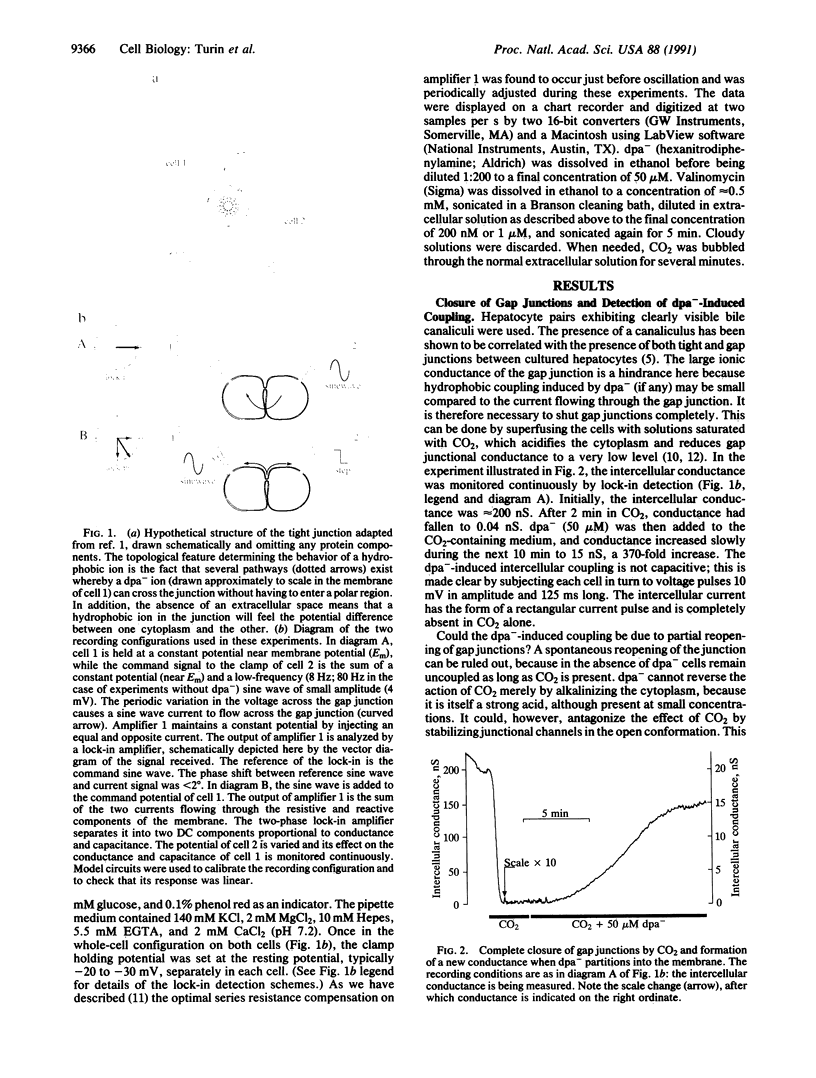
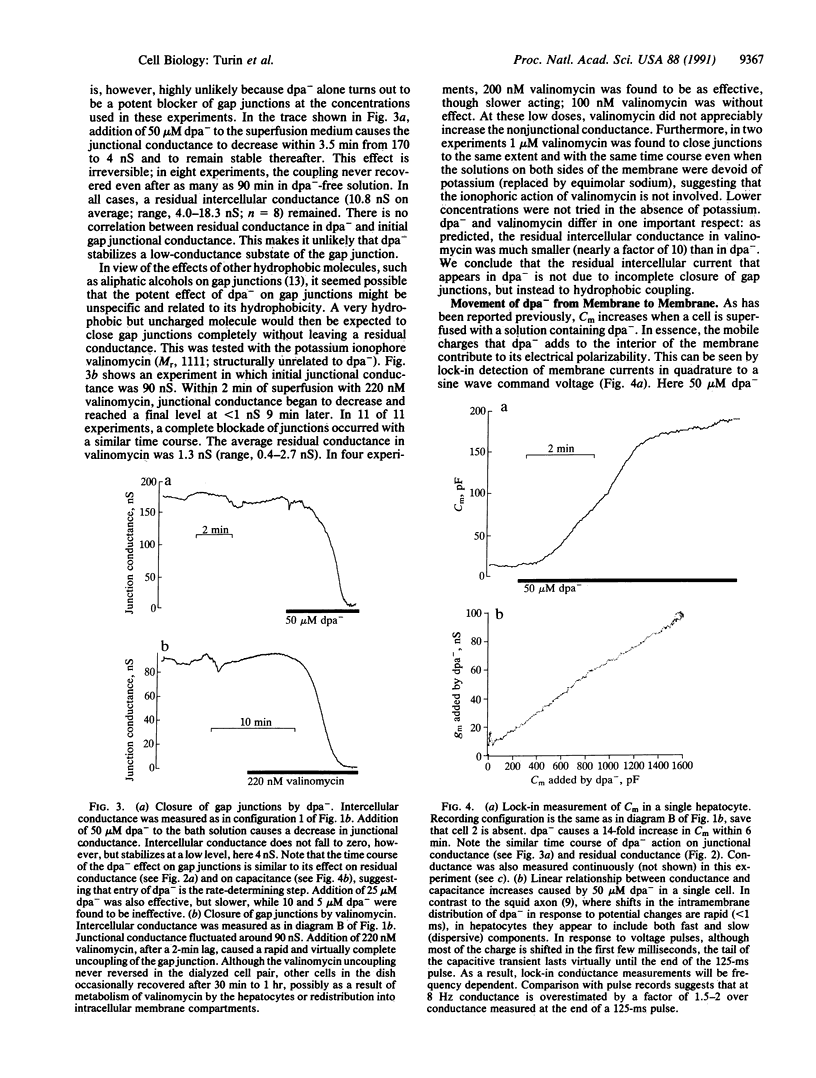
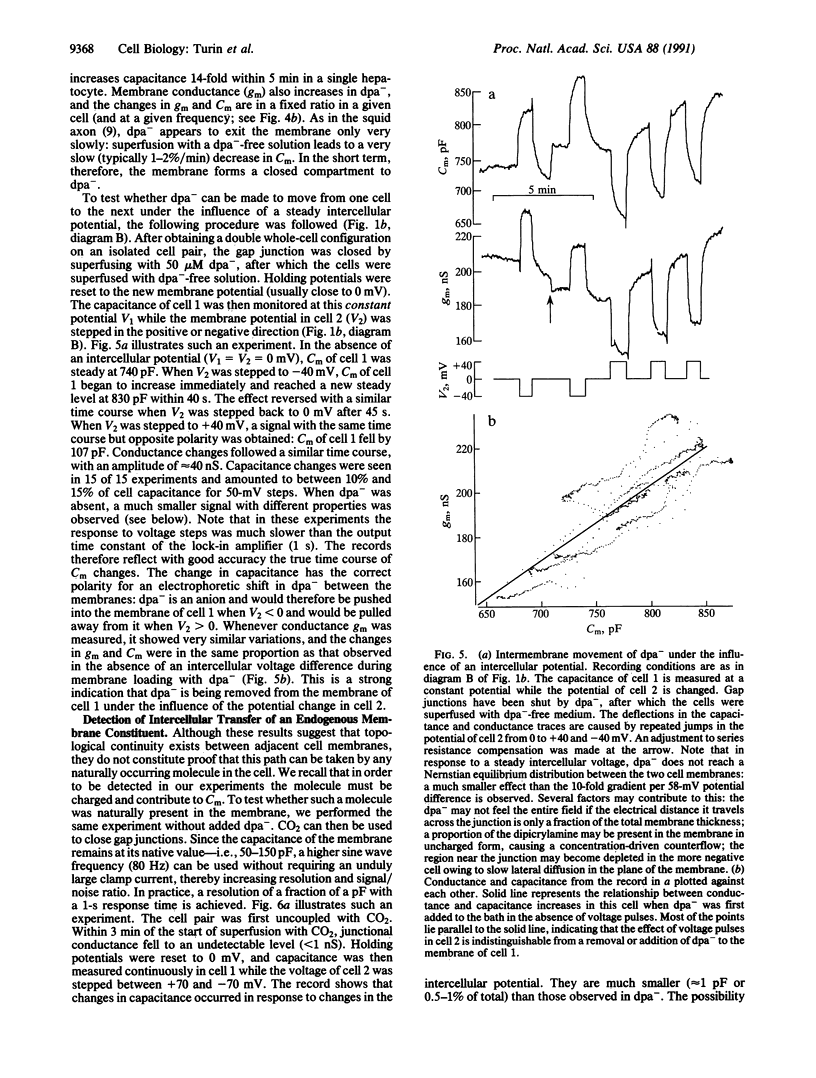
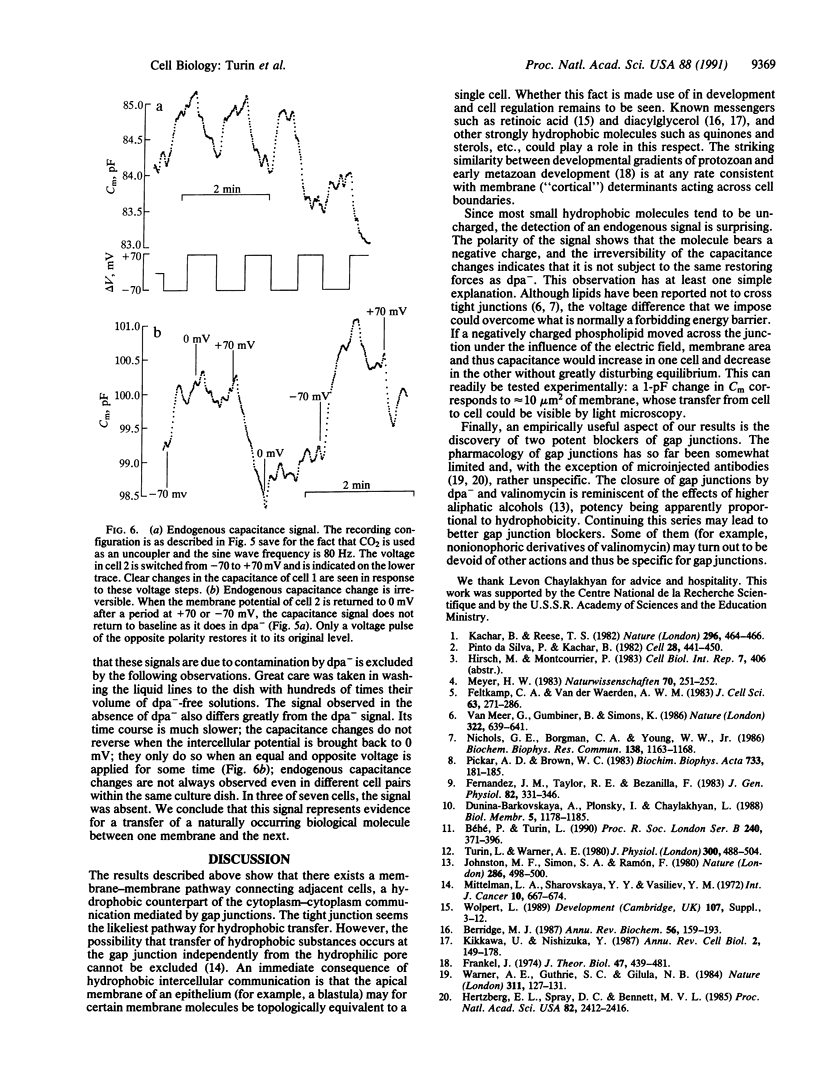
The topology of the tight junction is probed by introducing dipicrylamine (dpa-), a lipid-soluble anion, into the membranes of hepatocyte pairs in culture. Once partitioned into the membrane, dpa- ions are free to move in the hydrophobic core of the membrane, where their mobile charges greatly increase membrane capacitance. If tight junctions are lines of membrane fusion, dpa- will cross the tight junction without traversing a polar headgroup layer. Furthermore, the electric potential across the tight junction will be equal to the difference in membrane potentials of the two cells. dpa- can therefore be expected to move electrophoretically from cell membrane to cell membrane across the junction in response to an intercellular voltage difference. Experiments performed under double whole-cell clamp show that this transfer occurs as follows: First, dpa- causes an intercellular current unrelated to gap junctions to flow in response to an intercellular voltage difference. Second, this electrophoretic removal or addition of dpa- from a cell's membrane through the tight junction must reduce or increase its dpa- content and thus its capacitance. Experiments confirm this prediction: We detect rapid, symmetric, and reversible changes in membrane capacitance in response to changes in the membrane potential of the neighboring cell. Finally, we find that hepatocyte membranes contain a negatively charged endogenous molecule that contain a negatively charged endogenous molecule that can move from cell to cell like dpa- under the influence of an intercellular potential difference. We conclude that membrane fusion occurs at tight junctions and that this hydrophobic intercellular pathway can play a role in intercellular communication.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Feltkamp C. A., Van der Waerden A. W. Junction formation between cultured normal rat hepatocytes. An ultrastructural study on the presence of cholesterol and the structure of developing tight-junction strands. J Cell Sci. 1983 Sep;63:271–286. doi: 10.1242/jcs.63.1.271. [DOI] [PubMed] [Google Scholar]
- Fernández J. M., Taylor R. E., Bezanilla F. Induced capacitance in the squid giant axon. Lipophilic ion displacement currents. J Gen Physiol. 1983 Sep;82(3):331–346. doi: 10.1085/jgp.82.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel J. Positional information in unicellular organisms. J Theor Biol. 1974 Oct;47(2):439–481. doi: 10.1016/0022-5193(74)90209-4. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L., Spray D. C., Bennett M. V. Reduction of gap junctional conductance by microinjection of antibodies against the 27-kDa liver gap junction polypeptide. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2412–2416. doi: 10.1073/pnas.82.8.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M., Montcourrier P. A further iconographic argument in favour of the 'offset two-fibril' model of the tight junction. Cell Biol Int Rep. 1983 Jun;7(6):406–406. doi: 10.1016/0309-1651(83)90128-5. [DOI] [PubMed] [Google Scholar]
- Johnston M. F., Simon S. A., Ramón F. Interaction of anaesthetics with electrical synapses. Nature. 1980 Jul 31;286(5772):498–500. doi: 10.1038/286498a0. [DOI] [PubMed] [Google Scholar]
- Kachar B., Reese T. S. Evidence for the lipidic nature of tight junction strands. Nature. 1982 Apr 1;296(5856):464–466. doi: 10.1038/296464a0. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Nishizuka Y. The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- Meyer H. W. Tight junction strands are lipidic cylinders. Naturwissenschaften. 1983 May;70(5):251–252. doi: 10.1007/BF00405446. [DOI] [PubMed] [Google Scholar]
- Mittelman L. A., Sharovskaya J. J., Vasiliev J. M. Toxic effect of 7,12-dimethylbenz-alpha-anthracene on neoplastic cells grown in mixed cultures with normal fibroblasts. Int J Cancer. 1972 Nov;10(3):667–674. doi: 10.1002/ijc.2910100326. [DOI] [PubMed] [Google Scholar]
- Nichols G. E., Borgman C. A., Young W. W., Jr On tight junction structure: Forssman glycolipid does not flow between MDCK cells in an intact epithelial monolayer. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1163–1169. doi: 10.1016/s0006-291x(86)80404-1. [DOI] [PubMed] [Google Scholar]
- Pickar A. D., Brown W. C. Capacitance of bilayers in the presence of lipophilic ions. Biochim Biophys Acta. 1983 Aug 24;733(1):181–185. doi: 10.1016/0005-2736(83)90104-9. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Kachar B. On tight-junction structure. Cell. 1982 Mar;28(3):441–450. doi: 10.1016/0092-8674(82)90198-2. [DOI] [PubMed] [Google Scholar]
- Turin L., Warner A. E. Intracellular pH in early Xenopus embryos: its effect on current flow between blastomeres. J Physiol. 1980 Mar;300:489–504. doi: 10.1113/jphysiol.1980.sp013174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner A. E., Guthrie S. C., Gilula N. B. Antibodies to gap-junctional protein selectively disrupt junctional communication in the early amphibian embryo. Nature. 1984 Sep 13;311(5982):127–131. doi: 10.1038/311127a0. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information revisited. Development. 1989;107 (Suppl):3–12. doi: 10.1242/dev.107.Supplement.3. [DOI] [PubMed] [Google Scholar]
- van Meer G., Gumbiner B., Simons K. The tight junction does not allow lipid molecules to diffuse from one epithelial cell to the next. Nature. 1986 Aug 14;322(6080):639–641. doi: 10.1038/322639a0. [DOI] [PubMed] [Google Scholar]