Abstract
Carotenoids are thought to be the precursors of terpenoid volatile compounds that contribute to flavor and aroma. One such volatile, β-ionone, is important to fragrance in many flowers, including petunia (Petunia hybrida). However, little is known about the factors regulating its synthesis in vivo. The petunia genome contains a gene encoding a 9,10(9′,10′) carotenoid cleavage dioxygenase, PhCCD1. The PhCCD1 is 94% identical to LeCCD1A, an enzyme responsible for formation of β-ionone in tomato (Lycopersicon esculentum; Simkin AJ, Schwartz SH, Auldridge M, Taylor MG, Klee HJ [2004] Plant J [in press]). Reduction of PhCCD1 transcript levels in transgenic plants led to a 58% to 76% decrease in β-ionone synthesis in the corollas of selected petunia lines, indicating a significant role for this enzyme in volatile synthesis. Quantitative reverse transcription-PCR analysis revealed that PhCCD1 is highly expressed in corollas and leaves, where it constitutes approximately 0.04% and 0.02% of total RNA, respectively. PhCCD1 is light-inducible and exhibits a circadian rhythm in both leaves and flowers. β-Ionone emission by flowers occurred principally during daylight hours, paralleling PhCCD1 expression in corollas. The results indicate that PhCCD1 activity and β-ionone emission are likely regulated at the level of transcript.
Apocarotenoids are a class of compounds derived from oxidative cleavage of carotenoids that are important contributors to flavor and fragrance of foods (Walhberg and Eklund, 1998). Until recently, the derivation of many of these compounds from carotenoids was largely based on structural considerations and correlations between levels of substrates and products (Buttery et al., 1988). With more than 600 carotenoids identified to date, apocarotenoids constitute one of the largest classes of molecules in nature. Some of these apocarotenoids are essential and valuable constituents of color, flavor, and aroma (Winterhalter and Rouseff, 2002).
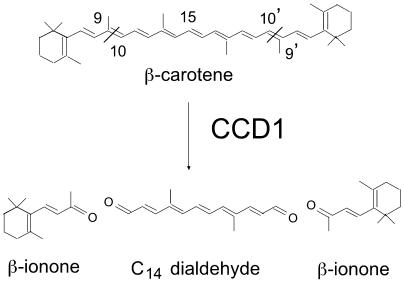
Recently, a family of enzymes that could potentially generate many apocarotenoids has been described. This family, the carotenoid cleavage dioxygenases (CCDs), has been shown to cleave multiple carotenoids at specific double bonds within the substrate (Schwartz et al., 2001; Giuliano et al., 2003). One of the best-characterized apocarotenoids is the hormone abscisic acid (ABA). ABA is a C15 compound derived from 11,12 cleavage of the epoxy-carotenoids 9-cis-violoaxanthin and 9-cis-neoxanthin by VP14 to produce xanthoxin (Schwartz et al., 1997; Tan et al., 1997). VP14 is the founding member of this unique family of dioxygenases. In Arabidopsis (Arabidopsis thaliana), there are nine members of the CCD family, five of which are believed to be involved in ABA synthesis (Tan et al., 2003). One member of the family, AtCCD1 that is not involved in ABA synthesis, symmetrically cleaves the 9,10(9′,10′) double bonds of multiple carotenoid substrates in vitro. Homologues of this enzyme, which generates a C14 dialdehyde and two C13 products, have been identified in Phaseolus vulgaris (Schwartz et al., 2001), crocus (Crocus sativus; Bouvier et al., 2003), and tomato (Lycopersicon esculentum; Simkin et al., 2004). When the substrate of CCD1 is β-carotene, the volatile apocarotenoid β-ionone is generated (Fig. 1; Schwartz et al., 2001). β-Ionone has been shown to be an important contributor to fragrance in the flowers of Boronia megastigma (Cooper et al., 2003) and Osmanthus fragrans (Kaiser and Lamparsky, 1980). In tomato fruit, β-ionone is present in very low concentrations (4 nL L−1), but due to its odor threshold (0.007 nL L−1), it is the second most important volatile contributing to fruit flavor (Baldwin et al., 2000).
Figure 1.
Scheme for the oxidative cleavage of β-carotene catalyzed by the recombinant CCD1 proteins, resulting in the formation of two molecules of β-ionone and one C14 dialdehyde.
In many flowers, fragrance is diurnally regulated (Loughrin et al., 1990; Kolosova et al., 2001; Pott et al., 2003; Underwood, 2003; Verdonk et al., 2003; Underwood et al., 2004). Since β-ionone is an important volatile fragrance component of many flowers, we were interested in determining whether it is synthesized in petunia (Petunia hybrida) and, if so, how its synthesis is regulated. We were also interested in determining the biochemical function of the CCD1 family of enzymes in vivo. Having identified both the CCD1 transcript and β-ionone in Mitchell Diploid (MD) petunia flowers, we determined how changing daily conditions influenced both gene expression and β-ionone formation in vivo.
RESULTS
Identification of PhCCD1 in Plants
In order to identify a petunia CCD1 homolog, the tomato LeCCD1A cDNA (AY576003) was used as a probe to screen a flower cDNA library of petunia (cv MD). Screening of this unamplified library yielded 17 independent clones. Sequence analysis revealed that all 17 clones were derived from the same gene, suggesting that only one gene is predominantly expressed in flowers. A full-length sequence was subsequently obtained by 5′RACE-PCR from flower-tissue RNA. The full-length cDNA has high sequence identity to the CCD genes AtCCD1 (74%) and LeCCD1A (92%). The petunia cDNA, PhCCD1, encodes an open reading frame of 1,641 nucleotides. A comparison of the deduced PhCCD1 protein with the proteins from tomato and Arabidopsis indicates the highest identity to LeCCD1A (94%; Simkin et al., 2004) and AtCCD1 (85%; Schwartz et al., 2001), enzymes that catalyze the symmetrical 9,10(9′,10′) cleavage of multiple linear and cyclized carotenoids (Fig. 2). Southern-blot analysis indicated that PhCCD1 likely exists as a single copy in the petunia genome (data not shown).
Figure 2.
Alignment of the predicted amino acid sequence of the petunia CCD and the related proteins from Arabidopsis (Neill et al., 1998: AJ005813) and tomato (Simkin et al., 2004: AY576001).
Activity of PhCCD1 in Escherichia coli
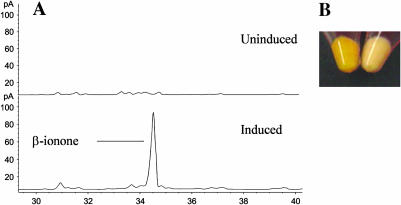
The predicted PhCCD1 enzyme is 94% identical to LeCCD1A, an enzyme that we have shown catalyzes symmetric 9,10(9′,10′) cleavage of multiple carotenoids, including β-carotene (Simkin et al., 2004). Cleavage of β-carotene by CCD1 enzymes releases two molecules of β-ionone as well as a central C14 dialdehyde (Fig. 1). To prove that PhCCD1 is also capable of generating β-ionone, a full-length cDNA was cloned into the E. coli expression vector pDEST14. This plasmid was then introduced into an E. coli host that had been previously engineered to accumulate β-carotene (Cunningham et al., 1996). Induction of PhCCD1 led to a loss of orange color, indicating catabolism of β-carotene, and synthesis of β-ionone (Fig. 3). Taken together, these results indicate that PhCCD1, like its Arabidopsis (Schwartz et al., 2001), tomato (Simkin et al., 2004), and crocus (Bouvier et al., 2003) homologs, cleaves β-carotene at the 9,10 double bond.
Figure 3.
Activity of E. coli-expressed PhCCD1 on β-carotene. A, GC trace showing β-ionone emitted by bacteria before (top) and following (bottom) induction of PhCCD1 expression. Collection and detection of volatile emissions were performed as described in “Materials and Methods.” B, Accumulation of β-carotene in E. coli cells without (U) and following (I) induction of PhCCD1 protein.
Overexpression of LeCCD1A and Analysis of Transgenic Plants
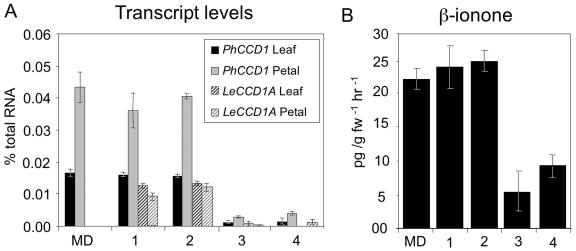
In order to verify the role of PhCCD1 in synthesis of β-ionone in vivo, petunia plants were transformed with a plasmid construct designed to overexpress LeCCD1A, encoding a tomato CCD1. We chose to express the tomato cDNA encoding a CCD1 enzyme to reduce the possibility of cosuppression of the endogenous petunia gene. The construct utilizes the figwort mosaic virus 35S promoter (Richins et al., 1987) to direct constitutive transcription of a tomato CCD1. To independently quantify mRNAs of PhCCD1 and LeCCD1A, gene-specific primers and probes were designed and tested for each of the two transcripts. Expression of LeCCD1A in the transgenic lines varied from 0% to 98% of wild-type PhCCD1 levels in the leaves and from 0% to 27% of wild-type levels in the flowers (Fig. 4A).
Figure 4.
A, Expression of PhCCD1 and LeCCD1A in the leaves and flowers of transgenic lines. Expression was determined by quantitative RT-PCR. B, β-Ionone emissions in two lines overexpressing LeCCD1A and two lines cosuppressed for PhCCD1.
Despite the use of a heterologous gene, several plants with significant reductions in the expression of the PhCCD1 gene were also identified, indicating that these lines had undergone cosuppression due to the high degree of identity (92%) between the gene sequences. Two independent transgenic lines showing the highest levels of expression of the tomato gene and two lines showing the greatest degree of cosuppression of the petunia gene (92% and 96%) were selected for further study (Fig. 4A).
The in Vivo Activity of PhCCD1
To quantitatively determine the roles of CCD1 enzymes in the formation of β-ionone in vivo, volatile analysis was performed. Each independent volatile extraction was carried out using a minimum of 16 newly opened flowers from at least 2 plants for each transgenic line collected at anthesis. The two transgenic lines showing the highest degree of overexpression of LeCCD1A (Fig. 4A) showed no significant difference in β-ionone emissions when compared to MD (Fig. 4B). The lack of significantly increased β-ionone production in flowers is likely due to the high expression of the endogenous petunia gene that constitutes approximately 80% of the CCD1 expression in these tissues. By contrast, the two cosuppressed lines produced only 24% and 42% of wild-type levels of β-ionone, indicating a major role for PhCCD1 in the formation of β-ionone in vivo.
PhCCD1 Is Expressed in All Tissues
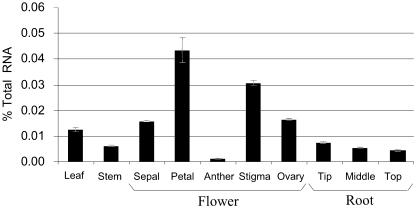
In order to quantify PhCCD1 transcript levels throughout the plant, a gene-specific assay based on fluorescent real-time reverse transcription (RT)-PCR (TaqMan; Applied Biosystems, Foster City, CA) was developed. Absolute mRNA levels were quantified against a standard curve of tritiated in vitro-transcribed sense-strand RNAs. Using TaqMan, we quantified the expression of the PhCCD1 in the leaves, stem, roots, and flower organs of petunia (Fig. 5). PhCCD1 mRNA was detected in all tissues tested. The highest transcript levels were detected in flower tissues, where it represented approximately 0.04% of total RNA in corollas, approximately 0.03% in the stigma/style, and approximately 0.02% in the ovary.
Figure 5.
Expression of PhCCD1 in different petunia tissues. Levels of expression were determined by quantitative RT-PCR.
PhCCD1 Transcript Levels Are Regulated by Circadian Rhythm and Light
A number of fragrance volatiles in petunia exhibit emissions peaking either at dusk or during the night (Underwood, 2003; Verdonk et al., 2003). In order to determine how PhCCD1 transcript levels change throughout the day, leaves were harvested from the growing tips of plants at 6-h intervals over 2 d. To test whether transcript levels are regulated by an internal oscillator, plants were then transferred to either constant light or constant darkness and leaf samples were collected for an additional 3 d. Flowering plants were maintained under the same conditions, and flowers were collected at the cognate time points so that transcript levels and volatiles could be compared.
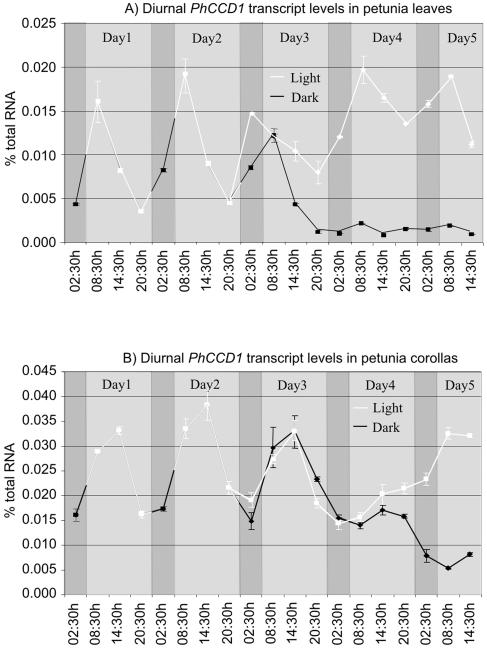
In leaves, PhCCD1 transcript levels increased approximately 3-fold between 2:30 am (in the middle of subjective night) and 8:30 am, reaching a peak at 8:30 am, 3 h after dawn. Following this increase, transcript levels decreased steadily during the day (Fig. 6A). When entrained plants were placed in constant darkness, the initial increase between 2:30 am and 8:30 am in PhCCD1 transcript levels was still observed, although at a reduced level. An increase was also observed in constant light. However, this increase was observed between 8:30 pm and 2:30 am, indicating that constant light is exerting an effect on the normal rhythm. In constant darkness, transcript levels decreased significantly and dampened to a low steady-state level. Minor peaks in transcript levels were still observed at 8:30 am on days 4 and 5 of the extended dark period, coinciding with the normal peak in the light. Furthermore, if the plants were placed in constant light, they continued to oscillate, although transcript levels steadily increased to levels slightly higher than those observed under normal conditions. Peaks in transcript levels were still observed at 8:30 am. These data indicate that PhCCD1 is regulated by both light and circadian mechanisms in leaves.
Figure 6.
Diurnal regulation of PhCCD1 transcript levels in leaves (A) and corollas (B) of petunia. The solid white line indicates when plants were exposed to the light, and the black line shows when plants were in dark conditions.
In corollas, PhCCD1 transcript levels increased rapidly in the early morning, reaching a peak at 2:30 pm. Subsequently, transcript levels decreased steadily in the evening, before the onset of darkness, and remained low during the night (Fig. 6B). After 8 h of darkness, transcript levels again increased, following the previous pattern of expression. This increase in PhCCD1 transcript level was also observed when the plants were placed in constant darkness. Furthermore, if the plants were placed in constant light, the decrease in transcript normally observed just prior to the onset of darkness (between 2:30 pm and 8:30 pm; plants having already missed the previous night cycle) was still observed. In constant light, transcript levels eventually increased and remained high, while in constant darkness transcript levels decreased and remained low. These data indicate that PhCCD1 expression in petunia corollas is regulated primarily by circadian rhythm, with light playing a secondary role. These different rhythms may indicate somewhat different roles for CCD1 in these different tissues.
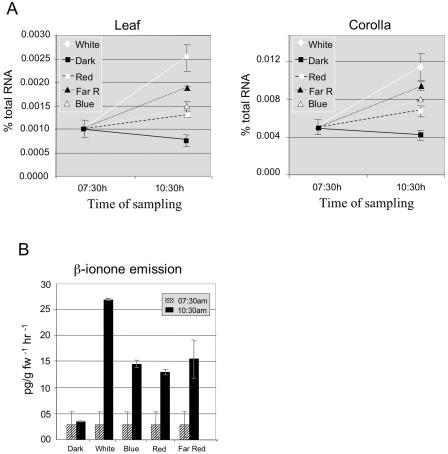
To test the influence of light on the induction of PhCCD1 and to confirm a link between transcript regulation and β-ionone emission, plants were placed in continuous darkness for 2 d. At 7:30 am on the third day, plants were exposed to strong white light (coinciding with the normal onset of the light period) for 10 min and returned to darkness. Transcript levels were determined 3 h later. White light induced a more than 2-fold increase in PhCCD1 transcript in both leaves and corollas (Fig. 7A). At the same time, plants of the same age and size were exposed to blue, red, or red/far-red light of equal fluence to the white light experiment. In all cases, PhCCD1 transcript levels increased when compared to untreated controls, although not to the same levels observed in white light treatment. These data indicate that exposure of a plant to a light source for a short period is sufficient to cause an up-regulation of transcript levels. Exposure of plants to far-red light immediately after red-light treatment did not inhibit the induction observed under red light only, suggesting that phytochrome is not involved in regulation of CCD1 expression. The induction of PhCCD1 transcript accumulation was also accompanied by an increase in β-ionone emission (Fig. 7B). Even this transient increase in RNA after a prolonged dark period was sufficient to bring about new synthesis of β-ionone. This result is consistent with gene expression and not substrate availability being limiting for β-ionone synthesis.
Figure 7.
Effects of transient exposure to light sources on PhCCD1 transcript and β-ionone emissions. A, PhCCD1 expression in petunia leaves and flower corollas following exposure to different light sources. Dark-adapted plants were treated with white, blue, red, or red/far-red light and returned to darkness. Transcript levels were determined 3 h postexposure. Dark-adapted, untreated plants were used as a control. B, Emission of β-ionone was measured from corollas of flowers treated in the same experiment as in A.
Circadian Emission of β-Ionone in the Corollas of Petunia
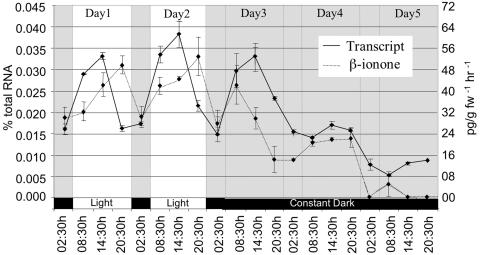
To determine if changes in PhCCD1 transcript are paralleled by changes in the emission of β-ionone, flower volatiles were collected and analyzed at the same time points used for RT-PCR analysis. At the onset of the light period, β-ionone levels increased throughout the day, reaching a peak at 8:30 pm (Fig. 8), 6 h after the peak in PhCCD1 transcript. Following this increase, β-ionone levels decreased during the night. After 8 h of darkness, β-ionone levels increased again. This increase was also observed when the plants were not returned to the light. After 14 h of prolonged darkness, β-ionone levels began to decrease prior to any decrease in transcript levels. By 8:30 pm of the first day of constant darkness, β-ionone levels had fallen to 25% of the normal 8:30 pm levels. During prolonged darkness, β-ionone levels continued to decrease until they became almost undetectable after 2 d of constant darkness. Note that samples for expression and volatile emission analyses shown in Figures 4 and 8 were collected in different seasons. We have observed seasonal variations in volatile emissions. Whether these variations are related to daylength, light intensity, or temperature remain to be determined.
Figure 8.
Circadian regulation of PhCCD1 transcript levels and β-ionone emissions in petunia corollas.
DISCUSSION
Although β-ionone is found in low concentrations when compared to other more abundant volatiles such as methylbenzoate and benzaldehyde, which have been detected at levels of 60 μg and 13 μg g fw−1 h−1, respectively (Underwood et al., 2004), β-ionone has a human odor threshold of 0.007 nL L−1 (Baldwin et al., 2000). This odor threshold is significantly lower than that observed for many of the other more abundant volatiles. By comparison, the human odor threshold for bezaldehyde, one of the most abundant petunia volatiles, is nearly five logs higher at 350 nL L−1. Thus, β-ionone has the potential to greatly impact flower aroma.
Using plants cosuppressed for PhCCD1 expression, we have demonstrated a role for this gene in the formation of β-ionone in petunia corollas. In transgenic lines, reduced PhCCD1 transcript levels resulted in significant decreases in β-ionone formation. This is consistent with previously reported in vitro results for the Arabidopsis homolog AtCCD1 (Schwartz et al., 2001), the crocus homolog CsCCD (Bouvier et al., 2003), and the tomato homologs LeCCD1A and LeCCD1B (Simkin et al., 2004). A 92% to 96% reduction in PhCCD1 transcript levels did not lead to complete loss of β-ionone synthesis in petunia corollas. Even with that level of transcript reduction, corollas still emitted β-ionone at approximately 25% of wild-type levels. These results suggest that while PhCCD1 must have a major role in β-ionone synthesis, there may be additional mechanisms to synthesize it in vivo. Although it seems unlikely, we cannot rule out that the residual PhCCD1 is responsible for this synthesis.
One possible source of the remaining β-ionone could be another CCD. Booker et al. (2004) have identified a second Arabidopsis CCD that can also cleave β-carotene (AtCCD7/AtMAX3). AtCCD7 catalyzes the oxidative cleavage of β-carotene at the 9,10 double bond, resulting in formation of β-ionone and β-apo-10-carotenal, a C27 aldehyde. In Arabidopsis, CCD1 is expressed at much higher levels than CCD7. There is significant sequence divergence between the CCD1 and CCD7 genes within both Arabidopsis and tomato (less than 30% identity). Thus, we do not expect any cosuppression of PhCCD7 in the plants generated for our studies. Furthermore, any loss of CCD7 activity would be expected to have an obvious morphological phenotype of increased lateral branching based on results in Arabidopsis (Booker et al., 2004). We also cannot exclude a role of nonenzymatic photooxidation of carotenoids as a source of β-ionone. The formation of β-ionone by nonenzymatic oxidative degradation has been demonstrated in vitro (Wache et al., 2003). It has been estimated that as much as 1 mg of carotenoids/g DW per day are oxidized in pepper (Capsicum annuum) leaves (Simkin et al., 2003a).
It is not surprising that loss of PhCCD1 activity did not completely eliminate β-ionone emission because most carotenoids are located within plastids. In Arabidopsis, every member of the CCD family, except CCD1, is targeted to plastids (Tan et al., 2003; Booker et al., 2004; M. Auldridge and H.J. Klee, unpublished data). While CCD7 is plastid localized, the evidence suggests that AtCCD1 (Tan et al., 2003; M. Auldridge and H.J. Klee, unpublished data), LeCCD1A. and LeCCD1B (Simkin et al., 2004) are located in the cytosol and/or attached to the outer envelope of the plastid. Bouvier et al. (2003) have used immunohistochemistry to show a cytoplasmic location for the crocus CCD1. Because of the high homology to these enzymes and the lack of an obvious transit peptide, we expect PhCCD1 to also be cytoplasmic. However, we cannot rule out a tight association with the outer envelope, as has been reported, for example, with tomato hydroperoxide lyase (Froehlich et al., 2001). PhCCD1 access to substrates most likely is limited to carotenoids located in the plastid outer envelope. Significant amounts of β-carotene have been identified in the outer envelopes of pea (Pisum sativum; Markwell et al., 1992) and spinach (Spinacia oleracea; Block et al., 1983) chloroplasts. Our results indicate that at least a certain level of carotenoid substrate must be available to CCD1. The relationship between substrate and enzymes, both inside and outside of the plastid, is an important and as yet unexplored aspect of the system.
In the context of substrate availability, it is interesting to note that within the normal day/night cycle, there is a correlation between mRNA abundance and β-ionone emissions. Peak emissions trail the peak of gene expression by several hours, presumably due to protein half-life. We did, however, observe that emissions are still rising in the afternoon, when transcript levels are decreasing. This suggests that there may be some limitation in substrate availability that changes throughout the day. It has been shown that carotenoid content of tomato leaves remains relatively constant throughout the day (Simkin et al., 2003b). Assuming that carotenoid content does not fluctuate substantially in petunia tissues, it appears that the steady-state level of PhCCD1 is a major but not the only determinant of β-ionone emissions. Further evidence supporting this conclusion is the transient light-induced increase in PhCCD1 expression (Fig. 7A) and the concomitant increase in β-ionone emissions (Fig. 7B). Thus, the regulation of β-ionone synthesis is at least in part controlled at the level of transcript. This result is consistent with the observation that emission of methylbenzoate by S-adenosylmethionine:benzoic acid/salicylic acid carboxyl methyltransferase is also transcriptionally regulated (Negre et al., 2003).
In order to understand how changing daily conditions influence β-ionone emissions, we studied transcript levels under different light conditions. In petunia leaves and corollas exposed to normal photoperiod (without artificial conditions of constant light or darkness), robust oscillations in the expression of PhCCD1 were observed. It is interesting to note that the period of PhCCD1 expression in corollas was 6 h longer than in leaves. This difference may be the result of tissue-specific clocks or due to a tissue-specific response to the same clock. In both leaves and corollas, a reduction in transcript was observed prior to the onset of darkness, while an increase was observed just before dawn. These rhythms persist in corollas when plants were transferred to either constant light or constant darkness. The regulation of PhCCD1 appears to fit with similar oscillations in the expression of phytoene desaturase and ξ-carotene desaturase (genes involved in the formation of β-carotene; Simkin et al., 2003b), which were also shown to increase just prior to the light period in tomato leaves. These oscillation patterns are indicative of a circadian mechanism. After 3 d of constant light, PhCCD1 transcript levels increased above the levels associated with a normal photoperiod in both leaves and corollas. Transcript levels were also shown to maintain robust oscillations in petunia leaves under these conditions. The decrease in transcript levels, in both corollas and leaves, and the residual oscillations in transcript levels observed in constant darkness suggest that light is required to drive the accumulation of the transcript and that the oscillator gates the circadian response.
The effect of light on PhCCD1 transcript accumulation in both leaves and corollas is complex. For example, a 10-min exposure of white light induces a more than 2-fold increase in PhCCD1 transcript in both leaves and corollas, when given after a 2-d dark period. Exposing plants to blue, red, and red/far-red light sources with equal fluence to that obtained from the white light source also led to an increase in PhCCD1 transcript levels, although not to the same levels as observed with white light. One interpretation is that the white light response involves coactivation of multiple photosensory systems. Furthermore, induction of PhCCD1 after red followed by far-red treatment implies that the induction of PhCCD1 is not solely due to an acute phytochrome response (Somers et al., 1998; Devlin and Kay, 2000). These data, taken together, show that β-ionone formation is diurnally regulated directly by light and indirectly through light entrainment of the circadian oscillator.
β-Ionone emission increased during the day and decreased to basal levels at night in petunia corollas. This pattern is somewhat different than emissions of other volatiles in MD, such as methylbenzoate, benzaldehyde, benzylbenzoate, phenylacetaldehyde, 2-phenylethanol, and isoeugenol (Underwood, 2003; Verdonk et al., 2003). Emissions of these other volatiles increase prior to the onset of darkness and remain high during the night before decreasing to low levels during the day. It has been suggested that this benzenoid volatile release coincides with the activity of the natural moth pollinators of one of the progenitors of line MD, Petunia axillaris (Verdonk et al., 2003). Comparable emission patterns are also observed in other moth-pollinated species, such as Nicotiana sylvestris (Loughrin et al., 1990, 1991; Knudsen and Tollsten, 1993; Levin et al., 2001). It is possible that β-ionone plays a role in attracting day-active insect pollinators, and it has previously been identified as an effective attractant for some insects (Hammack, 2001). This is further supported by the presence of β-ionone in the insect-pollinated progenitor of line MD, Petunia integrifolia. However, a link between β-ionone and petunia pollination remains untested. Also related to insect attraction, the C14 dialdehyde product of CCD1 activity (Fig. 1) is believed to be the precursor of rosafluene, a highly fluorescent compound found in some varieties of roses (Eugster and Marki-Fischer, 1991). Thus, PhCCD1 expression in flowers might have a double role in terms of both visual and olfactory cues.
Finally, our results indicate that PhCCD1 is present in all tissues tested, including roots, leaves, and flowers. This pattern of expression may indicate multiple functions for β-ionone in plants. CCD1 proteins catalyze the symmetric cleavage of multiple linear and cyclic carotenoids at the 9,10(9′,10′) double bonds. This cleavage results in formation of a diverse variety of C13 cyclohexone apocarotenoids and a C14 dialdehyde, corresponding to the central portion of the original carotenoid (Schwartz et al., 2001; Simkin et al., 2004). In the case of β-carotene, the C14 dialdehyde (Fig. 1) is thought to be the precursor of mycorradicin, a yellow pigment that accumulates in the roots of plants infected with arbuscular mycorrhizal fungi (Walter et al., 2000). Several C13 cyclohexone derivatives have also been identified in the same root tissue (Maier et al., 1995, 1997, 2000; Walter et al., 2000). It has been shown that exogenous application of blumenin, a C13 carotenoid-derived product that also accumulates in roots (Maier et al., 1995; Walter et al., 2000), strongly inhibits fungal colonization and arbuscule formation, implying that cyclohexenone derivatives might act to control fungal growth (Fester et al., 1999). β-Ionone has been shown to inhibit the growth of the pathogenic fungi Peronospora tabacina (Schiltz, 1974) and Colletotrichum musae (Utama et al., 2002). Also, Mikhlin et al. (1983) reported that β-ionone derivatives had antifungal activity against Fusarium solani, Botrytis cinerea, and Verticillum dahliae. Thus, it is possible that expression of PhCCD1 in vegetative tissues may have a role in protecting plants from fungal pathogens. Furthermore, the CCD1 C13 product of zeaxanthin or lutein cleavage, 3-hydroxy-β-ionone, accumulates in etiolated bean seedlings on exposure to light. It has been suggested that this compound functions in the light-induced inhibition of hypocotyl elongation (Kato-Noguchi, 1992; Kato-Noguchi et al., 1993).
In conclusion, we have shown that circadian and light regulation of PhCCD1 transcript levels integrally control the rhythmic pattern of transcript accumulation during normal photoperiodic conditions. The formation of β-ionone is temporally associated with steady-state PhCCD1 transcript levels. In contrast with many of the benzenoid volatiles, emission of β-ionone occurs principally during daylight hours. β-Ionone is known to be an important contributor to fragrance of flowers of many species, and the manipulation of its emission is an important step toward modifying fragrance in ornamentals.
MATERIALS AND METHODS
Plant Material and Treatment
Inbred cv MD petunia [Petunia axillaris × (P. axillaris × P. integrifolia)] plants were grown under greenhouse conditions with a day/night temperature regime of 25°C/18°C in commercial potting medium (Fafard 2B; Conrad Fafard, Agawam, MA) in 15-cm, 1.5-L pots, and were fertilized at each irrigation with 150 mg L−1 nitrogen from 15:7:14.1 soluble fertilizer (Peter's Fertilizer Products, Fogelsville, PA). Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
Gene Isolation and Vector Construction
A partial PhCCD1 clone from positions 376 bp to 1,641 bp (plus poly A tail) was recovered from a petunia flower cDNA library (Underwood et al., 2004), using LeCCD1A (AY576001) as a probe. The remaining 5′ sequence data was recovered by 5′RACE-PCR. The full-length cDNA sequence has been deposited in GenBank (accession no. AY576003). Transgenic MD plants were produced using Agrobacterium-mediated transformation of 5-week-old MD seedlings according to the method of Jorgensen et al. (1996). A full-length cDNA of LeCCD1A was introduced into the pDESTOE gateway vector (Booker et al., 2004) by recombination from the pENTRD vector (Invitrogen, Carlsbad, CA). The LeCCD1A cDNA was under transcriptional control of the figwort mosaic virus promoter (Richins et al., 1987) followed by the nos 3′ terminator. Introduction and inheritance of the transgenes were confirmed by PCR using primers specific for the NPTII marker gene.
Expression and Activity of PhCCD1 in Escherichia coli
The PhCCD1 cDNA from PhCCD1/pENTRD was put into pDEST14 (Invitrogen) by recombination. The plasmid containing the carotenoid biosynthetic genes (courtesy of F. Cunningham, University of Maryland) for β-carotene synthesis was cotransformed with PhCCD1/pDEST14 into the Ara-inducible E. coli strain BL21-AI (Invitrogen). Enzyme activity was measured as described by Booker et al. (2004). Briefly, cells were grown in Luria-Bertani medium with 0.2% Glc at 30°C until time of induction. Expression of PhCCD1 was induced by the addition of 0.2% Ara when cells reached an optical density of 0.4. Uninduced and induced 100-mL cultures were grown for 12 additional hours. Air was bubbled through the culture, and volatiles were collected onto a SuperQ filter trap. Volatiles were eluted off the trap with 150 μL of hexane. Volatiles were analyzed as described below. Injection volume was normalized to the optical density of bacterial culture.
Light Treatments
For light treatments, plants were exposed to various light sources for 10 min. Fluence rates were measured with a LI-COR LI-250 photometer using a PAR sensor (LI-COR, Lincoln, NE), and light qualities and far-red quantities were assessed using a StellarNet EPP2000 spectroradiometer (Apogee Instruments, Logan, UT). White light (380 μmol m−2 s−1) was used as a positive control. Blue- and red-light fluence rates were estimated to be 100 μmol m−2 s−1. Plants were then exposed to a blue-light source (100 μmol m−2 s−1) for 10 min or a red-light source (200 μmol m−2 s−1) for 5 min. A third set of plants was exposed to the same red-light source for 5 min followed by far-red light of a similar fluence for an additional 5 min. The plants were then returned to darkness (0.06 μmol m−2 s−1) along with controls and sampled at 3 h postexposure. Red and far-red light were generated from a Quantum Devices (Barneveld, WI) Q-Beam LED array. The emission spectra of light sources used in these experiments are viewable online at www.arabidopsisthaliana.com/lightsources.
Extraction of RNA and Real-Time Quantitative RT-PCR
Leaf tissue was taken from newly developing leaves at the growing tip of the plant every 6 h over a 5-d period. After 2 d, plants were transferred either to continuous light (380 μmol m−2 s−1) or dark (0.06 μmol m−2 s−1) for the remaining 3 d. Flowers were taken when fully open and roots from plants shortly after the development of the first flower.
Total RNA was isolated from tissues using the RNeasy plant mini kit (Qiagen, Valencia, CA). RNA samples were treated with RNase-free DNase (Qiagen) and purified using Qiagen mini-columns. Samples were checked for DNA contamination by TaqMan real-time RT-PCR in a reverse-minus transcription reaction. Concentration and purity of total plant RNA was determined by spectrophotometric analysis. The quantification was verified for all RNA samples in each experiment by formaldehyde agarose gel electrophoresis and visual inspection of rRNA bands upon ethidium bromide staining. To independently quantify mRNAs of PhCCD1 and LeCCD1A, gene-specific primers and probes were designed and tested for each of the two transcripts. The cross-specificity of the primers and probes is summarized in Table I. TaqMan one-step real-time RT-PCR was carried out as recommended by the manufacturer (Perkin-Elmer Applied Biosystems, Foster City, CA). All reactions contained 1× TaqMan buffer (Perkin-Elmer), 5 mm MgCl2, 200 μm each of dATP, dCTP, and dGTP, 400 μm dUTP, 0.625 units of AmpliTaq Gold polymerase, and 0.25 units of MultiScribe RNA reverse transcriptase and RNase inhibitor in a 25-μL volume. Reverse transcription was carried out using 250 ng of total RNA, 500 nm of each gene-specific primer, forward and reverse, and 250 nm TaqMan probe (see Table II). Reaction mixtures were incubated for 30 min at 48°C for reverse transcription, 10 min at 95°C, followed by 40 amplification cycles of 15 s at 95°C/1 min at 60°C. Samples were quantified in the GeneAmp 5700 Sequence Detection system (Perkin-Elmer). Absolute mRNA levels were quantified against a standard curve of tritiated in vitro-transcribed sense-strand RNAs.
Table I.
Specificity of each set of TaqMan real-time RT-PCR primers and probes in detecting the related sequence
| Transcript
|
||
|---|---|---|
| LeCCD1A | PhCCD1 | |
| LeCCD1A | 1 | <2 × 10−7 |
| PhCCD1 | <6 × 10−7 | 1 |
A total of 100 pg of each in vitro-transcribed mRNA was added per reaction in a pairwise test against each primer probe set. A standard curve was made from the corresponding messenger. The data represent the equivalent amount of signal produced by 100 pg of each specific gene.
Table II.
Primers and probes used in TaqMan real-time quantitative RT-PCR assay
| LeCCD1A | Forward | TTGATTACCTGCCGCCTTGT |
| Reverse | CATATAGCTCATTGCAGAAATTC | |
| Probea | AACCCAGACCTAGACATGGTCAATGGAGC | |
| PhCCD1 | Forward | TCATATTTCACAACGCCAATGC |
| Reverse | CGGAAGGCGGCAGGTAA | |
| Probea | TGGGAGGAGGGAGATGAAGTCGTGTTG |
Primers and probes were designed using PRIMER EXPRESS software (Applied Biosystems).
All probes were labeled at the 5′ with fluorescent reporter dye 6-carboxyfluorescein and at the 3′ with black hole quencher-1 from Integrated DNA Technologies (Coralville, IA).
Volatile Analysis
Four excised flowers were placed into glass vessels and assayed under normal light conditions or in the dark for dark-collected samples. Volatile emissions were collected for a period of 1 h in all experiments (Booker et al., 2004; Simkin et al., 2004; Underwood et al., 2004). Experiments utilized glass cylinders (17 mm i.d. × 61 cm long, 127-mL volume), and collection of volatiles followed Turlings et al. (1991). Briefly, clean humidified air was passed through the vessels (550 mL min−1) and volatiles were trapped on 30 mg of Super Q (80/100 mesh; Alltech, Deerfield, IL). The Super Q traps were eluted with 150 μL of dichloromethane. A total of 400 ng of nonyl acetate (in 5 μL of dichloromethane) was added to the trap as an internal standard immediately prior to elution. Quantification of volatiles was performed on an Agilent (Wilmington, DE) 6890N gas chromatograph as described in Schmelz et al. (2001). The identification of peaks, including β-ionone, was initially determined by mass spectrometry of the collected volatiles and subsequently by coelution with a known standard (Sigma, St. Louis). The presence of β-ionone in petunia volatiles was confirmed by gas chromatography (GC)-mass spectrometry on an ion trap mass spectrometer (MAT ITS40; Finnigan, Austin, TX) interfaced to a gas chromatograph (model 3400; Varian, Sunnyvale, CA) operated in the electron impact mode as described by Paré and Tumlinson (1997). Under these conditions, synthetic β-ionone produced the characteristic mass spectral fragments 177 (100%), 164 (8%), 149 (8%), 135 (10%), 121 (9%), and 43 (95%). With an identical GC retention time, the β-ionone present in the plant sample produced the same characteristic mass spectral fragments of 177 (100%), 164 (7%), 149 (10%), 135 (11%), 121 (12%), and 43 (100%). The parent ion of m/z = 192 was also detected at trace levels (1%) in both the synthetic and natural product samples.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number AY576003.
Acknowledgments
We would like to thank Richard Dexter for tissue collection and RNA extraction, Yec'han Laizet for sequence alignments, and Kevin Folta for critical reading of the manuscript as well as advice on light experiments. This is publication R-10461 of the Florida Agricultural Experiment Station.
This work was supported in part by National Science Foundation (grant no. IBN0115004 to H.J.K.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.049718.
References
- Baldwin EA, Scott JW, Shewmaker CK, Schuch W (2000) Flavor trivia and tomato aroma: biochemistry and possible mechanisms for control of important aroma components. HortScience 35: 1013–1021 [Google Scholar]
- Block M, Dorne A-J, Joyard J, Douce R (1983) Preparation and characterization of membrane fractions enriched in outer and inner membranes from spinach chloroplasts. J Biol Chem 258: 13281–13286 [PubMed] [Google Scholar]
- Booker J, Auldridge M, Wills S, McCarty D, Klee HJ, Leyser O (2004) MAX3 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signalling molecule. Curr Biol 14: 1232–1238 [DOI] [PubMed] [Google Scholar]
- Bouvier F, Suire C, Mutterer J, Camara B (2003) Oxidative remodelling of chromoplast carotenoids: identification of a carotenoid cleavage dioxygenase CsCCD and CsZCD genes involved in Crocus secondary metabolic biogenesis. Plant Cell 15: 47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery RG, Teranishi R, Ling LC, Turnbaugh JG (1988) Quantitative studies on origins of fresh tomato aroma volatiles. J Agric Food Chem 36: 1247–1250 [Google Scholar]
- Cooper CM, Davies NW, Menary RC (2003) C-27 apocarotenoids in the flowers of Boronia megastigma (Nees). J Agric Food Chem 51: 2384–2389 [DOI] [PubMed] [Google Scholar]
- Cunningham FX, Pogson B, Sun A, McDonald K, DellaPenna D, Gantt E (1996) Functional analysis of the β and ɛ lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. Plant Cell 8: 1613–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Kay SA (2000) Cryptochromes are required for phytochrome signalling to the circadian clock but not for rhythmicity. Plant Cell 12: 2499–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugster CH, Marki-Fischer E (1991) The chemistry of rose pigments. Angew Chem Int Ed Engl 30: 654–672 [Google Scholar]
- Fester T, Maier W, Strack D (1999) Accumulation of secondary compounds in barley and wheat roots in response to inoculation with an arbuscular mycorrhizal fungus and co-inoculation with rhizosphere bacteria. Mycorrhiza 8: 241–246 [Google Scholar]
- Froehlich J, Itoh A, Howe G (2001) Tomato allene oxide synthase and fatty acid hydroperoxide lyase, two cytochrome P450s involved in oxylipin metabolism, are targeted to different membranes of chloroplast envelope. Plant Physiol 125: 306–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano G, Al-Babili S, von Lintig J (2003) Carotenoid oxygenases: cleave it or leave it. Trends Plant Sci 8: 145–149 [DOI] [PubMed] [Google Scholar]
- Hammack L (2001) Single and blended maize volatiles as attractants for diabroticite corn rootworm beetles. J Chem Ecol 27: 1373–1390 [DOI] [PubMed] [Google Scholar]
- Jorgensen RA, Cluster PD, English J, Que QD, Napoli CA (1996) Chalcone synthase cosuppression phenotypes in petunia flowers: comparison of sense vs. antisense constructs and single-copy vs. complex T-DNA sequences. Plant Mol Biol 31: 957–973 [DOI] [PubMed] [Google Scholar]
- Kaiser R, Lamparsky D (1980) Volatile constituents of Osmanthus absolute. In BD Mookheijee, CJ Mussinan, eds, Essential Oils. Allured Publishing, Wheaton, IL, pp 159–192
- Kato-Noguchi H (1992) An endogenous growth inhibitor, 3-hydroxy-β-ionone. I. Its role in light-induced growth inhibition of hypocotyls of Phaseolus vulgaris. Physiol Plant 86: 583–586 [Google Scholar]
- Kato-Noguchi H, Kosemura S, Yamamura S, Hasegawa K (1993) A growth inhibitor, R-(-)-3-hydroxy-β-ionone, from light-grown shoots of a dwarf cultivar of Phaseolus vulgaris. Phytochemistry 33: 553–555 [Google Scholar]
- Knudsen JT, Tollsten L (1993) Trends in floral scent chemistry in pollination syndromes: floral scent composition in moth-pollinated taxa. Bot J Linn Soc 113: 263–284 [Google Scholar]
- Kolosova N, Gorenstein N, Kish CM, Dudareva N (2001) Regulation of circadian methyl benzoate emission in diurnally and nocturnally emitting plants. Plant Cell 13: 2333–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin RA, Raguso RA, McDade LA (2001) Fragrance chemistry and pollinator affinities in Nyctaginaceae. Phytochemistry 58: 429–440 [DOI] [PubMed] [Google Scholar]
- Loughrin JH, Hamilton-Kemp TR, Andersen RA, Hildebrand DF (1990) Volatiles from flowers of Nicotiana sylvestris, N. otophora and Malus×domestica: headspace components and day/night changes in their relative concentrations. Phytochemistry 29: 2473–2477 [Google Scholar]
- Loughrin JH, Hamilton-Kemp TR, Andersen RA, Hildebrand DF (1991) Circadian-rhythm of volatile emission from flowers of Nicotiana sylvestris and N. suaveolens. Physiol Plant 83: 492–496 [Google Scholar]
- Maier W, Peipp H, Schmidt J, Wray V, Strack D (1995) Levels of a terpenoid glycoside (Blumenin) and cell wall-bound phenolics in some cereal mycorrhizas. Plant Physiol 109: 465–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W, Hammer K, Dammann U, Schulz B, Strack D (1997) Accumulation of sesquiterpenoid cyclohexenone derivatives induced by an arbuscular mycorrhizal fungus in members of the Poaceae. Planta 202: 36–42 [Google Scholar]
- Maier W, Schmidt J, Nimtz M, Wray V, Strack D (2000) Secondary products of mycorrhizal roots of tobacco and tomato. Phytochemistry 54: 473–479 [DOI] [PubMed] [Google Scholar]
- Markwell J, Bruce B, Keegstra K (1992) Isolation of a carotenoid-containing sub-membrane particle from the chloroplastic envelope outer membrane of pea (Pisum sativum). J Biol Chem 267: 13933–13937 [PubMed] [Google Scholar]
- Mikhlin ED, Radina VP, Dmitrovskii AA, Blinkova LP, Butova LG (1983) Antifungal and antimicrobic activity of β-ionone and vitamin A derivatives (in Russian). Prikl Biokhim Mikrobiol 19: 795–803 [PubMed] [Google Scholar]
- Negre F, Kish C, Boatright J, Underwood B, Shibuya K, Wagner C, Clark D, Dudareva N (2003) Regulation of methylbenzoate emission after pollination in snapdragon and petunia flowers. Plant Cell 15: 2992–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill SJ, Burnett EC, Desikan R, Hancock JT (1998) Cloning of a wilt-responsive cDNA from Arabidopsis thaliana suspension culture cDNA library that encodes a putative 9-cis-epoxy-carotenoid dioxygenase. J Exp Bot 49: 1893–1894 [Google Scholar]
- Paré PW, Tumlinson JH (1997) De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol 114: 1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott MB, Effmert U, Piechulla B (2003) Transcriptional and post-translational regulation of S-adenosyl-L-methionine: salicylic acid carboxyl methyltransferase (SAMT) during Stephanotis floribunda flower development. J Plant Physiol 160: 635–643 [DOI] [PubMed] [Google Scholar]
- Richins RD, Scholthof HB, Shepard RJ (1987) Sequence of the figwort mosaic virus DNA (caulimovirus group). Nucleic Acids Res 15: 8451–8466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz P (1974) Action inhibitrice de la β-ionone au cours du développement de Peronospora tabacina. Ann Tabac 11: 207–216 [Google Scholar]
- Schmelz EA, Alborn HT, Tumlinson JH (2001) The influence of intact-plant and excised-leaf bioassay designs on volicitin- and jasmonic acid-induced sesquiterpene volatile release in Zea mays. Planta 214: 171–179 [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JAD, McCarty DR (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276: 1872–1874 [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Qin X, Zeevaart JAD (2001) Characterization of a novel carotenoid cleavage dioxygenase from plants. J Biol Chem 276: 25208–25211 [DOI] [PubMed] [Google Scholar]
- Simkin AJ, Changfu Z, Kuntz M, Sandmann G (2003. a) Light-dark regulation of carotenoid biosynthesis in pepper (Capsicum annuum) leaves. J Plant Physiol 160: 439–443 [DOI] [PubMed] [Google Scholar]
- Simkin AJ, Labouré AM, Kuntz M, Sandmann G (2003. b) Modulation of carotenoid biosynthesis in tomato leaves upon light-dark transition. Z Naturforsch [C] 58c: 371–380 [Google Scholar]
- Simkin AJ, Schwartz SH, Auldridge M, Taylor MG, Klee HJ (2004) The tomato CCD1 (CAROTENOID CLEAVAGE DIOXYGENASE 1) genes contribute to the formation of the flavor volatiles β-ionone, pseudoionone and geranylacetone. Plant J (in press) [DOI] [PubMed]
- Somers DE, Devlin PF, Kay SA (1998) Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science 282: 1488–1490 [DOI] [PubMed] [Google Scholar]
- Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty DR (2003) Molecular characterisation of the 9-cis epoxycarotenoid dioxygenase gene family. Plant J 35: 44–56 [DOI] [PubMed] [Google Scholar]
- Tan BC, Schwartz S, Zeevaart JAD, McCarty DR (1997) Genetic control of abscisic acid biosynthesis in maize. Proc Natl Acad Sci USA 94: 12235–12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlings TCJ, Tumlinson JH, Heath RR, Proveaux AT, Doolittle RE (1991) Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson) to the microhabitat of one of its hosts. J Chem Ecol 17: 2235–2251 [DOI] [PubMed] [Google Scholar]
- Underwood BA (2003) Effects of ethylene on floral fragrance in Petunia x hybrida Mitchell Diploid. PhD dissertation, University of Florida, Gainesville, FL
- Underwood BA, Tieman DM, Shibuya K, Loucas HM, Dexter RJ, Schmelz EA, Simkin AJ, Klee HJ, Clark DG (2004) Ethylene-regulated floral volatile synthesis in petunia corollas. Plant Physiol (in press) [DOI] [PMC free article] [PubMed]
- Utama IM, Wills RB, Ben-Yehoshua S, Kuek C (2002) In vitro efficacy of plant volatiles for inhibiting the growth of fruit and vegetable decay microorganisms. J Agric Food Chem 50: 6371–6377 [DOI] [PubMed] [Google Scholar]
- Verdonk JC, Ric de Vos CH, Verhoeven HA, Haring MA, van Tunen AJ, Schuurink RC (2003) Regulation of floral scent production in petunia revealed by targeted metabolomics. Phytochemistry 62: 997–1008 [DOI] [PubMed] [Google Scholar]
- Wache Y, Bosser-DeRatuld A, Lhuguenot JC, Belin JM (2003) Effect of cis/trans isomerism of beta-carotene on the ratios of volatile compounds produced during oxidative degradation. J Agric Food Chem 51: 1984–1987 [DOI] [PubMed] [Google Scholar]
- Walhberg I, Eklund A (1998) Degraded carotenoids. In G Britton, S Liaaen-Jensen, H Pfander, eds, Carotenoids. Birkhaüser Verlag, Boston
- Walter MH, Fester T, Strack D (2000) Arbuscular mycorrhizal fungi induce the nonmevalonate methylerythritol phosphate pathway of isoprenoid biosynthesis correlated with accumulation of the “yellow pigment” and other apocarotenoids. Plant J 21: 571–578 [DOI] [PubMed] [Google Scholar]
- Winterhalter P, Rouseff R (2002) Carotenoid-derived aroma compounds. American Chemical Society, Washington, DC