Abstract
We present cellular- and ultracellular-level studies here to show developmental programmed cell death (PCD) of placento-chalazal (P-C) cell layers in maternal pedicel tissue in developing caryopses of normal seed (Mn1) and in the invertase-deficient miniature (mn1) seed mutant in maize (Zea mays). PCD was evidenced by loss of nuclei and all subcellular membranous organizations in many P-C layers. The terminal deoxynucleotidyl transferase-mediated X-dUTP nick-end labeling (TUNEL) stain that is diagnostic of apoptotic-like PCD identified spatially and temporally two distinctive subdomains, which coincided with nucellar and integumental P-C layers based on their developmental origins. The early phase of PCD in the nucellar P-C was TUNEL negative and was specific to only the fertilized caryopses, indicating that the signaling for PCD in these maternal cells originated in the zygotic tissues. In fact, the initiation of PCD coincided with endosperm cellularization and was rapidly and coordinately completed prior to the beginning of the major storage phase in endosperm. Cell shape in these cell layers was also influenced by the genotype of filial endosperm. The later phase of PCD was restricted to the integumental P-C layers underneath the nucellar cells and was TUNEL positive in both genotypes. The two subdomains of the P-C layers were also distinguishable by unique cell wall-associated phenolic compounds. Based on collective evidence, we infer that the nucellar PCD may have osmolytic etiology and may lead to activation of the post-phloem transport function of the P-C layer, whereas the integumental PCD was senescent related, in particular, protecting the maturing seed against microbes that may be transported from the maternal tissue.
Pedicel, a maternal tissue at the base of developing seeds of all higher plants, provides the major structural bridge in the transfer of photoassimilates and nutrients from the mother plant to the filial generation, endosperm, and embryo. In maize (Zea mays), directly underneath the basal endosperm cells and just above the phloem termini in pedicel, is a mat of cells that constitute the placento-chalazal (P-C) layer (Fig. 1B), which is believed to play a critical role in post-phloem transport of water, sugars, and nutrients for developing seeds (Kiesselbach, 1949; Felker and Shannon, 1980; Schel et al., 1984). Although described in various plant species, a P-C region is known to exhibit a high level of anatomical variability in its structural adaptations (for review, see Thorne, 1985). P-C layers are perhaps best developed in tropical crops such as maize and sorghum (Sorghum bicolor), which show assimilate transport from only the base of the caryopsis. In fact, the functional importance of a normal P-C layer is best exemplified in the miniature 1 (mn1) seed mutation in maize that shows greatly reduced size of the endosperm due to a premature withdrawal of the P-C layer from developing seed, causing a physical discontinuity, a gap, between the source and sink tissues (Lowe and Nelson, 1946). The P-C layer in sorghum is largely comprised of a placental sac (Maness and McBee, 1986); how it is formed is unknown. In temperate cereals such as wheat (Triticum aestivum) and barley (Hordeum vulgare), where transport occurs along the entire length through a single vascular band embedded in the maternal pericarp, a P-C layer is modified into a diminutive form as a nucellar pad or a projection (Thorne, 1985; Olsen et al., 1999). Numerous structural adaptations of the P-C layer are also described in the dicots. In general, assimilate transport is through phloem elements that are embedded in the maternal seed coat. A single vascular bundle encircles the seed coat through funicular and chalazal vascular tissues, and an apoplast of intercellular space of several cell layers, presumably analogous to the P-C layer, separates the maternal and filial generations. Active transport systems at various points in filial tissues are known to provide controls in assimilate uptake in developing seed (Thorne, 1985; Weber et al., 1997; Rosche et al., 2002).
Figure 1.
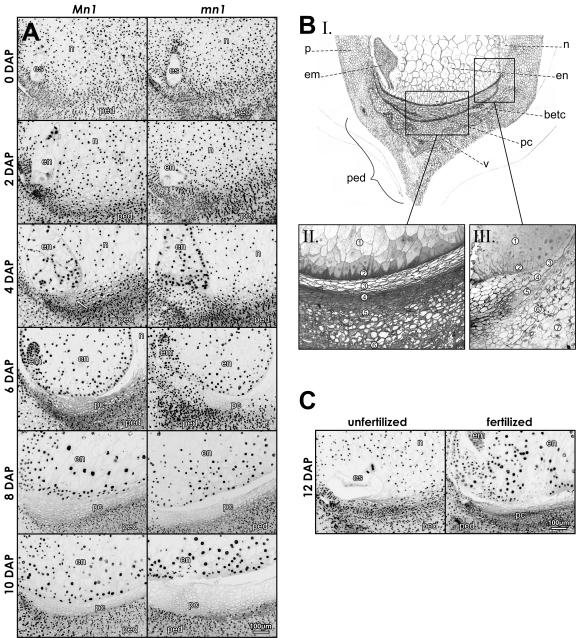
Evidence of the progressive loss of nuclei in the P-C layers of Mn1 and mn1 maize caryopses. A, DAPI-stained median longitudinal sections of the basal part of caryopses at 0 to 10 DAP; arrows in 4 DAP point to the first cells without visible nuclei. BI, A schematic drawing of the basal part of maize caryopsis at about 10 DAP. BII, Basal endosperm and pedicel can be subdivided into several zones: (1) endosperm; (2) BETC; (3) nucellar P-C layer; (4) closing layer; (4 and 5) integumental P-C layer; and (6) pedicel. BIII, Endosperm and the various layers at the base of the caryopsis that give rise to the P-C layers: (1) endosperm; (2) BETC; (3) nucellus epidermis; (4) semipermeable membrane; (5) outer integument; (6) inner pericarp; and (7) mid-pericarp. C, DAPI-stained 12-DAP fertilized caryopsis and unfertilized ovule from the same ear. em, embryo; en, endosperm; es, embryo sac; n, nucellus; p, pericarp; pc, P-C layer; ped, pedicel; v, vascular tissue.
An additional proposed function of the P-C layer, especially in maize, sorghum, and teosinte, is to prevent the entrance of microbes from maternal cells into the filial tissue through the accumulation of the antimicrobial peptide, basal layer-type antifungal protein 2 (BAP2; Serna et al., 2001). Whether or not BAP2, along with the collapsed or crushed mass of cellular tissue in the P-C cells at seed maturity, leads to the so-called black layer or an abscission layer (Felker and Shannon, 1980) is not clear. Similar cellular autolytic events, albeit not well characterized, are also reported in the nucellar projection in barley (Linnestad et al., 1998) and in thin-walled parenchymatous cells in developing cotyledons of fava bean (Weber et al., 1997). It is probable that these events are attributable to the phenomenon of programmed cell death (PCD), which is increasingly recognized as a major force in development and differentiation in plants and animals (for review, see Dangl et al., 2000; Krishnamurthy et al., 2000; Thomas et al., 2003). Despite the significant importance of PCD and much recent progress in understanding it at biochemical and molecular levels in certain select tissue systems (Ito and Fukuda, 2002; Milioni et al., 2002), very little is known on its role in seed development except for the studies in starchy endosperm cells of maize and wheat (Young et al., 1997; Young and Gallie, 1999, respectively). Recently, Wan et al. (2002) observed PCD-associated changes, including vacuolation, DNA fragmentation both by gel analysis and by in situ terminal deoxynucleotidyl transferase (TdT)-mediated X-dUTP nick-end labeling (TUNEL) staining, and eventual cell compression in the maternal inner integuments in rape seed (Brassica napus). Similarly, Giuliani et al. (2002) reported PCD during embryogenesis in maize, especially in organs that do not contribute to the adult plant body.
Our main objective of this study was to test a possibility that the physical gap in the P-C region of the mn1 seed mutation (Lowe and Nelson, 1946; Miller and Chourey, 1992) was formed through PCD in these cells. The homozygous recessive mn1 seeds are approximately 20% of the endosperm size as compared to the wild type, Mn1, and histological analyses indicate that the gap in P-C region results from an early (approximately at 9 d after pollination) withdrawal of maternal cells from the developing endosperm (Lowe and Nelson, 1946). Our recent studies have demonstrated that the causal basis of both the mn1 seed phenotype as well as the gap formation in the mutant is the loss of the Mn1-encoded cell wall invertase, INCW2, which is localized entirely in the basal endosperm transfer cells (BETC; Cheng et al., 1996). As a possible basis for the correlation between invertase deficiency and the gap formation in the mn1 seed mutant, we have suggested that the gap is a result of cellular destabilization presumably caused by a transient increase in Suc concentration in the P-C cells due to the lack of Suc hydrolysis in the mn1 BETC region (Cheng et al., 1996; Cheng and Chourey, 1999). Thus, our previous studies on the mn1 seed mutation have led us to suggest that structural stability of the maternal cells in the P-C region is dependent on the INCW2-mediated gradient of Suc utilization in the filial endosperm. The results in this study show that the gap in the P-C region of the mn1 seed mutant originated through PCD. In addition, we report an unexpected but important observation that the P-C layers of Mn1 caryopses also underwent PCD; thus, PCD in the P-C layer is a normal component of caryopsis development in maize. The PCD-mediated gap in the mn1 caryopsis appeared more prominently than the Mn1 due to the combined effect of a larger area of empty P-C cells in the mutant than the wild type and the detachment of the diminutive mn1 endosperm from the maternal tissue.
RESULTS
Loss of Nuclei in P-C Layers as Visualized by 4′,6′ Diamino-2-Phenylindole, Dihydrochloride Stain and Cellular Morphology of the P-C Cells in Mn1 and mn1 Caryopses
Figure 1A shows longitudinal sections of 4′,6′ diamino-2-phenylindole, dihydrochloride (DAPI)-stained Mn1 and mn1 caryopses at 0- to 10-d-after-pollination (DAP) stages along with a schematic drawing and two micrographs (Fig. 1B) that depict various cell layers that comprise the basal part of a developing caryopsis, including the pedicel. The P-C region (Fig. 1B) is composed of two distinct zones; the first zone, the nucellar P-C, immediately subtends the BETC and is derived from the nucellus epidermis, and the second, integumental P-C, is located immediately below the first and is derived from the inner integument (Esau, 1977). The DAPI fluorescence (Fig. 1A) marks the position of nuclei in the cell. At 2 DAP, both genotypes showed an extremely small vacuolated region of endosperm and embryo, lacking DAPI-positive area, inside a relatively large body of nuclei-filled nucellus. The PC layer underneath the endosperm cavity (i.e. female gametophyte) in both genotypes was also packed with DAPI-stained nuclei. At 4 DAP, the endosperm cavity was cellularized and a few cells without nuclei were already visible in the maternal tissue underneath the endosperm (Fig. 1A, arrows). Temporal increases in the number of cell layers lacking nuclei (also referred to here as enucleation) in the P-C layer were evident in the subsequent stages during 6 to 10 DAP (Fig. 1A). The gradual loss of nuclei in successive cell layers expanded coordinately, seemingly in a noncell autonomous fashion, toward the vascular tissue in the pedicel. By 10 DAP, the mn1 caryopsis showed much greater expansive region of the PC layer without nuclei relative to the wild type (however, see below, Fig. 2).
Figure 2.
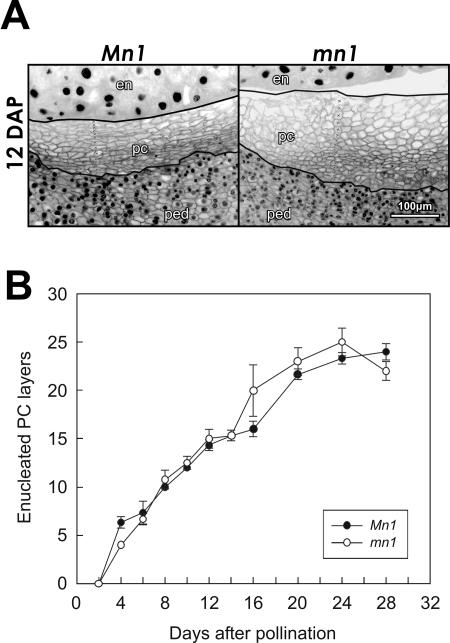
Cellular morphology of maternal P-C cells lacking nuclei is determined by the filial endosperm. A, DAPI-stained longitudinal sections of Mn1 and mn1 maize caryopses at 12 DAP. Manually drawn lines bracket the cells without visible nuclei in the P-C layer and the crosses (×) inside the stack of cells demonstrate how the counting of cell layers was done. B, Temporal changes in the number of P-C layers without nuclei in Mn1 and mn1 caryopses. Mean ± sd of number of layers in median longitudinal sections of three different caryopses is shown. en, endosperm; pc, P-C layer; ped, pedicel.
Significantly, the PC layers in ovules of unpollinated ears from homozygous Mn1 and mn1 plants did not exhibit such loss of nuclei during the same period of development (staged on the basis of days after anthesis; data not shown). A representative example that depicts an unfertilized ovule and a 12-DAP caryopsis from the same ear of a homozygous Mn1 plant is shown in Figure 1C. Based on DAPI stain, nearly all P-C cells in the unfertilized ovule were nucleated while the same region in fertilized kernels showed layers of cells without nuclei.
Lowe and Nelson (1946) reported the earliest detectable anatomical trait of the mn1 seed mutation, in particular, the premature withdrawal of pedicel from developing endosperm (i.e. the gap formation). We reexamined the P-C layer in Mn1 and mn1 caryopses here to better understand the possible basis for the gap region in the mutant seed. Although the two genotypes showed the same pattern of enucleation in the P-C region (Figs. 1A and 2B), there was a noteworthy morphological difference in P-C cells in the mutant relative to the wild type. Figure 2A illustrates a close-up view of DAPI-stained P-C layer of Mn1- and mn1-segregating caryopses from the same F2 ear at 12 DAP, a stage at which developing seeds of the two genotypes are readily identifiable. The enucleated P-C cells in the Mn1 kernels were distinctively compact and oblong, whereas the mn1 cells were irregular in shape and slightly larger than the Mn1 cells (Fig. 2A). Figure 2B depicts an actual estimate of the number of enucleated cell layers in homozygous Mn1 and mn1 kernels at various developmental stages, as shown. Both genotypes showed a range of 5 to 25 cell layers during 4 to 24 DAP duration. There was no significant difference in the number of P-C layers between the two genotypes. Thus, cellular morphology, but not the actual number of the cell layers, in the maternal P-C region was influenced by the genotype of the filial endosperm. Collectively, the gap in the mn1 seed mutation appeared to be a consequence of a detachment between the maternal tissue and the diminutive mn1 endosperm, presumably due to the reduced mitotic activities and cell expansions demonstrated previously (Vilhar et al., 2002). We suggest here that the gap formation is not due to cell degeneration in the P-C layers, as proposed previously (Lowe and Nelson, 1946; Miller and Chourey, 1992; Cheng et al., 1996).
TUNEL Reaction in the P-C Layers of Mn1 and mn1 Caryopses
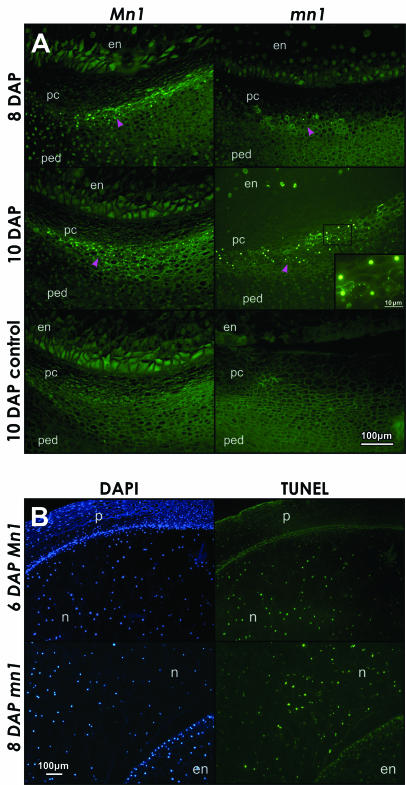
The TUNEL stain has been extensively used for in situ detection of DNA fragmentation sites associated with apoptotic PCD (Gavrieli et al., 1992). Green fluorescence in this study marks the site at which TdT-mediated incorporation of fluorescein-labeled dUTP occurs during the fragmentation of the DNA (Fig. 3). TUNEL staining of the same sections or serial sections of the same caryopses as shown in Figure 1A was performed to better understand the basis of enucleation and presumed cell death in the P-C layers in seed development. The earliest stage at which any TUNEL-positive nuclei could be seen was at 8 DAP and the number of such nuclei increased by 10 DAP; a similar pattern of TUNEL stain was also seen in the mn1 mutant at 10 DAP (Fig. 3A). No TUNEL-stained nuclei were seen at 2 to 6 DAP in either genotype (data not shown), preceding or coincident with substantial loss of nuclei seen at 6 DAP (Fig. 1A). Significantly, TUNEL-positive nuclei (green fluorescence) in the P-C layers of 8- to 12-DAP caryopses were spatially distinctive and restricted to the integumental P-C layer below the nucellar P-C layer that was already enucleated. In some instances, the TUNEL-staining pattern appeared as foci concentrated around the periphery of cell membrane (inset in Fig. 3A, 10-DAP mn1). These foci also stained positive with DAPI (data not shown), indicating that they were specifically due to DNA (also see Fig. 4, J–M). It is possible that such pattern of staining is due to the membrane-bound apoptotic-like bodies containing fragmented DNA as observed in animal cells undergoing apoptosis (Bursch et al., 1990).
Figure 3.
Apoptotic-like PCD is diagnosed by the TUNEL stain in A, P-C layers of Mn1 and mn1 maize caryopses at 8 and 10 DAP and B, nucellus tissue at 6 and 8 DAP. A, Green fluorescent dots mark nuclei containing fragmented DNA. Magenta arrowheads point to the layers of cells in the P-C region with TUNEL-positive nuclei. The inset in 10-DAP mn1 panel shows ringed foci of faint green fluorescence, indicative of apoptotic like bodies. The 10-DAP control panels, without the TdT enzyme, show only the background green autofluorescence, independent of the TUNEL stain. B, Comparison of DAPI and TUNEL staining in the apical part of the caryopsis. At 6 DAP, the nuclei in the nucellus reacted positively with both DAPI and TUNEL, whereas the pericarp nuclei stained much weaker with TUNEL compared to DAPI. At 8 DAP, the comparison of nucellar and endosperm tissue shows relatively much weaker TUNEL reaction in the endosperm compared to nucellus, indicating that endosperm and pericarp cells did not undergo PCD such as the nucellus cells at this early stage of development (both Mn1 and mn1 genotypes were similar). en, endosperm; n, nucellus; p, pericarp; pc, P-C layer; ped, pedicel.
Figure 4.
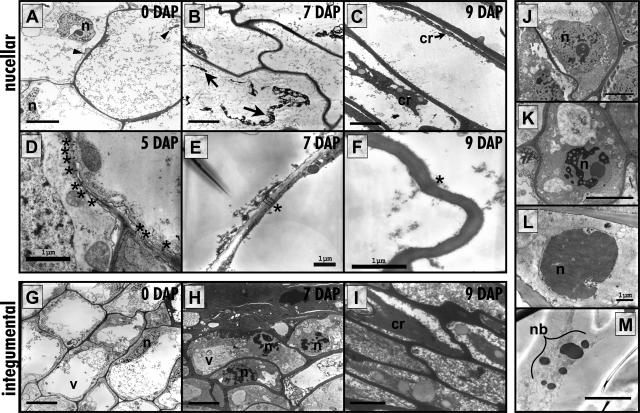
Transmission electron micrographs showing PCD in the P-C cells in maize caryopsis. A to C, Nucellar P-C at 0 to 9 DAP. A, At 0 DAP the cells are well differentiated showing mitochondria and nuclei that appear normal. B, At 7 DAP the cells are emptying and appear to plasmolyse. C, By 9 DAP the cells have completely emptied leaving only degenerated cytoplasmic contents. D to F, The number of visible plasmodesmata in nucellar P-C cell walls is diminishing from 5 to 9 DAP. G to I, Integumental P-C at 0 to 9 DAP. G, At 0 DAP the cells are well differentiated, similar to the nucellar P-C showing organelles and large vacuoles. H, At 7 DAP the nuclei in these cells, some of which form the closing layer, start displaying condensed chromatin and smaller vacuoles. I, By 9 DAP the cytoplasm is condensed and all organelles are lost; however, the cells subtending these are undergoing the morphological changes seen in H. J to M, Progressive morphological changes in the nuclei of the integumental P-C. J, Normal nucleus. K, Nucleus displaying condensing chromatin. L, Nucleus with completely condensed chromatin. M, The nucleus has broken up into nuclear bodies. cr, cytoplasmic remains; n, nucleus; nb, nuclear bodies; v, vacuole; arrows, leftover plasmolyzed protoplasm; arrowheads, mitochondria; asterisks, plasmodesmata. Bars in A to C, G to K, and M = 5 μm; bars in all the rest, as shown.
Nucellar Tissue
Nucellus in the ovule is a prominent nourishing tissue for the filial endosperm/embryo in the early stages of kernel development. We observed both DAPI and intense TUNEL staining throughout the autolysing nucellar cells during the early stages marked by rapid growth of endosperm in the nucellar cavity (Fig. 3B). Both Mn1 and mn1 nucelli showed a similar pattern of TUNEL staining. The pericarp (Fig. 3B; 6-DAP Mn1) and endosperm (Fig. 3B; 8 DAP mn1), however, showed no TUNEL stain (yet DAPI positive). PCD in developing endosperm does not initiate until approximately 16 DAP (Young et al., 1997). Giuliani et al. (2002) reported similar cellular-level data on PCD in suspensor cells, coleoptile, and scutellum during embryogenesis in developing maize seed. In developing canola seed, PCD in maternal seed, the inner integument, is associated with a Cys proteinase that may function in disposal of proteins related to the PCD (Wan et al., 2002). A nucellar protein in maize, nucellain, is developmentally correlated with autolysis, but whether or not it has any role in PCD remains to be elucidated (Linnestad et al., 1998).
Transmission Electron Microscopy Analyses
Ultrastructural analyses showed a progression of cellular change, apparently reflective of the two separate modes of PCD in the two P-C layers (Fig. 4). Both nucellar and integumental P-C cells showed well-differentiated intact nuclei, organelles, and large vacuoles up to 5 DAP. Thereafter, each zone appears to have a unique pattern of PCD. The nucellar P-C cells that are TUNEL negative (Fig. 4, A–C) underwent a rapid degeneration process during the 4- to 9-DAP period, all cellular contents were emptied by 9 DAP, and the cells were nothing more than cell corpses. Intermediate stages of degeneration proved to be extremely difficult to capture for transmission electron microscopy (TEM) analyses; in fact, the only intermediate stage of degeneration that was found was a cell in late stages of plasmolysis at 7 DAP (Fig. 4B). Another interesting observation is that of a decrease in the number of identifiable plasmodesmata in the cell walls of the nucellar P-C layer cells between 5 and 12 DAP. The plasmodesmata frequency up through 7 DAP was an average of 9.9 ± 1.99/cell transect perimeter, while this number decreased to 2.4 ± 0.85 between 9 and 12 DAP. A representative example of these changes in the plasmodesmatas is shown in Figure 4, D to F. The integumental P-C layer showed a PCD process that appeared to be much more gradual and was apoptoptic-like in nature, as seen from the changes in the nuclear morphology (Fig. 4, G–I) as well as by the TUNEL reactivity (Fig. 3A). This zone of the P-C includes the cells that will eventually contribute to the formation of the closing layer (Fig. 1B). Between 7 and 9 DAP, the uppermost layer of cells in this zone showed dense cytoplasm and contained only very small vacuoles along with condensing nuclei. By 9 DAP, these cells contained only fragmented vacuoles and showed no identifiable organelles or nuclei (Fig. 4I). The cells just subtending the closing layer, but still a part of the integumental P-C, displayed the most clear morphological nuclear changes. For example, by 7 DAP the chromatin began to condense and was delineated by sharp edges while the nucleolus remained intact (Fig. 4H). A series of nuclear changes were also detectable, especially at 10 through 12 DAP (Fig. 4, J–M). The nuclei in some cells were entirely condensed while in others these were breaking apart forming apoptotic bodies (Fig. 4, L and M, respectively).
Histochemical Evidence of Two Subdomains in the P-C Layer
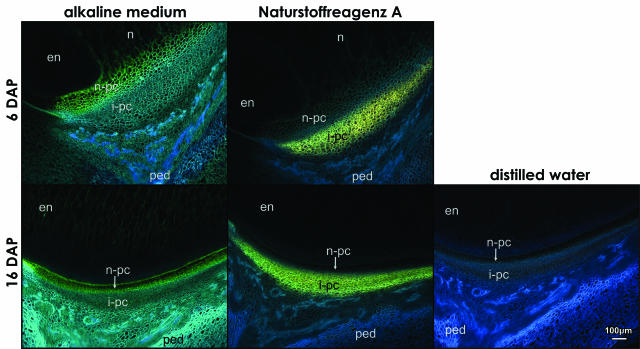
Additional differences in the two P-C layers as two distinctive zones are shown in Figure 5. The observed fluorescence patterns were the same in both genotypes examined. Both cell layers showed a dull-blue autofluorescence under UV when the caryopsis tissue sections were immersed in distilled water (Fig. 5, distilled water). However, in the alkaline medium, the nucellar P-C layer that is TUNEL negative fluoresced blue-green at 6 and 16 DAP (Fig. 5, alkaline medium); the blue-green fluorescence was detectable as early as at 2 to 4 DAP (data not shown) and reached a maximum of approximately 10 cell layers at 8 DAP. The autofluorescence of cell walls of the integumental cell layers shifted to bright blue. The observed shifts in the UV-induced autofluorescence in the alkaline medium indicate different phenolic compounds, namely hydroxycinamic acids; in particular, the blue-green coloration typifies sinapic acid, and bright blue indicates ferulic and caffeic acid (Harborne, 1998). Ferulic acid is bound to cell walls in relatively high quantities in plants of Poaceae family, as compared to dicots, and is the major substance responsible for blue-green emission from leaf cells (Lichtenthaler and Schweiger, 1998).
Figure 5.
Histochemical evidence for two subdomains in the P-C layer in the longitudinal sections of Mn1 maize caryopses at 6 and 16 DAP. The autofluorescence of cell walls in the alkaline medium is different in the nucellar and integumental P-C layer; the two colors, blue-green and bright blue, indicate different phenolic acids in each P-C layer. The Naturstoffreagenz A positive layer with yellow-green fluorescence indicates flavonoids, which are confined exclusively to the integumental P-C layer. Autofluorescence of cell walls in sections incubated in distilled water was blue. en, endosperm; i-pc, integumental P-C layer; n, nucellus; n-pc, nucellar P-C layer; ped, pedicel.
The Naturstoffreagenz A reagent is specific for flavonoids (Jork et al., 1989) and reacted uniquely with cells in the integumental P-C layer, the TUNEL-positive region. The flavonoid-positive cells fluoresced yellow-green in both examined genotypes at 6- and 16-DAP stages (Fig. 5, Naturstoffreagenz A). Significantly, the Naturstoffreagenz A fluorescence was also seen as early as 2 to 4 DAP (data not shown), which suggests that these cells utilized their own enzymatic machinery in the synthesis of the phenolic compounds prior to cell death.
DISCUSSION
PCD is referred to as “any process by which protoplasm, with or without the cell wall that encloses it, is eliminated as part of an adaptive event in the life cycle of the plant” (Dangl et al., 2000). Various forms of PCD based on biochemical, cellular, and molecular criteria have been described previously (for a comprehensive review, see Dangl et al., 2000). In animal cells, PCD is synonymous with apoptosis, which is mediated by a class of specific Cys proteases, caspases. No such functional homologs of animal caspases in plants are reported thus far (Woltering et al., 2002); however, TUNEL reactivity is now known to conform the general definition of apoptosis (Dangl et al., 2000). We report here two possible modes of PCDs that were spatially and temporally distinctive in the P-C layers of the developing maize caryopsis. The earliest manifestation of cell death was seen through the loss of nuclei and subcellular membranous organization; the dead cells, however, remained in situ providing a critical physical continuity between the mother plant and developing seed throughout the seed development. A temporal early phase of PCD in the nucellar P-C directly underneath the basal endosperm cells was TUNEL independent, whereas the later phase in a subset of cells underneath the nucellar P-C, the integumental P-C, was associated with the TUNEL stain. The loss of nuclei in both subdomains of the P-C cell layers occurred in a coordinate fashion, which suggests that the phenomenon was noncell autonomous in nature. Indeed, the evidence shows that signal for PCD may originate in the filial tissues (see below).
Cell Fate in the Maternal P-C Layer Is Dependent on Filial Tissues
One of the important observations here is that the symptoms of PCD in the maternal P-C layer appeared at an extremely early stage during the normal seed development. Initiation of PCD, first visualized through loss of nuclei, was detectable as early as 4 DAP (Fig. 1A), a stage that coincides with the reported stage for initiation of cellularization in the endosperm (Kiesselbach, 1949; Schel et al., 1984). By contrast, unpollinated ovaries showed no loss of nuclei as late as 12 DAP, the last stage examined here (Fig. 1C). Remarkably, the P-C layers in both Mn1 and mn1 caryopses were similar to the unfertilized ovaries until 4 DAP (Fig. 1A), indicating a delay in the initiation of PCD after fertilization. A possible basis for the lag in the disappearance of nuclei is not clear. Also unclear at present is how the signaling for PCD might occur between the maternal and filial generations that lack symplastic continuity between these two cell types in developing maize seed (Felker and Shannon, 1980). In fact, the lack of plasmodesmata between the maternal and filial generations is a common feature to several plant species (for review, see Fisher, 2000).
Additional evidence that the fate of maternal cells was controlled by the filial tissues was seen in the altered cellular morphology of P-C cells in the mn1 relative to the Mn1 kernels. These changes were detectable in caryopses from both lineage-related homozygous plants as well as in the selfed segregants of the Mn1 and mn1 seed phenotypes on the same F2 ear derived from Mn1/mn1 heterozygous plants (Fig. 2A). We suggest, among other possibilities, that these alterations in the P-C layers were signaled by several drastic metabolic changes in the mn1 endosperms relative to the wild type, Mn1. The invertase-deficient mn1 endosperm has greatly reduced cell size, cell number (Vilhar et al., 2002), and sink strength, and ultimately much reduced seed weight (Cheng et al., 1996; Cheng and Chourey, 1999). Thus, the P-C cells bearing the mutant endosperm, relative to the normal Mn1 endosperm, may be exposed to far less hydrostatic conductance, lesser mechanical pressure (i.e. lesser developmental compression), and greatly reduced transport activity. We suggest that these factors might affect cellular morphology of the P-C cells.
Possible Functional Role of PCD in the P-C Layers
As indicated previously, the P-C layer is believed to play a critical role in post-phloem transport of water and solutes to developing seeds. In fact, this region of pedicel constitutes the sole port of entry for assimilates for a developing seed in maize. It is thus logical to consider a possible functional role of the PCD in this region in the context of the transport activities. Noteworthy is that the TUNEL-independent, early phase of PCD in nucellar P-C layers was extremely rapid as evidenced by our TEM analyses. It also coincided with a major phase of cell division and expansion in the endosperm, which is dependent on increased turgor pressure and the sequestration of sugars, especially through sugar hydrolysis. In fact, Suc concentration in the P-C region is estimated to reach the 400 to 500 mm range (Shannon et al., 1986), which must cause significant osmotic stress and may lead to the PCD in these cells. Osmotic stress is known to cause PCD in animal cells (Edwards et al., 1998; Franco et al., 2002). In higher plants, however, there is thus far, to the best of our knowledge, only a single clear-cut report on osmotic stress-induced PCD (Wang et al., 1999).
The PCD in the P-C cells described here may actually be causal to the activation of the transport function of these cells. Unlike the nucellar cells that are autolysed, the dead P-C layer cells were present throughout the duration of seed development as a structural bridge for post-phloem transport of water, sugars, and other nutrients from vascular tissue in the pedicel to a developing seed. The P-C cells are thus similar to the transport cells in xylem, the tracheary elements (TEs), which also undergo a rapid PCD prior to becoming functionally mature (for review, see McCann, 1997). Xylogenesis is best analyzed in the TEs of Zinnia elegans; PCD in these cells is also through a TUNEL-independent pathway (Obara et al., 2001), specifically by vacuolar-lysis, which in turn leads to rapid nuclear degradation through an S1-type nuclease, ZEN1 (Ito and Fukuda, 2002). It is likely that the PCD in nucellar P-C layer is executed through a similar pathway. Despite the functional similarities between the P-C cells and the TEs, the former lacked the characteristic structural features of the mature TEs, which include secondary cell walls of annular, spiral reticulate, or pitted wall thickenings. A P-C layer thus appears to be a novel transport system between phloem termini in pedicel and basal endosperm. Overall, the PCD in P-C layers is an adaptive strategy to allow a clear passage of water through the dead cells while solutes may follow an apoplastic route.
A regulatory control of the transport function through these cells lacking plasmodesmata (Fig. 4, D–F) is most likely mediated by cellular and metabolic features of the filial and maternal cell layers that flank these P-C cells. One of the major factors, we believe, is the Mn1-encoded cell wall invertase, which is localized entirely in the basal endosperm cells, and the loss of this enzyme is the causal basis of the mn1 seed phenotype (Cheng et al., 1996). The temporal increases in cell wall invertase activity coincide with both the early stages of endosperm development (Cheng et al., 1996) and the first phase of PCD in the nucellar P-C layer described here. A key role of cell wall invertase is in the metabolic release of hexose sugars (i.e. steepening the gradient) for both downstream metabolic use and in osmoregulation through turgor-based signals that may integrate nutrient release and additional phloem import to the developing seed. This is consistent with Patrick and Offler (2001), who have suggested that sink demand for photoassimilates in short-distance transport may be communicated as a turgor signal. It is significant that the membrane-localized aquaporin that regulates transcellular flow of water is localized in both basal endosperm and pedicel region below the P-C layer, but not the P-C layer itself (Barrieu et al., 1998). In addition, the basal endosperm region is marked by Suc transporter (Carlson et al., 2002) and Suc synthase (Chen and Chourey, 1988), which presumably catalyze the Suc turnover reactions that are critical for the normal development of an endosperm (Cheng and Chourey, 1999).
The second phase of PCD (the TUNEL-positive mode) in the integumental P-C layer lasting until 24 DAP (Fig. 2B) is most likely related to the seed maturation process. Loss of nuclei and subcellular membranous organizations in the P-C cells has been reported previously in association with seed maturity in 22-DAP kernels (Felker and Shannon, 1980). Further, Serna et al. (2001) have reported on the intercellular secretion of the antifungal BAP2 protein from basal endosperm cells to the P-C layers of developing maize seeds during the later stages (12–15 DAP) of seed development, which is postulated to protect the developing seed from fungal infections. The deposition of flavonoids in the P-C layer is consistent with their proposed role in plant defense (Dixon and Steele, 1999), in particular as an antimicrobial protection of the maturing seed.
MATERIALS AND METHODS
Preparation of Plant Material for Light Microscopy
Maize (Zea mays) plants of the W22 inbred line were grown in the greenhouse. Selfed or sibbed developing kernels were harvested at 0 to 28 DAP and immediately fixed in cold formaldehyde-acetic acid fixative (3.7% formaldehyde, 5% acetic acid, and 50% ethanol) for 24 h, followed by dehydration in series of ethanol and tertiary butyl alcohol and embedding in Paraplast Plus (Fisher Scientific, Loughborough, UK). Paraffin-embedded kernels were sectioned to 8- to 12-μm thickness with a rotary microtome (Microm 325, Carl Zeiss, Jena, Germany).
DAPI Staining
Paraplast-embedded sections were dewaxed in xylene and rehydrated in an ethanol series, equilibrated in freshly prepared McIlvaine's buffer, pH 7.0 (0.02 m citric acid and 0.16 m Na2HPO4), followed by staining in 600 nm DAPI (Molecular Probes, Eugene, OR) in McIlvaine's buffer for 15 min at room temperature in dark, washed with distilled water, covered with coverslips, and observed with UV excitation. Cold-blue fluorescent nuclei were photographed with a color CCD digital camera using SPOT Insight, 3.5.1 software (Diagnostic Instruments, Sterling Heights, MI). Images of DAPI fluorescence were presented as black-and-white negatives to enhance the visibility of cellular components.
TUNEL Staining
TUNEL was performed using In Situ Cell Death Detection kit (Roche Diagnostics GmbH, Mannheim, Germany), essentially following the manufacturer's protocol. Briefly, sections were dewaxed in xylene and rehydrated in ethanol series, treated with 20 μg/mL Proteinase K (Gibco, Carlsbad, CA) in 10 mm Tris 7.5 and 5 mm EDTA for 15 min at room temperature, followed by incubation in mixture of fluorescein-labeled deoxynucleotides and TdT (TUNEL mix) for 60 min at 37°C. After washing the slides with phosphate buffered saline, the coverslips were mounted in GelMount (Biomedia via Fisher Scientific) with 600 nm DAPI. The fluorescein fluorescence of nuclei with fragmented DNA was observed with a microscope with blue-light excitation, and DAPI fluorescence of all nuclei was observed with UV excitation. Images were taken with a color CCD digital camera using SPOT Insight 3.5.1 software (Diagnostic Instruments).
TEM
All materials for electron microscopy processing were purchased from Electron Microscopy Sciences (Fort Washington, PA). Tissues were prepared for TEM by fixation in 4% glutaraldehyde (v/v) and 1% paraformaldehyde (v/v) in 0.1 m phosphate buffer (pH 7.2). Fresh kernels were harvested and immediately processed for fixation on site by placing a small aliquot of chilled fixative on a glass plate and hand sectioning a sagittal section of the kernel approximately 2 mm wide with a double-edged razor blade. The tissue was then put into a vial of ice-cold fixative and placed under vacuum for 5 to 10 min to aid fixative infiltration. After vacuuming, the samples were placed on a rotating plate overnight, approximately 12 h, at 4°C.
After fixation, the tissue was rinsed three times for 30 min each in chilled phosphate buffered saline then placed in 2% aqueous osmium tetroxide overnight at 4°C. Once the osmication was completed the samples were rinsed three times, 1 h each, in distilled water then dehydrated in a chilled acetone series at 20% increments for 1 h each. After 100% acetone the samples were sent through propylene oxide as a transition solvent, infiltrated and embedded with Spurr's resin, then polymerized at 56°C for 24 h.
Samples were sectioned on a Sorvall IIB Ultracut ultramicrotome (Ivan Sorvall, Norwalk, CT). The sections were cut to approximately 60 nm in thickness, indicated by the sections appearing pale gold in color, and picked up on formvar-coated copper grids (50 or 100 mesh). The sections were post stained 20 min in filtered 2% aqueous uranyl acetate, rinsed three times, 1 min each, in distilled water, then were allowed to dry. The sections were then stained 6 min in Reynold's lead citrate (Electron Microscopy Sciences), rinsed once in 0.02 m NaOH for 1 min, then three times more, 1 min each, in distilled water. Approximately four samples per developmental stage with an average of 20 P-C cells per section were used for counting plasmodesmata. The sections were examined and photographed in a Zeiss 109 or a Zeiss EM 10 transmission electron microscope. Negatives were scanned at 300 dpi using Epson Perfection 1650 scanner (Seiko Epson, Nagano-Ken, Japan) and digitally processed using Adobe Photoshop version 7.0 (Adobe Systems, Mountain View, CA).
Cell Walls Autofluorescence Shift in Alkaline Medium
Paraffin sections were dewaxed in xylene and rehydrated in ethanol series to water. Sections were then either treated with ammonia vapors by holding them briefly over the ammonia solution (Sigma-Aldrich, St. Louis) or covered with 0.15 m K2HPO4, pH 8.2. Control sections were incubated in distilled water only. Autofluorescence of cell walls was observed with UV excitation and photographed with AxioCam MRc color digital camera (Carl Zeiss).
Staining of Flavonoids with Naturstoffreagenz A
For Naturstoffreagenz A staining, dewaxed and rehydrated sections were stained with 1% methanol solution of Naturstoffreagenz A (diphenylboric acid 2-aminoethylester; Sigma-Aldrich). After a minute of incubation, a drop of distilled water was added to the sections to prevent them from drying out due to methanol evaporation. Preparations were immediately observed with UV excitation and photographed with AxioCam MRc color digital camera (Carl Zeiss).
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
Mention of trade names or commercial products in this publication is solely for the purpose of providing special information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Acknowledgments
We thank Drs. D.R. Pring and E.W. Taliercio for critical reading of the manuscript. Technical assistance from Ms. Shayna Southerland is gratefully acknowledged.
This work was supported by the Ministry of Education, Science and Sport, Republic of Slovenia (grant no. S1–487–001/20070/99) and by the USA-Slovenia Cooperation in Science and Technology (grant no. 3311–01–838050). This was a cooperative investigation of the U.S. Department of Agriculture, Agricultural Research Service, and the Institute of Food and Agricultural Science, University of Florida. This paper is Florida Agricultural Experiment Station Journal Series Number R–10415.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.045195.
References
- Barrieu F, Chaumont F, Chrispeels MJ (1998) High expression of the tonoplast aquaporin ZmTIP1 in epidermal and conducting tissues of maize. Plant Physiol 117: 1153–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursch W, Paffe S, Putz P, Barthel G, Schultehermann R (1990) Determination of the length of the histological stages of apoptosis in normal liver and in altered hepatic foci of rats. Carcinogenesis 11: 847–853 [DOI] [PubMed] [Google Scholar]
- Carlson SJ, Chourey PS, Helentjaris T, Datta R (2002) Gene expression studies on developing kernels of maize sucrose synthase (SuSy) mutants show evidence for a third SuSy gene. Plant Mol Biol 49: 15–29 [DOI] [PubMed] [Google Scholar]
- Chen Y-C, Chourey PS (1988) Spatial and temporal expression of the two sucrose synthase genes in maize: immunohistological evidence. Theor Appl Genet 78: 553–559 [DOI] [PubMed] [Google Scholar]
- Cheng W-H, Chourey PS (1999) Genetic evidence that invertase-mediated release of hexose is critical for appropriate carbon partitioning and normal seed development in maize. Theor Appl Genet 98: 485–495 [Google Scholar]
- Cheng W-H, Taliercio ET, Chourey PS (1996) The miniature1 seed locus of maize encodes a cell wall invertase required for normal development of endosperm and maternal cells in the pedicel. Plant Cell 8: 971–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl JL, Dietrich RA, Thomas H (2000) Senescence and programmed cell death. In B Buchanan, W Gruissem, R Jones, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Biologists, Rockville, MD, pp 1044–1100
- Dixon RA, Steele CL (1999) Flavonoids and isoflavonoids: a gold mine for metabolic engineering. Trends Plant Sci 4: 394–400 [DOI] [PubMed] [Google Scholar]
- Edwards YS, Sutherland LM, Power JHT, Nicholas TE, Murray AW (1998) Osmotic stress induces both secretion and apoptosis in rat alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 275: L670–L678 [DOI] [PubMed] [Google Scholar]
- Esau K (1977) Anatomy of Seed Plants, Ed 2. Malloy Lithographing, New York
- Felker FC, Shannon JC (1980) Movement of 14C-labeled assimilates into kernels of Zea mays L. Plant Physiol 65: 864–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DB (2000) Long-distance transport. In B Buchanan, W Gruissem, R Jones, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Biologists, Rockville, MD, pp 730–784
- Franco DL, Nojek IM, Molinero L, Coso OA, Costas MA (2002) Osmotic stress sensitizes naturally resistant cells to TNF-a-induced apoptosis. Cell Death Differ 9: 1090–1098 [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119: 493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani C, Consonni G, Gavazzi G, Colombo M, Dolfini S (2002) Programmed cell death during embryogenesis in maize. Ann Bot (Lond) 90: 287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne JB (1998) Phytochemical Methods, Ed 3. Chapmann and Hall, London, pp 40–106
- Ito J, Fukuda H (2002) ZEN1 is a key enzyme in the degradation of nuclear DNA during programmed cell death of tracheary elements. Plant Cell 14: 3201–3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jork H, Funk W, Fischer W, Wimmer H (1989) Dünnschicht-Chromatographie, Reagenzien und Nachweismethoden (Band 1a). VCH Verlagsgesellschaft mbH, Weinheim, Germany, pp 277–280
- Kiesselbach TA (1949) The Structure and Reproduction of Corn. Research Bulletin 161. University of Nebraska, College of Agriculture, Agricultural Experiment Station
- Krishnamurthy KV, Krishnaraj R, Chozhavendan R, Christopher FS (2000) The program of cell death in plants and animals: a comparison. Curr Sci 79: 1169–1181 [Google Scholar]
- Lichtenthaler HK, Schweiger J (1998) Cell wall bound ferulic acid, the major substance of the blue-green fluorescence emission of plants. J Plant Physiol 152: 272–282 [Google Scholar]
- Linnestad C, Doan DNP, Brown RC, Lemmon BE, Meyer DJ, Jung R, Olsen O-A (1998) Nucellain, a barley homolog of the dicot vacuolar-processing protease, is localized in nucellar cell walls. Plant Physiol 118: 1169–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J, Nelson OE (1946) Miniature seed: a study in the development of a defective caryopsis in maize. Genetics 31: 525–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness NO, McBee GG (1986) Role of placental sac in endosperm carbohydrate import in sorghum caryopses. Crop Sci 26: 1201–1207 [Google Scholar]
- McCann M (1997) Trachery element formation: building up to a dead end. Trends Plant Sci 2: 333–338 [Google Scholar]
- Milioni D, Sado P-E, Stacey NJ, Roberts K, McCann MC (2002) Early gene expression associated with the commitment and differentiation of a plant tracheary element is revealed by cDNA-amplified fragment polymorphism analysis. Plant Cell 14: 2813–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ME, Chourey PS (1992) The maize invertase-deficient miniature-1 seed mutation is associated with aberrant pedicel and endosperm development. Plant Cell 4: 297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara K, Kuriyama H, Fukuda H (2001) Direct evidence of active and rapid nuclear degradation triggered by vacuole rupture during programmed cell death in Zinnia. Plant Physiol 125: 615–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen O-A, Linnestad C, Scott EN (1999) Developmental biology of the cereal endosperm. Trends Plant Sci 4: 253–257 [DOI] [PubMed] [Google Scholar]
- Patrick JW, Offler CE (2001) Compartmentation of transport and transfer events in developing seeds. J Exp Bot 52: 551–564 [PubMed] [Google Scholar]
- Rosche E, Blackmore D, Tegeder M, Richardson T, Schroeder H, Higgins TJV, Frommer WB, Offler CE, Patrick JW (2002) Seed-specific over expression of potato sucrose transporter increases sucrose uptake and growth rates of developing pea cotyledons. Plant J 30: 165–175 [DOI] [PubMed] [Google Scholar]
- Schel JHN, Kieft H, Van Lammeren AAM (1984) Interaction between embryo and endosperm during early developmental stages maize caryopses. Can J Bot 62: 2842–2853 [Google Scholar]
- Serna A, Maitz M, O'Connell T, Santandrea G, Thevissen K, Tienens K, Hueros G, Faleri C, Cai G, Lottspeich F, et al (2001) Maize endosperm secretes a novel antifungal protein into adjacent maternal tissue. Plant J 25: 687–699 [DOI] [PubMed] [Google Scholar]
- Shannon JC, Porter GA, Knievel DP (1986) Phloem unloading and transfer of sugars into developing corn endosperm. In J Cronshaw, WJ Lucas, RT Giaquinta, eds, Phloem Transport. Alan Liss, New York, pp 265–277
- Thomas H, Ougham HJ, Stead AD (2003) Defining senescence and death. J Exp Bot 54: 1127–1132 [DOI] [PubMed] [Google Scholar]
- Thorne JH (1985) Phloem unloading of C and N assimilates in developing seeds. Annu Rev Plant Physiol 36: 317–343 [Google Scholar]
- Vilhar B, Kladnik A, Blejec A, Chourey PS, Dermastia M (2002) Cytometrical evidence that the loss of seed weight in the miniature1 seed mutant of maize is associated with reduced mitotic activity in the developing endosperm. Plant Physiol 129: 23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Xia Q, Qiu X, Selvaraj G (2002) Early stages of seed development in Brassica napus: a seed coat-specific cysteine proteinase associated with programmed cell death of the inner integument. Plant J 30: 1–10 [DOI] [PubMed] [Google Scholar]
- Wang M, Hoekstra S, van Bergen S, Lamers GEM, Oppedijk BJ, van der Heijden MW, de Priester W, Schilperoort RA (1999) Apoptosis in developing anthers and the role of ABA in this process during androgenesis in Hordeum vulgare L. Plant Mol Biol 39: 489–501 [DOI] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Wobus W (1997) Sugar import and metabolism during seed development. Trends Plant Sci 2: 169–174 [Google Scholar]
- Woltering EJ, van der Bent A, Hoeberichts FA (2002) Do plant caspases exist? Plant Physiol 130: 1764–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young TE, Gallie DR (1999) Analysis of programmed cell death in wheat endosperm reveals differences in endosperm development between cereals. Plant Mol Biol 39: 915–926 [DOI] [PubMed] [Google Scholar]
- Young TE, Gallie DR, DeMason DA (1997) Ethylene-mediated programmed cell death during maize endosperm development of wild type and shrunken2 genotypes. Plant Physiol 115: 737–751 [DOI] [PMC free article] [PubMed] [Google Scholar]