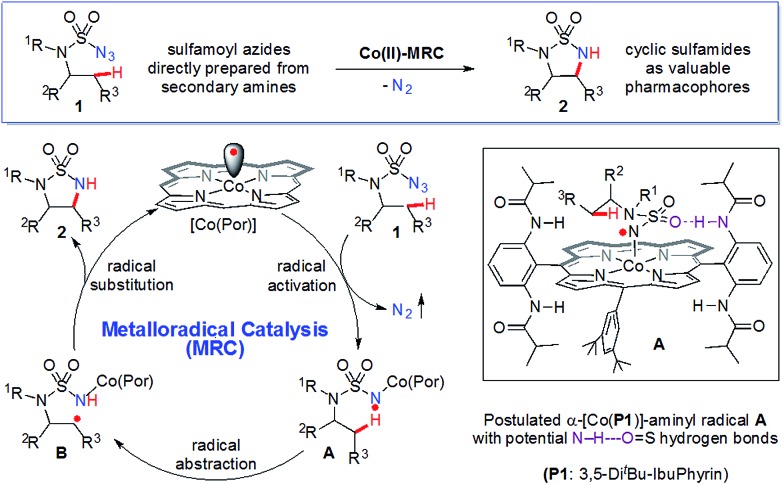
 Synthesis of strained five-membered cyclic sulfamides has been achieved for the first time by intramolecular 1,5-C(sp3)–H amination via Co(ii)-based metalloradical catalysis.
Synthesis of strained five-membered cyclic sulfamides has been achieved for the first time by intramolecular 1,5-C(sp3)–H amination via Co(ii)-based metalloradical catalysis.
Abstract
Co(ii)-based metalloradical catalysis (MRC) proves effective for intramolecular 1,5-C–H amination of sulfamoyl azides under neutral and nonoxidative conditions, providing a straightforward approach to access strained 5-membered cyclic sulfamides with nitrogen gas as the only byproduct. The metalloradical amination system is applicable to different types of C(sp3)–H bonds and has a high degree of functional group tolerance. Additional features of the Co(ii)-catalyzed 1,5-C–H amination include excellent chemoselectivity toward allylic and propargylic C–H bonds. The unique reactivity and selectivity profile of the Co(ii)-catalyzed 1,5-C–H amination is attributed to the underlying radical mechanism of MRC.
Introduction
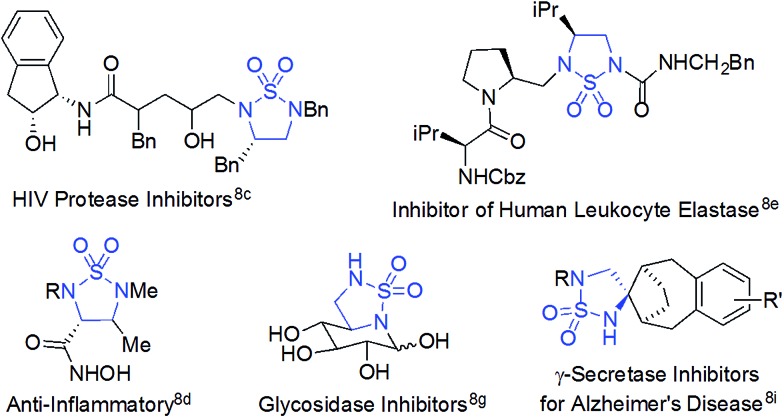
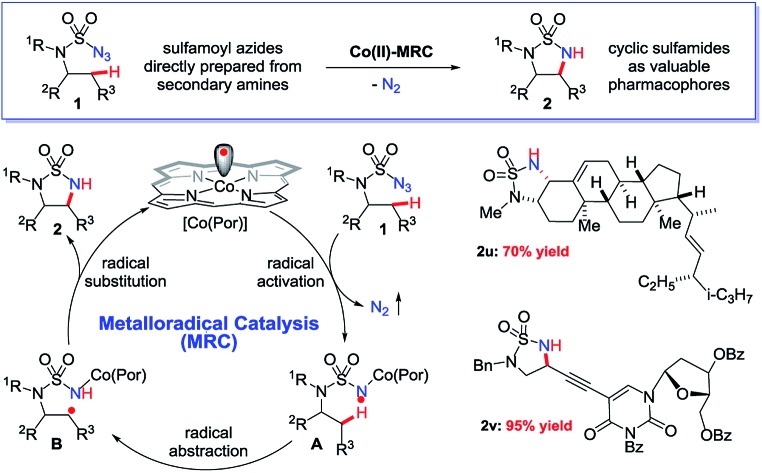
Radical chemistry has recently demonstrated increasing applications in organic synthesis, which has been traditionally dominated by the development of synthetic methods based on ionic chemistry.1 Among other approaches,2 metalloradical catalysis (MRC), which involves the development of metalloradical complexes as potential open-shell catalysts for initiating as well as controlling homolytic radical reactions, provides a fundamentally new strategy to address some long-standing challenges associated with radical chemistry.3,4 As stable metalloradicals, cobalt(ii) complexes of porphyrins [Co(Por)] have recently emerged as a unique class of catalysts for C–H amination through a 1e radical mechanism.5,6 Supported by porphyrin ligands bearing amide functionalities, the Co(ii)-based MRC has been shown to be particularly effective in activating various sulfamoyl azides for intramolecular radical amination of different types of C–H bonds, leading to the formation of 6-membered cyclic sulfamides with a high control of reactivities and selectivities.7 The success of the metalloradical system is attributed to the stabilization of the key α-Co(iii)-aminyl radical intermediates (also known as Co(iii)-nitrene radicals) through hydrogen-bonding interaction with the amide group of the porphyrin ligand as well as the high propensity of the α-metalloaminyl radicals toward 1,6-H-atom abstraction, followed by facile 6-exo-tet radical cyclization.5,7 To date, the feasibility of the α-metalloaminyl radicals for 1,5-H-atom abstraction to form the corresponding ε-Co(iii)-alkyl radicals (Scheme 1: A to B) and subsequent 5-exo-tet radical cyclization to generate the strained 5-membered cyclic sulfamides (Scheme 1: B to 2) remains unexplored. If successful, the catalytic C–H amination process (Scheme 1) would be highly attractive as the resulting 5-membered cyclic sulfamides have found important applications in the development of pharmaceutical agents (Fig. 1).8
Scheme 1. Metalloradical approach for 5-membered cyclic sulfamides via radical C(sp3)–H amination.
Fig. 1. Selected examples of biologically active molecules containing the 5-membered cyclic sulfamide motif.
A number of different metal-based catalytic systems have been developed for regioselective intramolecular C(sp3)–H amination to prepare N-heterocycles of variable ring size and functionality.9,10 Although considerable advances have been made, there has been no previous reports of the efficient synthesis of 5-membered cyclic sulfamides via metal-catalyzed intramolecular C–H amination.11–13 It is evident that catalytic 1,5-C(sp3)–H amination for the formation of 5-membered cyclic sulfamides is a challenging process, which is presumably attributable to the potentially strained [3.1.0]-bicyclic transition state associated with the asynchronous concerted mechanism that is shared by most catalytic C–H amination systems via metallonitrene intermediates.9j,14 Considering its stepwise radical mechanism through less-strained 6-membered monocyclic transition states (Scheme 1: A) followed by low-barrier radical substitution,5a we anticipated the possibility of applying MRC to address the challenges of this transformation (Scheme 1). Herein, we report that metalloradical catalysts [Co(Por)] are highly effective in activating sulfamoyl azides for intramolecular 1,5-C–H radical amination under neutral and nonoxidative conditions, affording the strained 5-membered cyclic sulfamides in high yields, with nitrogen gas as the only byproduct. In addition to its simple and practical protocol, the Co(ii)-based metalloradical system exhibits excellent chemoselectivity and high functional group tolerance.
Results and discussion
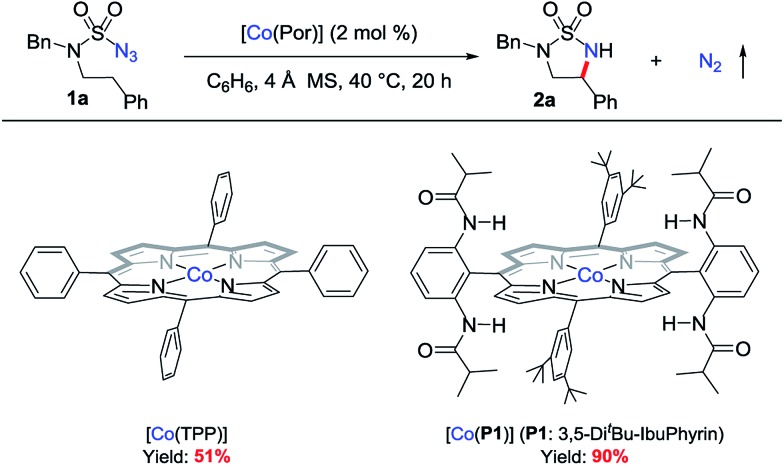
At the outset of this project, the sulfamoyl azide 1a,15 which contains benzylic C–H bonds for potential 1,5-H-atom abstraction, was selected as the model substrate to test the possibility of intramolecular 1,5-C(sp3)–H amination via MRC (Scheme 2). Initial experiments showed that the metalloradical complex [Co(TPP)] (TPP: tetraphenylporphyrin), which is commercially available, could activate azide 1a for the intramolecular radical amination of the benzylic C(sp3)–H bond, affording the strained 5-membered cyclic sulfamide 2a in 51% yield. Further optimization experiments indicated that [Co(P1)], which is supported by the D2h-symmetric amidoporphyrin 3,5-DitBu-IbuPhyrin (P1), was a superior metalloradical catalyst for the radical C–H amination reaction, leading to the formation of the desired 2a in 90% yield (Scheme 2). The enhanced catalytic activity of [Co(P1)] over [Co(TPP)] is ascribed to the stabilization of the key α-Co(iii)-aminyl radical intermediate by the amide functionalities through hydrogen-bonding interaction (Scheme 1, A).5a,b,6c,d,7
Scheme 2. Ligand effect on Co(ii)-catalyzed 1,5-C(sp3)–H metalloradical amination.
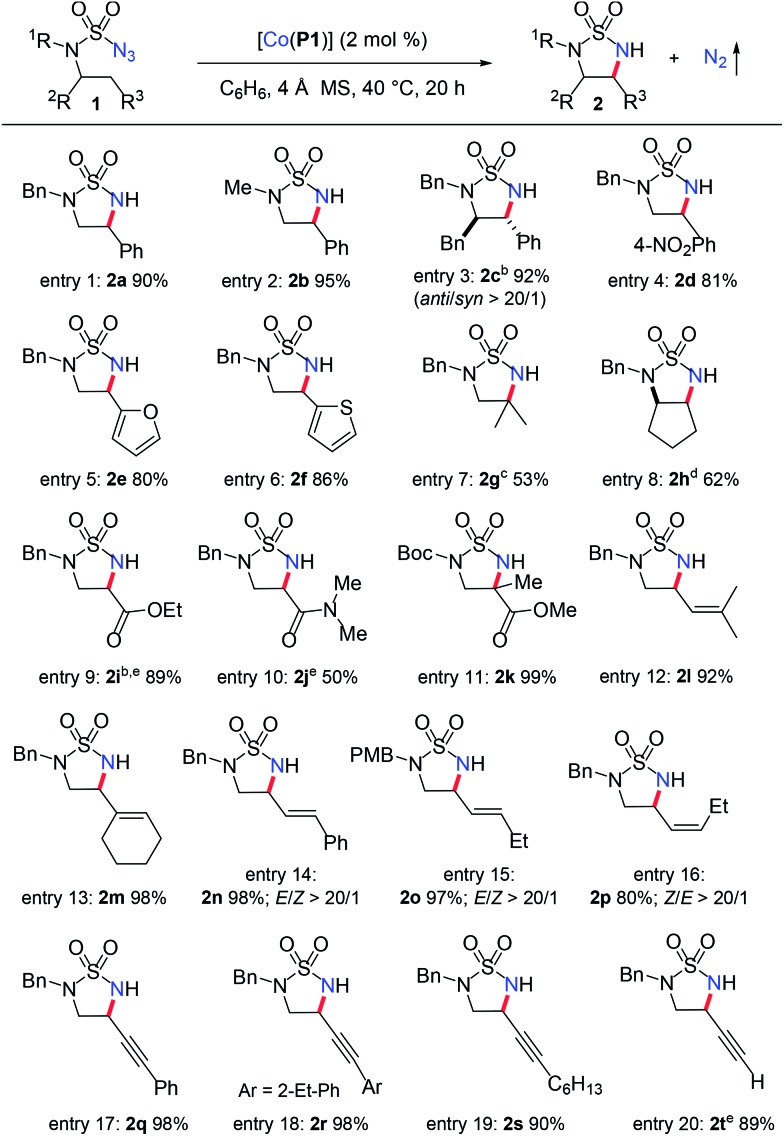
Under the optimized conditions, the [Co(P1)]-based metalloradical system was shown to be effective for intramolecular 1,5-C(sp3)–H radical amination of a wide range of sulfamoyl azide substrates (Table 1). In addition to effective amination reactions of benzylic C–H bonds with varied electronic properties (entries 1–4), the Co(ii)-based catalytic system could efficiently aminate α-C(sp3)–H bonds of heteroaromatic rings such as furan (entry 5) and thiophene (entry 6), without complication from potential reactions with the heteroatoms. As exemplified by the high-yielding formation of trans-cyclic sulfamide 2c, excellent diastereoselectivity could be achieved (entry 3). The metalloradical amination by [Co(P1)] could also be applied for non-benzylic C–H substrates, as demonstrated with the successful formation of the cyclic sulfamide 2g and bicyclic sulfamide 2h in respectable yields (entries 7 and 8), along with the corresponding 6-membered structure product formation.12g Moreover, even the challenging electron-deficient C(sp3)–H substrates, such as α-C–H bonds of esters and amides, could be aminated smoothly, producing α,β-diamino acid derivatives (entries 9 and 10).7c Besides secondary C–H bonds, this system proceeded successfully with more sterically hindered tertiary C(sp3)–H bonds as well (entries 7 and 11). It is notable that the α,β-diamino acid derivative 2k bearing a quaternary α-carbon center could be synthesized in near quantitative yield (entry 11). Different N-substituents in the azide substrates were effectively tolerated in the C–H amination process. For example, sulfamoyl azides containing both electron-donating and electron-withdrawing N-substituents, such as N-benzyl (entry 1), N-methyl (entry 2), N-Boc groups (entry 11), and N-4-methoxybenzyl (entry 15), groups proved to be suitable substrates.
Table 1. Intramolecular 1,5-C(sp3)–H radical amination of sulfamoyl azides by metalloradical catalyst [Co(P1)] a .
|
aPerformed in C6H6 at 40 °C for 20 h using 2 mol% [Co(P1)] under N2 in the presence of 4 Å MS; [azide 1a] = 0.10 M; isolated yields.
bConfirmed by X-ray crystallographic structure analysis.
cYield based on 1H NMR analysis of purified mixture of 1,5- and 1,6-products, 37% 6-membered ring product was also obtained.
d18% of the 6-membered ring product was obtained.
e5 mol% [Co(P1)].
The [Co(P1)]-catalyzed 1,5-C–H radical amination system exhibited excellent chemoselectivity towards allylic C–H bonds without affecting the C C π bonds.12e,f,16 For instance, the allylic C–H bonds of electron-rich trisubstituted alkenes were effectively aminated in high yields to afford the corresponding 5-membered cyclic sulfamides 2l and 2m without observation of the corresponding aziridination products (entries 12 and 13). Furthermore, this amination process was shown to be stereospecific regarding the stereochemistry of the alkene units as exemplified by the catalytic reactions of both trans- and cis-alkene-derived sulfamoyl azide substrates (entries 14–16). Under the standard conditions, the expected allylic C–H amination products 2n, 2o and 2p were formed in high yields with excellent stereospecificity as well as chemoselectivity. The fact that no olefin isomerization was observed during these catalytic amination reactions suggests the 5-exo-tet radical cyclization of the corresponding ε-Co(iii)-allylic radical (Scheme 1: B to 2) proceeds with a low barrier and even faster than the facile trans- and cis-C C π bond isomerization.17 The Co(ii)-based MRC is among the few catalytic systems that are effective for amination of propargylic C–H bonds without affecting the electron-rich C C π bonds.7d,18,19 Functionalization of propargylic C–H bonds of both aryl-conjugated and alkyl-substituted alkynes led to the formation of the desired 5-membered cyclic sulfamides in high yields (entries 17–19). Moreover, the propargylic C–H bond of unprotected terminal alkynes could be also aminated selectively without interference from the acidic terminal C(sp)–H bond, as demonstrated by the formation of the cyclic sulfamide 2t in 89% yield.
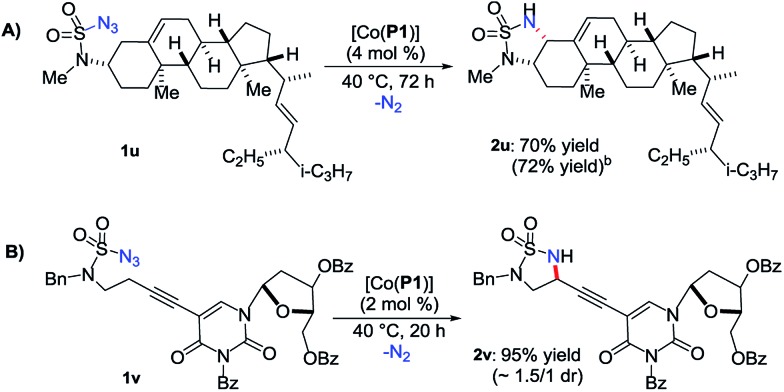
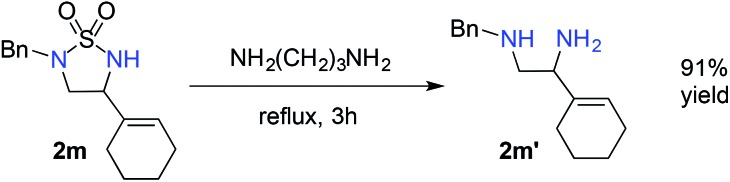
The demonstrated chemoselectivity and functional group tolerance of [Co(P1)]-catalyzed 1,5-C–H amination made it possible for late-stage functionalization of complex molecules in a predicable fashion. For example, when stigmasterol-based azide 1u, which was directly prepared from the corresponding amine by a one-step procedure (ESI†),15 was used as a substrate, 1,5-amination of the allylic C–H bond among various C–H and C C bonds was chemoselectively achieved, providing the fused multicyclic sulfamide 2u in 70% yield (Scheme 3A) with the cis-stereoisomer only.20 We also showed that the reaction could be effectively scaled up to 0.5 mmol in a similar 72% yield. As a further demonstration of the functional group tolerance of the current catalytic system, when the deoxyuridine-based substrate 1v, which was prepared directly from the corresponding deoxyuridine-based amine (ESI†),15 was treated with [Co(P1)], the propargylic C–H bond was selectively aminated to afford the deoxyuridine-derived 5-membered cyclic sulfamide 2v in 95% yield (Scheme 3B). Direct modification of highly functionalized amine compounds with known bioactivities may offer an attractive opportunity to obtain unexplored 5-membered cyclic sulfamides like 2u and 2v for the study of interesting biological activities.8 In addition, the resulting cyclic sulfamides 2 bearing various functionalities may also serve as efficient precursors for the preparation of the valuable corresponding 1,2-diamines.21 For example, cyclic sulfamide 2m was effectively converted to the corresponding unprotected 1,2-diamine derivative 2m′ in 91% yield (eqn (1)).
 |
1 |
Scheme 3. Late-stage functionalization of complex molecules by Co(ii)- based 1,5-C–H radical aminationa. (a) Isolated yields. (b) On 0.5 mmol scale.
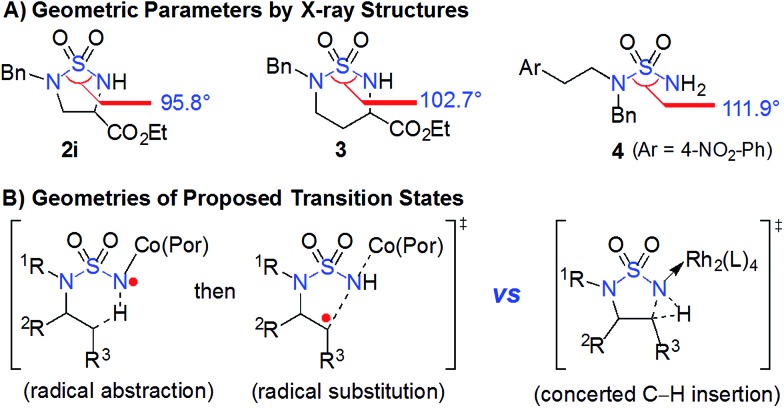
The catalytic capability of Co(ii)-based metalloradical catalysis (MRC) to facilitate intramolecular 1,5-C(sp3)–H amination to form 5-membered cyclic sulfamides is uniquely remarkable. The challenge of this type of transformation can be appreciated by examining the geometric parameters of 5-membered cyclic sulfamides in comparison with those of acyclic and 6-membered cyclic sulfamides. To this end, we synthesized and structurally characterized sulfamides 2i, 3 and 4 by X-ray crystallography (ESI†). As illustrated in Fig. 2A, while the N–S–N bond angle of the 6-membered cyclic sulfamide 3 is 9.2° smaller than that of acyclic sulfamide 4, the deviation in the N–S–N bond angle for 5-membered cyclic sulfamide 2i from 4 is considerably larger (16.1°),22 signifying the great angle strain inherent in the 5-membered cyclic sulfamide structure. It would be expected to have even greater ring strain for the corresponding [3.1.0]-bicyclic transition state (Fig. 2B; right) associated with intramolecular 1,5-C(sp3)–H amination via the asynchronous concerted mechanism through metallonitrene intermediates.12,14 This presumably accounts for the previous absence of effective catalytic systems for 1,5-C(sp3)–H amination towards formation of 5-membered cyclic sulfamides.11,12 Through a fundamentally different pathway involving the two-step radical cascade (Scheme 1: A to B and then B to 2), Co(ii)-based metalloradical catalysis (Fig. 2B; left) effectively obviates a highly strained [3.1.0]-bicyclic transition state, allowing for efficient construction of 5-membered cyclic sulfamides.6–8
Fig. 2. (A) Comparison of geometric parameters between acyclic and cyclic sulfamides based on X-ray structures. (B) Geometries of proposed transitional states for stepwise (left) and concerted (right) processes of intramolecular 1,5-C(sp3)–H amination.
Together with the previous reports on intramolecular 1,6-C(sp3)–H amination to form 6-membered cyclic sulfamides,7 the current work reveals the versatile pathways of Co(ii)-based MRC for selective amination.23 Although the key α-metalloaminyl radical intermediates are capable of undergoing both 1,5- and 1,6-H-atom abstraction followed by facile 5- and 6-exo-tet radical cyclization, respectively, the differentiation of the two pathways toward a selective catalytic process can be effectively achieved by Co(ii)-MRC for most substrates in a predictable fashion.7
Conclusions
In summary, by applying the concept of metalloradical catalysis (MRC), a new approach has been successfully demonstrated for addressing the challenges of intramolecular 1,5-C(sp3)–H amination to construct strained 5-membered cyclic sulfamides. The metalloradical complex [Co(P1)] is an effective catalyst with the capability of activating a broad scope of sulfamoyl azides for intramolecular 1,5-amination of different types of C(sp3)–H bonds with high stereospecificity, providing straightforward access to the potentially bioactive 5-membered cyclic sulfamide compounds in high yields. The Co(ii)-based catalytic system can be simply operated under neutral and non-oxidative conditions without the need for any additives, generating nitrogen gas as the only byproduct. Furthermore, this 1,5-C(sp3)–H amination process features excellent chemoselectivity and functional group tolerance, allowing for late-stage functionalization of complex molecules. The success in addressing this challenging amination process by Co(ii)-MRC is believed to be directly related to the underlying radical mechanism involving the key α-Co(iii)-aminyl radical intermediate.
Supplementary Material
Acknowledgments
We are grateful for financial support by NIH (R01-GM098777) and in part by NSF (CHE-1624216).
Footnotes
†Electronic supplementary information (ESI) available. CCDC 1480993–1480996. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6sc02231f
References
- For recent reviews, see: ; (a) Quiclet-Sire B., Zard S. Z. Pure Appl. Chem. 2011;83:519–551. [Google Scholar]; (b) Narayanam J. M. R., Stephenson C. R. J. Chem. Soc. Rev. 2011;40:102–113. doi: 10.1039/b913880n. [DOI] [PubMed] [Google Scholar]; (c) Prier C. K., Rankic D. A., MacMillan D. W. C. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected recent examples, see: ; (a) Du J., Skubi K. L., Schultz D. M., Yoon T. P. Science. 2014;344:392–396. doi: 10.1126/science.1251511. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hoyt J. M., Schmidt V. A., Tondreau A. M., Chirik P. J. Science. 2015;349:960–963. doi: 10.1126/science.aac7440. [DOI] [PubMed] [Google Scholar]; (c) Jeffrey J. L., Terrett J. A., MacMillan D. W. C. Science. 2015;349:1532–1536. doi: 10.1126/science.aac8555. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kainz Q. M., Matier C. D., Bartoszewicz A., Zultanski S. L., Peters J. C., Fu G. C. Science. 2016;351:681–684. doi: 10.1126/science.aad8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reviews on applications of Co(ii)-based MRC, see: ; (a) Fantauzzi S., Caselli A., Gallo E. Dalton Trans. 2009:5434–5443. doi: 10.1039/b902929j. [DOI] [PubMed] [Google Scholar]; (b) Driver T. G. Org. Biomol. Chem. 2010;8:3831–3846. doi: 10.1039/c005219c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lu H., Zhang X. P. Chem. Soc. Rev. 2011;40:1899–1909. doi: 10.1039/c0cs00070a. [DOI] [PubMed] [Google Scholar]; (d) Che C. M., Lo V. K. Y., Zhou C. Y., Huang J. S. Chem. Soc. Rev. 2011;40:1950–1975. doi: 10.1039/c0cs00142b. [DOI] [PubMed] [Google Scholar]; (e) Pellissier H., Clavier H. Chem. Rev. 2014;114:2775–2823. doi: 10.1021/cr4004055. [DOI] [PubMed] [Google Scholar]
- For a recent review on Ti(iii)-based MRC, see: . For the first demonstration of Ti(iii)-based metalloradical non-catalytic process, see: . For select examples of catalytic processes via Ti(III)-based MRC, see: ; (a) Gansäuer A., Hildebrandt S., Vogelsang E., Flowers II R. A. Dalton Trans. 2016;45:448–452. doi: 10.1039/c5dt03891j. [DOI] [PubMed] [Google Scholar]; (b) Nugent W. A., RajanBabu T. V. J. Am. Chem. Soc. 1988;110:8561–8562. [Google Scholar]; (c) Gansäuer A., Rinker B., Pierobon M., Grimme S., Gerenkamp M., Mück-Lichtenfeld C. Angew. Chem., Int. Ed. 2003;42:3687–3690. doi: 10.1002/anie.200351240. [DOI] [PubMed] [Google Scholar]; (d) Gansäuer A., Fan C.-A., Keller F., Keil J. J. Am. Chem. Soc. 2007;129:3484–3485. doi: 10.1021/ja0686211. [DOI] [PubMed] [Google Scholar]; (e) Gansäuer A., Fleckhaus A., Lafont M. A., Okkel A., Kotsis K., Anoop A., Neese F. J. Am. Chem. Soc. 2009;131:16989–16999. doi: 10.1021/ja907817y. [DOI] [PubMed] [Google Scholar]; (f) Gansäuer A., Hildebrandt S., Michelmann A., Dahmen T., von Laufenberg D., Kube C., Fianu G. D., Flowers II R. A. Angew. Chem., Int. Ed. 2015;54:7003–7006. doi: 10.1002/anie.201501955. [DOI] [PubMed] [Google Scholar]
- For detailed studies on the radical mechanism of [Co(Por)]-catalyzed C–H amination, including EPR observation of α-Co(iii)-aminyl radical intermediates (also known as Co(iii)-nitrene radicals), see: . For related DFT studies on the radical mechanism of [Co(Por)]-catalyzed olefin aziridination and the ligand hydrogen-bonding effect, see: ; for detailed studies on a similar radical mechanism involving α-Co(iii)-alkyl radical intermediates (also known as Co(iii)-carbene radicals), see: ; (a) Lyaskovskyy V., Olivos Suarez A. I., Lu H., Jiang H., Zhang X. P., de Bruin B. J. Am. Chem. Soc. 2011;133:12264–12273. doi: 10.1021/ja204800a. [DOI] [PubMed] [Google Scholar]; (b) Olivos Suarez A. I., Jiang H., Zhang X. P., de Bruin B. Dalton Trans. 2011;40:5697–5705. doi: 10.1039/c1dt10027k. [DOI] [PubMed] [Google Scholar]; (c) Hopmann K. H., Ghosh A. ACS Catal. 2011;1:597–600. [Google Scholar]; (d) Dzik W. I., Xu X., Zhang X. P., Reek J. N. H., de Bruin B. J. Am. Chem. Soc. 2010;132:10891–10902. doi: 10.1021/ja103768r. [DOI] [PubMed] [Google Scholar]; (e) Belof J. L., Cioce C. R., Xu X., Zhang X. P., Space B., Woodcock H. L. Organometallics. 2011;30:2739–2746. doi: 10.1021/om2001348. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Lu H., Dzik W. I., Xu X., Wojtas L., de Bruin B., Zhang X. P. J. Am. Chem. Soc. 2011;133:8518–8521. doi: 10.1021/ja203434c. [DOI] [PubMed] [Google Scholar]
- For select examples of [Co(Por)]-catalyzed C–H amination, see: . For a review on C–H amination via Co(ii)-based MRC, see: ; (a) Ruppel J. V., Kamble R. M., Zhang X. P. Org. Lett. 2007;9:4889–4892. doi: 10.1021/ol702265h. [DOI] [PubMed] [Google Scholar]; (b) Lu H., Subbarayan V., Tao J., Zhang X. P. Organometallics. 2009;29:389–393. [Google Scholar]; (c) Lu H., Tao J., Jones J. E., Wojtas L., Zhang X. P. Org. Lett. 2010;12:1248–1251. doi: 10.1021/ol100110z. [DOI] [PubMed] [Google Scholar]; (d) Jin L.-M., Lu H., Cui Y., Lizardi C. L., Arzua T. N., Wojtas L., Cui X., Zhang X. P. Chem. Sci. 2014;5:2422–2427. doi: 10.1039/C4SC00697F. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Cenini S., Gallo E., Penoni A., Ragaini F., Tollari S. Chem. Commun. 2000:2265–2266. [Google Scholar]; (f) Ragaini F., Penoni A., Gallo E., Tollari S., Li Gotti C., Lapadula M., Mangioni E., Cenini S. Chem.–Eur. J. 2003;9:249–259. doi: 10.1002/chem.200390018. [DOI] [PubMed] [Google Scholar]; (g) Zardi P., Intrieri D., Caselli A., Gallo E. J. Organomet. Chem. 2012;716:269–274. [Google Scholar]; (h) Lu H., Zhang X. P. Chem. Soc. Rev. 2011;40:1899–1909. doi: 10.1039/c0cs00070a. [DOI] [PubMed] [Google Scholar]
- (a) Lu H., Jiang H., Wojtas L., Zhang X. P. Angew. Chem., Int. Ed. 2010;49:10192–10196. doi: 10.1002/anie.201005552. [DOI] [PubMed] [Google Scholar]; (b) Lu H., Jiang H., Hu Y., Wojtas L., Zhang X. P. Chem. Sci. 2011;2:2361–2366. [Google Scholar]; (c) Lu H., Hu Y., Jiang H., Wojtas L., Zhang X. P. Org. Lett. 2012;14:5158–5161. doi: 10.1021/ol302511f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lu H., Li C., Jiang H., Lizardi C. L., Zhang X. P. Angew. Chem., Int. Ed. 2014;53:7028–7032. doi: 10.1002/anie.201400557. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) for a study of porphyrin ligand effect on Co(ii)-based C–H amination, see ref. 6a; (f) for the use of different amide-containing porphyrins to support Co(ii)-catalyzed radical C–H amination, see ref. 6c
- For selected reviews, see: ; for selected examples, see: ; (a) Winum J.-Y., Scozzafava A., Montero J.-L., Supuran C. T. Med. Res. Rev. 2006;26:767–792. doi: 10.1002/med.20068. [DOI] [PubMed] [Google Scholar]; (b) Reitz A. B., Smith G. R., Parker M. H. Expert Opin. Ther. Pat. 2009;19:1449–1453. doi: 10.1517/13543770903185920. [DOI] [PubMed] [Google Scholar]; (c) Tung R. D., Salituro F. G., Deininger D. D., Bhisetti G. R., Baker C. T. and Spaltenstein A., US Pat., 5945413, 1999Chem. Abst. 1999, 131, 185247. [Google Scholar]; (d) Cherney R. J. and King B. W., WO-0228846, 2002Chem. Abst., 2002, 136, 309923. [Google Scholar]; (e) Zhong J., Gan X., Alliston K. R., Groutas W. C. Bioorg. Med. Chem. 2004;12:589–593. doi: 10.1016/j.bmc.2003.10.059. [DOI] [PubMed] [Google Scholar]; (f) Sparey T., Beher D., Best J., Biba M., Castro J. L., Clarke E., Hannam J., Harrison T., Lewis H., Madin A., Shearman M., Sohal B., Tsou N., Welch C., Wrigley J. Bioorg. Med. Chem. Lett. 2005;15:4212–4216. doi: 10.1016/j.bmcl.2005.06.084. [DOI] [PubMed] [Google Scholar]; (g) Benltifa M., García Moreno M. I., Ortiz Mellet C., García Fernández J. M., Wadouachi A. Bioorg. Med. Chem. Lett. 2008;18:2805–2808. doi: 10.1016/j.bmcl.2008.04.004. [DOI] [PubMed] [Google Scholar]; (h) Yang Q., Li Y., Dou D., Gan X., Mohan S., Groutas C. S., Stevenson L. E., Lai Z., Alliston K. R., Zhong J., Williams T. D., Groutas W. C. Arch. Biochem. Biophys. 2008;475:115–120. doi: 10.1016/j.abb.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Brodney M. A., Barreiro G., Ogilvie K., Hajos-Korcsok E., Murray J., Vajdos F., Ambroise C., Christoffersen C., Fisher K., Lanyon L., Liu J., Nolan C. E., Withka J. M., Borzilleri K. A., Efremov I., Oborski C. E., Varghese A., O'Neill B. T. J. Med. Chem. 2012;55:9224–9239. doi: 10.1021/jm3009426. [DOI] [PubMed] [Google Scholar]
- For selected reviews on catalytic C(sp3)–H amination, see: ; (a) Müller P., Fruit C. Chem. Rev. 2003;103:2905–2920. doi: 10.1021/cr020043t. [DOI] [PubMed] [Google Scholar]; (b) Li Z. G., He C. Eur. J. Org. Chem. 2006:4313–4322. [Google Scholar]; (c) Davies H. M. L., Manning J. R. Nature. 2008;451:417–424. doi: 10.1038/nature06485. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Collet F., Dodd R. H., Dauban P. Chem. Commun. 2009:5061–5074. doi: 10.1039/b905820f. [DOI] [PubMed] [Google Scholar]; (e) Zalatan D. N., Du Bois J. Top. Curr. Chem. 2010;292:347–378. doi: 10.1007/128_2009_19. [DOI] [PubMed] [Google Scholar]; (f) Collet F., Lescot C., Dauban P. Chem. Soc. Rev. 2011;40:1926–1936. doi: 10.1039/c0cs00095g. [DOI] [PubMed] [Google Scholar]; (g) Chang J. W. W., Ton T. M. U., Chan P. W. H. Chem. Rec. 2011;11:331–357. doi: 10.1002/tcr.201100018. [DOI] [PubMed] [Google Scholar]; (h) Davies H. M. L., Du Bois J., Yu J. Q. Chem. Soc. Rev. 2011;40:1855–1856. doi: 10.1039/c1cs90010b. [DOI] [PubMed] [Google Scholar]; (i) Du Bois J. Org. Process Res. Dev. 2011;15:758–762. doi: 10.1021/op200046v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Roizen J. L., Harvey M. E., Du Bois J. Acc. Chem. Res. 2012;45:911–922. doi: 10.1021/ar200318q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Ramirez T. A., Zhao B., Shi Y. Chem. Soc. Rev. 2012;41:931–942. doi: 10.1039/c1cs15104e. [DOI] [PubMed] [Google Scholar]; (l) Yamaguchi J., Yamaguchi A. D., Itami K. Angew. Chem., Int. Ed. 2012;51:8960–9009. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]; (m) Dequirez G., Pons V., Dauban P. Angew. Chem., Int. Ed. 2012;51:7384–7395. doi: 10.1002/anie.201201945. [DOI] [PubMed] [Google Scholar]
- For selected reviews and highlights on catalytic intramolecular C–H amination, see: ; (a) Davies H. M. L., Long M. S. Angew. Chem., Int. Ed. 2005;44:3518–3520. doi: 10.1002/anie.200500554. [DOI] [PubMed] [Google Scholar]; (b) Driver T. G. Nat. Chem. 2013;5:736–738. doi: 10.1038/nchem.1739. [DOI] [PubMed] [Google Scholar]; (c) Jeffrey J. L., Sarpong R. Chem. Sci. 2013;4:4092–4106. [Google Scholar]; (d) Zatolochnaya O. V., Gevorgyan V. Nat. Chem. 2014;6:661–663. doi: 10.1038/nchem.2018. [DOI] [PubMed] [Google Scholar]
- (a) For favored formation of 6-membered cyclic sulfamides through catalytic intramolecular C–H amination, see: Kurokawa T., Kim M., Bois J. D., Angew. Chem., Int. Ed., 2009, 48 , 2777 –2779 . [Google Scholar]; (b) to the best of our knowledge, only two substrates were reported for the formation of 5-membered cyclic sulfamides through catalytic intramolecular benzylic 1,5-C(sp3)–H amination using an Ir-based catalyst in moderate yields, see: Ichinose M., Suematsu H., Yasutomi Y., Nishioka Y., Uchida T., Katsuki T., Angew. Chem., Int. Ed., 2011, 50 , 9884 –9887 . [DOI] [PubMed] [Google Scholar]
- For favored formation of analogous 6-membered cyclic sulfamates through catalytic intramolecular C–H amination, see ref. 9 and 10. For limited examples of the formation of 5-membered cyclic sulfamates through catalytic intramolecular C–H amination (mostly benzylic C–H amination), see: ; for allylic C(sp3)–H amination with one specific substrate, see ref. 12e and ; (a) Espino C. G., Wehn P. M., Chow J., Du Bois J. J. Am. Chem. Soc. 2001;123:6935–6936. [Google Scholar]; (b) Liang J. L., Yuan S. X., Huang J. S., Yu W. Y., Che C. M. Angew. Chem., Int. Ed. 2002;41:3465–3468. doi: 10.1002/1521-3773(20020916)41:18<3465::AID-ANIE3465>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]; (c) Cui Y., He C. Angew. Chem., Int. Ed. 2004;43:4210–4212. doi: 10.1002/anie.200454243. [DOI] [PubMed] [Google Scholar]; (d) Milczek E., Boudet N., Blakey S. Angew. Chem., Int. Ed. 2008;47:6825–6828. doi: 10.1002/anie.200801445. [DOI] [PubMed] [Google Scholar]; (e) Liu Y., Guan X., Wong E. L.-M., Liu P., Huang J.-S., Che C.-M. J. Am. Chem. Soc. 2013;135:7194–7204. doi: 10.1021/ja3122526. [DOI] [PubMed] [Google Scholar]; (f) Paradine S. M., White M. C. J. Am. Chem. Soc. 2012;134:2036–2039. doi: 10.1021/ja211600g. [DOI] [PubMed] [Google Scholar]; (g) Paradine S. M., Griffin J. R., Zhao J., Petronico A. L., Miller S. M., White M. C. Nat. Chem. 2015;7:987–994. doi: 10.1038/nchem.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5-Membered cyclic sulfamides have been traditionally synthesized through interconversion of functional groups. For selected examples, see: ; For transition-metal promoted/catalyzed diamination of alkenes, see: ; (a) Nicolaou K. C., Snyder S. A., Longbottom D. A., Nalbandian A. Z., Huang X. H. Chem.–Eur. J. 2004;10:5581–5606. doi: 10.1002/chem.200400503. [DOI] [PubMed] [Google Scholar]; (b) McDonald R. I., Stahl S. S. Angew. Chem., Int. Ed. 2010;49:5529–5532. doi: 10.1002/anie.200906342. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Olson D. E., Su J. Y., Roberts D. A., Du Bois J. J. Am. Chem. Soc. 2014;136:13506–13509. doi: 10.1021/ja506532h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zabawa T. P., Kasi D., Chemler S. R. J. Am. Chem. Soc. 2005;127:11250–11251. doi: 10.1021/ja053335v. [DOI] [PubMed] [Google Scholar]; (e) Muñiz K., Streuff J., Höevelmann C. H., Núñez A. Angew. Chem., Int. Ed. 2007;46:7125–7127. doi: 10.1002/anie.200702160. [DOI] [PubMed] [Google Scholar]; (f) Zhao B. G., Yuan W. C., Du H. F., Shi Y. Org. Lett. 2007;9:4943–4945. doi: 10.1021/ol702061s. [DOI] [PubMed] [Google Scholar]; (g) Muñiz K., Hövelmann C. H., Streuff J. J. Am. Chem. Soc. 2008;130:763–773. doi: 10.1021/ja075041a. [DOI] [PubMed] [Google Scholar]; (h) Wang B., Du H. F., Shi Y. Angew. Chem., Int. Ed. 2008;47:8224–8227. doi: 10.1002/anie.200803184. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Cornwall R. G., Zhao B. G., Shi Y. Org. Lett. 2013;15:796–799. doi: 10.1021/ol303469a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Muñiz K., Martínez C. J. Org. Chem. 2013;78:2168–2174. doi: 10.1021/jo302472w. [DOI] [PubMed] [Google Scholar]
- For mechanistic studies of Rh2-catalyzed intramolecular C(sp3)–H amination, see: ; (a) Fiori K. W., Espino C. G., Brodsky B. H., Du Bois J. Tetrahedron. 2009;65:3042–3051. [Google Scholar]; (b) Wehn P. M., Lee J. H., DuDu BoisBois J. Org. Lett. 2003;5:4823–4826. doi: 10.1021/ol035776u. [DOI] [PubMed] [Google Scholar]
- N,N-Disubstituted sulfamoyl azides were directly prepared from secondary amines by a one-step procedure (see ESI). The DSC experiment indicated that these sulfamoyl azides were thermally stable without decomposition up to at least 100 °C, see ESI for a representative DSC plot of a sulfamoyl azide
- For the preference of intramolecular [6,3]-C C aziridination of sulfamides over 1,5-allylic C(sp3)–H amination of sulfamides in Rh2-based catalytic system, see ref. 11a. For the preference of catalytic intramolecular [6,3]-C C aziridination over 1,5-allylic C–H amination of analogous sulfamates, see: ; (a) Duran F., Leman L., Ghini A., Burton G., Dauban P., Dodd R. H. Org. Lett. 2002;4:2481–2483. doi: 10.1021/ol0200899. [DOI] [PubMed] [Google Scholar]; (b) ref. 14b ; (c) Guthikonda K., Wehn P. M., Caliando B. J., Du Bois J. Tetrahedron. 2006;62:11331–11342. [Google Scholar]; (d) Estéoule A., Durán F., Retailleau P., Dodd R. H., Dauban P. Synthesis. 2007:1251–1260. [Google Scholar]; (e) Malik G., Estéoule A., Retailleau P., Dauban P. J. Org. Chem. 2011;76:7438–7448. doi: 10.1021/jo201209x. [DOI] [PubMed] [Google Scholar]
- For a computational study on rotational isomerization of allylic radicals, see: ; for observation of olefin isomerization in a parallel study on Co(ii)-based metalloradical catalysis for 1,5-C–H alkylation of α-methoxycarbonyl-α-diazosulfones, see: ; (a) Pasto D. J. J. Phys. Org. Chem. 1997;10:475–483. [Google Scholar]; (b) Cui X., Xu X., Wojtas L.-M., Jin L., Zhang X. P. Chem. Sci. 2015;6:1219–1224. doi: 10.1039/c4sc02610a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For examples of intramolecular 1,6-addition of C C π bonds of analogous sulfamates without observation of 1,5-propargulic C–H amination, see: ; (a) Thornton A. R., Blakey S. B. J. Am. Chem. Soc. 2008;130:5020–5021. doi: 10.1021/ja7111788. [DOI] [PubMed] [Google Scholar]; (b) Thornton A. R., Martin V. I., Blakey S. B. J. Am. Chem. Soc. 2009;131:2434–2435. doi: 10.1021/ja809078d. [DOI] [PubMed] [Google Scholar]; (c) Mace N., Thornton A. R., Blakey S. B. Angew. Chem., Int. Ed. 2013;52:5836–5839. doi: 10.1002/anie.201301087. [DOI] [PubMed] [Google Scholar]
- For Rh2-catalyzed propargylic C(sp3)–H amination of carbamates, see: ; (a) Grigg R. D., Rigoli J. W., Pearce S. D., Schomaker J. M. Org. Lett. 2012;14:280–283. doi: 10.1021/ol203055v. [DOI] [PubMed] [Google Scholar]; (b) Lebel H., Trudel C., Spitz C. Chem. Commun. 2012;48:7799–7801. doi: 10.1039/c2cc33689h. [DOI] [PubMed] [Google Scholar]
- Stigmasterol-based sulfamoyl amine was produced in 22% yield as the only side product (Scheme S1 in ESI), which could be smoothly converted back to the starting azide 1u under diazo transfer conditions (Scheme S2 in ESI)
- For the methods to convert 5-membered cyclic sulfamides to 1,2-diamines, see ref. 13b–f and i
- For corresponding O–S–N bond angles of acyclic sulfamate (103°), 5-membered cyclic sulfamate (95°), and 6-membered cyclic sulfamate (104°), see ref. 9j
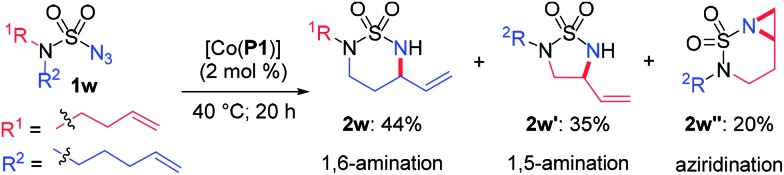
- To further demonstrate the versatility of metalloradical amination, the sulfamoyl azide 1w, which incorporates both N-homoallyl and N-bishomoallyl groups into a single substrate, was designed and synthesized as a substrate for the catalytic reaction. Considering that the two potential reactive arms in the azide are both flexible and differ only by a single methylene unit, they were anticipated to have near equal probability to react with the resulting α-Co(iii)-aminyl radical intermediate without any electronic and steric bias. As expected, the catalytic reaction of azide 1w by [Co(P1)] gave comparable yields of both the 6-membered cyclic sulfamide 2w (44%) and the 5-membered cyclic sulfamide 2w′ (35%) along with [4.1.0]bicyclic aziridine 2w′′ (20%) under standard conditions. This experiment further confirmed the competence of the α-Co(iii)-aminyl radical intermediate to proceed via multiple reaction pathways, including 1,5- and 1,6-H-atom abstraction as well as 1,6-C
C addition. It is important to emphasize that these multiple reaction pathways of Co(ii)-based MRC can be effectively differentiated toward a selective catalytic process for substrates with specific electronic and steric properties.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.