Abstract
In this review, we address selected areas that are central to the state-of-the-art of cancer prevention science. The emphasis on prevention as a viable and critical approach to decreasing cancer mortality has gained traction in recent years, evidenced by its inclusion in the US Vice President's Cancer Initiative (also termed ‘Moonshot’). Cancer prevention occurs by arresting, slowing down, or reversing the carcinogenic process before invasion into surrounding tissue or by avoiding or blocking causative exposure. An important challenge is to identify individuals who will benefit most from preventive interventions with the least possible harm. Preventive interventions range from avoiding known carcinogens (e.g., tobacco or asbestos) to intervening with anticarcinogenic strategies (behavioral modifications , such as diet and exercise; medications; nutritional agents; and vaccination against causative agents). Here, we focus on active intervention with measures involving pharmaceutical and immunological agents.
Cancer Prevention: Its History and Progress in the Modern Molecular Era
The importance of the prevention of cancer as a viable option to cancer management is evidenced by the inclusion of prevention in the US Vice President's Cancer Initiative [1] (also termed ‘Moonshot’). Prevention of cancer is accomplished by arresting, slowing down, or reversing the carcinogenic process before invasion into surrounding tissue or by avoiding or blocking causative exposure [2–4].
In 1966, Lee Wattenberg coined the term ‘chemoprophylaxis’ to denote the experimental chemical inhibition of chemically induced carcinogenesis in animals [5]. The word ‘chemoprevention’ (see Glossary) was introduced in 1976 by Sporn et al. [3] to describe ‘a new pharmacologic approach to the prevention of cancer. . .’, administered during a pre-invasive stage. Although chemoprevention sometimes includes natural and/or nutritional agents (occasionally referred to as ‘bioactive food components’ or ‘nutriceuticals’), such as selenium and vitamin E for prostate cancer prevention, here we focus on synthetic pharmaceuticals. In current usage ‘chemoprevention’ encompasses ‘molecular prevention’ (Figure 1, Key Figure) [6], emphasizing targeting high-risk individuals via ‘precision medicine’ [7]. A ‘Pre-Cancer Genome Atlas’ (PCGA) [8], analogous to The Cancer Genome Atlas (TCGA) project for established invasive cancers, involves determining genomic profiles of premalignant high-risk lesions, enabling stratification for prognosis and targeting [7]. The Erlotinib Prevention of Oral Cancer (EPOC) randomized, placebo-controlled trial (RCT), for example, includes genomic analyses of oral premalignant lesions for loss-of-heterozygosity (LOH) to classify patients into high-risk (LOH-positive) or low-risk (LOH-negative) cohorts [9]. Molecular classification of high-risk chemoprevention candidates should enable the refining of risk models based on traditional epidemiological and clinical attributes, such as those used to formulate the ‘Gail’ model of breast cancer risk [10].
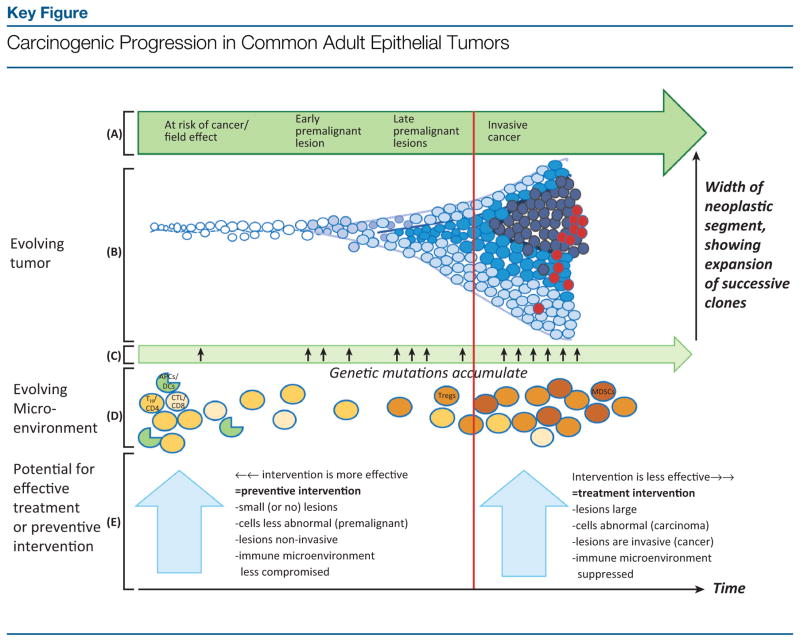
Figure 1.
Evolution of a tumor from normal tissue through progressively advanced premalignant lesions to invasive cancer [going from left to right in (A)] is depicted by the cartoon in (B). Normal cells are shown in white. Progressively abnormal cells appear in gradually darker shades of blue, with dark-blue circles representing invasive cancer cells and red circles depicting cancer cells with metastatic potential. This progression results from the accumulation of oncogenic genetic mutations (C). The resulting mutational accumulation confers growth advantages, leading to clonal expansion over time of genetically more complex cells, as shown in cells spreading out along the Y axis (B). In parallel with the neoplastic progression (B), the microenvironment, in particular the immune environment (D), evolves. In normal-appearing or at-risk tissue, the immune component of the microenvironment is populated in large part by immunocompetent cells capable of fighting cancer cells; in (D), these are represented by TH/CD4 and cytolytic/CD8 T cells and antigen-presenting cells (APCs), such as dendritic cells (DCs). Among others, these cells are capable of carrying out immuno-surveillance and eliminating incipient premalignant cells. As lesions progress to more advanced premalignant and, ultimately, invasive malignant states, the immune environment becomes progressively suppressed and less able to eliminate the abnormal cells. This evolving immunosuppression is represented in (D) by increased abundance of T regulatory cells (Tregs) and myeloid-derived suppressor cells (MDSCs), which antagonize beneficial immune responses, allowing the tumor to grow. Administering anticancer drugs or vaccines before invasion (vertical red line) constitutes ‘preventive’ intervention. This approach, especially early during progression, is likely to be more effective than administering preventive agents late in premalignancy or ‘treatment’ interventions after invasion. The more intact state of the immune system early during premalignancy facilitates a robust response to cancer-preventive vaccination and may also enhance anticancer responses to chemopreventive agents. Additional factors contributing to the relative efficacy of preventive versus treatment interventions are shown in the large light-blue arrows. The progressive changes observed in the tumor microenvironment during carcinogenesis, including evolving immunosuppression, are reviewed in [93,138,139]. Key:
 , normal-appearing/at-risk cell;
, normal-appearing/at-risk cell;
 ,
,
 , progressively abnormal premalignant cells;
, progressively abnormal premalignant cells;
 , invasive cancer cell;
, invasive cancer cell;
 , cancer cell with metastatic potential;
, cancer cell with metastatic potential;
 , helper T cell (TH/CD4];
, helper T cell (TH/CD4];
 , cytolytic T cell (CTL/CD8];
, cytolytic T cell (CTL/CD8];
 , APC, for example, DC.
, APC, for example, DC.
Asymptomatic high-risk individuals show low acceptance of adverse drug effects, even for minor, bothersome symptoms , such as hot flushes [11–13]. Even ostensibly molecularly targeted agents can elicit off-target interactions, resulting in undesirable clinical effects. The goal in developing preventive agents is to optimize the benefit:risk ratio regardless of the specificity of the targeting [2].
Chemoprevention: Using Drugs and Nutrients to Decrease Cancer Risk
Chemoprevention at Specific Disease Sites
Cancer-preventive agent development often ‘repurposes’ drugs used for other disease indications. The selective estrogen receptor modulators (SERMs), tamoxifen and raloxifene [14], both approved by the US Food and Drug Administration (FDA) as breast cancer risk-reducing agents, were suggested for breast cancer prevention based on their reduction of new breast cancer events as secondary endpoints in seminal trials establishing their use in therapeutic settings (tamoxifen as adjuvant breast cancer therapy [15]; raloxifene to treat [16] and/or prevent [17] osteoporosis). In the National Surgical Breast and Bowel Project (NSABP) Breast Cancer Prevention Trial (BCPT) [18] , tamoxifen reduced invasive breast cancer risk by 49% (P < 0.00001; ER-positive tumors by 69%) at 69 months [18] and 43% (P < 0.0001; ER-positive tumors by 63%) at 7 years follow-up [19] among the 13 388 high-risk (≥1.67% 5-year risk by Gail model criteria) women. Combined data from four tamoxifen-versus-placebo trials strengthened these findings (original data were included for all four trials), with 38% (P < 0.0001] reduction in breast cancer incidence overall and 48% (P < 0.0001] in ER-positive tumors [20]. Although the risk-reducing benefits of tamoxifen led to FDA approval of tamoxifen for this indication in 1998, concerns over its toxicities, especially endometrial cancer and venous thromboemboli, present barriers to its use for prevention [11–13], encouraging the search for less toxic alternatives. The osteoporosis drug raloxifene, compared head-to-head with tamoxifen in the NSABP Study of Tamoxifen and Raloxifene (STAR) trial in postmenopausal women, proved less toxic, especially regarding endometrial cancer, and approximately as efficacious as tamoxifen in lowering the risk for invasive breast cancer [21–23], leading to FDA approval for breast cancer risk reduction in 2007. Subsequent meta-analyses of SERM trials, including tamoxifen and raloxifene, continue to demonstrate their efficacy in reducing breast cancer incidence [24–26]. However, none of the trials was statistically powerful enough to show a reduction in breast cancer mortality.
The third-generation aromatase inhibitors (AIs) selectively inhibit aromatase, which converts androgens to estrogens. Similar to tamoxifen, AI adjuvant trials in postmenopausal women reduced new, contralateral breast cancers [exemestane: Intergroup Exemestane Study (IES) trial [27]; anastrozole: Anastrozole and Tamoxifen Alone and in Combination (ATAC) trial [28]], suggesting repurposing AIs for prevention. The NCI Canada Mammary Prevention 3 (MAP-3) trial showed a 65% (P = 0.002] relative reduction in breast cancer in high-risk women with exemestane-versus-placebo [29], a benefit confined to ER-positive breast cancers (73% reduction; P < 0.001). Although supporting exemestane for prevention in high-risk postmenopausal women, the short trial duration, among other features, limited its ability to adequately assess the long-term benefit:risk balance in treated women [30]. Anastrozole showed comparable benefits in the ‘high-risk’ arm of International Breast Cancer Intervention Study (IBIS)-II [hazard ratio (HR ), 0.47; 95% confidence interval (CI ), 0.32–0.58; P < 0.0001; invasive ER-positive cancers: HR, 0.42, 95%CI, 0.25–0.71; P < 0.001 ] [31]. In an independent ‘arm’ of IBIS-II comparing anastrozole to tamoxifen in women with hormone receptor (HR)-positive ductal carcinoma in situ (DCIS), a strong risk factor for invasive breast cancer, no definitive efficacy differences emerged [32]. By contrast, the NSABP B-35 trial in postmenopausal women with hormone-positive DCIS showed that anastrozole treatment was superior to tamoxifen in terms of breast-cancer free interval (HR, 0.73; 95% CI, 0.56–0.96; P = 0.0234) [33].
The antiestrogens (SERMs and aromatase inhibitors) have in common their ability to reduce contralateral breast cancers in the adjuvant treatment of ER-positive cancers. This form of ‘secondary’ prevention illustrates how activity in one context can suggest a repurposed application in another setting. All SERMs and aromatase inhibitors that were subsequently tested in well-conducted Phase 3 prevention trials have proved effective in reducing primary (ER-positive) breast cancers. The major concern that remains for the two FDA-approved agents, tamoxifen and to a lesser extent raloxifene, is toxicity, which has resulted in the limited uptake of these agents for prevention. Aromatase inhibitors may be less challenging, but to date these are not approved for prevention and they also have adverse effects that may prove unacceptable to healthy, high-risk women. These classes of breast cancer preventive drug illustrate the challenges to prevention for all cancer types and all classes of potentially preventive pharmaceutical agent: the balancing of toxicity against efficacy.
Repurposing of the type II 5α-reductase inhibitor finasteride, indicated for benign prostatic hyperplasia (and for male-pattern baldness), was seen in the Prostate Cancer Prevention Trial (PCPT) in 18 882 men ≥55 years with PSA (prostate specific antigen) ≤3 ng/ml [34]. Period prevalence, the primary endpoint, was reduced at 7 years by 24.8% with finasteride relative to placebo [34]. Yet, because the rate of high-grade cancers was increased with finasteride, the FDA did not approve this drug (or the similarly acting dutasteride, tested in the REDUCE/Reduction by Dutasteride of Prostate Cancer Events trial [35]) for prostate cancer risk reduction [36]. No effect was seen on long-term survival, although statistical power was limited [37]. Explanations for the high-grade observation centered on possible bias in the pathological analyses [38,39].
Aspirin and other Nonsteroidal Anti-Inflammatory Drugs
Numerous epidemiological and experimental studies, as well as clinical trials, point to the cancer-preventive properties of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs ). Although generally attributed to cyclooxygenase inhibition resulting in decreased prostaglandin E2 (PGE2] and its proinflammatory, procarcinogenic effects, recent data suggest additional, more global beneficial effects on the immune microenvironment [40–42].
Several meta-analyses of observational studies and clinical trials suggest selective benefits for particular cancers in terms of reduction in cancer incidence, risk of metastases , and cancer deaths [43–46]. Colorectal cancer (CRC) stands out as benefitting the most [47], with a pooled analysis of four randomized trials showing a HR of 0.76 (95% CI, 0.60–0.96; P = 0.02) for the 20-year risk of this cancer with aspirin versus control [48]. The Colorectal Adenoma Prevention Study showed fewer recurrent adenomas with aspirin 325 mg/day [adjusted relative risk (RR ), 0.65; 95% CI, 0.46–0.91] [49] and the Aspirin/Folate Polyp Prevention Study showed a moderate effect on recurrence (RR, 0.81; 95% CI, 0.69–0.96 at 81 mg/day and RR, 0.96; 95% CI, 0.81–1.13 at 325 mg/day) [50]. There is no clear dose–response gradient over the range of 81–325 mg/day [49,50]. In high-risk CRC syndromes, aspirin showed a nonsignificant 23% reduction in polyp count in familial adenomatous polyposis (FAP) [51] , but a 59% decrease in CRCs in individuals with Lynch syndrome [52].
Other NSAIDs also reduce CRC risk [40]. In a preliminary study, sulindac with difluoromethylornithine (DFMO) reduced adenoma recurrence (RR, 0.30; 95% CI, 0.18–0.49; P < 0.001), especially advanced adenomas (RR, 0.085; 95% CI, 0.011–0.65; P < 0.001) [53]. The cyclooxygenase-2 (COX-2]-selective inhibitors also reduce adenoma incidence, as seen in the Adenoma Prevention with Celecoxib (APC) trial, but the associated cardiovascular toxicities [54,55] limit their use for cancer prevention. Given the gastrointestinal bleeding associated with aspirin, the current US Preventive Services Task Force (USPSTF) recommendations for aspirin (low-dose) to prevent cardiovascular disease as well as CRC have been carefully crafted to limit its use to individuals at low risk of bleeding but at elevated risk of cardiovascular disease (estimated risk of ≥10% over 10 years) [56,57].
Retinoids and Rexinoids
Retinoids, natural and synthetic derivatives of vitamin A (retinol), are felt to exert their anticancer effects via activation of nuclear retinoic acid receptors (RARs) [58]. Although structurally resembling retinoids, rexinoids bind to retinoid X receptors (RXRs) to activate the transcription of target genes. Retinoids were among the first agents tested for chemoprevention [3,4]. Short-term 13-cis-retinoic acid (isotretinoin) for premalignant oral leukoplakia elicited decreases in the size of lesions in 67% of patients [59]. Isotretinoin showed activity in head and neck squamous cell carcinoma, with high doses preventing second primary tumors in previously treated patients [60]. Adverse events with retinoids, especially at high doses, include mucocutaneous toxicity and hypertriglyceridemia. Rexinoids are less toxic than most retinoids [61]. Bexarotene reduced mammary tumorigenesis by 75% in p53-null mammary epithelium [62] and prevented the development of preinvasive mammary lesions in MMTV-erbB2 mice [63]; unfortunately, it elevates triglycerides [64,65]. UAB30, a novel synthetic analog of 9-cis-retinoic acid that lacks the hypertriglyceridemic effect of other rexinoids, is currently in pilot studies in healthy volunteers ( clinicaltrials.gov identifiers: NCT00896974, NCT01935960). The new class III rexinoids, UAB125 and UAB126, show promise for preventing mammary cancers, while potentially lowering lipid levels; however, the evidence to date is limited to animal studies [64,65].
Metformin
Metformin is an example of a drug approved for another disease (diabetes ) ‘repurposed’ as a possible cancer preventive agent [66]. It also exemplifies the targeting of the obesity–diabetes–physical activity–energy balance axis, disruption of which by onset of the metabolic syndrome is associated with risk for certain cancers [67–70]. Observational studies have shown metformin use by diabetics to correlate with reduced incidence of overall cancer, specific cancers (pancreatic, hepatocellular, colorectal, lung , and breast cancers [71]), and cancer mortality [72–75]. However, results from observational studies are not consistent [73,76,77]. The DPP Observational Study (DPPOS) [78] ( clinicaltrials.gov identifier: NCT00038727i ); an ongoing follow-up to the randomized placebo-controlled Diabetes Prevention Program (DPP) assessing metformin-versus-lifestyle in prediabetics [79], is a stronger study design. Results from this study are pending.
Reducing Toxicity while Maintaining Efficacy
Two strategies to circumvent undesirable drug effects are lowering the dose [80] and using local administration [81], both of which have been implemented with tamoxifen. Reducing the usual 20-mg/day dose by 75% [80,82] was comparable to full doses in reduction of total cholesterol, IGF-1 , and other circulating biomarkers in healthy women [83]. Three tamoxifen doses in a randomized presurgical trial in women with early-stage ER-positive breast cancer exhibited the same degree of Ki-67 reduction in tumor tissue [84] and were associated with recurrence-free survival [85]. Topical administration of active tamoxifen metabolites to the breast circumvents hepatic metabolism, thereby avoiding the excretion of large amounts of drug before it reaches the target site [81,86] and may avoid systemic distribution with possible associated adverse effects (endometrial cancer, thromboembolism, hot flushes, night sweats, and menstrual irregularity). In a Phase 2 presurgical trial in 27 women with ER-positive DCIS, 4-hydroxy-tamoxifen [4-OHT) gel applied to the breast skin (2 g/day to each breast) decreased Ki-67 by 3.4% compared with 5.1% with oral tamoxifen (P ≤ 0.03 in both groups, P = 0.99 between groups) [86]. Importantly, mean plasma 4-OHT levels differed significantly : 0.2 ng/ml with topical 4-OHT versus 1.1 ng/ml with oral tamoxifen.
Immunoprevention: Vaccines
Immunological approaches, mainly vaccines [87,88], are especially applicable to cancer prevention [89–93], where the favorable safety profile is well suited to the healthy recipients (Figure 1). Cancer-prevention vaccines either prevent infection with cancer-causing organisms or directly target non-infection-associated cancers [88,94].
Vaccines to prevent infectious cancers [95] are ‘prophylactic’ when administered before exposure to prevent infection by the carcinogenic agent [96]. The immunogenic antigen is derived from the infectious agent, generally an oncovirus. The recombinant hepatitis B virus (HBV) vaccine, approved in 1981 [97], decreased HBV-associated hepatocellular carcinoma (HCC) following implementation of population-based HBV vaccination programs (first in children in Taiwan [98]).
Similarly, the carcinogenic role of specific serotypes of human papillomavirus (HPV) (especially HPV 16 and 18 , which cause 70% of cervical cancers) [99] underlay the development of prophylactic HPV vaccines targeting known viral antigens [100–102]. Three prophylactic vaccines, all containing the major capsid protein L1 of specific HPV strains, have been FDA approved: a bivalent vaccine (HPV 16/18); a quadrivalent vaccine (HPV 6/11/16/18); and a nonavalent vaccine (HPV 6/11/16/18/31/33/45/52/58) [103]. National vaccination programs have resulted in decreases in HPV prevalence in girls and women in the targeted age group, approximately 16–26 years [104,105]. In terms of our ultimate goal of cancer prevention, the decreases in HPV prevalence have translated into reductions in the prevalence of downstream precancerous cervical lesions. Thus, vaccination against HPV types 16/18 resulted in a significant decrease in HPV 16/18-attributable lesions in women with pre-existing cervical intra-epithelial neoplasia (CIN) 2 (diagnosed on abnormal Papanicolaou/Pap test ; i.e. , the ‘trigger’ Pap) who initiated vaccination at least 24 months before their trigger Pap [106,107]. This type-specific reduction in high-grade cervical lesions was apparent in women receiving at least one vaccine dose but not in unvaccinated women, suggesting an early impact of HPV vaccination on vaccine-type disease [107]. Furthermore, application of the existing vaccines is expanding to include boys. The vaccines may also offer protection against a broad array of other HPV-associated cancers (vulva, vagina, penis, anus , and a subset of head-and-neck cancers, particularly of the oropharynx [88]). In fact, Gardasil, the quadrivalent vaccine, is indicated for the prevention of a variety of precancerous lesions caused by HPV types 6, 11, 16, and 18ii.
Hepatitis C virus (HCV), an RNA virus that causes chronic hepatitis, cirrhosis, and HCC, has proven more elusive in terms of the development of effective prophylactic vaccines [108,109]. However, drugs , such as ombitasvir, dasabuvir, ledipasvir , and sofosbuvir , have demonstrated efficacy in inducing high rates of sustained virological responses in individuals with chronic infection [110–112]. Until HCV vaccines are developed, these antiviral agents, paired with regular screening for HCV infection, offer promise for the prevention of downstream sequelae , including virus-associated HCC.
Selection of immunogenic antigens for vaccines to prevent noninfectious cancers is more challenging. Unlike the inherently ‘non-self’ nature of virus-derived antigens, the ‘neoantigens’/tumor-associated antigens (TAAs) produced by noninfectious tumors have some component of ‘self’. TAAs develop by modification of normal host antigens via a variety of mechanisms, including mutation, abnormal post-translational modification, and overexpression [88]. The goal is to break tolerance to ‘self’ [87], while avoiding autoimmune responses against normal tissue. Autoimmune adverse effects have been rare with immunopreventive vaccines [113].
Overexpression of the neoantigen hypoglycosylated MUC1 occurs in colonic epithelium in inflammatory conditions such as inflammatory bowel disease (IBD) and CRC, exposing the peptide backbone as an immunostimulatory epitope. A vaccine targeting MUC1 has shown promise in preventing both IBD and CRC in a mouse model [114]. Her2 overexpression in 20–30% of breast cancers prompted development of a Her2-derived peptide vaccine (E75/Nelipepimut-S) [115]. Nelipepimut-S is being tested in the preventive context in women with DCIS in the VADIS trial ( clinicaltrials.gov identifier: NCT02636582).
Together with Her2, insulin-like growth factor-binding protein 2 (IGFBP-2) and insulin-like growth factor receptor-I (IGF-IR) are overexpressed in many breast cancers and DCIS and are associated with a poor prognosis [116–118]. Immunization of transgenic mice engineered to develop mammary tumors overexpressing Her2-IGFBP-2-IGFR-1 with a multi-antigen vaccine effectively inhibited tumor growth; the tri-antigen vaccine showed greater efficacy compared with the individual antigens [116]. Combined with the rexinoid bexarotene, this vaccine showed even better disease-free survival. Given this preclinical outcome, vaccination with the same antigens may hold promise for breast cancer prevention in high-risk women.
In a final example, exploitation of a neoantigen encoded by a KrasC12D mutation, present in > 90% of pancreatic ductal adenocarcinomas (PDA), is the basis for a Listeria vaccine (LM-Kras) administered to KPC mice. These mice express this mutated gene in pancreatic tissues, mimicking carcinogenic progression seen in human PDAs. Immunization of KPC mice at early stages of progression, together with depletion of T regulatory cells, reduced the progression of premalignant lesions to cancer and prolonged survival [119,120], suggesting that this vaccine has potential for PDA prevention in the clinical setting.
The immune checkpoint inhibitors are currently among the most promising immunological interventions [121]. Members of this class are being investigated or have been FDA- approved for several metastatic and advanced cancers. Although these agents may ultimately prove valuable for cancer prevention, the associated toxicities in their current usage preclude this application.
Concluding Remarks
Contrary to the view that ‘chemoprevention of cancer is an almost universal failure’ [122], successes in cancer prevention have been achieved [18,21,22,29,31,123]. For several agents, preclinical models have accurately predicted efficacy in humans [2], with both negative [124–128] and positive outcomes [29,31,129]. Nevertheless, new animal models must be developed with long enough latency periods to enable preventive intervention and to explore optimal timing of administration [130,131]. Engineered mouse strains should effectively model the genetic diversity of human cancer to enable ‘precision’ cancer prevention [132]. There are interventions at the interface of primary prevention and screening. Cervical cancer screening, for example, should ideally detect pre-invasive disease, enabling resection of cervical intraepithelial neoplastic lesions and thereby ‘preventing’ progression to invasive cancer. Similarly, detection and removal of adenomas during screening colonoscopy is a form of primary prevention. The development of a prophylactic vaccine to prevent chronic HCV infection has proven challenging, given the many ways in which this RNA virus has evolved to evade the immune system [133]. However, several laboratories are addressing this problem and , hopefully , an effective vaccine that ultimately prevents HCV-associated HCC will eventually be available.
Among areas ripe for investigation are combinations of preventive agents, exemplified by the Her2-IGFBP-2-IGFR-1 vaccine plus bexarotene for mammary cancer [116]. Even with ‘successes’, such as FDA approval of tamoxifen for risk reduction, hurdles in acceptance by the at-risk and physician community [11,134,135] abound due to concerns about toxicity [136]. Therefore, risk models must be refined [137] to better delineate candidates for preventive interventions in whom the risk:benefit balance is optimized. These should improve the entire decision-making process [12,13]. Key challenges that are currently being addressed in the field of cancer prevention are summarized in the Outstanding Questions.
Outstanding Questions.
The key challenge to developing agents for chemoprevention is the need to minimize toxicity while enhancing efficacy, allowing for an optimal benefit:risk ratio.
Risk models for each of the common cancers must be developed to determine which individuals are at high enough risk to benefit from preventive interventions.
Analysis of genomic, epigenomic , and proteomic profiles of premalignant lesions is a promising direction for future research. The resulting PCGA database will provide the molecular backdrop against which targeted preventive agents can be developed.
The development and validation of additional preclinical prevention models that can generate accurate and reliable mechanistic and responsive predictors of human preventive efficacy and toxicity are key concerns in the field. Although investigators in some cases rely on agent effects against cancer cell lines or xenografted tumors for early preclinical studies regarding the effective concentrations for preventive agents, such models are not appropriate for prevention, since they involve existing cancer. The need is for animal models exhibiting a precancerous continuum during which tested agents can be administered and cancer prevention evaluated.
The question of whether immunological interventions, especially vaccines, that target non-infection-associated cancers will be effective and nontoxic needs to be addressed.
Trends.
The development of small-molecule pharmaceutical agents, especially those that target known molecular abnormalities associated with specific cancers, reflects the application of ‘precision medicine’ to chemoprevention and is an area of current interest.
Increasing emphasis is being placed on immunological interventions, mainly vaccines, for cancer prevention.
Repurposing of drugs approved for noncancer prevention indications is a promising area of research, since toxicities for such agents are already known.
Combining agents, including vaccines, to enhance their preventive efficacy is an approach to chemoprevention that is currently being investigated.
Glossary
- Cancer prevention
the arrest, slowing down, or reversal of the carcinogenic process before invasion into surrounding tissue by avoiding or blocking causative exposure
- Chemoprevention
the application of a pharmacological approach for the prevention of cancer
- Pre-Cancer Genome Atlas (PCGA)
a database of genomic and epigenomic abnormalities that are observed in pre-invasive lesions and potentially contribute to carcinogenesis. The PCGA, which is in the early stages of development, will provide information that enables more accurate prediction of prognosis and drug response as well as providing new targets for preventive agent development
Footnotes
References
- 1.The White House, Office of the Press Secretary. Fact Sheet: Investing in the National Cancer Moonshot. US Government; 2016. [Google Scholar]
- 2.Meyskens F, et al. Cancer prevention: obstacles, challenges and the road ahead. J Natl Cancer Inst. 2016;108:djv309. doi: 10.1093/jnci/djv309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sporn MB, et al. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids) Fed Proc. 1976;35:1332–1328. [PubMed] [Google Scholar]
- 4.Lippman SM, Hawk ET. Cancer prevention: from 1727 to milestones of the past 100 years. Cancer Res. 2009;69:5269–5284. doi: 10.1158/0008-5472.CAN-09-1750. [DOI] [PubMed] [Google Scholar]
- 5.Wattenberg LW. Chemoprophylaxis of carcinogenesis: a review. Cancer Res. 1966;26:1520–1526. [PubMed] [Google Scholar]
- 6.Maresso KC, et al. Molecular cancer prevention: current status and future directions. CA Cancer J Clin. 2015;65:345–383. doi: 10.3322/caac.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kensler TW, et al. Transforming cancer prevention through precision medicine and immune-oncology. Cancer Prev Res. 2016;9:2–10. doi: 10.1158/1940-6207.CAPR-15-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell JD, et al. The case for a pre-cancer genome atlas (PCGA) Cancer Prev Res. 2016;9:119–124. doi: 10.1158/1940-6207.CAPR-16-0024. [DOI] [PubMed] [Google Scholar]
- 9.William WN, Jr, et al. Erlotinib and the risk of oral cancer: the Erlotinib Prevention of Oral Cancer (EPOC) Randomized clinical trial. JAMA Oncol. 2015;2:209–216. doi: 10.1001/jamaoncol.2015.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gail MH, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 11.DeCensi A, et al. Barriers to preventive therapy for breast and other major cancers and strategies to improve uptake. Ecancermedicalscience. 2015;9:595. doi: 10.3332/ecancer.2015.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bober SL, et al. Decision-making about tamoxifen in women at high risk for breast cancer: clinical and psychological factors. J Clin Oncol. 2004;22:4951–4957. doi: 10.1200/JCO.2004.05.192. [DOI] [PubMed] [Google Scholar]
- 13.Ropka ME, et al. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28:3090–3095. doi: 10.1200/JCO.2009.27.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan VC. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat Rev Cancer. 2007;7:46–53. doi: 10.1038/nrc2048. [DOI] [PubMed] [Google Scholar]
- 15.Nayfield SG, et al. Potential role of tamoxifen in prevention of breast cancer. J Natl Cancer Inst. 1991;83:1450–1459. doi: 10.1093/jnci/83.20.1450. [DOI] [PubMed] [Google Scholar]
- 16.Ettinger B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. J Am Med Assoc. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 17.Delmas PD, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641–1647. doi: 10.1056/NEJM199712043372301. [DOI] [PubMed] [Google Scholar]
- 18.Fisher B, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 19.Fisher B, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 20.Cuzick J, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 21.Vogel VG, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. J Am Med Assoc. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 22.Vogel VG, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila) 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Provinciali N, et al. Raloxifene hydrochloride for breast cancer risk reduction in postmenopausal women. Expert Rev Clin Pharmacol. 2016:1–10. doi: 10.1080/17512433.2016.1231575. [DOI] [PubMed] [Google Scholar]
- 24.Cuzick J, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827–1834. doi: 10.1016/S0140-6736(13)60140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cummings SR, et al. Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. J Natl Cancer Inst. 2009;101:384–398. doi: 10.1093/jnci/djp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mocellin S, et al. Breast cancer chemoprevention: a network meta-analysis of randomized controlled trials. J Natl Cancer Inst. 2016:108. doi: 10.1093/jnci/djv318. [DOI] [PubMed] [Google Scholar]
- 27.Coombes RC, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 28.Baum M, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of post-menopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 29.Goss PE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 30.Decensi A, et al. Exemestane for breast cancer prevention: a critical shift? Cancer Discov. 2012;2:25–40. doi: 10.1158/2159-8290.CD-11-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuzick J, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041–1048. doi: 10.1016/S0140-6736(13)62292-8. [DOI] [PubMed] [Google Scholar]
- 32.Forbes JF, et al. Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): a double-blind, randomised controlled trial. Lancet. 2016;387:866–873. doi: 10.1016/S0140-6736(15)01129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margolese RG, et al. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet. 2016;387:849–856. doi: 10.1016/S0140-6736(15)01168-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson IM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 35.Andriole GL, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 36.Theoret MR, et al. The risks and benefits of 5alpha-reductase inhibitors for prostate-cancer prevention. N Engl J Med. 2011;365:97–99. doi: 10.1056/NEJMp1106783. [DOI] [PubMed] [Google Scholar]
- 37.Thompson IM, et al. Long-term survival of participants in the prostate cancer prevention trial. N Engl J Med. 2013;369:603–610. doi: 10.1056/NEJMoa1215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucia MS, et al. Finasteride and high-grade prostate cancer in the prostate cancer prevention trial. J Natl Cancer Inst. 2007;99:1375–1383. doi: 10.1093/jnci/djm117. [DOI] [PubMed] [Google Scholar]
- 39.Redman MW, et al. Finasteride does not increase the risk of high-grade prostate cancer: a bias-adjusted modeling approach. Cancer Prev Res (Phila) 2008;1:174–181. doi: 10.1158/1940-6207.CAPR-08-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Umar A, et al. Mechanisms of nonsteroidal anti-inflammatory drugs in cancer prevention. Semin Oncol. 2016;43:65–77. doi: 10.1053/j.seminoncol.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Zelenay S, et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell. 2015;162:1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho JY. Immunomodulatory effect of nonsteroidal anti-inflammatory drugs (NSAIDs) at the clinically available doses. Arch Pharm Res. 2007;30:64–74. doi: 10.1007/BF02977780. [DOI] [PubMed] [Google Scholar]
- 43.Rothwell PM, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 44.Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–527. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 45.Rothwell PM, et al. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 46.Rothwell PM, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 47.Strum WB. Colorectal adenomas. N Engl J Med. 2016;374:1065–1075. doi: 10.1056/NEJMra1513581. [DOI] [PubMed] [Google Scholar]
- 48.Rothwell PM, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 49.Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 50.Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 51.Burn J, et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev Res (Phila) 2011;4:655–665. doi: 10.1158/1940-6207.CAPR-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burn J, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081–2087. doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyskens FL, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila Pa) 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bresalier RS, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 55.Bertagnolli MM, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 56.Dehmer SP, et al. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:777–786. doi: 10.7326/M15-2129. [DOI] [PubMed] [Google Scholar]
- 57.Bibbins-Domingo K Force USPS.T. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836–845. doi: 10.7326/M16-0577. [DOI] [PubMed] [Google Scholar]
- 58.Uray IP, et al. Retinoids and rexinoids in cancer prevention: from laboratory to clinic. Semin Oncol. 2016;43:49–64. doi: 10.1053/j.seminoncol.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hong WK, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315:1501–1505. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 60.Hong WK, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323:795–801. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- 61.Miller VA, et al. Initial clinical trial of a selective retinoid X receptor ligand, LGD1069. J Clin Oncol. 1997;15:790–795. doi: 10.1200/JCO.1997.15.2.790. [DOI] [PubMed] [Google Scholar]
- 62.Medina D, et al. Prevention of tumorigenesis in p53-null mammary epithelium by rexinoid bexarotene, tyrosine kinase inhibitor gefitinib, and celecoxib. Cancer Prev Res (Phila) 2009;2:168–174. doi: 10.1158/1940-6207.CAPR-08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y, et al. The rexinoid, bexarotene, prevents the development of premalignant lesions in MMTV-erbB2 mice. Br J Cancer. 2008;98:1380–1388. doi: 10.1038/sj.bjc.6604320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grubbs CJ, et al. Efficacy of new retinoids in the prevention of mammary cancers and correlations with short-term bio-markers. Carcinogenesis. 2006;27:1232–1239. doi: 10.1093/carcin/bgi308. [DOI] [PubMed] [Google Scholar]
- 65.Atigadda VR, et al. Conformationally defined rexinoids and their efficacy in the prevention of mammary cancers. J Med Chem. 2015;58:7763–7774. doi: 10.1021/acs.jmedchem.5b00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heckman-Stoddard BM, et al. Update on phase I/II breast cancer prevention trials. Curr Breast Cancer Rep. 2011;3:131–141. [Google Scholar]
- 67.Roberts DL, et al. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–316. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 68.Braun S, et al. The link between the metabolic syndrome and cancer. Int J Biol Sci. 2011;7:1003–1015. doi: 10.7150/ijbs.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Howe LR, et al. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin Cancer Res. 2013;19:6074–6083. doi: 10.1158/1078-0432.CCR-12-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcia-Jimenez C, et al. From obesity to diabetes and cancer: epidemiological links and role of therapies. Br J Cancer. 2016;114:716–722. doi: 10.1038/bjc.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Umar A, et al. Future directions in cancer prevention. Nat Rev Cancer. 2012;12:835–848. doi: 10.1038/nrc3397. [DOI] [PubMed] [Google Scholar]
- 72.Noto H, et al. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 2012;7:e33411. doi: 10.1371/journal.pone.0033411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Franciosi M, et al. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One. 2013;8:e71583. doi: 10.1371/journal.pone.0071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu L, et al. Pharmacologic therapy of diabetes and overall cancer risk and mortality: a meta-analysis of 265 studies. Sci Rep. 2015;5:10147. doi: 10.1038/srep10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heckman-Stoddard BM, et al. Repurposing old drugs to chemoprevention: the case of metformin. Semin Oncol. 2016;43:123–133. doi: 10.1053/j.seminoncol.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsilidis KK, et al. Metformin does not affect cancer risk: a cohort study in the U.K. clinical practice research datalink analyzed like an intention-to-treat trial. Diabetes Care. 2014;37:2522–2532. doi: 10.2337/dc14-0584. [DOI] [PubMed] [Google Scholar]
- 77.Thakkar B, et al. Metformin and sulfonylureas in relation to cancer risk in type II diabetes patients: a meta-analysis using primary data of published studies. Metabolism. 2013;62:922–934. doi: 10.1016/j.metabol.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 78.Diabetes Prevention Program Research Group. Knowler WC, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Knowler WC, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lazzeroni M, DeCensi A. Alternate dosing schedules for cancer chemopreventive agents. Semin Oncol. 2016;43:116–122. doi: 10.1053/j.seminoncol.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 81.Lee O, Khan SA. Novel routes for administering chemoprevention: local transdermal therapy to the breasts. Semin Oncol. 2016;43:107–115. doi: 10.1053/j.seminoncol.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 82.Lazzeroni M, et al. Oral low dose and topical tamoxifen for breast cancer prevention: modern approaches for an old drug. Breast Cancer Res. 2012;14:214. doi: 10.1186/bcr3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Decensi A, et al. Biologic activity of tamoxifen at low doses in healthy women. J Natl Cancer Inst. 1998;90:1461–1467. doi: 10.1093/jnci/90.19.1461. [DOI] [PubMed] [Google Scholar]
- 84.Decensi A, et al. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. 2003;95:779–790. doi: 10.1093/jnci/95.11.779. [DOI] [PubMed] [Google Scholar]
- 85.DeCensi A, et al. Prognostic significance of Ki-67 labeling index after short-term presurgical tamoxifen in women with ER-positive breast cancer. Ann Oncol. 2011;22:582–587. doi: 10.1093/annonc/mdq427. [DOI] [PubMed] [Google Scholar]
- 86.Lee O, et al. A Randomized phase II presurgical trial of transdermal 4-hydroxytamoxifen gel versus oral tamoxifen in women with ductal carcinoma in situ of the breast. Clin Cancer Res. 2014;20:3672–3682. doi: 10.1158/1078-0432.CCR-13-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Finn OJ, Beatty PL. Cancer immunoprevention. Curr Opin Immunol. 2016;39:52–58. doi: 10.1016/j.coi.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wojtowicz ME, et al. Immunologic approaches to cancer prevention-current status, challenges, and future perspectives. Semin Oncol. 2016;43:161–172. doi: 10.1053/j.seminoncol.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 89.Mellman I, et al. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Umar A. Cancer immunoprevention: a new approach to intercept cancer early. Cancer Prev Res (Phila) 2014;7:1067–1071. doi: 10.1158/1940-6207.CAPR-14-0213. [DOI] [PubMed] [Google Scholar]
- 91.van der Burg SH, et al. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16:219–233. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- 92.Schreiber RD, et al. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 93.Lollini PL, et al. Vaccines for tumour prevention. Nat Rev Cancer. 2006;6:204–216. doi: 10.1038/nrc1815. [DOI] [PubMed] [Google Scholar]
- 94.Roeser JC, et al. Emerging strategies for cancer immunoprevention. Oncogene. 2015;34:6029–6039. doi: 10.1038/onc.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.zur Hausen H, de Villiers EM. Cancer ‘causation’ by infections–individual contributions and synergistic networks. Semin Oncol. 2014;41:860–875. doi: 10.1053/j.seminoncol.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 96.Schiller JT, Lowy DR. Vaccines to prevent infections by oncoviruses. Annu Rev Microbiol. 2010;64:23–41. doi: 10.1146/annurev.micro.112408.134019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hilleman MR. Critical overview and outlook: pathogenesis, prevention, and treatment of hepatitis and hepatocarcinoma caused by hepatitis B virus. Vaccine. 2003;21:4626–4649. doi: 10.1016/s0264-410x(03)00529-2. [DOI] [PubMed] [Google Scholar]
- 98.Chang MH, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 99.Schiffman M, Solomon D. Clinical practice. Cervical-cancer screening with human papillomavirus and cytologic cotesting. N Engl J Med. 2013;369:2324–2331. doi: 10.1056/NEJMcp1210379. [DOI] [PubMed] [Google Scholar]
- 100.Koutsky LA, et al. A controlled trial of a human papillo-mavirus type 16 vaccine. N Engl J Med. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 101.Munoz N, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373:1949–1957. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 102.Wang JW, et al. Immunoprevention of human papillo-mavirus-associated malignancies. Cancer Prev Res (Phila) 2015;8:95–104. doi: 10.1158/1940-6207.CAPR-14-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.FDA. FDA Approves Gardasil 9 for Prevention of Certain Cancers Caused by Five Additional Types of HPV. FDA; 2014. [Google Scholar]
- 104.Tabrizi SN, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645–1651. doi: 10.1093/infdis/jis590. [DOI] [PubMed] [Google Scholar]
- 105.Osborne SL, et al. Assessing genital human papilloma-virus genoprevalence in young Australian women following the introduction of a national vaccination program. Vaccine. 2015;33:201–208. doi: 10.1016/j.vaccine.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 106.Powell SE, et al. Impact of human papillomavirus (HPV) vaccination on HPV 16/18-related prevalence in precancerous cervical lesions. Vaccine. 2012;31:109–113. doi: 10.1016/j.vaccine.2012.10.092. [DOI] [PubMed] [Google Scholar]
- 107.Hariri S, et al. Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States – 2008–2012. Vaccine. 2015;33:1608–1613. doi: 10.1016/j.vaccine.2015.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Law LMJ, et al. Progress towards a hepatitis C virus vaccine. Emerg Microbes Infect. 2013;2:e79. doi: 10.1038/emi.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fauvelle C, et al. Hepatitis C virus vaccines–progress and perspectives. Microb Pathog. 2013;58:66–72. doi: 10.1016/j.micpath.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 110.Feld JJ, et al. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med. 2015;373:2599–2607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 111.Kowdley KV, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 112.Afdhal N, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 113.Weber JS, et al. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33:2092–2099. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beatty PL, et al. Vaccine against MUC1 antigen expressed in inflammatory bowel disease and cancer lessens colonic inflammation and prevents progression to colitis-associated colon cancer. Cancer Prev Res (Phila) 2010;3:438–446. doi: 10.1158/1940-6207.CAPR-09-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mittendorf EA, et al. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann Oncol. 2014;25:1735–1742. doi: 10.1093/annonc/mdu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Disis ML, et al. A multiantigen vaccine targeting neu, IGFBP-2, and IGF-IR prevents tumor progression in mice with preinvasive breast disease. Cancer Prev Res (Phila) 2013;6:1273–1282. doi: 10.1158/1940-6207.CAPR-13-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Probst-Hensch NM, et al. IGFBP2 and IGFBP3 protein expressions in human breast cancer: association with hormonal factors and obesity. Clin Cancer Res. 2010;16:1025–1032. doi: 10.1158/1078-0432.CCR-09-0957. [DOI] [PubMed] [Google Scholar]
- 118.Marquez JP, et al. The antigenic repertoire of premalignant and high-risk lesions. Cancer Prev Res (Phila) 2015;8:266–270. doi: 10.1158/1940-6207.CAPR-14-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chu NJ, et al. Nonviral oncogenic antigens and the inflammatory signals driving early cancer development as targets for cancer immunoprevention. Clin Cancer Res. 2015;21:1549–1557. doi: 10.1158/1078-0432.CCR-14-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smit MA, et al. Cancer immunoprevention–the next frontier. Cancer Prev Res (Phila) 2014;7:1072–1080. doi: 10.1158/1940-6207.CAPR-14-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Potter JD. The failure of cancer chemoprevention. Carcinogenesis. 2014;35:974–982. doi: 10.1093/carcin/bgu063. [DOI] [PubMed] [Google Scholar]
- 123.Adhami VM, et al. Cancer chemoprevention is not a failure. Carcinogenesis. 2014;35:2154–2155. doi: 10.1093/carcin/bgu141. [DOI] [PubMed] [Google Scholar]
- 124.Lippman SM, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Am Med Assoc. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Klein EA, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Am Med Assoc. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang CS, et al. Does vitamin E prevent or promote cancer? Cancer Prev Res (Phila) 2012;5:701–705. doi: 10.1158/1940-6207.CAPR-12-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McCormick DL, et al. Null activity of selenium and vitamin e as cancer chemopreventive agents in the rat prostate. Cancer Prev Res (Phila) 2010;3:381–392. doi: 10.1158/1940-6207.CAPR-09-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nicastro H, Dunn B. The Selenium and Vitamin E Cancer Prevention Trial (SELECT): prevention of prostate cancer using selenium and/or vitamin E in the SELECT cancer prevention trial. In: Preedy V, editor. Food and Nutritional Components in Focus No 9: Selenium: Chemistry, Analysis, Function and Effects. The Royal Society of Chemistry; 2015. pp. XXX–YYY. [Google Scholar]
- 129.Christov K, et al. Short-term modulation of cell proliferation and apoptosis and preventive/therapeutic efficacy of various agents in a mammary cancer model. Clin Cancer Res. 2007;13:5488–5496. doi: 10.1158/1078-0432.CCR-07-0404. [DOI] [PubMed] [Google Scholar]
- 130.Keaney JF, Solomon CG. Postmenopausal hormone therapy and atherosclerosis–time is of the essence. N Engl J Med. 2016;374:1279–1280. doi: 10.1056/NEJMe1602846. [DOI] [PubMed] [Google Scholar]
- 131.Umar A, et al. Further thoughts on preclinical animal models for cancer prevention: when is it best to start treatment? What are potential histopathologic endpoints? Semin Oncol. 2010;37:339–344. doi: 10.1053/j.seminoncol.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 132.Le Magnen C, et al. Optimizing mouse models for precision cancer prevention. Nat Rev Cancer. 2016;16:187–196. doi: 10.1038/nrc.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Eisenstein M. Vaccines: a moving target. Nature. 2011;474:S16–S17. doi: 10.1038/474S16a. [DOI] [PubMed] [Google Scholar]
- 134.Melnikow J, et al. Preferences of Women Evaluating Risks of Tamoxifen (POWER) study of preferences for tamoxifen for breast cancer risk reduction. Cancer. 2005;103:1996–2005. doi: 10.1002/cncr.20981. [DOI] [PubMed] [Google Scholar]
- 135.Waters EA, Cronin KA, Graubard BI, Han PK, Freedman AN, et al. Prevalence of tamoxifen use for breast cancer chemoprevention among U.S. women. Cancer Epidemiol Biomarkers Prev. 2010;19:443–446. doi: 10.1158/1055-9965.EPI-09-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Port ER, et al. Patient reluctance toward tamoxifen use for breast cancer primary prevention. Ann Surg Oncol. 2001;8:580–585. doi: 10.1007/s10434-001-0580-9. [DOI] [PubMed] [Google Scholar]
- 137.Gail MH, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91:1829–1846. doi: 10.1093/jnci/91.21.1829. [DOI] [PubMed] [Google Scholar]
- 138.Dunn GP, et al. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 139.Zitvogel L, et al. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]