Abstract
Brief periods of ischaemia followed by reperfusion of one tissue such as skeletal muscle can confer subsequent protection against ischaemia-induced injury in other organs such as the heart. Substantial evidence of this effect has been accrued in experimental animal models. However, the translation of this phenomenon to its use as a therapy in ischaemic disease has been largely disappointing without clear evidence of benefit in humans. Recently, innovative experimental observations have suggested that remote ischaemic preconditioning (RIPC) may be largely mediated through hypoxic inhibition of the oxygen-sensing enzyme PHD2, leading to enhanced levels of alpha-ketoglutarate and subsequent increases in circulating kynurenic acid (KYNA). These observations provide vital insights into the likely mechanisms of RIPC and a route to manipulating this mechanism towards therapeutic benefit by direct alteration of KYNA, alpha-ketoglutarate levels, PHD inhibition, or pharmacological targeting of the incompletely understood cardioprotective mechanism activated by KYNA.
Keywords: ischaemia, reperfusion, remote ischaemic preconditioning, cardioprotection
Introduction
Ischaemia followed by reperfusion of one tissue such as muscle can confer subsequent protection against ischaemia-induced injury in other organs such as the heart. Substantial evidence of this effect has been accrued in experimental animal models, but the translation to a therapy in ischaemic disease has not been definitively achieved in humans. Furthermore, experimental evidence for a large number of potential mediators and mechanisms has been obtained, but a clear understanding of the mechanisms is lacking. This commentary focuses on recent work examining a novel mechanism that may underlie remote ischaemic preconditioning (RIPC) 1.
What is ischaemic preconditioning?
Ischaemic preconditioning is the phenomenon whereby brief periods of ischaemia followed by tissue reperfusion confer subsequent protection against ischaemia-induced injury. The concept, proposed 30 years ago by Murry et al., demonstrated that brief cycles of ischaemia and reperfusion of the coronary arteries protect the myocardium from subsequent prolonged ischaemia and reperfusion, leading to a reduction in infarct size 2.
What is remote ischaemic preconditioning?
The concept was developed further with the observation that ischaemia in one coronary territory could protect cardiac tissue supplied by other epicardial arteries 3. Birnbaum et al. went on to demonstrate that “remote” transient ischaemia of non-myocardial tissues could also be associated with reductions in the extent of myocardial infarction. They combined partial reduction of blood flow to the hindlimb with increased oxygen demand by rapid electrical stimulation of the gastrocnemius muscle and showed reduced myocardial infarct size in rabbits 4. Subsequently, Kharbanda et al. showed similar beneficial effects in a porcine model of myocardial infarction and applied the concept of RIPC to healthy human volunteers by inducing transient non-invasive ischaemia with the use of a blood pressure cuff applied to one arm and demonstrated improved endothelial function in the contralateral arm 5.
Does it benefit patients?
The important clinical question has emerged of whether RIPC can be used therapeutically in the wide range of medical conditions in which ischaemic injury occurs. RIPC has been applied in elective cardiac surgery, vascular surgery, percutaneous coronary intervention, and organ transplantation in attempts to improve cardiac, renal, and other outcomes. Individual, small randomised controlled trials have been reported to show potential benefit 6– 9. Hu et al. undertook a systematic review of 30 randomised controlled trials to investigate the effects of RIPC on the incidence and outcomes of acute kidney injury (AKI) and found evidence of benefit in preventing contrast-induced AKI 10. However, there was not benefit in ischaemia reperfusion-induced AKI 10, and more recent trials have also failed to see clear benefit in that setting 11. The REmote preconditioning for Protection Against Ischaemia-Reperfusion in renal transplantation (REPAIR) trial found some evidence that RIPC using transient arm ischaemia-reperfusion improved renal transplant function 12.
In the setting of cardiac surgery, meta-analyses have not confirmed any therapeutic benefit from RIPC 13 nor have more recent larger-scale studies. The Effect of RIPC on Clinical Outcomes in Coronary Artery Bypass Graft (CABG) Surgery (ERICCA) study, a randomised controlled clinical trial in 1,612 patients, showed no effect of RIPC on clinical outcomes 14. RIPC consisting of four 5-minute cycles of ischaemia-reperfusion of the upper arm did not improve clinical outcomes in patients undergoing elective CABG. No differences were seen in mortality, stroke, myocardial infarction, or AKI. The RIPC for Heart Surgery (RIPHeart) trial of 1,385 patients used a similar upper limb ischaemia protocol but also failed to see benefit 15.
Overall, these results are disappointing but convincing in their failure to see a therapeutic benefit of RIPC in most patients. The optimum type, duration, and timing of the ischaemic intervention is uncertain; skeletal muscle mass, hepatic function, concurrent medications, choice of anaesthetic, and the effect on different target organs may also vary and influence the effect of the intervention. How can the benefits seen in experimental studies be translated to a useful therapy, and does RIPC operate in humans? Understanding the mechanism of effect might enable optimisation of the clinical use of RIPC.
What is the mechanism of remote ischaemic preconditioning?
Whilst definitive evidence for therapeutic benefit in humans is lacking, evidence that experimental manipulations can have a protective benefit is strong (for review, see 16). A large number of different mechanisms have been suggested, including roles for neurally mediated mechanisms and hormonal mediators (for selected examples, see Table 1), with a recent workshop suggesting that the mechanisms underlying RIPC remain unclear 17. Recent work has implicated the hypoxia response and the generation of circulating molecular mediators. Hypoxia is a central component of ischaemia, and the hypoxia-inducible factor (HIF) transcription factors play a dominant role in co-ordinating the transcriptional response to hypoxia. The abundance of the HIF-α factors is controlled by oxygen-dependent prolyl hydroxylation by the PHD family of 2-oxoglutarate dioxygenases 18– 20 (PHD1, 2, and 3, also known as EGLN2, 1, and 3, respectively) and their transcriptional potency by the FIH-1 asparaginyl hydroxylase 21, 22. Several studies have implicated the HIF–PHD system in the mechanism of RIPC. These include impaired RIPC in mice heterozygous for a knockout allele encoding HIF-1α 23, activation of HIF-1α by ischaemic preconditioning, and enhancement of cardiac protection by pharmacological and genetic enhancement of HIF-1α 24. Mice with genetically reduced levels of PHD2 (and hence enhanced HIF-1α levels) showed greater resistance to cardiac ischaemia 25, 26, as did animals with activation of HIF by pharmacological PHD inhibition or VHL deficiency 27, though other studies have suggested that HIF-1α upregulation is unnecessary in acute RIPC 28.
Table 1. Selected animal studies that have implicated potential mechanisms and mediators of remote ischaemic preconditioning of the heart (RIPC).
| Potential Mechanism/Mediator |
Species | RIPC model | Reference |
|---|---|---|---|
| Neurally mediated erythropoietin release |
Mice | Hindlimb ischaemia |
29 |
| MicroRNA-144 | Mice | Hindlimb ischaemia |
36 |
| Neurally mediated bradykinin release |
Rat | Mesenteric artery occlusion |
37 |
| Adenosine | Rat | Mesenteric artery occlusion |
38 |
| Bradykinin and epoxyeicosatrienoic acids |
Dog | Abdominal skin incision |
39 |
| Endogenous opioids | Rat | Mesenteric artery occlusion |
40 |
| SDF-1/CXCR4 | Rat | Hindlimb ischaemia |
31 |
| Adenosine and ATP- sensitive potassium (KATP) channels |
Rabbit | Renal ischaemia | 41 |
| Haem oxygenase-1 | Rat | Hindlimb ischaemia |
42 |
| Interleukin-10 | Mice | Hindlimb ischaemia |
23 |
| Nitrite | Mice | Hindlimb ischaemia |
43 |
| Apolipoprotein A-I | Rat | Hindlimb ischaemia |
44 |
| Glucagon-like peptide-1 |
Rat | Hindlimb ischaemia |
45 |
| Hypoxia inducible factor (HIF) |
Mice | Hindlimb ischaemia |
23 |
There are a broad array of HIF-mediated responses to hypoxia that might help mediate ischaemic preconditioning, including the promotion of anaerobic metabolism, vascularity, and vasodilatation, reactive oxygen species protection, and alterations in cell survival and cell cycle. Some of these HIF-dependent hypoxic responses include the release of circulating mediators by ischaemic tissue, such as its canonical target erythropoietin 29, 30 and others including CXCL12 (SDF-1) 31, that might act as circulating mediators of RIPC. Whilst these studies do suggest a role for the HIF–PHD system in RIPC, they have not fully disentangled the requirement for HIF activation in the remote ischaemic tissue versus that in the target protected organ nor the relative contributions of neural or hormonal mediators.
Is there a role for kynurenic acid as the mediator of remote ischaemic preconditioning?
A major insight into the mechanism of RIPC and the role of the HIF–PHD system and circulating mediators has come from the recent work of Kaelin and colleagues 1. They initially provided further evidence for the protective effects of HIF activation by showing that genetic and chronic PHD2 inactivation in mice hearts conferred protective benefit against permanent and transient cardiac ischemia. Similar beneficial effects were also seen with acute systemic PHD2 genetic inactivation and with systemic administration of a pharmacological PHD inhibitor. To determine whether manipulations of the HIF–PHD system in the remote ischaemic tissue (but not the target heart) affected RIPC, they studied mice with PHD2 inactivated only in skeletal muscle. Such mice again showed enhanced myocardial protection following ischaemia. They then undertook parabiotic experiments to provide important evidence that this protective effect was mediated by a circulating factor. To determine the nature of this circulating factor, they tested for cytokine and metabolite differences in the blood of mice with and without PHD2 skeletal muscle inactivation. No significant changes were seen in cytokines or molecules such as erythropoietin, which has previously been suggested to act as a circulating mediator of RIPC. Similarly, no plausible secreted candidates were identified from genetic expression analyses between mice with and without PHD2 skeletal muscle inactivation. However, when blood was compared by analysis with liquid chromatography and mass spectroscopy, significant differences in tryptophan metabolites were observed. Similar alterations were also seen in blood shortly after pharmacological PHD inhibition with significant elevations in the level of the tryptophan metabolite kynurenic acid (KYNA).
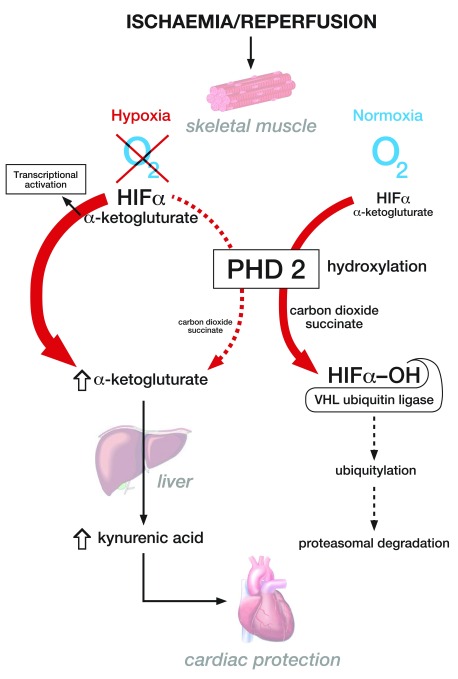
Further evidence implicating KYNA as a mediator of ischaemic preconditioning were obtained by abrogating RIPC with inhibitors of the tryptophan pathway and from the beneficial effects of administration of KYNA itself. Studies were then undertaken to explore the mechanism by which PHD2 inactivation in muscle resulted in increases in circulating KYNA and mediation via an increase in levels of the obligatory PHD co-substrate alpha-ketoglutarate with subsequent hepatic generation of KYNA ( Figure 1). Systemic alpha-ketoglutarate administration also protected hearts from ischaemia-reperfusion injury. PHD2 inhibition appeared to increase alpha-ketoglutarate levels as a consequence of its reduced decarboxylation, with evidence provided of a high rate of PHD2-dependent alpha-ketoglutarate conversion to succinate. This is superficially surprising given its well-understood role as an oxygen-sensing enzyme as opposed to one with significant roles in metabolic flux. Some support for a role for the kynurenine pathway in the mechanism of RIPC has been provided by studies in humans and rats in which circulating metabolites, including kynurenine and glycine, that demonstrated elevated levels after RIPC were injected prior to myocardial infarction and had a protective effect 32.
Figure 1. Schematic illustration of the pathways involved in enhanced kynurenic acid (KYNA) generation by the hypoxia-inducible factor (HIF) hydroxylase PHD2 during hypoxia.
The figure demonstrates the mechanism by which muscle hypoxia results in the inhibition of PHD2 function leading to enhanced alpha-ketoglutarate generation and kynurenic acid production, which may mediate a cardioprotective effect. It also shows the canonical role of PHD2 in normoxia in the oxygen-dependent degradation of the transcription factor HIF. HIFα, hypoxia inducible factor α; PHD2, prolyl hydroxylase domain 2; VHL, von Hippel Lindau.
Conclusions
These findings provide vital insights into a potential mechanism of RIPC and generate intriguing questions ( Box 1). Notably, what is the mechanism of the cardioprotective effect and does it operate in other tissues? Is it mediated through metabolic effects, via effects on specific G-protein-coupled receptors 33, or by the known influence of KYNA on the aryl hydrocarbon receptor (AHR) response 34 (which shares with the HIF pathway the heterodimeric transcription factor AHR nuclear translocator [ARNT])? Whilst protective effects of AHR activation have been suggested in some models of ischaemia, in others activation of the AHR response by tryptophan metabolites can have deleterious effects 35.
Box 1. Outstanding questions concerning remote ischaemia preconditioning (RIPC) and the role of kynurenic acid (KYNA).
What is the mechanism of the cardioprotective effect, and does it operate in other tissues?
Can the manipulation of KYNA or alpha-ketoglutarate levels or direct pharmacological targeting of the cardioprotective mechanism activated by KYNA produce therapeutic benefit in patients with ischaemic diseases?
What mass of tissue ischaemia is necessary to achieve sufficient perturbations in the levels of circulating KYNA?
Will the emerging PHD inhibitors currently being trialled for their erythropoietic effect 46 have protective benefits?
Do the other known influences on PHD function, such as oxygen availability, iron and ascorbate, or perturbations of alpha-ketoglutarate metabolism, influence protective mechanisms via this effect in vivo?
What effect is produced by acute versus chronic elevations in the levels of such molecules, and to what extent do metabolic compensations or the complex feedback loops operating in the PHD–hypoxia-inducible factor (HIF) system affect the operation of RIPC?
Does ischaemia operate locally to mediate protective effects through this mechanism?
Does this mechanism operate in other situations, such as hypoxic tumours?
Are there associations between levels of KYNA and outcomes in ischaemic diseases?
Is KYNA the dominant mediator of RIPC in humans, or are other mediators/mechanisms more important?
What is the relative importance of this newly defined mechanism of RIPC to other pathways, how is it related to neurally mediated effects, and how do they interact? Can manipulation of KYNA or alpha-ketoglutarate levels or direct pharmacological targeting of the cardioprotective mechanism activated by KYNA produce therapeutic benefit in patients with ischaemic diseases? In contrast to the impressive protective benefits seen in the work of Olenchock and colleagues 1 and other animal studies, does the failure of RIPC to achieve improved clinical outcomes reflect inadequate suppression of PHD2 activity and/or insufficient increases in levels of KYNA? Improved understanding of the transduction of the RIPC signal from remote tissue to protected target may now allow improvements in clinical strategies to deliver the enormous potential benefits of RIPC and the development of new pharmacological approaches that directly activate the protective pathway.
Acknowledgements
Thanks to David Heinrich for his expertise in the preparation of Figure 1.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Tienush Rassaf, Department for Cardiology and Vascular Medicine (T.R.); Gerd Heusch - Institute for Pathophysiology (G.H.) and West German Heart and Vascular Center, University School of Medicine Essen, Essen, Germany
Fabrice Prunier, Institut MITOVASC, Service de Cardiologie, CHU Angers, Université d’Angers, Angers, France
Alexander Gourine, Centre for Cardiovascular and Metabolic Neuroscience, Neuroscience, Physiology and Pharmacology, University College London, London, UK
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 3 approved]
References
- 1. Olenchock BA, Moslehi J, Baik AH, et al. : EGLN1 Inhibition and Rerouting of α-Ketoglutarate Suffice for Remote Ischemic Protection. Cell. 2016;164(5):884–95. 10.1016/j.cell.2016.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murry CE, Jennings RB, Reimer KA: Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–36. 10.1161/01.CIR.74.5.1124 [DOI] [PubMed] [Google Scholar]
- 3. Przyklenk K, Bauer B, Ovize M, et al. : Regional ischemic 'preconditioning' protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87(3):893–9. 10.1161/01.CIR.87.3.893 [DOI] [PubMed] [Google Scholar]
- 4. Birnbaum Y, Hale SL, Kloner RA: Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96(5):1641–6. 10.1161/01.CIR.96.5.1641 [DOI] [PubMed] [Google Scholar]
- 5. Kharbanda RK, Mortensen UM, White PA, et al. : Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106(23):2881–3. 10.1161/01.CIR.0000043806.51912.9B [DOI] [PubMed] [Google Scholar]
- 6. Candilio L, Malik A, Ariti C, et al. : Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing cardiac bypass surgery: a randomised controlled clinical trial. Heart. 2015;101(3):185–92. 10.1136/heartjnl-2014-306178 [DOI] [PubMed] [Google Scholar]
- 7. Thielmann M, Kottenberg E, Kleinbongard P, et al. : Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382(9892):597–604. 10.1016/S0140-6736(13)61450-6 [DOI] [PubMed] [Google Scholar]
- 8. Hoole SP, Heck PM, Sharples L, et al. : Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119(6):820–7. 10.1161/CIRCULATIONAHA.108.809723 [DOI] [PubMed] [Google Scholar]
- 9. Bøtker HE, Kharbanda R, Schmidt MR, et al. : Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375(9716):727–34. 10.1016/S0140-6736(09)62001-8 [DOI] [PubMed] [Google Scholar]
- 10. Hu J, Liu S, Jia P, et al. : Protection of remote ischemic preconditioning against acute kidney injury: a systematic review and meta-analysis. Crit Care. 2016;20(1):111. 10.1186/s13054-016-1272-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gallagher SM, Jones DA, Kapur A, et al. : Remote ischemic preconditioning has a neutral effect on the incidence of kidney injury after coronary artery bypass graft surgery. Kidney Int. 2015;87(2):473–81. 10.1038/ki.2014.259 [DOI] [PubMed] [Google Scholar]
- 12. MacAllister R, Clayton T, Knight R, et al. : REmote preconditioning for Protection Against Ischaemia–Reperfusion in renal transplantation (REPAIR): a multicentre, multinational, double-blind, factorial designed randomised controlled trial.2015. 10.3310/eme02030 [DOI] [PubMed] [Google Scholar]
- 13. Remote Preconditioning Trialists' Group, . Healy DA, Khan WA, et al. : Remote preconditioning and major clinical complications following adult cardiovascular surgery: systematic review and meta-analysis. Int J Cardiol. 2014;176(1):20–31. 10.1016/j.ijcard.2014.06.018 [DOI] [PubMed] [Google Scholar]
- 14. Hausenloy DJ, Candilio L, Evans R, et al. : Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N Engl J Med. 2015;373(15):1408–17. 10.1056/NEJMoa1413534 [DOI] [PubMed] [Google Scholar]
- 15. Meybohm P, Bein B, Brosteanu O, et al. : A Multicenter Trial of Remote Ischemic Preconditioning for Heart Surgery. N Engl J Med. 2015;373(15):1397–407. 10.1056/NEJMoa1413579 [DOI] [PubMed] [Google Scholar]
- 16. Heusch G, Bøtker HE, Przyklenk K, et al. : Remote ischemic conditioning. J Am Coll Cardiol. 2015;65(2):177–95. 10.1016/j.jacc.2014.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pickard JM, Botker HE, Crimi G, et al. : Remote ischemic conditioning: from experimental observation to clinical application: report from the 8th Biennial Hatter Cardiovascular Institute Workshop. Basic Res Cardiol. 2015;110(1):453. 10.1007/s00395-014-0453-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Epstein AC, Gleadle JM, McNeill LA, et al. : C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107(1):43–54. 10.1016/S0092-8674(01)00507-4 [DOI] [PubMed] [Google Scholar]
- 19. Bruick RK, McKnight SL: A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294(5545):1337–40. 10.1126/science.1066373 [DOI] [PubMed] [Google Scholar]
- 20. Ivan M, Haberberger T, Gervasi DC, et al. : Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci U S A. 2002;99(21):13459–64. 10.1073/pnas.192342099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lando D, Peet DJ, Gorman JJ, et al. : FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16(12):1466–71. 10.1101/gad.991402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hewitson KS, McNeill LA, Riordan MV, et al. : Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J Biol Chem. 2002;277(29):26351–5. 10.1074/jbc.C200273200 [DOI] [PubMed] [Google Scholar]
- 23. Cai Z, Luo W, Zhan H, et al. : Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc Natl Acad Sci U S A. 2013;110(43):17462–7. 10.1073/pnas.1317158110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eckle T, Köhler D, Lehmann R, et al. : Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118(2):166–75. 10.1161/CIRCULATIONAHA.107.758516 [DOI] [PubMed] [Google Scholar]
- 25. Hyvärinen J, Hassinen IE, Sormunen R, et al. : Hearts of hypoxia-inducible factor prolyl 4-hydroxylase-2 hypomorphic mice show protection against acute ischemia-reperfusion injury. J Biol Chem. 2010;285(18):13646–57. 10.1074/jbc.M109.084855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hölscher M, Silter M, Krull S, et al. : Cardiomyocyte-specific prolyl-4-hydroxylase domain 2 knock out protects from acute myocardial ischemic injury. J Biol Chem. 2011;286(13):11185–94. 10.1074/jbc.M110.186809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ong SG, Lee WH, Theodorou L, et al. : HIF-1 reduces ischaemia-reperfusion injury in the heart by targeting the mitochondrial permeability transition pore. Cardiovasc Res. 2014;104(1):24–36. 10.1093/cvr/cvu172 [DOI] [PubMed] [Google Scholar]
- 28. Kalakech H, Tamareille S, Pons S, et al. : Role of hypoxia inducible factor-1α in remote limb ischemic preconditioning. J Mol Cell Cardiol. 2013;65:98–104. 10.1016/j.yjmcc.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 29. Oba T, Yasukawa H, Nagata T, et al. : Renal Nerve-Mediated Erythropoietin Release Confers Cardioprotection During Remote Ischemic Preconditioning. Circ J. 2015;79(7):1557–67. 10.1253/circj.CJ-14-1171 [DOI] [PubMed] [Google Scholar]
- 30. Diwan V, Jaggi AS, Singh M, et al. : Possible involvement of erythropoietin in remote renal preconditioning-induced cardioprotection in rats. J Cardiovasc Pharmacol. 2008;51(2):126–30. 10.1097/FJC.0b013e31815d88c9 [DOI] [PubMed] [Google Scholar]
- 31. Davidson SM, Selvaraj P, He D, et al. : Remote ischaemic preconditioning involves signalling through the SDF-1α/CXCR4 signalling axis. Basic Res Cardiol. 2013;108(5):377. 10.1007/s00395-013-0377-6 [DOI] [PubMed] [Google Scholar]
- 32. Chao de la Barca JM, Bakhta O, Kalakech H, et al. : Metabolic Signature of Remote Ischemic Preconditioning Involving a Cocktail of Amino Acids and Biogenic Amines. J Am Heart Assoc. 2016;5(9): pii: e003891. 10.1161/JAHA.116.003891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang J, Simonavicius N, Wu X, et al. : Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem. 2006;281(31):22021–8. 10.1074/jbc.M603503200 [DOI] [PubMed] [Google Scholar]
- 34. DiNatale BC, Murray IA, Schroeder JC, et al. : Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115(1):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cuartero MI, Ballesteros I, de La Parra J, et al. : L-kynurenine/aryl hydrocarbon receptor pathway mediates brain damage after experimental stroke. Circulation. 2014;130(23):2040–51. 10.1161/CIRCULATIONAHA.114.011394 [DOI] [PubMed] [Google Scholar]
- 36. Li J, Rohailla S, Gelber N, et al. : MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol. 2014;109(5):423. 10.1007/s00395-014-0423-z [DOI] [PubMed] [Google Scholar]
- 37. Schoemaker RG, van Heijningen CL: Bradykinin mediates cardiac preconditioning at a distance. Am J Physiol Heart Circ Physiol. 2000;278(5):H1571–6. [DOI] [PubMed] [Google Scholar]
- 38. Liem DA, Verdouw PD, Ploeg H, et al. : Sites of action of adenosine in interorgan preconditioning of the heart. Am J Physiol Heart Circ Physiol. 2002;283(1):H29–37. 10.1152/ajpheart.01031.2001 [DOI] [PubMed] [Google Scholar]
- 39. Gross GJ, Baker JE, Moore J, et al. : Abdominal surgical incision induces remote preconditioning of trauma (RPCT) via activation of bradykinin receptors (BK2R) and the cytochrome P450 epoxygenase pathway in canine hearts. Cardiovasc Drugs Ther. 2011;25(6):517–22. 10.1007/s10557-011-6321-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patel HH, Moore J, Hsu AK, et al. : Cardioprotection at a distance: mesenteric artery occlusion protects the myocardium via an opioid sensitive mechanism. J Mol Cell Cardiol. 2002;34(10):1317–23. 10.1006/jmcc.2002.2072 [DOI] [PubMed] [Google Scholar]
- 41. Pell TJ, Baxter GF, Yellon DM, et al. : Renal ischemia preconditions myocardium: role of adenosine receptors and ATP-sensitive potassium channels. Am J Physiol. 1998;275(5 Pt 2):H1542–7. [DOI] [PubMed] [Google Scholar]
- 42. Zhou C, Li L, Li H, et al. : Delayed remote preconditioning induces cardioprotection: role of heme oxygenase-1. J Surg Res. 2014;191(1):51–7. 10.1016/j.jss.2014.03.054 [DOI] [PubMed] [Google Scholar]
- 43. Rassaf T, Totzeck M, Hendgen-Cotta UB, et al. : Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114(10):1601–10. 10.1161/CIRCRESAHA.114.303822 [DOI] [PubMed] [Google Scholar]
- 44. Hibert P, Prunier-Mirebeau D, Beseme O, et al. : Apolipoprotein a-I is a potential mediator of remote ischemic preconditioning. PLoS one. 2013;8(10):e77211. 10.1371/journal.pone.0077211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Basalay MV, Mastitskaya S, Mrochek A, et al. : Glucagon-like peptide-1 (GLP-1) mediates cardioprotection by remote ischaemic conditioning. Cardiovasc Res. 2016; pii: cvw216. 10.1093/cvr/cvw216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lenihan CR, Winkelmayer WC: The Dawning of a New Day in CKD Anemia Care? J Am Soc Nephrol. 2016;27(4):968–70. 10.1681/ASN.2015091009 [DOI] [PMC free article] [PubMed] [Google Scholar]